Identification of Crustacean Female Sex Hormone Receptor Involved in Sexual Differentiation of a Hermaphroditic Shrimp
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
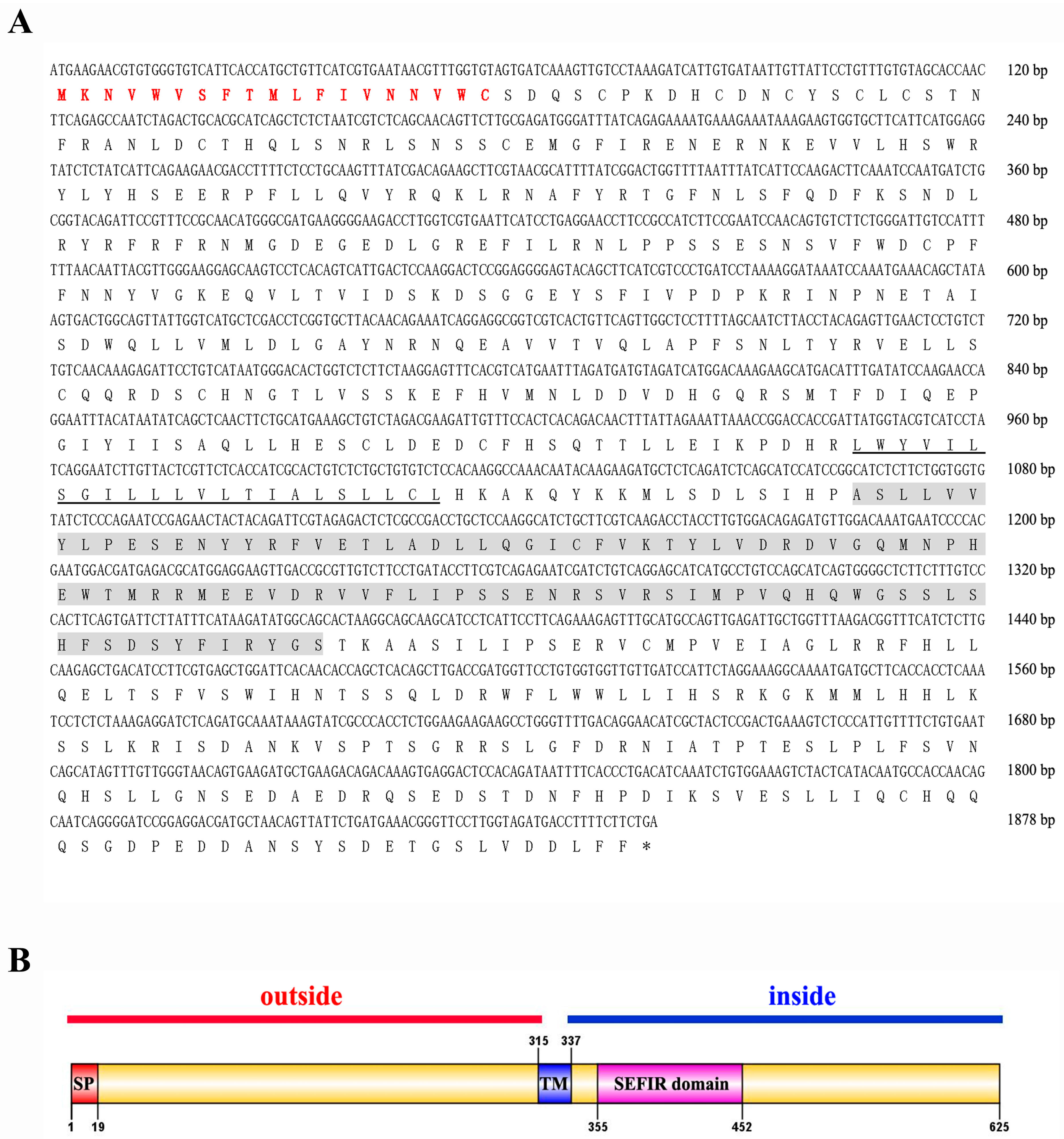
2.2. cDNA Cloning of Lvit-IL-17R
2.3. The qRT-PCR Assays
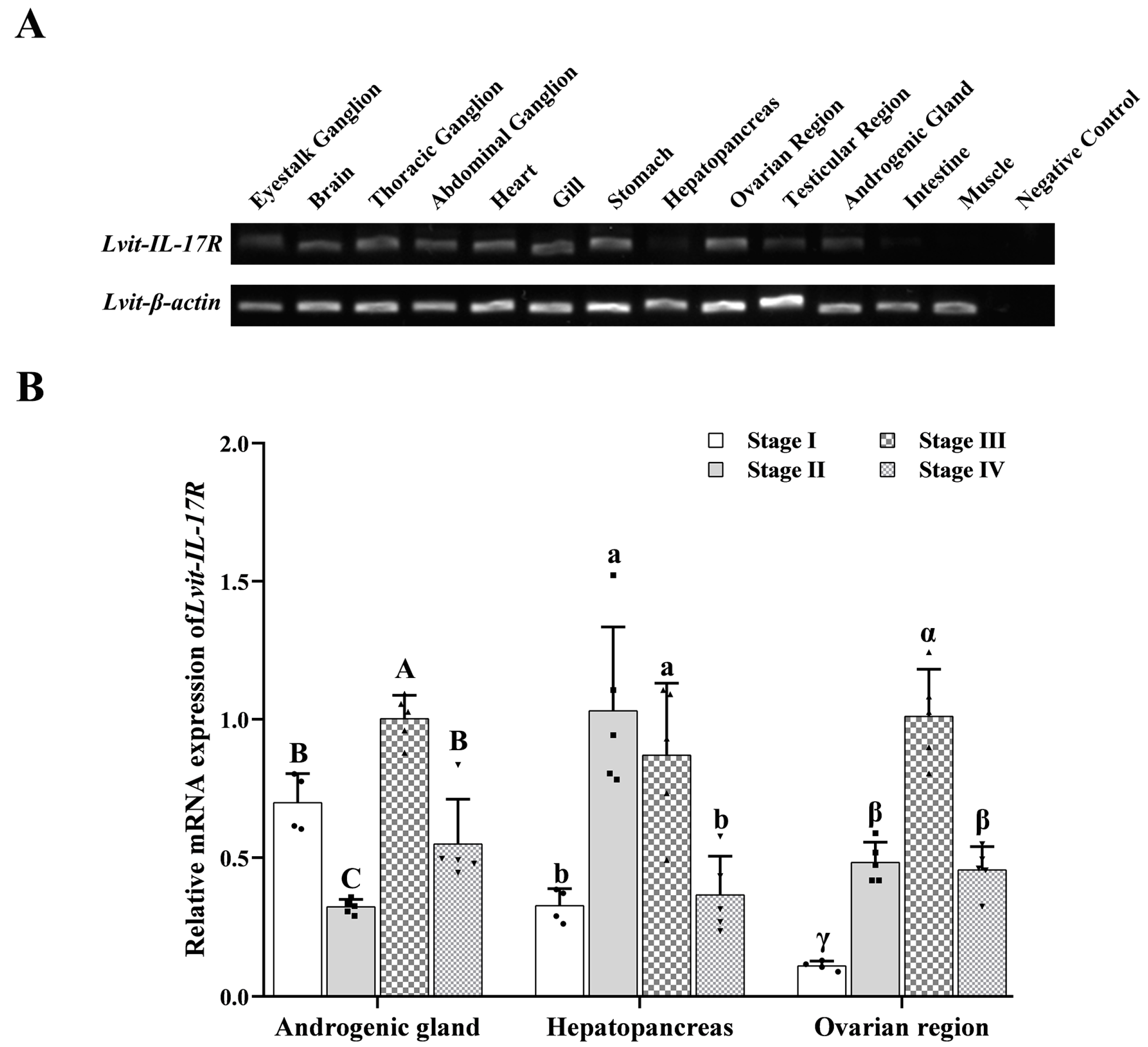
2.4. Expression Profiles of Lvit-IL-17R
2.5. GST Pulldown Assays
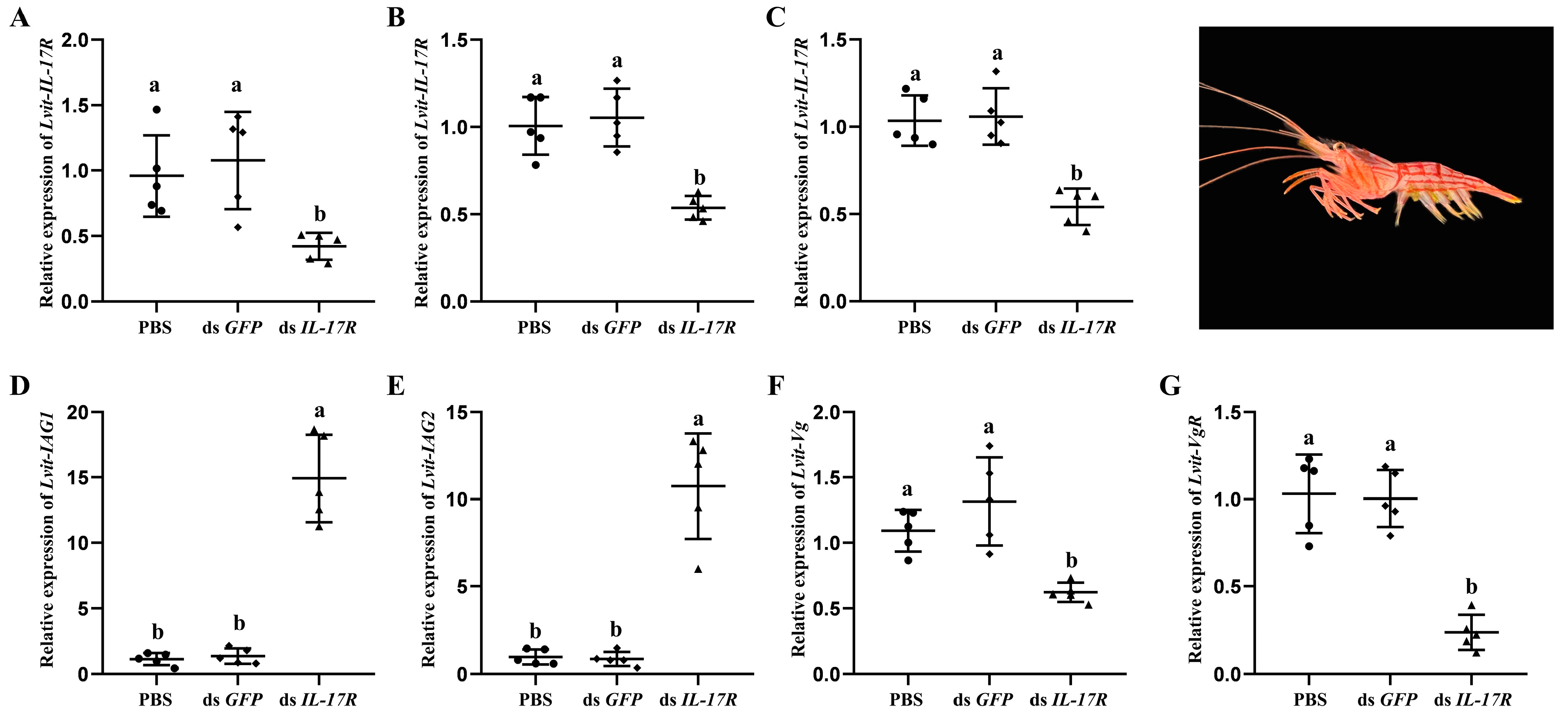
2.6. Short-Term Silencing Experiment
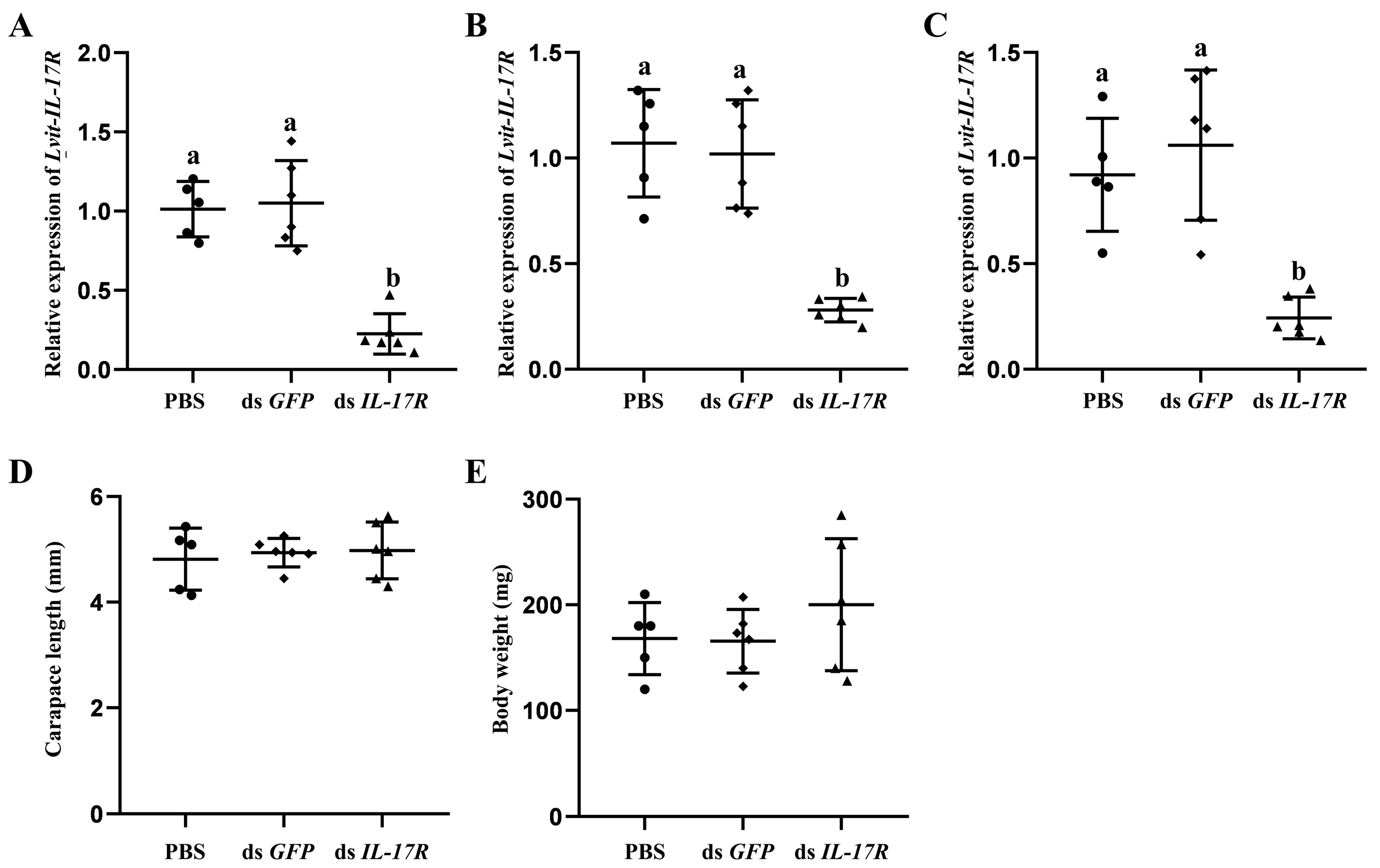
2.7. Long-Term Silencing Experiment
2.8. Bioinformatics and Statistical Analyses
3. Results
3.1. Sequence Analysis of Lvit-IL-17R
3.2. Phylogenetic Analysis of IL-17 Domain-Contained Proteins from Decapods
3.3. Expression Profiles of Lvit-IL-17R
3.4. Ligand–Receptor Interaction Analysis
3.5. Short-Term Silencing Experiment In Vivo
3.6. Long-Term Silencing Experiment In Vivo
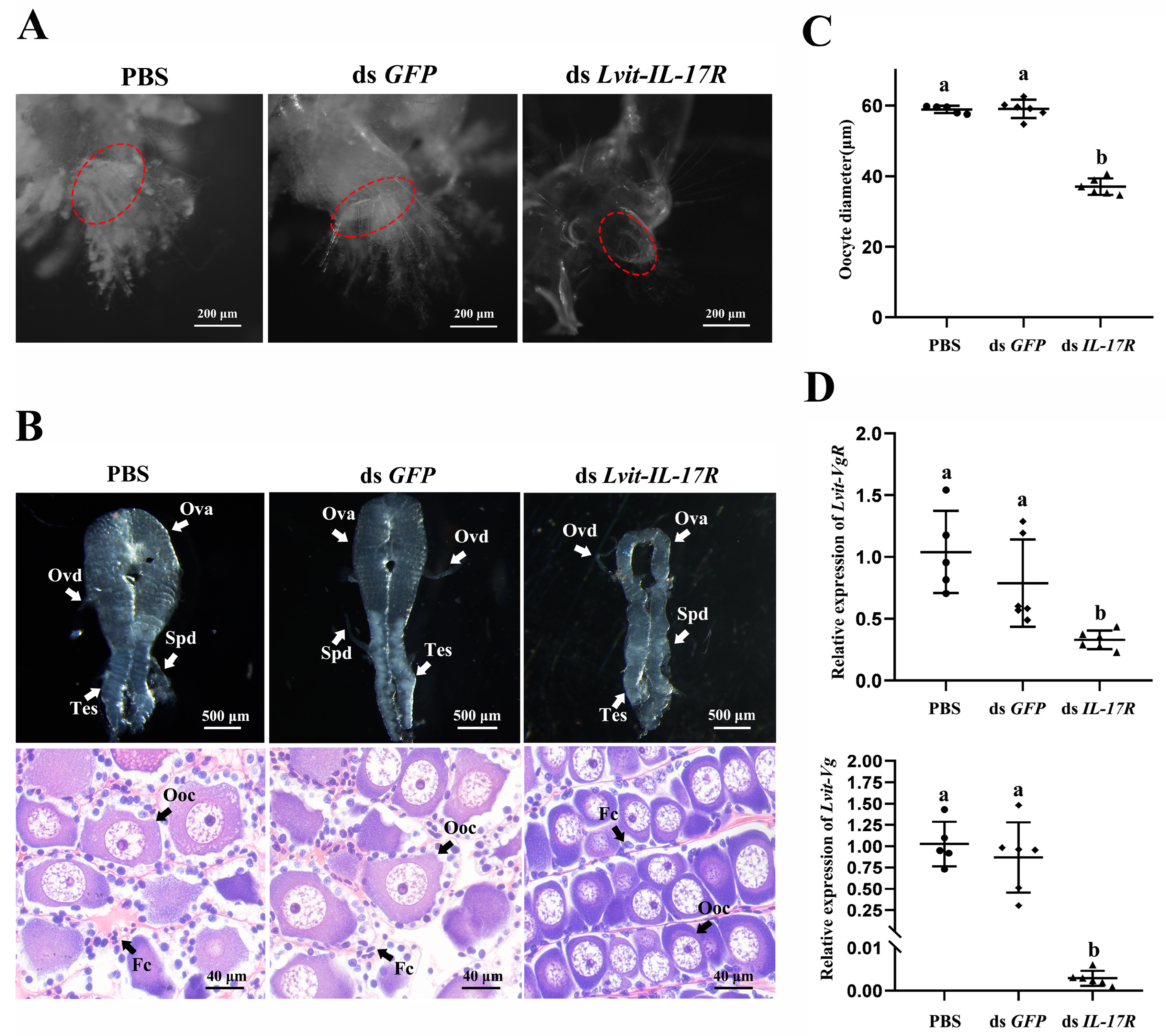
3.6.1. Effects of Lvit-IL-17R Silencing on Female Sexual Differentiation
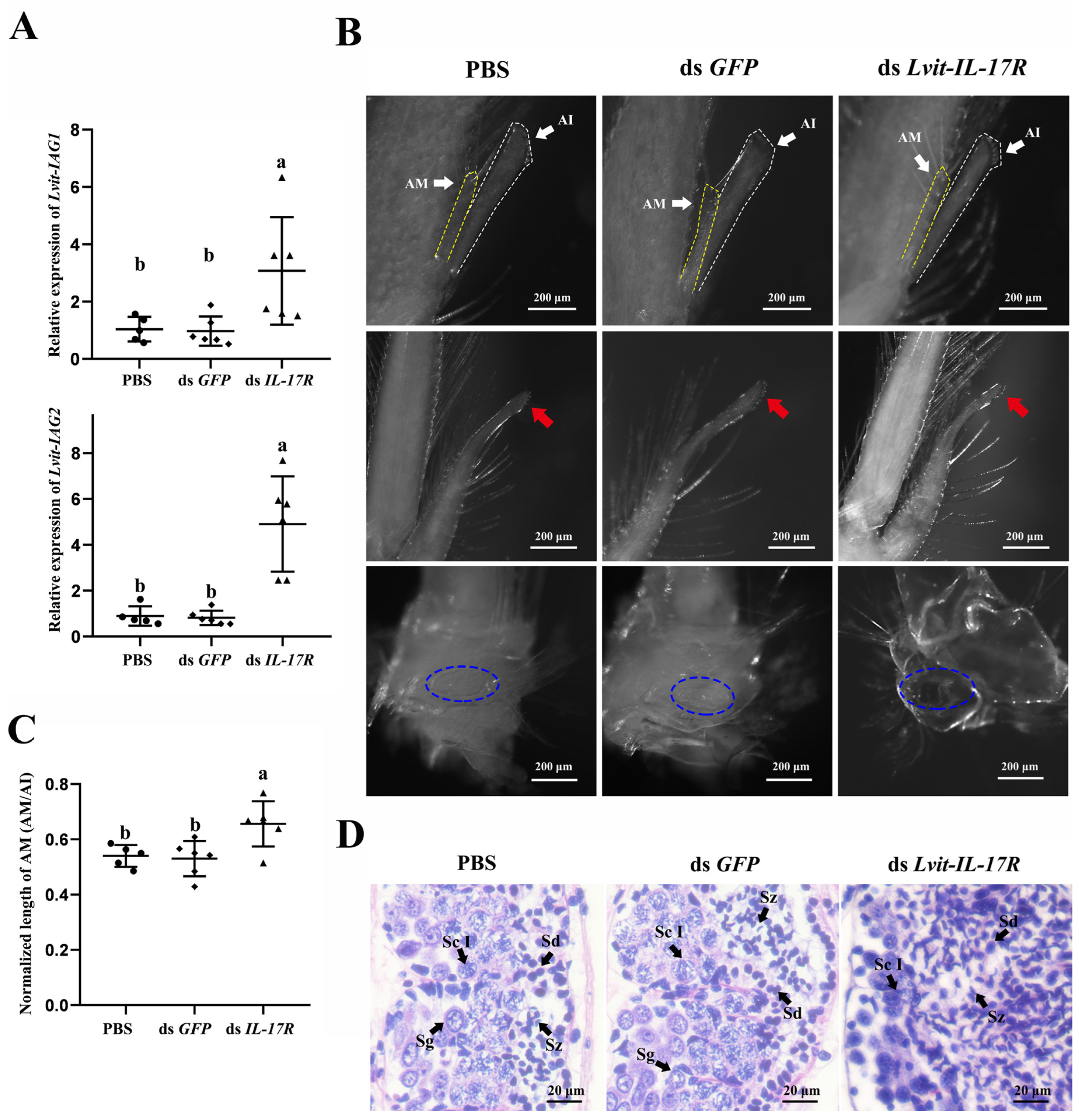
3.6.2. Effects of Lvit-IL-17R Silencing on Male Sexual Differentiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Primer | Sequence (5′-3′) | Application |
|---|---|---|
| IL17RF | TTCGTCGCCTCGTCTCTCTTCACAC | Fragment validation |
| IL17RR | AAAGGTCATCTACCAAGGAACCCG | |
| UPM | CTAATACGACTCACTATAGGGCAAGCAGTGGTATCAACGCAGAGT | 5′RACE |
| 5IL17Rout | ACGAAGCAGATGCCTTGGAGCAGG | |
| 5IL17Rin | GTGCGATGGTGAGAACGAGTAACAAG | |
| CFSH1aQF | CAAGGACGGCGATGATGA | RT-PCR/qRT-PCR |
| CFSH1aQR | GCGAAGGATCTGAGATGTGTA | |
| CFSH1bQF | ATCCACACCTCAGAACTCATC | |
| CFSH1bQR | GCACAGGCTACGGTTATCT | |
| IAG1QF | CTAATCTTGCTGCCTCATTCTAC | |
| IAG1QR | GCGTCGTTCTCTGTAATAATCG | |
| IAG2QF | TCAGTCTCAGCCATCTCCT | |
| IAG2QR | TGAACCGACCACCTCTAATG | |
| VgQF | GCAAAAGTGGGAGCCGAAAG | |
| VgQR | ATCACCCGTAGAGGGTAGGG | |
| VgRQF | CTGCGTCTCGGAACTCAA | |
| VgRQR | GTGCTGGTGGTGAAGATGA | |
| IL17RQF | GGTTCCTGTGGTGGTTGT | |
| IL17RQR | GAGTAGCGATGTTCCTGTCA | |
| actinF | CGTGACCTGACTGATTACC | |
| actinR | CGTTACCGATAGTGATTACCT | |
| IL17RdsF | GTGTAGCACCAACTTCAGAGC | dsRNA synthesis |
| IL17RdsR | AACAGTGACGACCGCCTC | |
| GFPdsF | TGGGCGTGGATAGCGGTTTG | |
| GFPdsR | GGTCGGGGTAGCGGCTGAAG | |
| T7primer | TAATACGACTCACTATAGGG | |
| SP6primer | ATTTAGGTGACACTATAG | |
| IL17RyhF | CGCGGATCCAGTGATCAAAGTTGTCCTAAAGATC | Prokaryotic expression |
| IL17RyhR | CTAGCTAGCTCGGTGGTCCGGTTTAATTTCTAAT |
| Sequence | Genebank Accession Number | NR Description |
|---|---|---|
| IL-17RA Homo sapiens | NP_055154.3 | interleukin-17 receptor A isoform 1 precursor [Homo sapiens] |
| IL-17RA Trichosurus vulpecula | XP_036616840.1 | interleukin-17 receptor A [Trichosurus vulpecula] |
| IL-17RA Anas platyrhynchos | XP_027319183.2 | interleukin-17 receptor A [Anas platyrhynchos] |
| IL-17RA Corvus cornix cornix | XP_039426789.1 | interleukin-17 receptor A [Corvus cornix cornix] |
| IL-17RA Ctenopharyngodon idella | AXF94778.1 | IL17RA [Ctenopharyngodon idella] |
| IL-17RA Xiphophorus hellerii | XP_032445247.1 | interleukin-17 receptor A [Xiphophorus hellerii] |
| IL-17RA Labrus bergylta | XP_020500355.1 | interleukin-17 receptor A [Labrus bergylta] |
| IL-17RA Crotalus tigris | XP_039218521.1 | interleukin-17 receptor A [Crotalus tigris] |
| IL-17RA Thamnophis elegans | XP_032077075.1 | interleukin-17 receptor A [Thamnophis elegans] |
| IL-17RB Bos taurus | AAI33637.1 | IL17RB protein [Bos taurus] |
| IL-17RB Mus musculus | NP_062529.2 | interleukin-17 receptor B precursor [Mus musculus] |
| IL-17RB Homo sapiens | NP_061195.2 | interleukin-17 receptor B precursor [Homo sapiens] |
| IL-17RB Protobothrops mucrosquamatus | XP_015671688.1 | interleukin-17 receptor B [Protobothrops mucrosquamatus] |
| IL-17RB Notechis scutatus | XP_026532517.1 | interleukin-17 receptor B isoform X1 [Notechis scutatus] |
| IL-17RB Pantherophis guttatus | XP_034293105.1 | interleukin-17 receptor B isoform X2 [Pantherophis guttatus] |
| IL-17RB Cygnus atratus | XP_035414138.1 | LOW QUALITY PROTEIN: interleukin-17 receptor B [Cygnus atratus] |
| IL-17RB Gavia stellata | XP_009818499.1 | PREDICTED: interleukin-17 receptor B [Gavia stellata] |
| IL-17RB Crocodylus porosus | XP_019392467.1 | PREDICTED: interleukin-17 receptor B isoform X3 [Crocodylus porosus] |
| IL-17RB Mauremys reevesii | XP_039402548.1 | interleukin-17 receptor B [Mauremys reevesii] |
| IL-17RB Chelonoidis abingdonii | XP_032650202.1 | interleukin-17 receptor B isoform X1 [Chelonoidis abingdonii] |
| IL-17RB Trachemys scripta elegans | XP_034631495.1 | interleukin-17 receptor B isoform X1 [Trachemys scripta elegans] |
| IL-17RC Mus musculus | AAH04759.1 | Il17rc protein [Mus musculus] |
| IL-17RC Homo sapiens | NP_001190193.2 | interleukin-17 receptor C isoform 5 precursor [Homo sapiens] |
| IL-17RC Kryptolebias marmoratus | XP_017290980.1 | interleukin-17 receptor C [Kryptolebias marmoratus] |
| IL-17RC Cyprinodon tularosa | XP_038135791.1 | interleukin-17 receptor C isoform X1 [Cyprinodon tularosa] |
| IL-17RC Nematolebias whitei | XP_037535779.1 | interleukin-17 receptor C [Nematolebias whitei] |
| IL-17RC Oryzias melastigma | XP_024125869.1 | interleukin-17 receptor C [Oryzias melastigma] |
| IL-17RC Passer montanus | XP_039590153.1 | interleukin-17 receptor C [Passer montanus] |
| IL-17RC Anas platyrhynchos | XP_038041828.1 | interleukin-17 receptor C [Anas platyrhynchos] |
| IL-17RC Falco rusticolus | XP_037241131.1 | interleukin-17 receptor C [Falco rusticolus] |
| IL-17RC Parus major | XP_033373328.1 | interleukin-17 receptor C [Parus major] |
| IL-17RC Crotalus tigris | XP_039189296.1 | interleukin-17 receptor C [Crotalus tigris] |
| IL-17RC Lacerta agilis | XP_032997296.1 | interleukin-17 receptor C [Lacerta agilis] |
| IL-17RC Thamnophis elegans | XP_032068166.1 | interleukin-17 receptor C [Thamnophis elegans] |
| IL-17RC Chelonoidis abingdonii | XP_032645769.1 | interleukin-17 receptor C [Chelonoidis abingdonii] |
| IL-17RC Rana temporaria | XP_040214946.1 | interleukin-17 receptor C [Rana temporaria] |
| IL-17RC Xenopus tropicalis | XP_002941718.2 | interleukin-17 receptor C [Xenopus tropicalis] |
| IL-17RD Homo sapiens | AAI11703.2 | Interleukin 17 receptor D [Homo sapiens] |
| IL-17RD Camelus dromedarius | KAB1264165.1 | Interleukin-17 receptor D [Camelus dromedarius] |
| IL-17RD Mus pahari | XP_021059177.1 | interleukin-17 receptor D [Mus pahari] |
| IL-17RD Epinephelus coioides | ASU91965.1 | interleukin 17 receptor D [Epinephelus coioides] |
| IL-17RD Oncorhynchus mykiss | XP_021422760.1 | interleukin-17 receptor D [Oncorhynchus mykiss] |
| IL-17RD Oryzias melastigma | XP_024149401.1 | interleukin-17 receptor D [Oryzias melastigma] |
| IL-17RD Larimichthys crocea | TMS19695.1 | Interleukin-17 receptor D [Larimichthys crocea] |
| IL-17RD Serinus canaria | XP_009089302.2 | interleukin-17 receptor D [Serinus canaria] |
| IL-17RD Parus major | XP_015496904.1 | interleukin-17 receptor D [Parus major] |
| IL-17RD Phasianus colchicus | XP_031464092.1 | interleukin-17 receptor D [Phasianus colchicus] |
| IL-17RD Geospiza fortis | XP_014163114.1 | interleukin-17 receptor D [Geospiza fortis] |
| IL-17RD Protobothrops mucrosquamatus | XP_029142521.1 | interleukin-17 receptor D [Protobothrops mucrosquamatus] |
| IL-17RD Pseudonaja textilis | XP_026579511.1 | interleukin-17 receptor D [Pseudonaja textilis] |
| IL-17RD Notechis scutatus | XP_026533350.1 | interleukin-17 receptor D [Notechis scutatus] |
| IL-17RD Crassostrea gigas | XP_034308350.1 | interleukin-17 receptor D [Crassostrea gigas] |
| IL-17RD Mizuhopecten yessoensis | OWF39523.1 | Interleukin-17 receptor D [Mizuhopecten yessoensis] |
| IL-17RD Mytilus coruscus | CAC5416786.1 | IL17RD [Mytilus coruscus] |
| IL-17RD Sepia pharaonis | CAE1176245.1 | IL17RD [Sepia pharaonis] |
| IL-17RE Ochotona princeps | XP_004581715.1 | interleukin-17 receptor E [Ochotona princeps] |
| IL-17RE Mastomys coucha | XP_031236803.1 | interleukin-17 receptor E [Mastomys coucha] |
| IL-17RE Bos indicus | XP_019840332.1 | PREDICTED: interleukin-17 receptor E [Bos indicus] |
| IL-17RE Epinephelus coioides | ASU91966.1 | interleukin 17 receptor E [Epinephelus coioides] |
| IL-17RE Anabarilius grahami | ROI74335.1 | Interleukin-17 receptor E [Anabarilius grahami] |
| IL-17RE Periophthalmus magnuspinnatus | XP_033824756.1 | interleukin-17 receptor E [Periophthalmus magnuspinnatus] |
| IL-17RE Syngnathus acus | XP_037134538.1 | interleukin-17 receptor E [Syngnathus acus] |
| IL-17RE Calypte anna | XP_030314349.1 | interleukin-17 receptor E [Calypte anna] |
| IL-17RE Manacus vitellinus | XP_029814192.1 | interleukin-17 receptor E [Manacus vitellinus] |
| IL-17RE Gallus gallus | XP_015148829.1 | interleukin-17 receptor E [Gallus gallus] |
| IL-17RE Amazona aestiva | KQK84533.1 | interleukin-17 receptor E [Amazona aestiva] |
| IL-17RE Crotalus tigris | XP_039189291.1 | interleukin-17 receptor E isoform X2 [Crotalus tigris] |
| IL-17RE Thamnophis elegans | XP_032065471.1 | interleukin-17 receptor E [Thamnophis elegans] |
| IL-17RE Alligator sinensis | XP_006036694.2 | interleukin-17 receptor E [Alligator sinensis] |
| IL-17R1 Penaeus monodon | XP_037788272.1 | uncharacterized protein LOC119583705 isoform X1 [Penaeus monodon] |
| IL-17R1 Penaeus vannamei | XP_027209688.1 | uncharacterized protein LOC113803161 isoform X3 [Penaeus vannamei] |
| IL-17R Homarus americanus | XP_042224392.1 | uncharacterized protein LOC121868143 [Homarus americanus] |
| IL-17R Penaeus japonicus | XP_042872695.1 | uncharacterized protein LOC122253547 isoform X1 [Penaeus japonicus] |
| IL-17R2 Penaeus vannamei | XP_027210315.1 | uncharacterized protein LOC113803705 [Penaeus vannamei] |
| IL-17R2 Penaeus monodon | XP_037791606.1 | uncharacterized protein LOC119586945 [Penaeus monodon] |
| IL-17R Scylla paramamosain | ON787957 | female sex hormone receptor [Scylla paramamosain] |
| IL-17R Lysmata vittata | MZ367742 | interleukin 17 receptor [Lysmata vittata] |
| Sequence | Genebank Accession Number | NR Description |
|---|---|---|
| CFSH1a L. vittata | QOD42431.1 | crustacean female sex hormone 2 [Lysmata vittata] |
| CFSH1b L. vittata | QOD42430.1 | crustacean female sex hormone 1 [Lysmata vittata] |
| CFSH1c P. japonicus | XP_042859036.1 | uncharacterized protein LOC122245152 [Penaeus japonicus] |
| CFSH1c P. vannamei | XP_027234405.1 | uncharacterized protein LOC113825772 [Penaeus vannamei] |
| CFSH1c P. monodon | XP_037783148.1 | uncharacterized protein LOC119579417 [Penaeus monodon] |
| CFSH1c1 P. chinensis | XP_047497145.1 | uncharacterized protein LOC125044498 [Penaeus chinensis] |
| CFSH1c2 P. chinensis | XP_047496354.1 | uncharacterized protein LOC125043989 [Penaeus chinensis] |
| CFSH1b P. vannamei | XP_027214658.1 | uncharacterized protein LOC113807568 [Penaeus vannamei] |
| CFSH1b P. japonicus | XP_042860457.1 | uncharacterized protein LOC122246162 [Penaeus japonicus] |
| CFSH1a P. japonicus | XP_042860456.1 | uncharacterized protein LOC122246161 [Penaeus japonicus] |
| CFSH1a P. vannamei | XP_027214657.1 | uncharacterized protein LOC113807567 [Penaeus vannamei] |
| CFSH1a P. monodon | XP_037787591.1 | uncharacterized protein LOC119583157 [Penaeus monodon] |
| CFSH1d P. japonicus | XP_042858179.1 | uncharacterized protein LOC122244361 [Penaeus japonicus] |
| CFSH1d P. vannamei | XP_027219797.1 | uncharacterized protein LOC113812164 [Penaeus vannamei] |
| CFSH1d P. chinensis | XP_047496468.1 | uncharacterized protein LOC125044066 [Penaeus chinensis] |
| CFSH1d P. monodon | XP_037783482.1 | uncharacterized protein LOC119579641 [Penaeus monodon] |
| CFSH1 P. clarkii | XP_045612404.1 | uncharacterized protein LOC123766959 [Procambarus clarkii] |
| CFSH1 S. paramamosain | QIH29133.1 | crustacean female sex hormone [Scylla paramamosain] |
| CFSH1 P. trituberculatus | XP_045110383.1 | uncharacterized protein LOC123504127 [Portunus trituberculatus] |
| CFSH1 C. sapidus | ADO00266.1 | crustacean female hormone [Callinectes sapidus] |
| CFSH2b C. quadricarinatus | XP_053635083.1 | uncharacterized protein LOC128690428 [Cherax quadricarinatus] |
| CFSH2b P. clarkii | XP_045592388.1 | uncharacterized protein LOC123754223 [Procambarus clarkii] |
| CFSH2b E. sinensis | XP_050718041.1 | uncharacterized protein LOC126999466 [Eriocheir sinensis] |
| CFSH2a E. sinensis | XP_050685866.1 | uncharacterized protein LOC126980239 [Eriocheir sinensis] |
| CFSH2a P. clarkii | XP_045612404.1 | uncharacterized protein LOC123766959 [Procambarus clarkii] |
| CFSH2a C. quadricarinatus | XP_053655536.1 | uncharacterized protein LOC128704410 [Cherax quadricarinatus] |
| CFSH2 P. japonicus | XP_042863675.1 | uncharacterized protein LOC122248000 [Penaeus japonicus] |
| CFSH2 P. vannamei | XP_027237919.1 | uncharacterized protein LOC113829039 [Penaeus vannamei] |
| CFSH2 P. monodon | XP_037797660.1 | uncharacterized protein LOC119592827 [Penaeus monodon] |
| IL-17F H. sapiens | AAH70124.1 | Interleukin 17F [Homo sapiens] |
| IL-17F M. musculus | AAQ88439.1 | interleukin 17F [Mus musculus] |
| IL-17A H. sapiens | AAH67504.1 | Interleukin 17A [Homo sapiens] |
| IL-17A M. musculus | EDL14378.1 | interleukin 17A [Mus musculus] |
| IL-17F G. gallus | ADR73041.1 | interleukin 17F [Gallus gallus] |
| IL-17A G. gallus | CAO79600.1 | interleukin-17A [Gallus gallus] |
| IL-17A O. mykiss | CCG00933.1 | Interleukin 17A [Oncorhynchus mykiss] |
| IL-17F O. mykiss | XP_036818687.1 | interleukin-17F [Oncorhynchus mykiss] |
| IL-17D F. rusticolus | XP_037233120.1 | interleukin-17D [Falco rusticolus] |
| IL-17D H. sapiens | AAH36243.1 | Interleukin 17D [Homo sapiens] |
| IL-17D C. idella | AGW43284.1 | interleukin 17D [Ctenopharyngodon idella] |
| IL-17B A. chrysaetos chrysaetos | XP_029853932.1 | interleukin-17B [Aquila chrysaetos chrysaetos] |
| IL-17B M. musculus | EDL09761.1 | interleukin 17B [Mus musculus] |
| IL-17B H. sapiens | AAF28104.1 | interleukin 17B [Homo sapiens] |
| IL-17B F. heteroclitus | XP_012710165.2 | interleukin-17B [Fundulus heteroclitus] |
| IL-17C C. idella | AKM20918.1 | interleukin 17C [Ctenopharyngodon idella] |
| IL-17E O. niloticus | XP_005447866.2 | interleukin-25 isoform X3 [Oreochromis niloticus] |
| IL-17C M. musculus | NP_665833.4 | interleukin-17C [Mus musculus] |
| IL-17C H. sapiens | AAF28105.1 | interleukin 17C [Homo sapiens] |
| IL-17C M. ater | XP_036246432.1 | interleukin-17C [Molothrus ater] |
| IL-17E A. sinensis | XP_025048580.1 | interleukin-25 [Alligator sinensis] |
| IL-17E T. vulpecula | XP_036592492.1 | interleukin-25 [Trichosurus vulpecula] |
| IL-17E M. musculus | NP_542767.1 | interleukin-25 [Mus musculus] |
| IL-17E S. scrofa | AXF36091.1 | interleukin 17E [Sus scrofa] |
| IL-17E H. sapiens | AAG40848.1 | interleukin 17E [Homo sapiens] |
| IL-6 H. sapiens | AAD13886.1 | interleukin-6 [Homo sapiens] |
Appendix B
References
- Juchault, P. Hermaphroditism and gonochorism. A new hypothesis on the evolution of sexuality in Crustacea. C. R. Acad. Sci. III 1999, 322, 423–427. [Google Scholar] [CrossRef]
- Bauer, R.T. Simultaneous hermaphroditism in caridean shrimps: A unique and puzzling sexual system in the Decapoda. J. Crustac. Biol. 2000, 20, 116–128. [Google Scholar] [CrossRef]
- Ford, A.T. Intersexuality in Crustacea: An environmental issue? Aquat. Toxicol. 2012, 108, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Levy, T.; Tamone, S.L.; Manor, R.; Bower, E.D.; Sagi, A. The protandric life history of the Northern spot shrimp Pandalus platyceros: Molecular insights and implications for fishery management. Sci. Rep. 2020, 10, 1287. [Google Scholar] [CrossRef] [PubMed]
- Levy, T.; Tamone, S.L.; Manor, R.; Aflalo, E.D.; Sklarz, M.Y.; Chalifa-Caspi, V.; Sagi, A. The IAG-switch and further transcriptomic insights into sexual differentiation of a protandric shrimp. Front. Mar. Sci. 2020, 7, 587454. [Google Scholar] [CrossRef]
- Chen, D.M.; Liu, F.; Zhu, Z.H.; Lin, Q.; Zeng, C.S.; Ye, H.H. Ontogenetic development of gonads and external sexual characters of the protandric simultaneous hermaphrodite peppermint shrimp, Lysmata vittata (Caridea: Hippolytidae). PLoS ONE 2019, 14, e0215406. [Google Scholar] [CrossRef]
- Alves, D.F.R.; Greco, L.S.L.; Barros-Alves, S.D.; Hirose, G.L. Sexual system, reproductive cycle and embryonic development of the red-striped shrimp Lysmata vittata, an invader in the western Atlantic Ocean. PLoS ONE 2019, 14, e0210723. [Google Scholar] [CrossRef]
- Toyota, K.; Miyakawa, H.; Hiruta, C.; Sato, T.; Katayama, H.; Ohira, T.; Iguchi, T. Sex determination and differentiation in decapod and cladoceran crustaceans: An overview of endocrine regulation. Genes 2021, 12, 305. [Google Scholar] [CrossRef]
- Levy, T.; Sagi, A. The “IAG-switch”—A key controlling element in decapod crustacean sex differentiation. Front. Endocrinol. 2020, 11, 651. [Google Scholar] [CrossRef]
- Liu, F.; Liu, A.; Zhu, Z.; Wang, Y.; Ye, H. Crustacean female sex hormone: More than a female phenotypes-related hormone in a protandric simultaneous hermaphroditism shrimp. Int. J. Biol. Macromol. 2023, 238, 124181. [Google Scholar] [CrossRef]
- Liu, F.; Shi, W.Y.; Ye, H.H.; Liu, A.; Zhu, Z.H. RNAi Reveals Role of insulin-like androgenic gland hormone 2 (IAG2) in sexual differentiation and growth in hermaphrodite shrimp. Front. Mar. Sci. 2021, 8, 666763. [Google Scholar] [CrossRef]
- Martin, G.; Sorokine, O.; Moniatte, M.; Vandorsselaer, A. The androgenic hormone of the crustacean isopod Armadillidium vulgare. In Trends in Comparative Endocrinology and Neurobiology: From Molecular to Integrative Biology; Vaudry, H., Tonon, M.C., Roubos, E.W., de Loof, A., Eds.; Annals of the New York Academy of Sciences: New York, NY, USA, 1998; Volume 839, pp. 111–117. [Google Scholar]
- Martin, G.; Sorokine, O.; Moniatte, M.; Bulet, P.; Hetru, C.; Van Dorsselaer, A. The structure of a glycosylated protein hormone responsible for sex determination in the isopod, Armadillidium vulgare. Eur. J. Biochem. 1999, 262, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Manor, R.; Weil, S.; Oren, S.; Glazer, L.; Aflalo, E.D.; Ventura, T.; Chalifa-Caspi, V.; Lapidot, M.; Sagi, A. Insulin and gender: An insulin-like gene expressed exclusively in the androgenic gland of the male crayfish. Gen. Comp. Endocrinol. 2007, 150, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Ventura, T.; Manor, R.; Aflalo, E.D.; Weil, S.; Raviv, S.; Glazer, L.; Sagi, A. Temporal silencing of an androgenic gland-specific insulin-like gene affecting phenotypical gender differences and spermatogenesis. Endocrinology 2009, 150, 1278–1286. [Google Scholar] [CrossRef]
- Rosen, O.; Manor, R.; Weil, S.; Gafni, O.; Linial, A.; Aflalo, E.D.; Ventura, T.; Sagi, A. A sexual shift induced by silencing of a single insulin-like gene in crayfish: Ovarian upregulation and testicular degeneration. PLoS ONE 2010, 5, e15281. [Google Scholar] [CrossRef]
- Fu, C.P.; Li, F.J.; Wang, L.F.; Wu, F.R.; Wang, J.M.; Fan, X.L.; Liu, T. Molecular characteristics and abundance of insulin-like androgenic gland hormone and effects of RNA interference in Eriocheir sinensis. Anim. Reprod. Sci. 2020, 215, 106332. [Google Scholar] [CrossRef]
- Aizen, J.; Chandler, J.C.; Fitzgibbon, Q.P.; Sagi, A.; Battaglene, S.C.; Elizur, A.; Ventura, T. Production of recombinant insulin-like androgenic gland hormones from three decapod species: In Vitro testicular phosphorylation and activation of a newly identified tyrosine kinase receptor from the Eastern spiny lobster, Sagmariasus verreauxi. Gen. Comp. Endocrinol. 2016, 229, 8–18. [Google Scholar] [CrossRef]
- Sharabi, O.; Manor, R.; Weil, S.; Aflalo, E.D.; Lezer, Y.; Levy, T.; Aizen, J.; Ventura, T.; Mather, P.B.; Khalaila, I.; et al. Identification and characterization of an insulin-like receptor involved in crustacean reproduction. Endocrinology 2016, 157, 928–941. [Google Scholar] [CrossRef]
- Guo, Q.; Li, S.H.; Lv, X.J.; Xiang, J.H.; Sagi, A.; Manor, R.; Li, F.H. A putative insulin-like androgenic gland hormone receptor gene specifically expressed in male Chinese shrimp. Endocrinology 2018, 159, 2173–2185. [Google Scholar] [CrossRef]
- Tan, K.; Li, Y.; Zhou, M.; Wang, W. siRNA knockdown of MrIR induces sex reversal in Macrobrachium rosenbergii. Aquaculture 2020, 523, 735172. [Google Scholar] [CrossRef]
- Zmora, N.; Chung, J.S. A novel hormone is required for the development of reproductive phenotypes in adult female crabs. Endocrinology 2014, 155, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Feng, T.; Mo, N.; Han, R.; Lu, W.; Shao, S.; Cui, Z. New insights for the regulatory feedback loop between type 1 crustacean female sex hormone (CFSH-1) and insulin-like androgenic gland hormone (IAG) in the Chinese mitten crab (Eriocheir sinensis). Front. Physiol. 2022, 13, 1054773. [Google Scholar] [CrossRef] [PubMed]
- Ventura, T.; Cummins, S.F.; Fitzgibbon, Q.; Battaglene, S.; Elizur, A. Analysis of the central nervous system transcriptome of the eastern rock lobster Sagmariasus verreauxi reveals its putative neuropeptidome. PLoS ONE 2014, 9, e97323. [Google Scholar] [CrossRef] [PubMed]
- Veenstra, J.A. The power of next-generation sequencing as illustrated by the neuropeptidome of the crayfish Procambarus clarkii. Gen. Comp. Endocrinol. 2015, 224, 84–95. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Cummins, S.F.; Elizur, A.; Ventura, T. Transcriptomic characterization and curation of candidate neuropeptides regulating reproduction in the eyestalk ganglia of the Australian crayfish, Cherax quadricarinatus. Sci. Rep. 2016, 6, 38658. [Google Scholar] [CrossRef] [PubMed]
- Veenstra, J.A. Similarities between decapod and insect neuropeptidomes. PeerJ 2016, 4, e2043. [Google Scholar] [CrossRef]
- Kotaka, S.; Ohira, T. cDNA cloning and in situ localization of a crustacean female sex hormone-like molecule in the kuruma prawn Marsupenaeus japonicus. Fish. Sci. 2018, 84, 53–60. [Google Scholar] [CrossRef]
- Tsutsui, N.; Kotaka, S.; Ohira, T.; Sakamoto, T. Characterization of distinct ovarian isoform of crustacean female sex hormone in the kuruma prawn Marsupenaeus japonicus. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2018, 217, 7–16. [Google Scholar] [CrossRef]
- Thongbuakaew, T.; Suwansa-ard, S.; Sretarugsa, P.; Sobhon, P.; Cummins, S.F. Identification and characterization of a crustacean female sex hormone in the giant freshwater prawn, Macrobrachium rosenbergii. Aquaculture 2019, 507, 56–68. [Google Scholar] [CrossRef]
- Saco, A.; Rey-Campos, M.; Rosani, U.; Novoa, B.; Figueras, A. The evolution and diversity of interleukin-17 highlight an expansion in marine invertebrates and its conserved role in mucosal immunity. Front. Immunol. 2021, 12, 692997. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, C.; Chaves-Pozo, E.; Cuesta, A. Identification and regulation of interleukin-17 (IL-17) family ligands in the teleost fish European sea bass. Int. J. Mol. Sci. 2020, 21, 2439. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.F.; Wu, L.; Li, X.X. IL-17 family: Cytokines, receptors and signaling. Cytokine 2013, 64, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Rast, J.P.; Smith, L.C.; Loza-Coll, M.; Hibino, T.; Litman, G.W. Review—Genomic insights into the immune system of the sea urchin. Science 2006, 314, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Hibino, T.; Loza-Coll, M.; Messier, C.; Majeske, A.J.; Cohen, A.H.; Terwilliger, D.P.; Buckley, K.M.; Brockton, V.; Nair, S.V.; Berney, K.; et al. The immune gene repertoire encoded in the purple sea urchin genome. Dev. Biol. 2006, 300, 349–365. [Google Scholar] [CrossRef]
- Huang, X.D.; Zhang, H.; He, M.X. Comparative and evolutionary analysis of the Interleukin 17 gene family in invertebrates. PLoS ONE 2015, 10, e0132802. [Google Scholar] [CrossRef]
- Liu, F.; Huang, L.; Liu, A.; Jiang, Q.; Huang, H.; Ye, H. Identification of a putative CFSH receptor inhibiting IAG expression in crabs. Int. J. Mol. Sci. 2023, 24, 12240. [Google Scholar] [CrossRef]
- Baeza, J.A.; Behringer, D.C. Integrative taxonomy of the ornamental ‘peppermint’ shrimp public market and population genetics of Lysmata boggessi, the most heavily traded species worldwide. PeerJ 2017, 5, e3786. [Google Scholar] [CrossRef]
- Gaffen, S.L. Structure and signalling in the IL-17 receptor family. Nat. Rev. Immunol. 2009, 9, 556–567. [Google Scholar] [CrossRef]
- Amatya, N.; Garg, A.V.; Gaffen, S.L. IL-17 signaling: The Yin and the Yang. Trends. Immunol. 2017, 38, 310–322. [Google Scholar] [CrossRef]
- Cao, W.Q.; Wang, W.L.; Fan, S.Q.; Li, J.L.; Li, Q.; Wu, S.S.; Wang, L.L.; Song, L.S. The receptor CgIL-17R1 expressed in granulocytes mediates the CgIL-17 induced haemocytes proliferation in Crassostrea gigas. Dev. Comp. Immunol. 2022, 131, 104376. [Google Scholar] [CrossRef]
- Rosani, U.; Varotto, L.; Gerdol, M.; Pallavicini, A.; Venier, P. IL-17 signaling components in bivalves: Comparative sequence analysis and involvement in the immune responses. Dev. Comp. Immunol. 2015, 52, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Terajima, D.; Yamada, S.; Uchino, R.; Ikawa, S.; Ikeda, M.; Shida, K.; Arai, Y.; Wang, H.G.; Satoh, N.; Satake, M. Identification and sequence of seventy-nine new transcripts expressed in hemocytes of Ciona intestinalis, three of which may be involved in characteristic cell-cell communication. DNA Res. 2003, 10, 203–212. [Google Scholar] [CrossRef] [PubMed][Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Liu, A.; Ye, H. Identification of Crustacean Female Sex Hormone Receptor Involved in Sexual Differentiation of a Hermaphroditic Shrimp. Biomolecules 2023, 13, 1456. https://doi.org/10.3390/biom13101456
Liu F, Liu A, Ye H. Identification of Crustacean Female Sex Hormone Receptor Involved in Sexual Differentiation of a Hermaphroditic Shrimp. Biomolecules. 2023; 13(10):1456. https://doi.org/10.3390/biom13101456
Chicago/Turabian StyleLiu, Fang, An Liu, and Haihui Ye. 2023. "Identification of Crustacean Female Sex Hormone Receptor Involved in Sexual Differentiation of a Hermaphroditic Shrimp" Biomolecules 13, no. 10: 1456. https://doi.org/10.3390/biom13101456
APA StyleLiu, F., Liu, A., & Ye, H. (2023). Identification of Crustacean Female Sex Hormone Receptor Involved in Sexual Differentiation of a Hermaphroditic Shrimp. Biomolecules, 13(10), 1456. https://doi.org/10.3390/biom13101456








