In Vitro and In Vivo Chaperone Effect of (R)-2-amino-6-(1R, 2S)-1,2-dihydroxypropyl)-5,6,7,8-tetrahydropterin-4(3H)-one on the C1473G Mutant Tryptophan Hydroxylase 2
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drug and Treatment
2.3. Experimental Design and Treatment
2.4. Tail Suspension Test
2.5. Tissue Preparation
2.6. Assay of BH4, 5-HT and 5-HIAA Levels
2.7. Assay of TPH Activity
2.8. Assay of BH4 Effect on TPH2 Thermal Stability
2.9. Statistics
3. Results
3.1. Hind-Limb Dystonia in Balb/c and C57BL/6 Mice (Experiment 1)
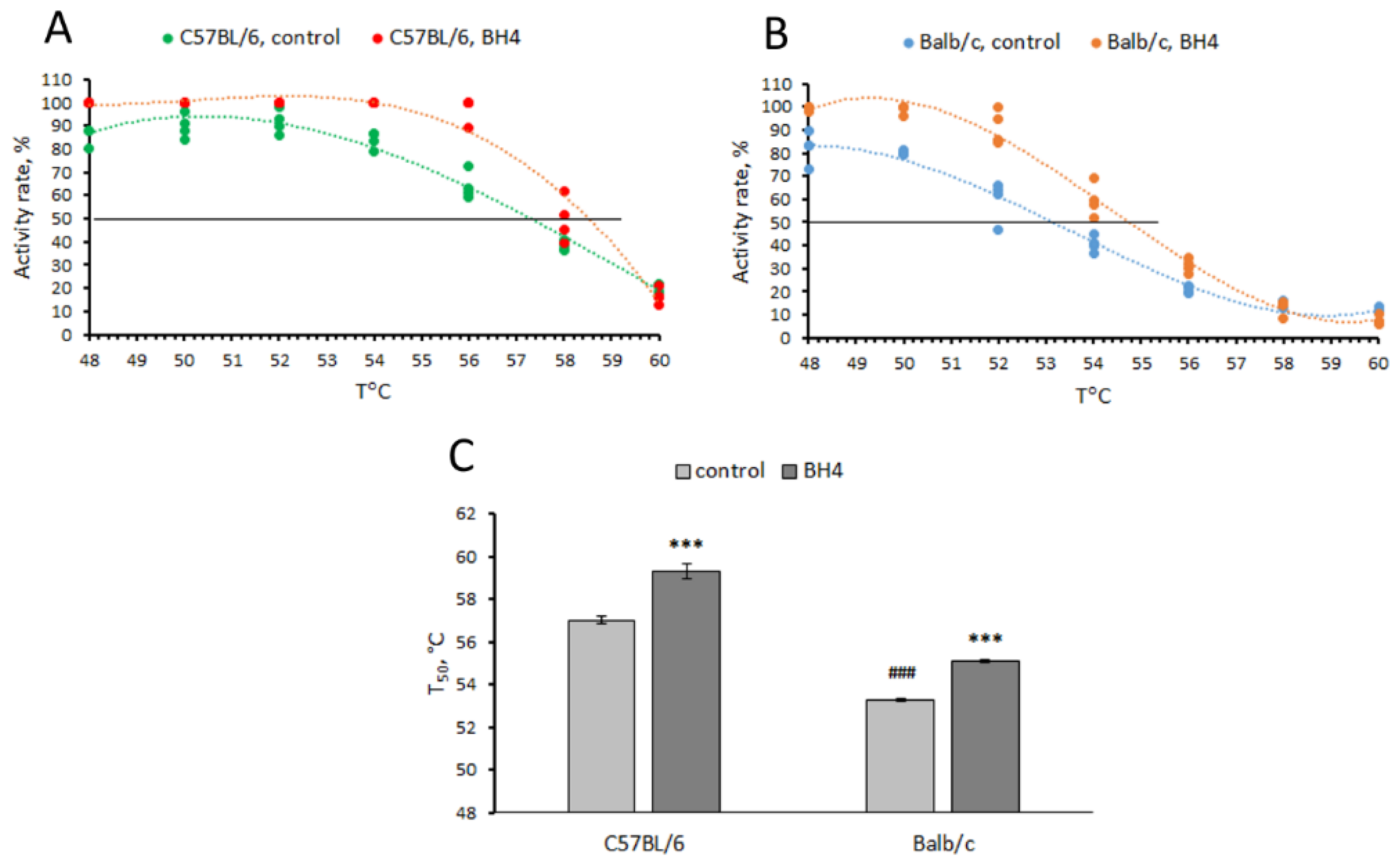
3.2. Effect of BH4 on Thermal Stability (T50) of TPH2 Extracted from Midbrain of C57BL/6 and Balb/c Mice (Experiment 2)
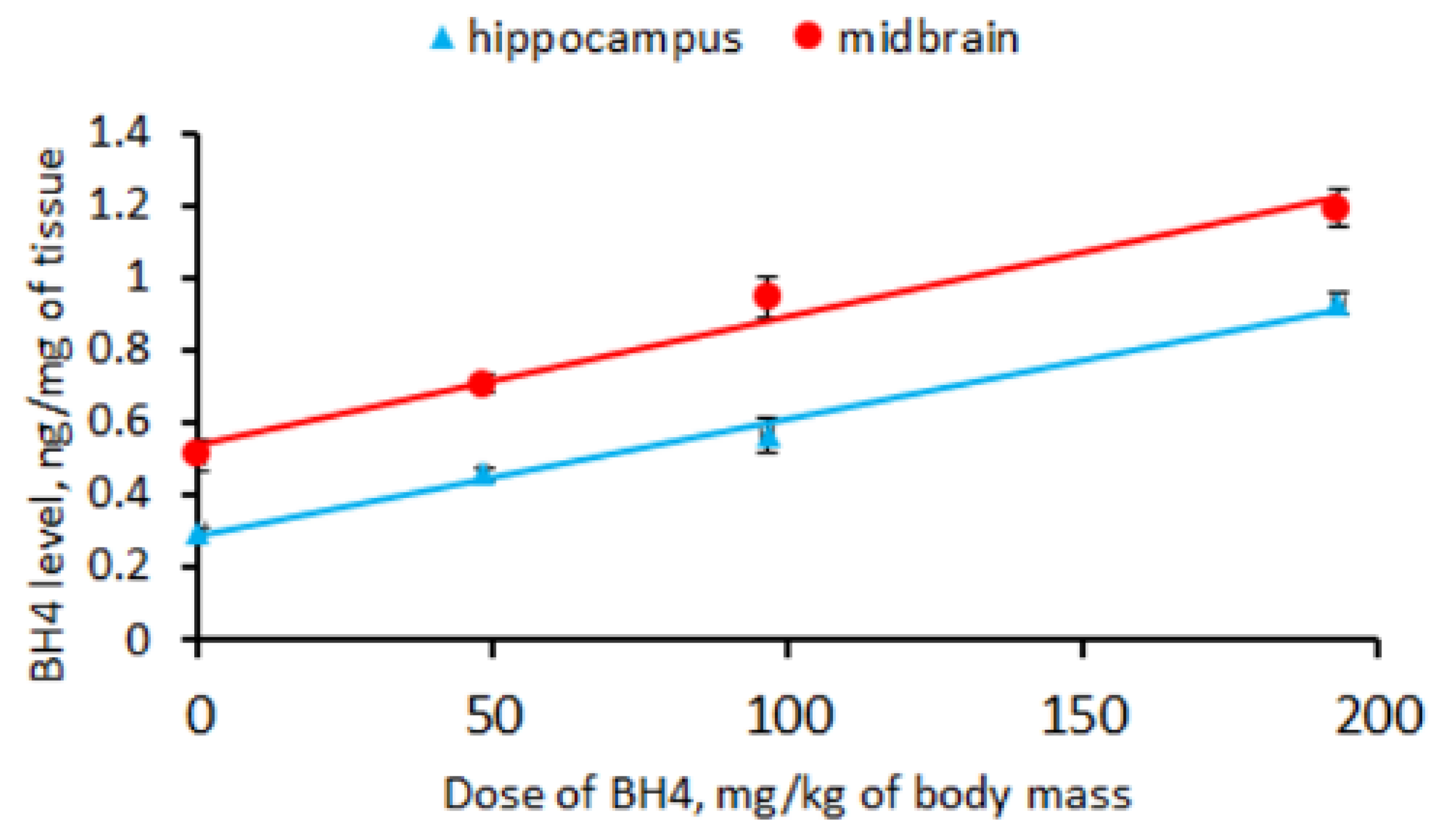
3.3. BH4 Level in Hippocampus and Midbrain of CD-1 Mice an Hour after a Single Intraperitoneal Administration of This Drug (Experiment 3)
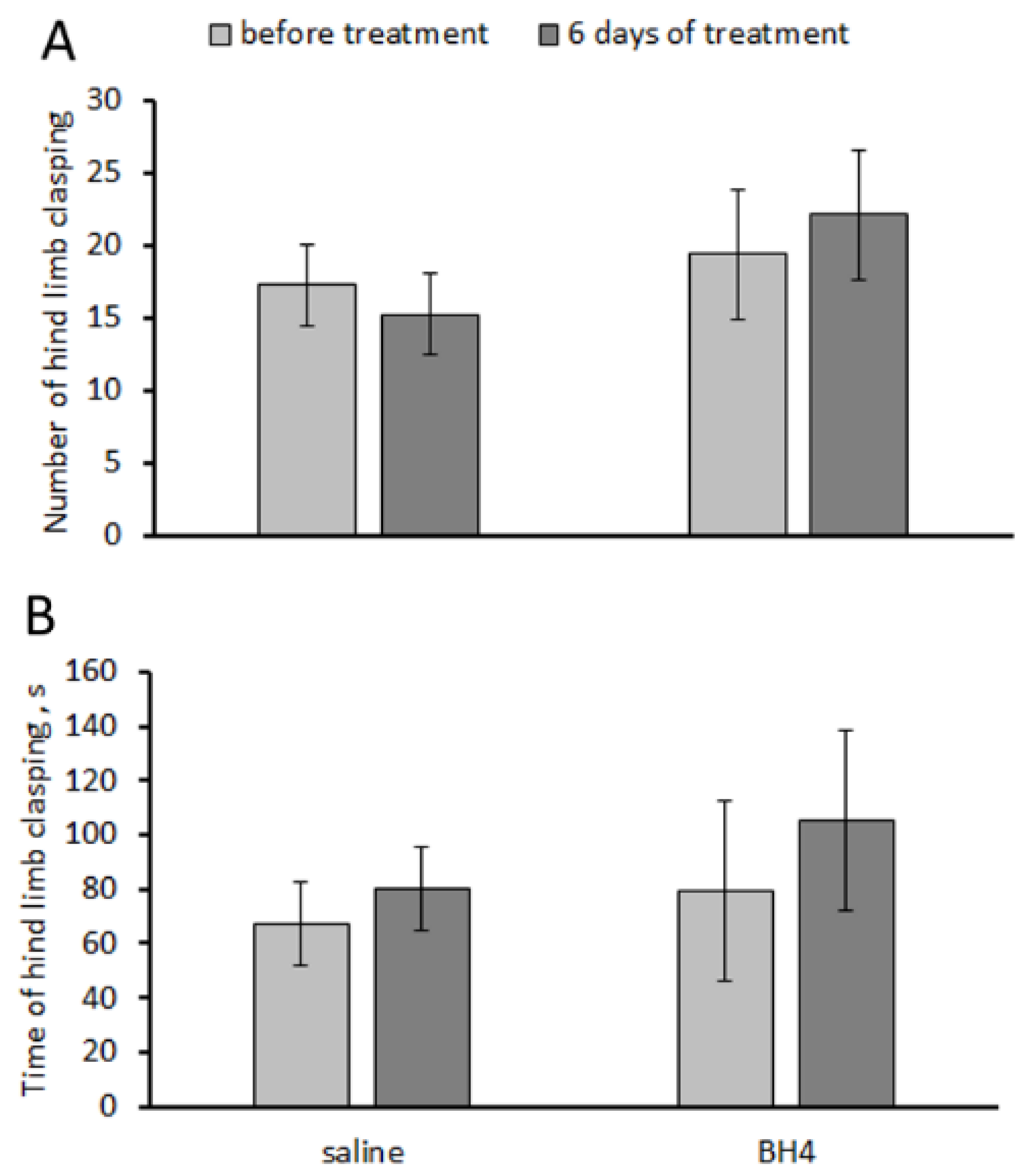
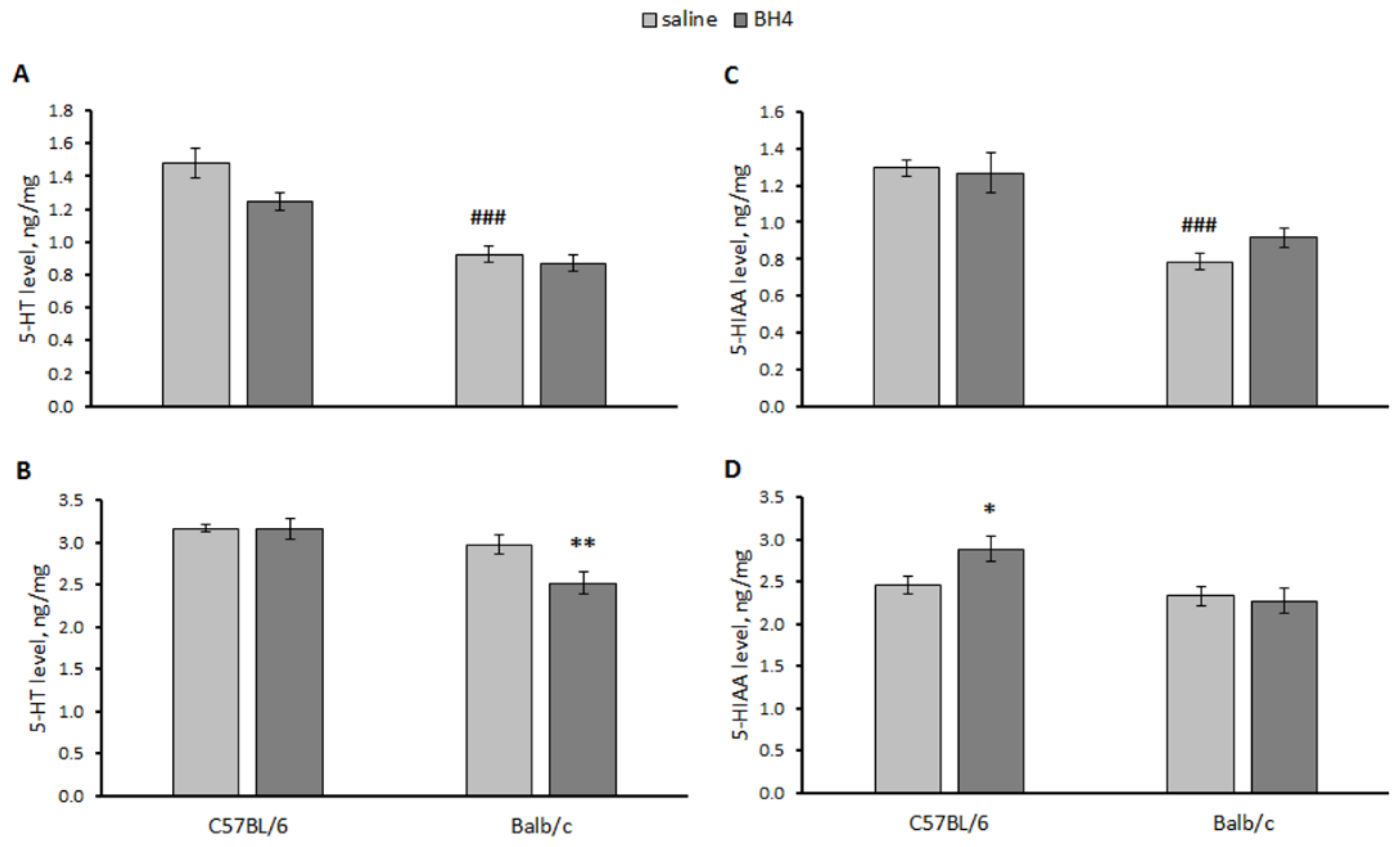
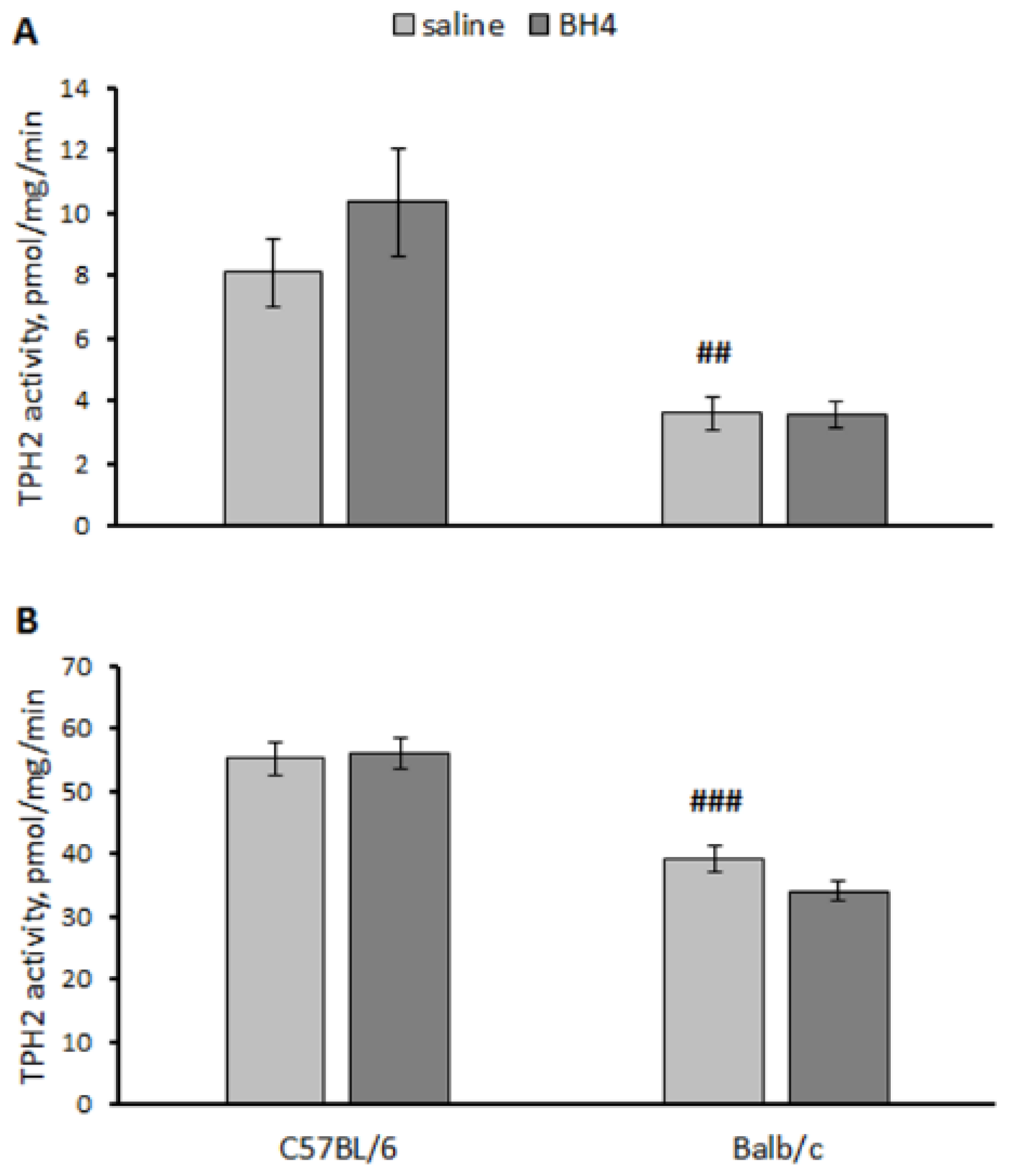
3.4. Effect of Chronic BH4 Intraperitoneal Administration on Hind-Limb Dystonia; BH4, 5-HT and 5-HIAA Levels; and TPH2 Activity in the Hippocampus and Midbrain of Balb/c Mice (Experiment 4)
3.5. Effect of Acute BH4 Intraventricular Administration on BH4, 5-HT and 5-HIAA Levels and TPH2 Activity in yjr Hippocampus and Midbrain of Balb/c and C57BL/6 Mice (Experiment 5)
4. Discussion
5. Conclusions
- In the tail suspension test, the mutant 1473G allele increased the frequency and duration of hind-limb dystonia in Balb/c mice compared to the wild-type 1473C allele in C57BL/6 mice.
- In vitro, the 1473G allele decreased the thermal stability of the TPH2 molecule (decreased T50 value) compared to the 1473C allele. BH4 at a concentration of 0.2 mM increased the thermal stability (increases T50 value) of mutant and wild-type TPH2 molecules.
- Only 0.32–0.35% of BH4 penetrated the murine brain an hour after intraperitoneal drug administration.
- Chronic intraperitoneal administration of 48.3 mg/kg of BH4 twice per day for 7 days failed to decrease hind-leg dystonia and alter 5-HT and 5-HIAA levels and TPH2 activity in the hippocampus and midbrain of Balb/c mice (homozygous for the 1473G allele).
- A single intraventricular administration of 60 μg of BH4 failed to alter the TPH2 activity in the hippocampus and the midbrain in Balb/c and C57BL/6 mice.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walther, D.J.; Peter, J.U.; Bashammakh, S.; Hörtnagl, H.; Voits, M.; Fink, H.; Bader, M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 2003, 299, 76. [Google Scholar] [CrossRef] [PubMed]
- Walther, D.J.; Bader, M. A unique central tryptophan hydroxylase isoform. Biochem. Pharmacol. 2003, 66, 1673–1680. [Google Scholar] [CrossRef]
- Gutknecht, L.; Waider, J.; Kraft, S.; Kriegebaum, C.; Holtmann, B.; Reif, A.; Schmitt, A.; Lesch, K.P. Deficiency of brain 5-HT synthesis but serotonergic neuron formation in Tph2 knockout mice. J. Neural. Transm. 2008, 115, 1127–1132. [Google Scholar] [CrossRef]
- Savelieva, K.V.; Zhao, S.; Pogorelov, V.M.; Rajan, I.; Yang, Q.; Cullinan, E.; Lanthorn, T.H. Genetic disruption of both tryp-tophan hydroxylase genes dramatically reduces serotonin and affects behavior in models sensitive to antidepressants. PLoS ONE 2008, 3, e3301. [Google Scholar] [CrossRef] [PubMed]
- Alenina, N.; Kikic, D.; Todiras, M.; Mosienko, V.; Qadri, F.; Plehm, R.; Boyé, P.; Vilianovitch, L.; Sohr, R.; Tenner, K.; et al. Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc. Natl. Acad. Sci. USA 2009, 106, 10332–10337. [Google Scholar] [CrossRef]
- Cervo, L.; Canetta, A.; Calcagno, E.; Burbassi, S.; Sacchetti, G.; Caccia, S.; Fracasso, C.; Albani, D.; Forloni, G.; Invernizzi, R.W. Genotype-dependent activity of tryptophan hydroxylase-2 determines the response to citalopram in a mouse model of depression. J. Neurosci. 2005, 25, 8165–8172. [Google Scholar] [CrossRef]
- Kulikov, A.V.; Osipova, D.V.; Naumenko, V.S.; Terenina, E.; Mormède, P.; Popova, N.K. A pharmacological evidence of positive association between mouse intermale aggression and brain serotonin metabolism. Behav. Brain Res. 2012, 233, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Popova, N.K.; Kulikov, A.V. Targeting tryptophan hydroxylase 2 in affective disorder. Expert Opin. Ther. Targets 2010, 14, 1259–1271. [Google Scholar] [CrossRef]
- Ottenhof, K.W.; Sild, M.; Lévesque, M.L.; Ruhé, H.G.; Booij, L. TPH2 polymorphisms across the spectrum of psychiatric morbidity: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2018, 92, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, P.F. The aromatic amino acid hydroxylases: Structures, catalysis, and regulation of phenylalanine hydroxylase, tyrosine hydroxylase, and tryptophan hydroxylase. Arch. Biochem. Biophys. 2023, 735, 109518. [Google Scholar] [CrossRef]
- Pey, A.L.; Ying, M.; Cremades, N.; Velazquez-Campoy, A.; Scherer, T.; Thöny, B.; Sancho, J.; Martinez, A. Identification of pharmacological chaperones as potential therapeutic agents to treat phenylketonuria. J. Clin. Investig. 2008, 118, 2858–2867. [Google Scholar] [CrossRef] [PubMed]
- Calvo, A.C.; Scherer, T.; Pey, A.L.; Ying, M.; Winge, I.; McKinney, J.; Haavik, J.; Thöny, B.; Martinez, A. Effect of pharmaco-logical chaperones on brain tyrosine hydroxylase and tryptophan hydroxylase 2. J. Neurochem. 2010, 114, 853–863. [Google Scholar] [CrossRef]
- Waløen, K.; Kleppe, R.; Martinez, A.; Haavik, J. Tyrosine and tryptophan hydroxylases as therapeutic targets in human disease. Expert Opin. Ther. Targets 2017, 21, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Thöny, B.; Calvo, A.C.; Scherer, T.; Svebak, R.M.; Haavik, J.; Blau, N.; Martinez, A. Tetrahydrobiopterin shows chaperone activity for tyrosine hydroxylase. J. Neurochem. 2008, 106, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Nicholls, P.J.; Laje, G.; Sotnikova, T.D.; Gainetdinov, R.R.; Albert, P.R.; Rajkowska, G.; Stockmeier, C.A.; Speer, M.C.; Steffens, D.C.; et al. A functional alternative splicing mutation in human tryptophan hydroxylase-2. Mol. Psychiatry 2011, 16, 1169–1176. [Google Scholar] [CrossRef][Green Version]
- Lin, Y.M.; Chao, S.C.; Chen, T.M.; Lai, T.J.; Chen, J.S.; Sun, H.S. Association of functional polymorphisms of the human tryptophan hydroxylase 2 gene with risk for bipolar disorder in Han Chinese. Arch. Gen. Psychiatry 2007, 64, 1015–1024. [Google Scholar] [CrossRef]
- Scheuch, K.; Lautenschlager, M.; Grohmann, M.; Stahlberg, S.; Kirchheiner, J.; Zill, P.; Heinz, A.; Walther, D.J.; Priller, J. Characterization of a functional promoter polymorphism of the human tryptophan hydroxylase 2 gene in serotonergic raphe neurons. Biol. Psychiatry 2007, 62, 1288–1294. [Google Scholar] [CrossRef]
- Zhang, X.; Gainetdinov, R.R.; Beaulieu, J.M.; Sotnikova, T.D.; Burch, L.H.; Williams, R.B.; Schwartz, D.A.; Krishnan, K.R.; Caron, M.G. Loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron 2005, 45, 11–16. [Google Scholar] [CrossRef]
- McKinney, J.; Johansson, S.; Halmøy, A.; Dramsdahl, M.; Winge, I.; Knappskog, P.M.; Haavik, J. A loss-of-function mutation in tryptophan hydroxylase 2 segregating with attention-deficit/hyperactivity disorder. Mol. Psychiatry 2008, 13, 365–367. [Google Scholar] [CrossRef]
- McKinney, J.A.; Turel, B.; Winge, I.; Knappskog, P.M.; Haavik, J. Functional properties of missense variants of human tryptophan hydroxylase 2. Hum. Mutat. 2009, 30, 787–794. [Google Scholar] [CrossRef]
- Beaulieu, J.M.; Zhang, X.; Rodriguiz, R.M.; Sotnikova, T.D.; Cools, M.J.; Wetsel, W.C.; Gainetdinov, R.R.; Caron, M.G. Role of GSK3 beta in behavioral abnormalities induced by serotonin deficiency. Proc. Natl. Acad. Sci. USA 2008, 105, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Beaulieu, J.M.; Sotnikova, T.D.; Gainetdinov, R.R.; Caron, M.G. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science 2004, 305, 217. [Google Scholar] [CrossRef] [PubMed]
- Kulikov, A.V.; Osipova, D.V.; Naumenko, V.S.; Popova, N.K. Association between Tph2 gene polymorphism, brain tryptophan hydroxylase activity and aggressiveness in mouse strains. Genes Brain Behav. 2005, 4, 482–485. [Google Scholar] [CrossRef]
- Kulikova, E.A.; Kulikov, A.V. Tryptophan hydroxylase 2 as a therapeutic target for psychiatric disorders: Focus on animal models. Expert Opin. Ther. Targets 2019, 23, 655–667. [Google Scholar] [CrossRef]
- Osipova, D.V.; Kulikov, A.V.; Mekada, K.; Yoshiki, A.; Moshkin, M.P.; Kotenkova, E.V.; Popova, N.K. Distribution of the C1473G polymorphism in tryptophan hydroxylase 2 gene in laboratory and wild mice. Genes Brain Behav. 2010, 9, 537–543. [Google Scholar] [CrossRef]
- Kulikov, A.V.; Osipova, D.V.; Popova, N.K. The C1473G polymorphism in gene tph2 is the main factor mediating the genetically defined variability of tryptophan hydroxylase-2 activity in the mouse brain. Genetika 2007, 43, 1676–1681. [Google Scholar] [CrossRef] [PubMed]
- Komleva, P.D.; Alhalabi, G.; Izyurov, A.E.; Khotskin, N.V.; Kulikov, A.V. Effects of the Combination of the C1473G Mutation in the Tph2 Gene and Lethal Yellow Mutations in the Raly-Agouti Locus on Behavior, Brain 5-HT and Melanocortin Systems in Mice. Biomolecules 2023, 13, 963. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Sumi-Ichinose, C.; Kaji, R.; Ikemoto, K.; Nomura, T.; Nagatsu, I.; Ichinose, H.; Ito, M.; Sako, W.; Nagahiro, S.; et al. Differential involvement of striosome and matrix dopamine systems in a transgenic model of dopa-responsive dystonia. Proc. Natl. Acad. Sci. USA 2008, 105, 12551–12556. [Google Scholar] [CrossRef]
- Eichwald, T.; da Silva, L.B.; Staats Pires, A.C.; Niero, L.; Schnorrenberger, E.; Filho, C.C.; Espíndola, G.; Huang, W.L.; Guillemin, G.J.; Abdenur, J.E.; et al. Tetrahydrobiopterin: Beyond Its Traditional Role as a Cofactor. Antioxidants 2023, 12, 1037. [Google Scholar] [CrossRef]
- Opladen, T.; López-Laso, E.; Cortès-Saladelafont, E.; Pearson, T.S.; Sivri, H.S.; Yildiz, Y.; Assmann, B.; Kurian, M.A.; Leuzzi, V.; Heales, S.; et al. Consensus guideline for the diagnosis and treatment of tetrahydrobiopterin (BH4 deficiencies. Orphanet J. Rare Dis. 2020, 15, 126. [Google Scholar] [CrossRef]
- Blau, N.; Bélanger-Quintana, A.; Demirkol, M.; Feillet, F.; Giovannini, M.; MacDonald, A.; Trefz, F.K.; van Spronsen, F.J. Optimizing the use of sapropterin (BH(4)) in the management of phenylketonuria. Mol. Genet. Metab. 2009, 96, 158–163. [Google Scholar] [CrossRef]
- Pan, L.; McKain, B.W.; Madan-Khetarpal, S.; Mcguire, M.; Diler, R.S.; Perel, J.M.; Vockley, J.; Brent, D.A. GTP-cyclohydrolase deficiency responsive to sapropterin and 5-HTP supplementation: Relief of treatment-refractory depression and suicidal behaviour. BMJ Case Rep. 2011, 2011, bcr0320113927. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; DeLatorre, R.; Taylor, H.B.; Slattery, J.; Melnyk, S.; Chowdhury, N.; James, S.J. Metabolic effects of sapropterin treatment in autism spectrum disorder: A preliminary study. Transl. Psychiatry 2013, 3, e237. [Google Scholar] [CrossRef] [PubMed]
- Winn, S.R.; Scherer, T.; Thöny, B.; Harding, C.O. High dose sapropterin dihydrochloride therapy improves monoamine neurotransmitter turnover in murine phenylketonuria (PKU). Mol. Genet. Metab. 2016, 117, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Muntau, A.C.; Burlina, A.; Eyskens, F.; Freisinger, P.; Leuzzi, V.; Sivri, H.S.; Gramer, G.; Pazdírková, R.; Cleary, M.; Lotz-Havla, A.S.; et al. Long-term efficacy and safety of sapropterin in patients who initiated sapropterin at <4 years of age with phenylketonuria: Results of the 3-year extension of the SPARK open-label, multicentre, randomised phase IIIb trial. Orphanet J. Rare Dis. 2021, 16, 341. [Google Scholar] [CrossRef]
- Meek, J.L.; Neff, N.H. Tryptophan 5-hydroxylase: Approximation of half-life and rate of axonal transport. J. Neurochem. 1972, 19, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Aboagye, B.; Weber, T.; Merdian, H.L.; Bartsch, D.; Lesch, K.P.; Waider, J. Serotonin deficiency induced after brain maturation rescues consequences of early life adversity. Sci. Rep. 2021, 11, 5368. [Google Scholar] [CrossRef]
- Korovesi, A.G.; Anagnostopoulos, A.K.; Pierros, V.; Stravopodis, D.J.; Tsangaris, G.T. Normal Mouse Brain Proteome II: Analysis of Brain Regions by High-resolution Mass Spectrometry. Cancer Genom. Proteom. 2020, 17, 757–767. [Google Scholar] [CrossRef]
- Feillet, F.; Clarke, L.; Meli, C.; Lipson, M.; Morris, A.A.; Harmatz, P.; Mould, D.R.; Green, B.; Dorenbaum, A.; Giovannini, M.; et al. Pharmacokinetics of sapropterin in patients with phenylketonuria. Clin. Pharmacokinet. 2008, 47, 817–825. [Google Scholar] [CrossRef]
- Fanet, H.; Capuron, L.; Castanon, N.; Calon, F.; Vancassel, S. Tetrahydrobioterin (BH4) Pathway: From Metabolism to Neuropsychiatry. Curr. Neuropharmacol. 2021, 19, 591–609. [Google Scholar] [CrossRef]
- Fanet, H.; Tournissac, M.; Leclerc, M.; Caron, V.; Tremblay, C.; Vancassel, S.; Calon, F. Tetrahydrobiopterin Improves Recognition Memory in the Triple-Transgenic Mouse Model of Alzheimer’s Disease, Without Altering Amyloid-β and Tau Pathologies. J. Alzheimers Dis. 2021, 79, 709–727. [Google Scholar] [CrossRef] [PubMed]


| Factor | Number | Accumulated Time |
|---|---|---|
| “Treatment” | F(1,14) = 1.2, p = 0.30 | F(1,14) < 1 |
| “Duration” | F(1,14) < 1 | F(1,14) < 1 |
| “Treatment” × “Duration” | F(1,14) < 1 | F(1,14) < 1 |
| Factor | 5-HT | 5-HIAA |
|---|---|---|
| Hippocampus | ||
| “Genotype” | F(1,27) = 60.4, p < 0.001 | F(1,27) = 37.2, p < 0.001 |
| “Treatment” | F(1,27) = 5.67, p = 0.025 | F(1,27) < 1 |
| “Genotype” × “Treatment” | F(1,27) = 2.16, p = 0.15 | F(1,27) = 1.22, p = 0.22 |
| Midbrain | ||
| “Genotype” | F(1,27) = 13.34, p = 0.001 | F(1,27) = 7.38, p = 0.011 |
| “Treatment” | F(1,27) = 4.21, p = 0.049 | F(1,27) = 1.88, p = 0.18 |
| “Genotype” × “Treatment” | F(1,27) = 4.0, p = 0.055 | F(1,27) = 2.16, p = 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arefieva, A.B.; Komleva, P.D.; Naumenko, V.S.; Khotskin, N.V.; Kulikov, A.V. In Vitro and In Vivo Chaperone Effect of (R)-2-amino-6-(1R, 2S)-1,2-dihydroxypropyl)-5,6,7,8-tetrahydropterin-4(3H)-one on the C1473G Mutant Tryptophan Hydroxylase 2. Biomolecules 2023, 13, 1458. https://doi.org/10.3390/biom13101458
Arefieva AB, Komleva PD, Naumenko VS, Khotskin NV, Kulikov AV. In Vitro and In Vivo Chaperone Effect of (R)-2-amino-6-(1R, 2S)-1,2-dihydroxypropyl)-5,6,7,8-tetrahydropterin-4(3H)-one on the C1473G Mutant Tryptophan Hydroxylase 2. Biomolecules. 2023; 13(10):1458. https://doi.org/10.3390/biom13101458
Chicago/Turabian StyleArefieva, Alla B., Polina D. Komleva, Vladimir S. Naumenko, Nikita V. Khotskin, and Alexander V. Kulikov. 2023. "In Vitro and In Vivo Chaperone Effect of (R)-2-amino-6-(1R, 2S)-1,2-dihydroxypropyl)-5,6,7,8-tetrahydropterin-4(3H)-one on the C1473G Mutant Tryptophan Hydroxylase 2" Biomolecules 13, no. 10: 1458. https://doi.org/10.3390/biom13101458
APA StyleArefieva, A. B., Komleva, P. D., Naumenko, V. S., Khotskin, N. V., & Kulikov, A. V. (2023). In Vitro and In Vivo Chaperone Effect of (R)-2-amino-6-(1R, 2S)-1,2-dihydroxypropyl)-5,6,7,8-tetrahydropterin-4(3H)-one on the C1473G Mutant Tryptophan Hydroxylase 2. Biomolecules, 13(10), 1458. https://doi.org/10.3390/biom13101458









