Exploring the Potential Link between Acute Central Serous Chorioretinopathy and Trimethylamine N-Oxide, Phoenixin, Spexin, and Alarin Molecules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Measurement of Molecules with ELISA
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaye, R.; Chandra, S.; Sheth, J.; Boon, C.J.; Sivaprasad, S.; Lotery, A. Central serous chorioretinopathy: An update on risk factors, pathophysiology and imaging modalities. Prog. Retin. Eye Res. 2020, 79, 100865. [Google Scholar] [CrossRef] [PubMed]
- Celik, F.; Coteli, E.; Gul, F.; Ozsoy, E.; Kobat, S.G.; Akkoc, R.; Yardim, M.; Sahin, I.; Yalcin, M.; Aydin, S. Laboratory evidence on a direct correlation between acute central serous chorioretinopathy and tenascin C, metalloprotein 1, BAX, BCL2, subfatin and asprosin. J. Français D’ophtalmologie 2022, 45, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Schneider, U.; Gelisken, F.; Inhoffen, W. Klinische Charakteristika der idiopathischen polipoiden choroidalen Vaskulopathie. Der Ophthalmol. 2001, 98, 1186–1191. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Tripathy, K. Central Serous Chorioretinopathy. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Daruich, A.; Matet, A.; Dirani, A.; Bousquet, E.; Zhao, M.; Farman, N.; Jaisser, F.; Behar-Cohen, F. Central serous chorioretinopathy: Recent findings and new physiopathology hypothesis. Prog. Retin. Eye Res. 2015, 48, 82–118. [Google Scholar] [CrossRef] [PubMed]
- Liew, G.; Quin, G.; Gillies, M.; Fraser-Bell, S. Central serous chorioretinopathy: A review of epidemiology and pathophysiology. Clin. Exp. Ophthalmol. 2013, 41, 201–214. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, J.; Li, D. Functions and diseases of the retinal pigment epithelium. Front. Pharmacol. 2021, 12, 727870. [Google Scholar] [CrossRef]
- Chrapek, O.; Spackova, K.; Rehak, J. Lecba centralni serozni chorioretinopatie betablokatory. Czech Slovak Ophthalmol. 2002, 58, 382–386. [Google Scholar]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current understanding of dysbiosis in disease in human and animal models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef]
- Gatarek, P.; Kaluzna-Czaplinska, J. Trimethylamine N-oxide (TMAO) in human health. EXCLI J. 2021, 20, 301. [Google Scholar]
- Rajakovich, L.J.; Fu, B.; Bollenbach, M.; Balskus, E.P. Elucidation of an anaerobic pathway for metabolism of l-carnitine–derived γ-butyrobetaine to trimethylamine in human gut bacteria. Proc. Natl. Acad. Sci. USA 2021, 118, e2101498118. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, Y.J.; Won, J.Y. Molecular mechanisms of retinal pigment epithelium dysfunction in age-related macular degeneration. Int. J. Mol. Sci. 2021, 22, 12298. [Google Scholar] [CrossRef] [PubMed]
- Yosten, G.L.; Lyu, R.M.; Hsueh, A.J.; Avsian-Kretchmer, O.; Chang, J.K.; Tullock, C.W.; Dun, S.; Dun, N.; Samson, W.K. A novel reproductive peptide, phoenixin. J. Neuroendocrinol. 2013, 25, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Zhao, Q.; Lv, S.; Ji, X. Regulation and physiological functions of phoenixin. Front. Mol. Biosci. 2022, 9, 956500. [Google Scholar] [CrossRef]
- Hayashida, K.; Bartlett, A.H.; Chen, Y.; Park, P.W. Molecular and cellular mechanisms of ectodomain shedding. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2010, 293, 925–937. [Google Scholar] [CrossRef]
- Prinz, P.; Scharner, S.; Friedrich, T.; Schalla, M.; Goebel-Stengel, M.; Rose, M.; Stengel, A. Central and peripheral expression sites of phoenixin-14 immunoreactivity in rats. Biochem. Biophys. Res. Commun. 2017, 493, 195–201. [Google Scholar] [CrossRef]
- Billert, M.; Rak, A.; Nowak, K.W.; Skrzypski, M. Phoenixin: More than reproductive peptide. Int. J. Mol. Sci. 2020, 21, 8378. [Google Scholar] [CrossRef]
- Zhang, B.; Li, J. Phoenixin-14 protects human brain vascular endothelial cells against oxygen-glucose deprivation/reoxygenation (OGD/R)-induced inflammation and permeability. Arch. Biochem. Biophys. 2020, 682, 108275. [Google Scholar] [CrossRef]
- Mirabeau, O.; Perlas, E.; Severini, C.; Audero, E.; Gascuel, O.; Possenti, R.; Birney, E.; Rosenthal, N.; Gross, C. Identification of novel peptide hormones in the human proteome by hidden Markov model screening. Genome Res. 2007, 17, 320–327. [Google Scholar] [CrossRef]
- Lim, C.H.; Lee, M.Y.M.; Soga, T.; Parhar, I. Evolution of structural and functional diversity of spexin in mammalian and non-mammalian vertebrate species. Front. Endocrinol. 2019, 10, 379. [Google Scholar] [CrossRef]
- Gu, L.; Ma, Y.; Gu, M.; Zhang, Y.; Yan, S.; Li, N.; Wang, Y.; Ding, X.; Yin, J.; Fan, N. Spexin peptide is expressed in human endocrine and epithelial tissues and reduced after glucose load in type 2 diabetes. Peptides 2015, 71, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Alcaraz, A.; Lee, Y.-N.; Yun, S.; Hwang, J.-I.; Seong, J.Y. Conformational signatures in β-arrestin2 reveal natural biased agonism at a G-protein-coupled receptor. Commun. Biol. 2018, 1, 128. [Google Scholar] [CrossRef] [PubMed]
- Santic, R.; Schmidhuber, S.M.; Lang, R.; Rauch, I.; Voglas, E.; Eberhard, N.; Bauer, J.W.; Brain, S.D.; Kofler, B. Alarin is a vasoactive peptide. Proc. Natl. Acad. Sci. USA 2007, 104, 10217–10222. [Google Scholar] [CrossRef] [PubMed]
- Abebe, E.C.; Mengstie, M.A.; Seid, M.A.; Malik, T.; Dejenie, T.A. The evolving roles of alarin in physiological and disease conditions, and its future potential clinical implications. Front. Endocrinol. 2022, 13, 1028982. [Google Scholar] [CrossRef] [PubMed]
- Gül, F.C.; Kobat, S.G.; Çelik, F.; Aydin, S.; Akkoç, R.F. Plasma and aqueous levels of alarin and adipsin in patients with and without diabetic retinopathy. BMC Ophthalmol. 2022, 22, 176. [Google Scholar] [CrossRef] [PubMed]
- Celik, F.; Aydin, S. Blood and aqueous humor phoenixin, endocan and spexin in patients with diabetes mellitus and cataract with and without diabetic retinopathy. Peptides 2022, 150, 170728. [Google Scholar] [CrossRef] [PubMed]
- Aksoyalp, Z.S.; Erdogan, B.R.; Aksun, S. Optimization of enzyme-linked immunosorbent assay kit protocol to detect trimethylamine N-oxide levels in humans. EXCLI J. 2023, 22, 263. [Google Scholar]
- Zarnegar, A.; Ong, J.; Matsyaraja, T.; Arora, S.; Chhablani, J. Pathomechanisms in central serous chorioretinopathy: A recent update. Int. J. Retin. Vitr. 2023, 9, 3. [Google Scholar] [CrossRef]
- Macpherson, M.E.; Hov, J.R.; Ueland, T.; Dahl, T.B.; Kummen, M.; Otterdal, K.; Holm, K.; Berge, R.K.; Mollnes, T.E.; Trøseid, M. Gut microbiota-dependent trimethylamine N-oxide associates with inflammation in common variable immunodeficiency. Front. Immunol. 2020, 11, 574500. [Google Scholar] [CrossRef]
- Matet, A.; Jaworski, T.; Bousquet, E.; Canonica, J.; Gobeaux, C.; Daruich, A.; Zhao, M.; Zola, M.; Meester-Smoor, M.; Mohabati, D. Lipocalin 2 as a potential systemic biomarker for central serous chorioretinopathy. Sci. Rep. 2020, 10, 20175. [Google Scholar] [CrossRef]
- Eom, Y.; Oh, J.; Kim, S.-W.; Huh, K. Systemic factors associated with central serous chorioretinopathy in Koreans. Korean J. Ophthalmol. 2012, 26, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Shui, Y.; Cui, Y.; Tang, C.; Wang, X.; Qiu, X.; Hu, W.; Fei, L.; Li, Y.; Zhang, S. Gut microbiota dependent trimethylamine N-oxide aggravates angiotensin II–induced hypertension. Redox Biol. 2021, 46, 102115. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.Z.; Mackay, C.R.; Kaye, D.M. Beyond gut feelings: How the gut microbiota regulates blood pressure. Nat. Rev. Cardiol. 2018, 15, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, T.; Schalla, M.; Lommel, R.; Goebel-Stengel, M.; Kobelt, P.; Rose, M.; Stengel, A. Restraint stress increases the expression of phoenixin immunoreactivity in rat brain nuclei. Brain Res. 2020, 1743, 146904. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Li, Y.; Ma, S.; Tang, Y.; Li, H. Phoenixin-20 ameliorates lipopolysaccharide-induced activation of microglial NLRP3 inflammasome. Neurotox. Res. 2020, 38, 785–792. [Google Scholar] [CrossRef]
- Friedrich, T.; Stengel, A. Role of the novel peptide phoenixin in stress response and possible interactions with nesfatin-1. Int. J. Mol. Sci. 2021, 22, 9156. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, B.; Yang, S.; Tang, X.; Wang, J.; Wei, D. The protective effects of phoenixin-14 against lipopolysaccharide-induced inflammation and inflammasome activation in astrocytes. Inflamm. Res. 2020, 69, 779–787. [Google Scholar] [CrossRef]
- Daruich, A.; Sauvain, J.-J.; Matet, A.; Eperon, S.; Schweizer, C.; Berthet, A.; Danuser, B.; Behar-Cohen, F. Levels of the oxidative stress biomarker malondialdehyde in tears of patients with central serous chorioretinopathy relate to disease activity. Mol. Vis. 2020, 26, 722. [Google Scholar]
- Li, J.; Ding, H.; Li, Y.; Zhou, H.; Wang, W.; Mei, Y.; Zhang, R. Alarin alleviated cardiac fibrosis via attenuating oxidative stress in heart failure rats. Amino Acids 2021, 53, 1079–1089. [Google Scholar] [CrossRef]
- Mikó, A.; Füredi, N.; Tenk, J.; Rostás, I.; Soós, S.; Solymár, M.; Székely, M.; Balaskó, M.; Brunner, S.M.; Kofler, B. Acute central effects of alarin on the regulation on energy homeostasis. Neuropeptides 2017, 64, 117–122. [Google Scholar] [CrossRef]
- Yu, M.; Ju, M.; Fang, P.; Zhang, Z. Emerging central and peripheral actions of spexin in feeding behavior, leptin resistance and obesity. Biochem. Pharmacol. 2022, 202, 115121. [Google Scholar] [CrossRef] [PubMed]
- Daruich, A.; Matet, A.; Moulin, A.; Kowalczuk, L.; Nicolas, M.; Sellam, A.; Rothschild, P.-R.; Omri, S.; Gélizé, E.; Jonet, L. Mechanisms of macular edema: Beyond the surface. Prog. Retin. Eye Res. 2018, 63, 20–68. [Google Scholar] [CrossRef] [PubMed]
- Sassek, M.; Kolodziejski, P.A.; Szczepankiewicz, D.; Pruszynska-Oszmalek, E. Spexin in the physiology of pancreatic islets—Mutual interactions with insulin. Endocrine 2019, 63, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.-Y.; Zhou, Y.-C.; Zhang, X.-M.; Chen, W.-D.; Wang, Y.-D. Emerging roles of NPQ/spexin in physiology and pathology. Front. Pharmacol. 2019, 10, 457. [Google Scholar] [CrossRef]
- El-Saka, M.H.; Abo El Gheit, R.E.; El Saadany, A.; Alghazaly, G.M.; Marea, K.E.; Madi, N.M. Effect of spexin on renal dysfunction in experimentally obese rats: Potential mitigating mechanisms via galanin receptor-2. Arch. Physiol. Biochem. 2023, 129, 933–942. [Google Scholar] [CrossRef]
- Mohan, A.; Soman, M.; Nair, R.U. Commentary: Our understanding of central serous chorioretinopathy—Coming a full circle? Indian J. Ophthalmol. 2020, 68, 858. [Google Scholar] [CrossRef]
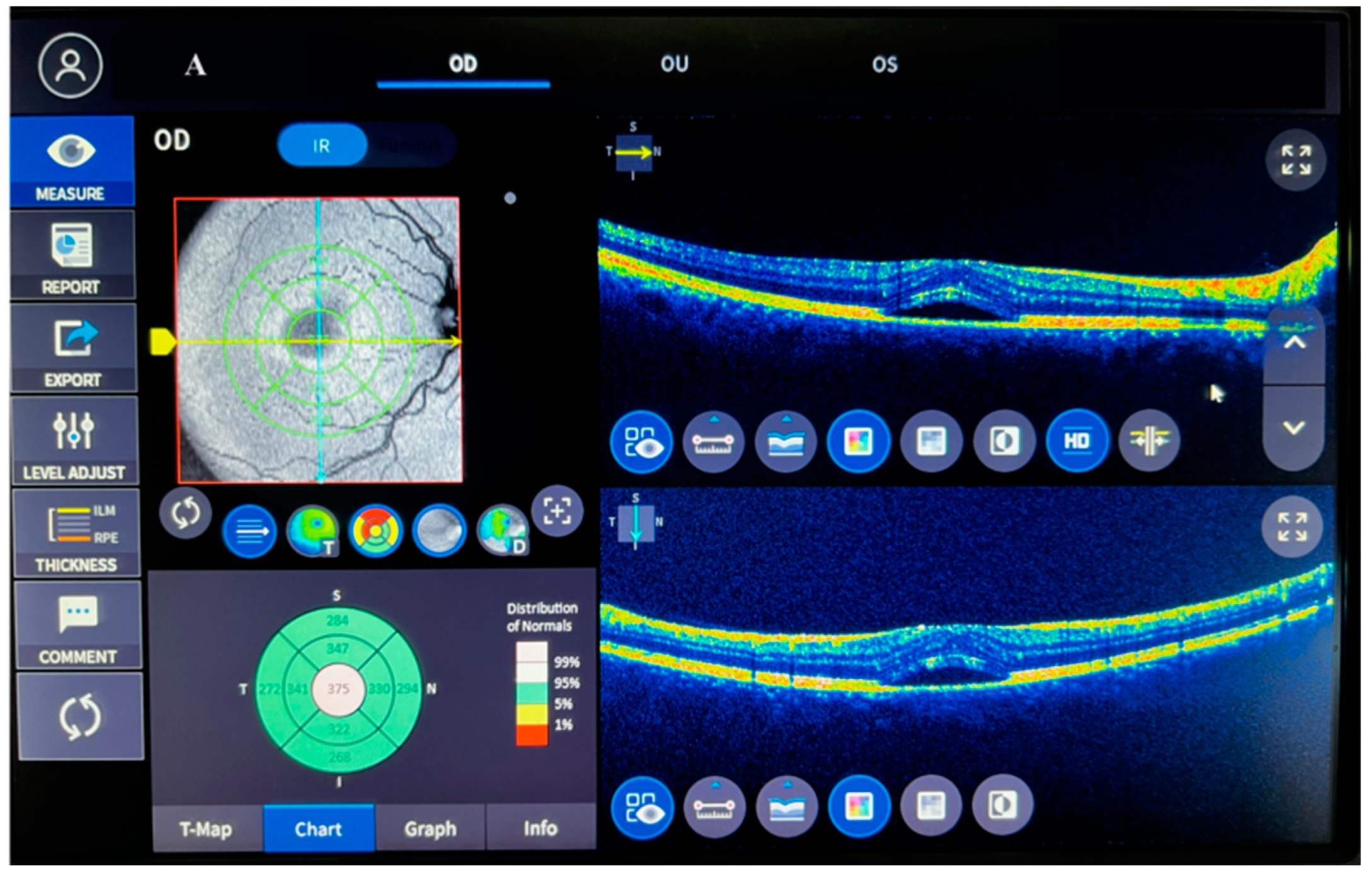
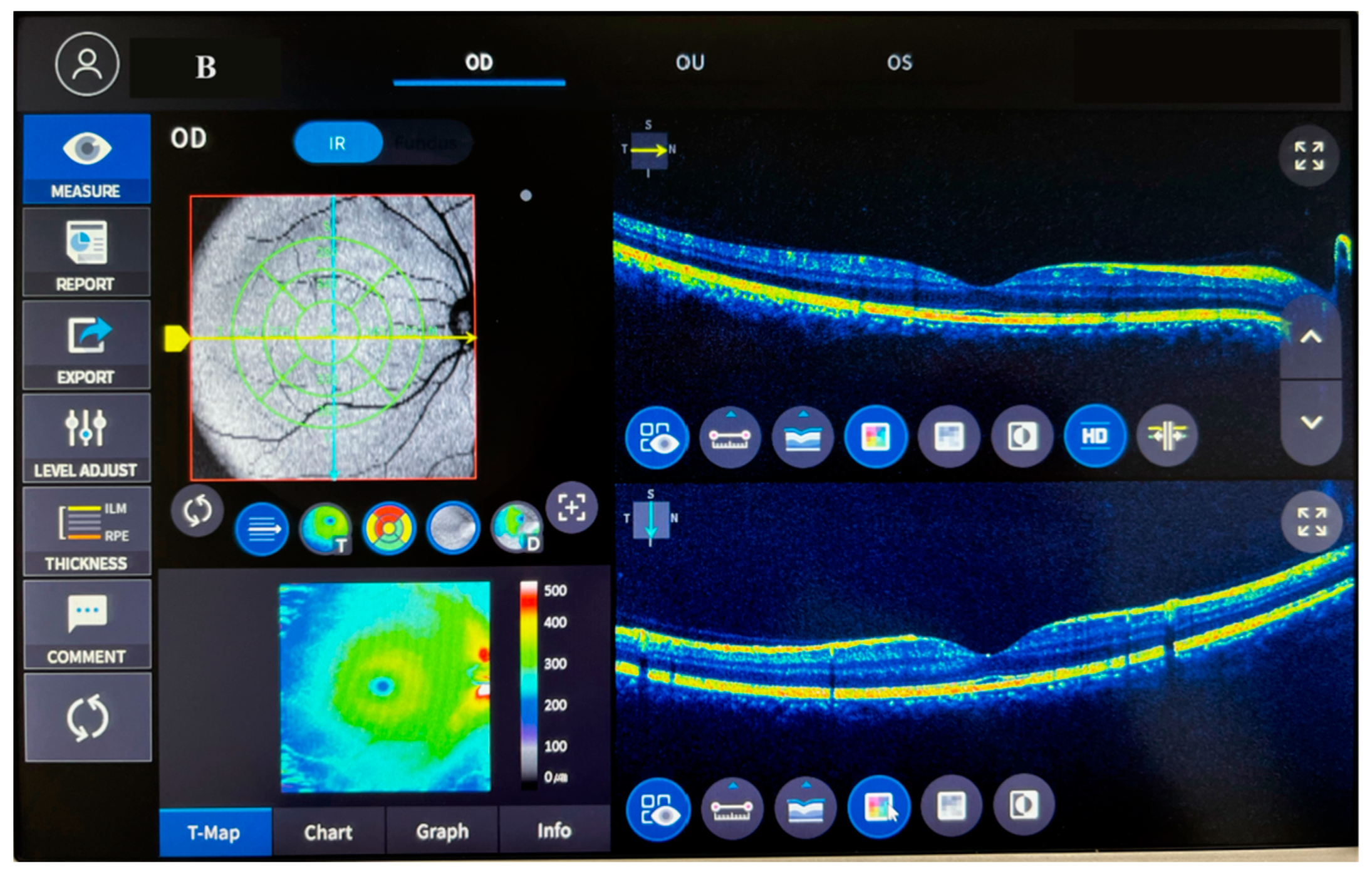
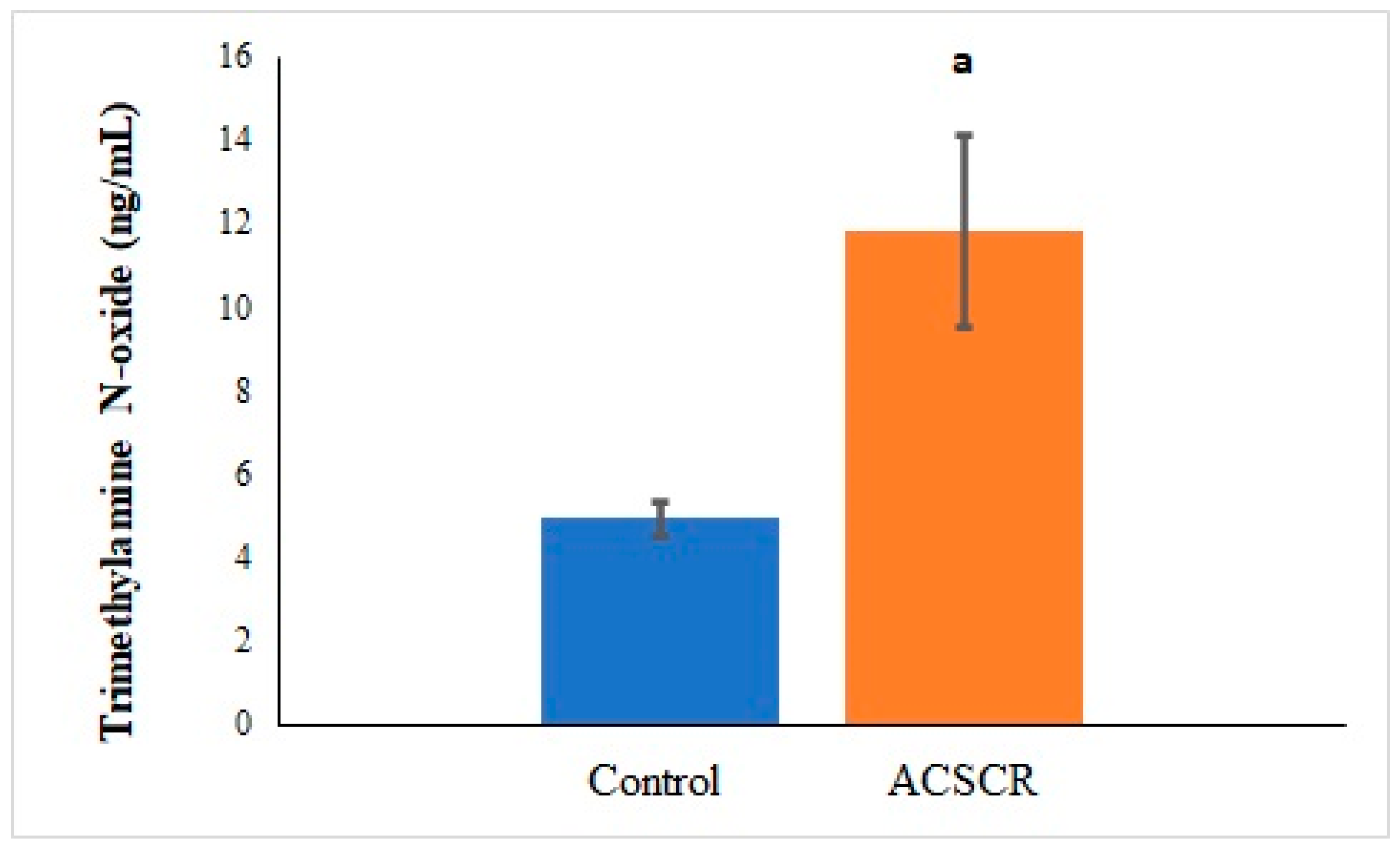
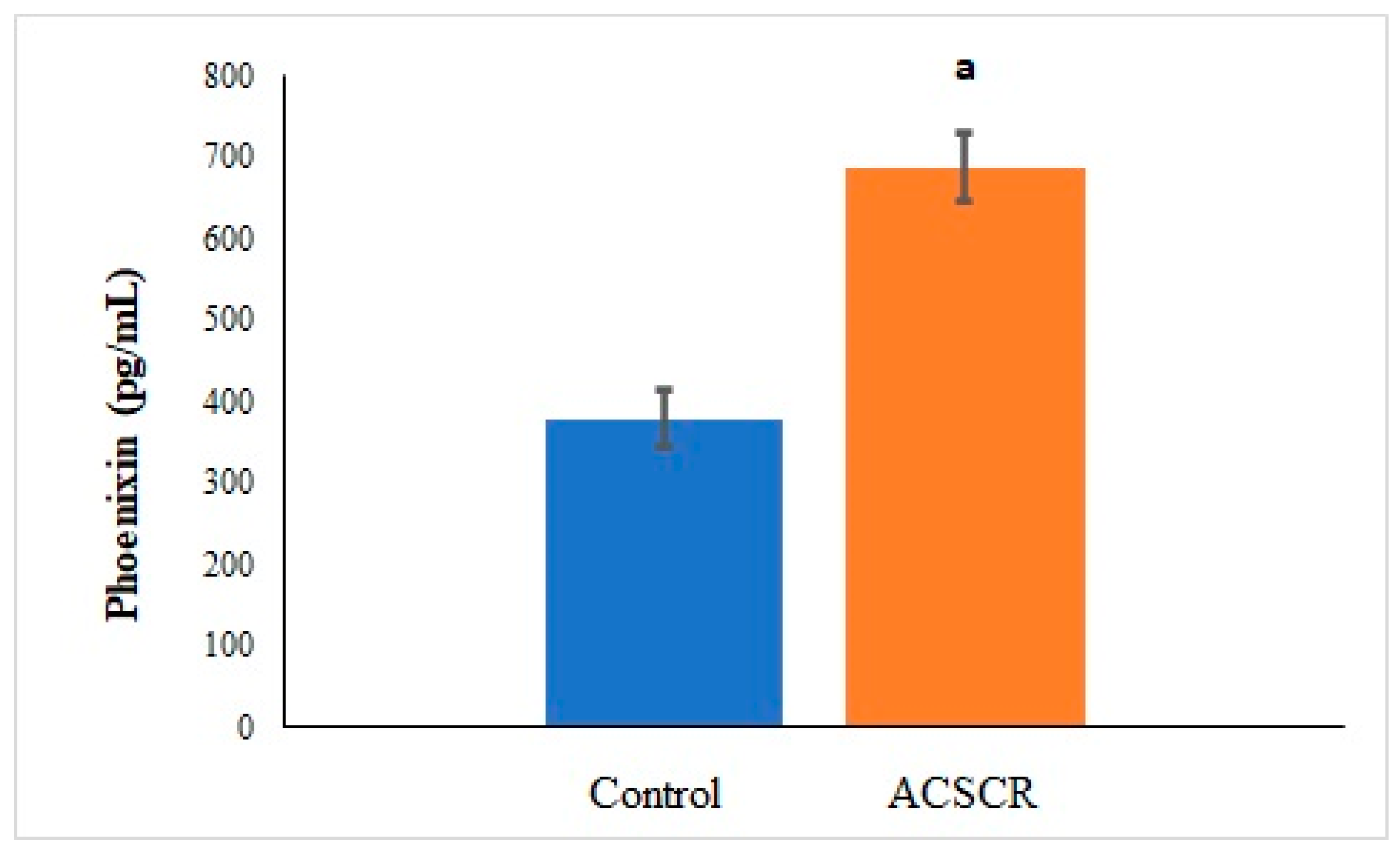
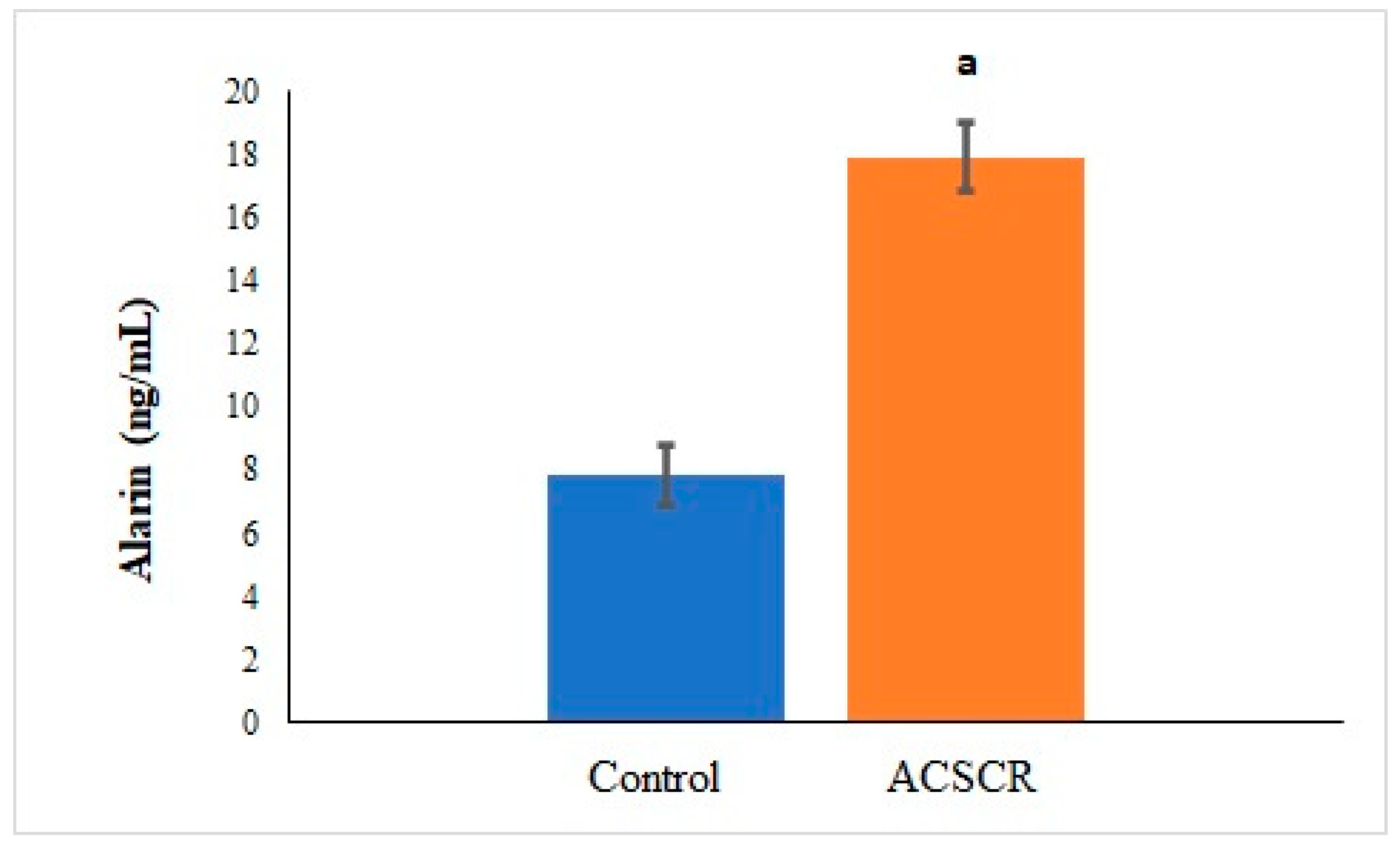
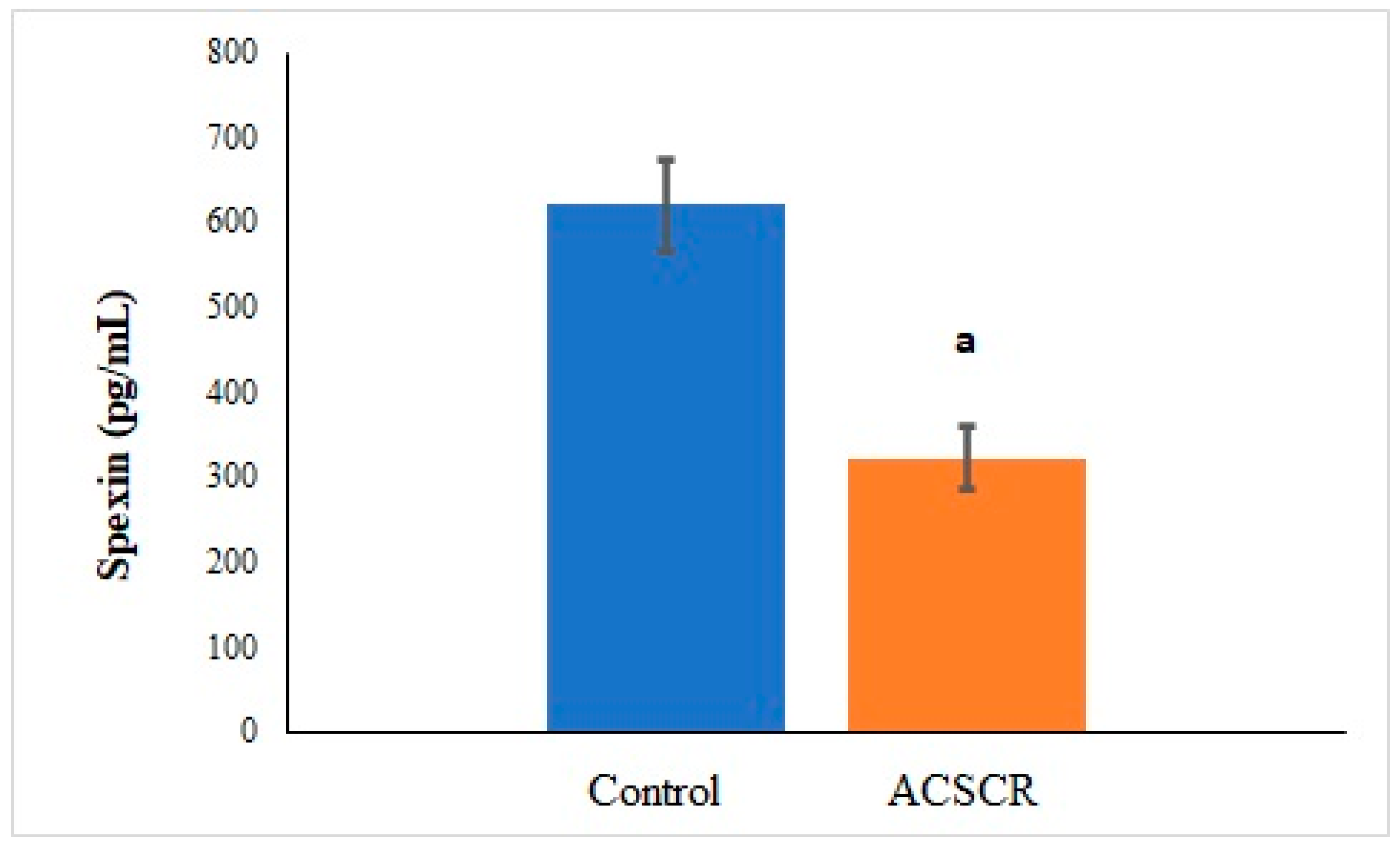
| Parameters | Control | ACSCR | p |
|---|---|---|---|
| Age | 47.4 ± 8.22 | 49.32 ± 5.16 | 0.81 |
| Gender (male/female) | 22/8 | 23/7 | 0.94 |
| BMI | 24.7 ± 1.6 | 24.9 ± 2.3 | 0.87 |
| Glucose (mg/dL) | 94.8 ± 5.33 | 99.6 ± 5.22 | 0.77 |
| CMT (μm) | 216.92 ± 12.34 | 476.11 ± 72.41 | 0.001 |
| VA | 1.00 ± 0.00 | 0.52 ± 0.31 | 0.026 |
| Correlations | r Value | p Value |
|---|---|---|
| TMAO-PNX | 0.494 | 0.011 |
| TMAO-SPX | −0.582 | 0 |
| TMAO-ALA | 0.528 | 0 |
| PNX-ALA | 0.622 | 0 |
| PNX-SPX | −0.764 | 0 |
| SPX-ALA | −0.587 | 0 |
| TMAO-CMT | 0.612 | 0 |
| PNX-CMT | 0.498 | 0.011 |
| ALA-CMT | 0.576 | 0.001 |
| SPX-CMT | −0.542 | 0.001 |
| TMAO-VA | 0.498 | 0.011 |
| PNX- VA | 0.578 | 0.001 |
| ALA-VA | 0.612 | 0 |
| SPX-VA | 0.562 | 0 |
| CMT-VA | −0.596 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaya, M.K.; Arslan, S. Exploring the Potential Link between Acute Central Serous Chorioretinopathy and Trimethylamine N-Oxide, Phoenixin, Spexin, and Alarin Molecules. Biomolecules 2023, 13, 1459. https://doi.org/10.3390/biom13101459
Kaya MK, Arslan S. Exploring the Potential Link between Acute Central Serous Chorioretinopathy and Trimethylamine N-Oxide, Phoenixin, Spexin, and Alarin Molecules. Biomolecules. 2023; 13(10):1459. https://doi.org/10.3390/biom13101459
Chicago/Turabian StyleKaya, Mehmet Kaan, and Sermal Arslan. 2023. "Exploring the Potential Link between Acute Central Serous Chorioretinopathy and Trimethylamine N-Oxide, Phoenixin, Spexin, and Alarin Molecules" Biomolecules 13, no. 10: 1459. https://doi.org/10.3390/biom13101459
APA StyleKaya, M. K., & Arslan, S. (2023). Exploring the Potential Link between Acute Central Serous Chorioretinopathy and Trimethylamine N-Oxide, Phoenixin, Spexin, and Alarin Molecules. Biomolecules, 13(10), 1459. https://doi.org/10.3390/biom13101459






