Silencing of Glutamine: Fructose-6-Phosphate Aminotransferase Impairs Growth and Development in Sogatella furcifera (Hemiptera: Delphacidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing and Sample Collection
2.2. Primer Design
2.3. RNA Isolation and cDNA Synthesis
2.4. Cloning of SfGFAT
2.5. Bioinformatics Analyses
2.6. Real-Time Quantitative PCR (RT-qPCR) Analysis of SfGFAT Expression Levels
2.7. Functional Analysis of SfGFAT Using RNAi
2.8. Statistical Analysis
3. Results
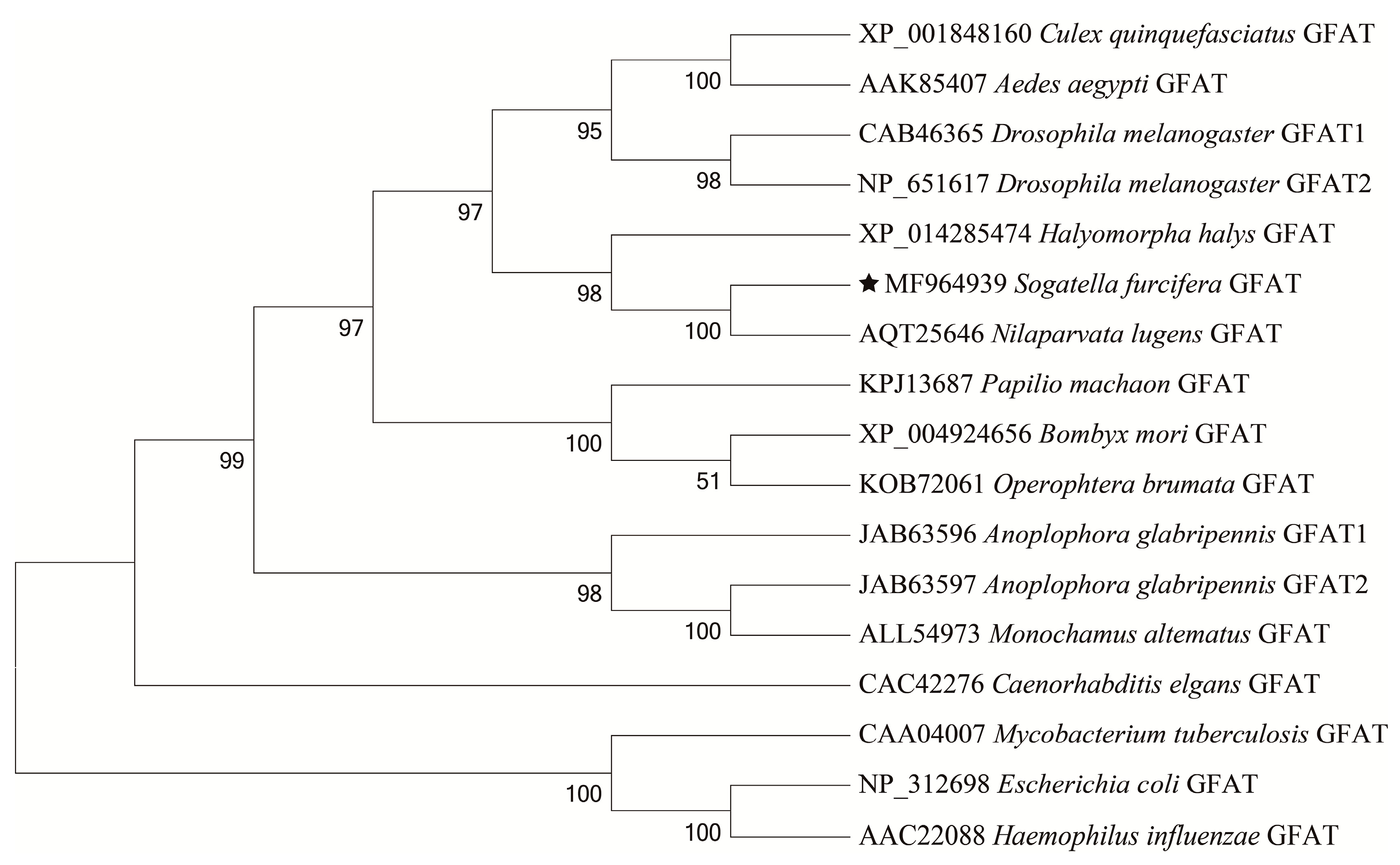
3.1. Identification and Sequence Analysis of SfGFAT
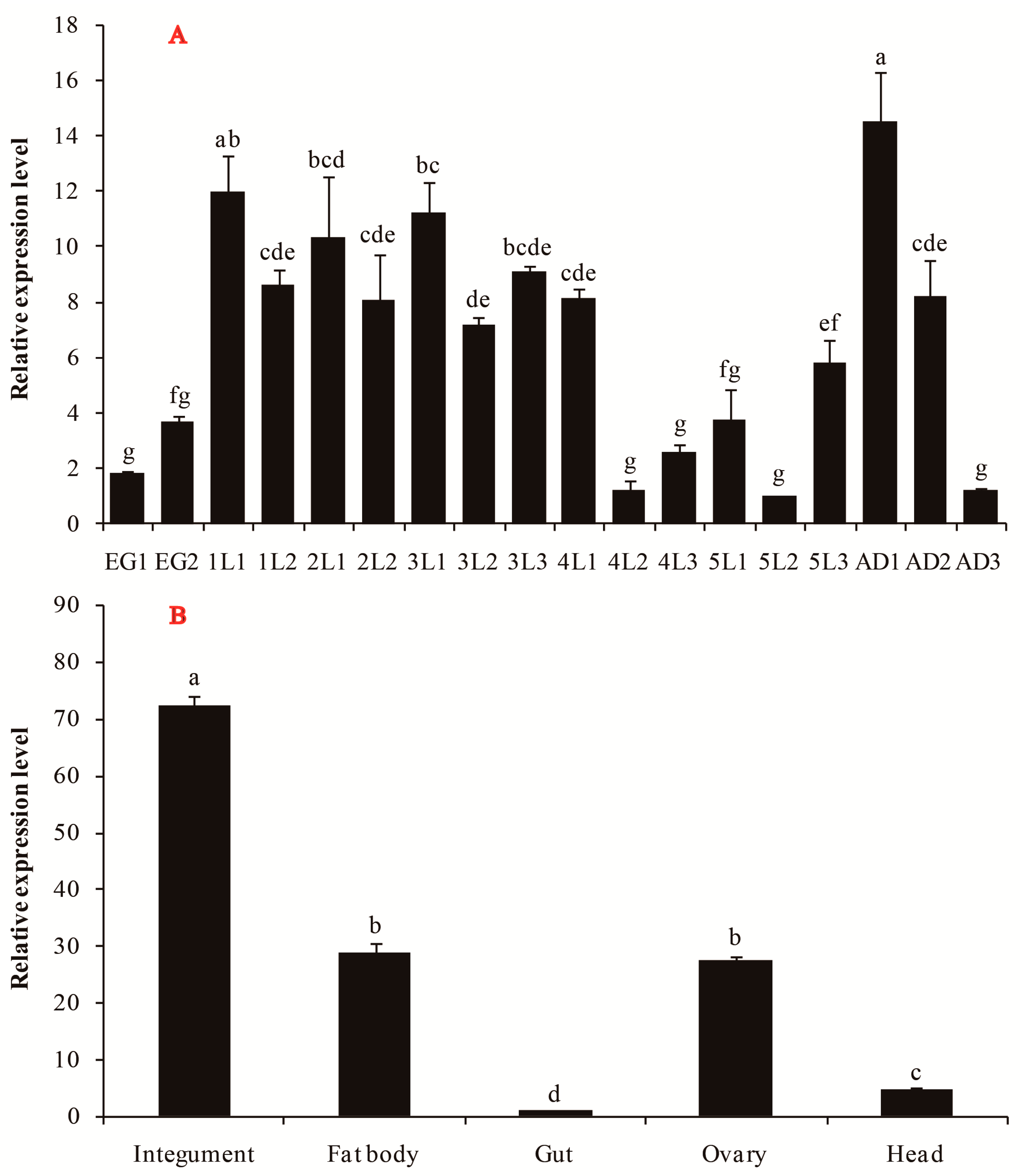
3.2. Spatiotemporal Expression Profile of SfGFAT in S. furcifera
3.3. Functional Analysis of SfGFAT
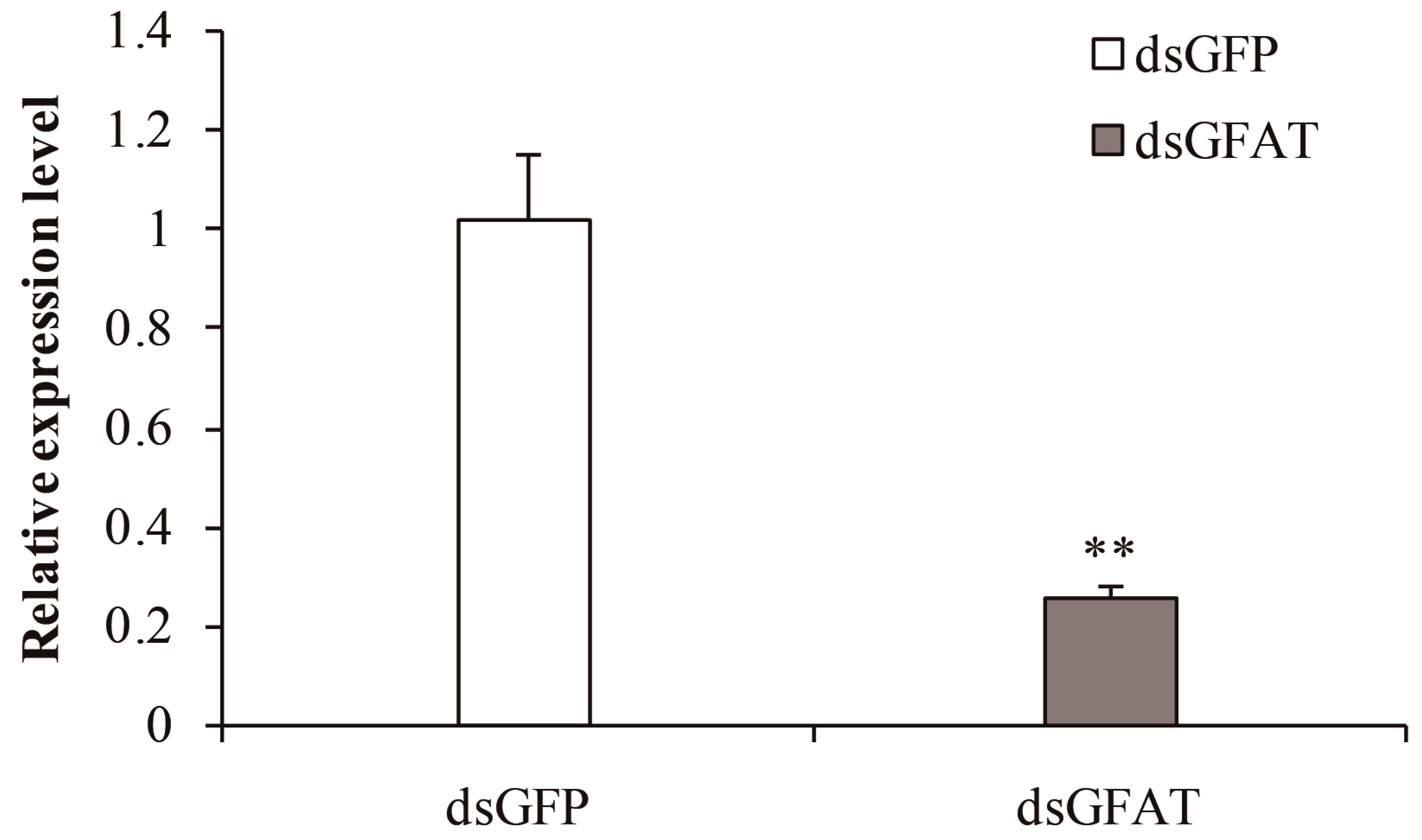
3.3.1. Analysis of SfGFAT mRNA Levels and Survival after RNAi Exposure
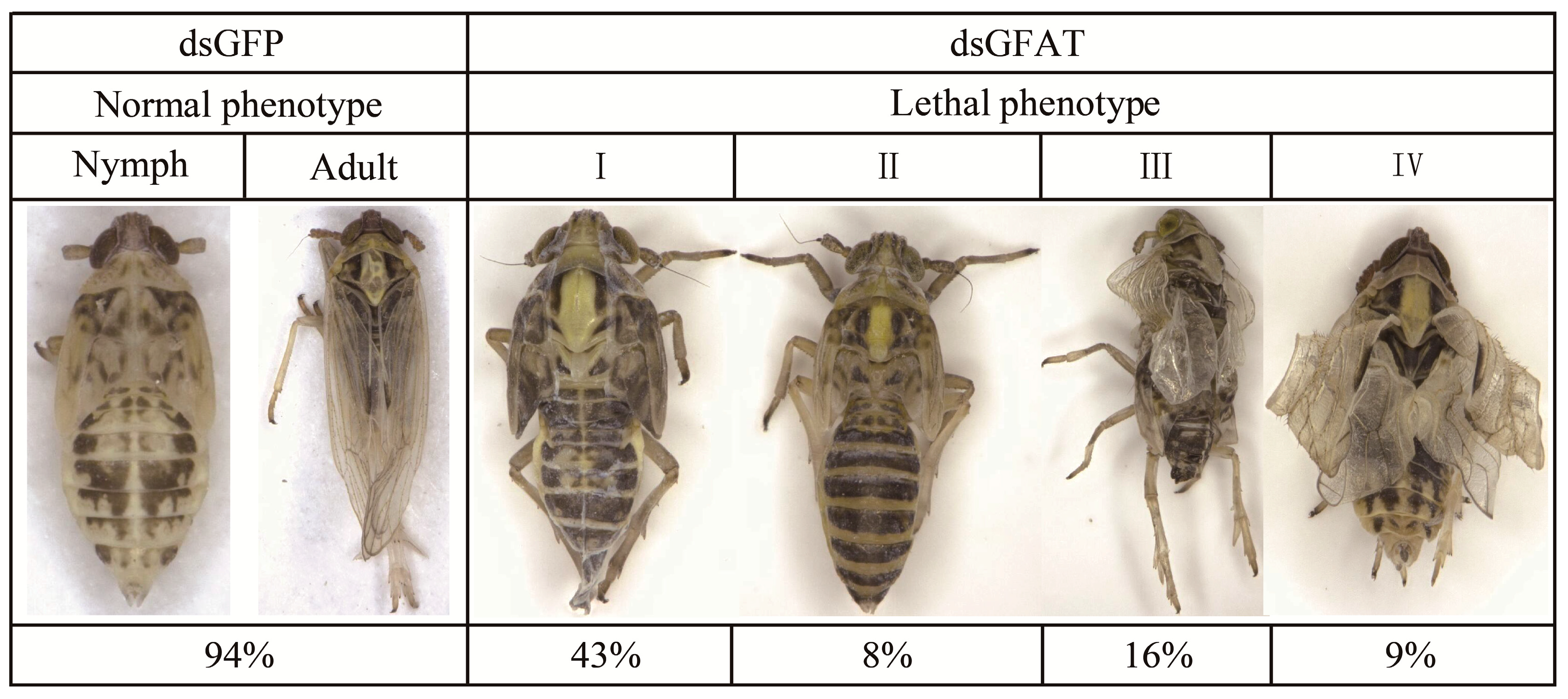
3.3.2. Phenotype Analysis after RNAi
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, S.H.; Cheng, C.H.; Chen, C.N.; Wu, W.J.; Otuka, A. Estimating the immigration source of rice planthoppers, Nilaparvata lugens (Stål) and Sogatella furcifera (Horváth) (Homoptera: Delphacidae), in Taiwan. Appl. Entomol. Zool. 2010, 45, 521–531. [Google Scholar] [CrossRef][Green Version]
- Mao, K.K.; Ren, Z.J.; Li, W.H.; Liu, C.Y.; Xu, P.F.; He, S.; Li, J.H.; Wan, H. An insecticide resistance diagnostic kit for whitebacked planthopper Sogatella furcifera (Horváth). J. Pest. Sci. 2021, 94, 531–540. [Google Scholar] [CrossRef]
- Long, G.Y.; Yang, J.P.; Jin, D.C.; Yang, H.; Zhou, C.; Wang, Z.; Yang, X.B. Silencing of Decapentaplegic (Dpp) gene inhibited the wing expansion in the white-backed planthopper, Sogatella furcifera (Horváth) (Hemiptera: Delphacidae). Arch. Insect Biochem. Physiol. 2022, 110, e21879. [Google Scholar] [CrossRef]
- Zhu, Z.R.; Cheng, J.A. Sucking rates of the white-backed planthopper Sogatella furcifera (Horv.) (Homoptera: Delphacidae) and yield loss of rice. J. Pest Sci. 2002, 75, 113–117. [Google Scholar] [CrossRef]
- Zheng, D.B.; Hu, G.; Yang, F.; Du, X.D.; Yang, H.B.; Zhang, G.; Qi, G.J.; Liang, Z.L.; Zhang, X.X.; Cheng, X.N.; et al. Ovarian development status and population characteristics of Sogatella furcifera (Horváth) and Nilaparvata lugens (Stål): Implications for pest forecasting. J. Appl. Entomol. 2014, 138, 67–77. [Google Scholar] [CrossRef]
- Ruan, Y.W.; Wang, X.G.; Xiang, X.; Xu, X.; Guo, Y.Q.; Liu, Y.H.; Yin, Y.; Wu, Y.Q.; Cheng, Q.H.; Gong, C.W.; et al. Status of insecticide resistance and biochemical characterization of chlorpyrifos resistance in Sogatella furcifera (Hemiptera: Delphacidae) in Sichuan Province, China. Pestic. Biochem. Phys. 2021, 171, 104723. [Google Scholar] [CrossRef]
- Liu, Y.T.; Song, X.Y.; Zeng, B.; Zhang, W.J.; Chen, X.Y.; Feng, Z.R.; Yu, H.Y.; Gao, C.F.; Wu, S.F. The evolution of insecticide resistance in the white backed planthopper Sogatella furcifera (Horváth) of China in the period 2014–2022. Crop Prot. 2023, 172, 106312. [Google Scholar] [CrossRef]
- Zhou, G.H.; Wen, J.J.; Cai, D.J.; Li, P.; Xu, D.L.; Zhang, S.G. Southern rice black-streaked dwarf virus: A new proposed Fijivirus species in the family Reoviridae. Chin. Sci. Bull. 2008, 53, 3677–3685. [Google Scholar] [CrossRef]
- Matsukura, K.; Towata, T.; Yoshida, K.; Sakai, J.; Okuda, M.; Onuki, M.; Matsumura, M. Quantitative analysis of southern rice black-streaked dwarf virus in Sogatella furcifera and virus threshold for transmission. Phytopathology 2015, 105, 550–554. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, W.W.; Zhang, X.W.; Li, L.; Wang, X.F. Southern rice black-streaked dwarf virus hijacks SNARE complex of its insect vector for its effective transmission to rice. Mol. Plant Pathol. 2021, 22, 1256–1270. [Google Scholar] [CrossRef]
- Long, G.Y.; Liu, L.L.; Yang, H.; Wang, Z.; Jin, D.C.; Zhou, C. Sublethal effects of pymetrozine on the development, reproduction and insecticidal susceptibility of Sogatella furcifera (Hemiptera:Delphacidae). Acta Entomol. Sinic. 2017, 60, 790–798. [Google Scholar]
- Chen, L.; Wang, X.G.; Zhang, Y.Z.; Yang, Y.; Zhang, S.R.; Xu, X.; Zhu, M.J.; Gong, C.W.; Hasnain, A.; Shen, L.T.; et al. The population growth, development and metabolic enzymes of the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae) under the sublethal dose of triflumezopyrim. Chemosphere 2020, 247, 125865. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Chen, H.S.; Wu, S.; Xia, F.J.; He, M.R.; Yang, L.; Li, R.Y.; Liao, X.; Li, M. Sublethal effects of nitenpyram on the biological traits and metabolic enzymes of the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae). Crop Prot. 2022, 155, 105931. [Google Scholar] [CrossRef]
- Zeng, Q.H.; Long, G.Y.; Yang, H.; Zhou, C.; Yang, X.B.; Wang, Z.; Jin, D.C. SfDicer1 participates in the regulation of molting development and reproduction in the white-backed planthopper, Sogatella furcifera. Pestic. Biochem. Phys. 2023, 191, 105347. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.; Zhou, C.; Yang, W.J.; Jin, D.C.; Long, G.Y. Molecular cloning, expression, and functional analysis of the chitin synthase 1 gene and its two alternative splicing variants in the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae). Sci. Rep. 2019, 9, 1087. [Google Scholar] [CrossRef]
- Dong, W.; Gao, Y.H.; Zhang, X.B.; Moussian, B.; Zhang, J.Z. Chitinase 10 controls chitin amounts and organization in the wing cuticle of Drosophila. Insect Sci. 2020, 27, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.H.; Mu, L.L.; Jin, L.; Anjum, A.A. RNAi for chitin synthase 1 rather than 2 causes growth delay and molting defect in Henosepilachna vigintioctopunctata. Pestic. Biochem. Phys. 2021, 178, 104934. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Merzendorfer, H.; Zhang, W.Q.; Zhang, J.Z.; Muthukrishnan, S. Biosynthesis, turnover, and functions of chitin in insects. Annu. Rev. Entomol. 2016, 61, 177–196. [Google Scholar] [CrossRef]
- Lu, Z.J.; Huang, Y.L.; Yu, H.Z.; Li, N.Y.; Xie, Y.X.; Zhang, Q.; Zeng, X.D.; Hu, H.; Huang, A.J.; Yi, L.; et al. Silencing of the chitin synthase gene is lethal to the Asian citrus psyllid, Diaphorina citri. Int. J. Mol. Sci. 2019, 20, 3734. [Google Scholar] [CrossRef]
- Cohen, E. Chitin synthesis and inhibition: A revisit. Pest Manag. Sci. 2001, 57, 946–950. [Google Scholar] [CrossRef]
- Merzendorfer, H.; Zimoch, L. Chitin metabolism in insects: Structure, function and regulation of chitin synthases and chitinases. J. Exp. Biol. 2003, 206, 4393–4412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.C.; Zou, H.; Geng, N.N.; Ding, N.; Wang, Y.J.; Zhang, J.; Zou, C.S. Fenoxycarb and methoxyfenozide (RH-2485) affected development and chitin synthesis through disturbing glycometabolism in Lymantria dispar larvae. Pestic. Biochem. Phys. 2020, 163, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Cooper, A.M.W.; Zhang, J.Z.; Zhu, K.Y. Biosynthesis, modifications and degradation of chitin in the formation and turnover of peritrophic matrix in insects. J. Insect Physiol. 2019, 114, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Palaka, B.K.; Ilavarasi, A.V.; Sapam, T.D.; Kotapati, K.V.; Nallala, V.S.; Khan, M.B.; Ampasala, D.R. Molecular cloning, gene expression analysis, and in silico characterization of UDP-N-acetylglucosamine pyrophosphorylase from Bombyx mori. Biotechnol. Appl. Biochem. 2019, 66, 880–899. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.H.; Mu, L.L.; Jin, L.; Anjum, A.A.; Li, G.Q. Silencing uridine diphosphate N-acetylglucosamine pyrophosphorylase gene impairs larval development in Henosepilachna vigintioctopunctata. Pest Manag. Sci. 2022, 78, 3894–3902. [Google Scholar] [CrossRef]
- Ranganathan, S.; Ilavarasi, A.V.; Palaka, B.K.; Kuppusamy, D.; Ampasala, D.R. Cloning, functional characterization and screening of potential inhibitors for Chilo partellus chitin synthase A using in silico, in vitro and in vivo approaches. J. Biomol. Struct. Dyn. 2022, 40, 1416–1429. [Google Scholar] [CrossRef]
- Neyman, V.; Francis, F.; Matagne, A.; Dieu, M.; Michaux, C.; Perpète, E.A. Purification and characterization of trehalase from Acyrthosiphon pisum, a target for pest control. Prot. J. 2022, 41, 189–200. [Google Scholar] [CrossRef]
- Denzel, M.S.; Antebi, A. Hexosamine pathway and (ER) protein quality control. Curr. Opin. Cell Biol. 2015, 33, 14–18. [Google Scholar] [CrossRef]
- Merzendorfer, H. Insect chitin synthases: A review. J. Comp. Physiol. B 2006, 176, 1–15. [Google Scholar] [CrossRef]
- Graack, H.R.; Cinque, U.; Kress, H. Functional regulation of glutamine: Fructose-6-phosphate aminotransferase 1 (GFAT1) of Drosophila melanogaster in a UDP-N-acetylglucosamine and cAMP-dependent manner. Biochem. J. 2001, 360, 401–412. [Google Scholar] [CrossRef]
- Kato, N.; Dasgupta, R.; Smartt, C.T.; Christensen, B.M. Glucosamine: Fructose-6-phosphate aminotransferase: Gene characterization, chitin biosynthesis and peritrophic matrix formation in Aedes aegypti. Insect Mol. Biol. 2002, 11, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.D.; Liu, Y.K.; Qiu, L.Y.; Wang, S.S.; Pan, B.Y.; Li, Y.; Wang, S.G.; Tang, B. GFAT and PFK genes show contrasting regulation of chitin metabolism in Nilaparvata lugens. Sci. Rep. 2021, 11, 5246. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Zhang, B.W.; Zou, C.S.; Ma, W.H.; Zhang, S.Y.; Wang, Z.; Bi, B.; Li, S.Y.; Gao, J.H.; Zhang, C.X.; et al. Knockdown of GFAT disrupts chitin synthesis in Hyphantria cunea larvae. Pestic. Biochem. Phys. 2022, 188, 105245. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Mueller, C.R.; Fuchs, J.F.; Wessely, V.; Lan, Q.; Christensen, B.M. Regulatory mechanisms of chitin biosynthesis and roles of chitin in peritrophic matrix formation in the midgut of adult Aedes aegypti. Insect Biochem. Molec. 2006, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Long, G.Y.; Jin, D.C.; Yang, H.; Zhou, C.; Yang, X.B. Knockdown of two trehalase genes by RNA interference is lethal to the white-backed planthopper Sogatella furcifera (Horváth) (Hemiptera: Delphacidae). Biomolecules 2022, 12, 1699. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using realtime quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wang, Z.; Long, G.Y.; Zhou, C.; Jin, D.C.; Yang, H.; Yang, W.J. Molecular characterization of UDP-N-acetylglucosamine pyrophosphorylase and its role in the growth and development of the white-backed planthopper Sogatella furcifera (Hemiptera: Delphacidae). Genes 2022, 13, 1340. [Google Scholar] [CrossRef]
- Kelkenberg, M.; Odman-Naresh, J.; Muthukrishnan, S.; Merzendorfer, H. Chitin is a necessary component to maintain the barrier function of the peritrophic matrix in the insect midgut. Insect Biochem. Mol. Biol. 2015, 56, 21–28. [Google Scholar] [CrossRef]
- Chen, P.; Visokay, S.; Abrams, J.M. Drosophila GFAT1 and GFAT2 enzymes encode obligate developmental functions. Fly 2020, 4, 3–9. [Google Scholar] [CrossRef]
- Huang, X.H.; Tsuji, N.; Miyoshi, T.; Motobu, M.; Islam, M.K.; Alim, M.A.; Fujisaki, K. Characterization of glutamine: Fructose-6-phosphate amidotransferase from the ixodid tick, Haemaphysalis longicornis, and its critical role in host blood feeding. Int. J. Parasitol. 2007, 37, 383–392. [Google Scholar] [CrossRef]
- Durand, P.; Golinelli-Pimpaneau, B.; Mouilleron, S.; Badet, B.; Badet-Denisot, M.A. Highlights of glucosamine-6P synthase catalysis. Arch. Biochem. Biophys. 2008, 474, 302–317. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, S.; Oshiro, N.; Miyamoto, T.; Yoshino, K.; Okamoto, S.; Ono, T.; Kikkawa, U.; Yonezawa, K. AMP-activated protein kinase phosphorylates glutamine: Fructose-6-phosphate amidotransferase 1 at Ser243 to modulate its enzymatic activity. Genes Cells 2009, 14, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.F.; Dos Santos, A.S.; Marotta, H.R.; Mansur, J.F.; Ramos, I.; Machado, E.A.; Souza, G.H.M.F.; Eberlin, M.N.; Kaiser, C.R.; Kramer, K.J. A chitin-like component in Aedes aegypti eggshells, eggs and ovaries. Insect Biochem. Mol. Biol. 2007, 37, 1249–1261. [Google Scholar] [CrossRef]
- Liu, X.J.; Cooper, A.M.W.; Yu, Z.T.; Silver, K.; Zhang, J.Z.; Zhu, K.Y. Progress and prospects of arthropod chitin pathways and structures as targets for pest management. Pestic. Biochem. Phys. 2019, 161, 33–46. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, H.W.; Huang, H.J.; Xue, J.; Wu, W.J.; Bao, Y.Y.; Xu, H.J.; Zhu, Z.R.; Cheng, J.A.; Zhang, C.X. Chitin synthase 1 gene and its two alternative splicing variants from two sap-sucking insects, Nilaparvata lugens and Laodelphax striatellus (Hemiptera: Delphacidae). Insect Biochem. Mol. Biol. 2012, 42, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.N.; Yang, M.M.; Shen, Q.D.; Liu, X.J.; Shi, Z.K.; Wang, S.G.; Tang, B. Functional characterization of three trehalase genes regulating the chitin metabolism pathway in rice brown planthopper using RNA interference. Sci. Rep. 2016, 6, 27841. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, D.W.; Yao, Q.; Zhang, J.; Dong, X.; Tian, H.; Chen, J.; Zhang, W.Q. Feeding-based RNA interference of a trehalose phosphate synthase gene in the brown planthopper, Nilaparvata lugens. Insect Mol. Biol. 2010, 19, 777–786. [Google Scholar] [CrossRef]



| Gene | Notes | Primer Name | Primer Sequence (5′–3′) |
|---|---|---|---|
| SfGFAT | SfGFAT | SfGFAT-F | CGAGCAAGTCATCCAACA |
| cloning | SfGFAT-R | GGTCAACAAGAGCCAGAG | |
| 5′GFAT-R1 | TTTGGTGGGTTCCTCTTTAC | ||
| 5′GFAT-R2 | ACTTCCTCTCCTTGTTGCT | ||
| 3′GFAT-F1 | TGCCAGTGATAATGATTGTC | ||
| 3′GFAT-F2 | GAAGATGGAGACACTGAGAC | ||
| RT-qPCR | qGFAT-F | CGAAGATGGAGACACTGAG | |
| for SfGFAT | qGFAT-R | CGGCAATGTGATAGGAGAG | |
| dsGFAT | dsGFAT-F | TAATACGACTCACTATAGGGGTAGCAACAAGGAGAGGAAG | |
| synthesis | dsGFAT-R | TAATACGACTCACTATAGGGACAGCCAATCAGCATCAAG | |
| Sf18S | RT-qPCR for | q18S-F | CGGAAGGATTGACAGATTGAT |
| rRNA | reference gene | q18S-R | CACGATTGCTGATACCACATAC |
| GFP | dsGFP | dsGFP-F | TAATACGACTCACTATAGGGAAGGGCGAGGAGCTGTTCACCG |
| synthesis | dsGFP-R | TAATACGACTCACTATAGGGCAGCAGGACCATGTGATCGCGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Long, G.; Zhu, H.; Jin, D.; Yang, H.; Zhou, C. Silencing of Glutamine: Fructose-6-Phosphate Aminotransferase Impairs Growth and Development in Sogatella furcifera (Hemiptera: Delphacidae). Biomolecules 2023, 13, 1433. https://doi.org/10.3390/biom13101433
Wang Z, Long G, Zhu H, Jin D, Yang H, Zhou C. Silencing of Glutamine: Fructose-6-Phosphate Aminotransferase Impairs Growth and Development in Sogatella furcifera (Hemiptera: Delphacidae). Biomolecules. 2023; 13(10):1433. https://doi.org/10.3390/biom13101433
Chicago/Turabian StyleWang, Zhao, Guiyun Long, Huan Zhu, Daochao Jin, Hong Yang, and Cao Zhou. 2023. "Silencing of Glutamine: Fructose-6-Phosphate Aminotransferase Impairs Growth and Development in Sogatella furcifera (Hemiptera: Delphacidae)" Biomolecules 13, no. 10: 1433. https://doi.org/10.3390/biom13101433
APA StyleWang, Z., Long, G., Zhu, H., Jin, D., Yang, H., & Zhou, C. (2023). Silencing of Glutamine: Fructose-6-Phosphate Aminotransferase Impairs Growth and Development in Sogatella furcifera (Hemiptera: Delphacidae). Biomolecules, 13(10), 1433. https://doi.org/10.3390/biom13101433







