The Role of Adenosine in γδ T-Cell Regulation of Th17 Responses in Experimental Autoimmune Uveitis
Abstract
1. Introduction
2. Adenosine and Inflammation
3. Adenosine Effect in γδ T Cell Activation
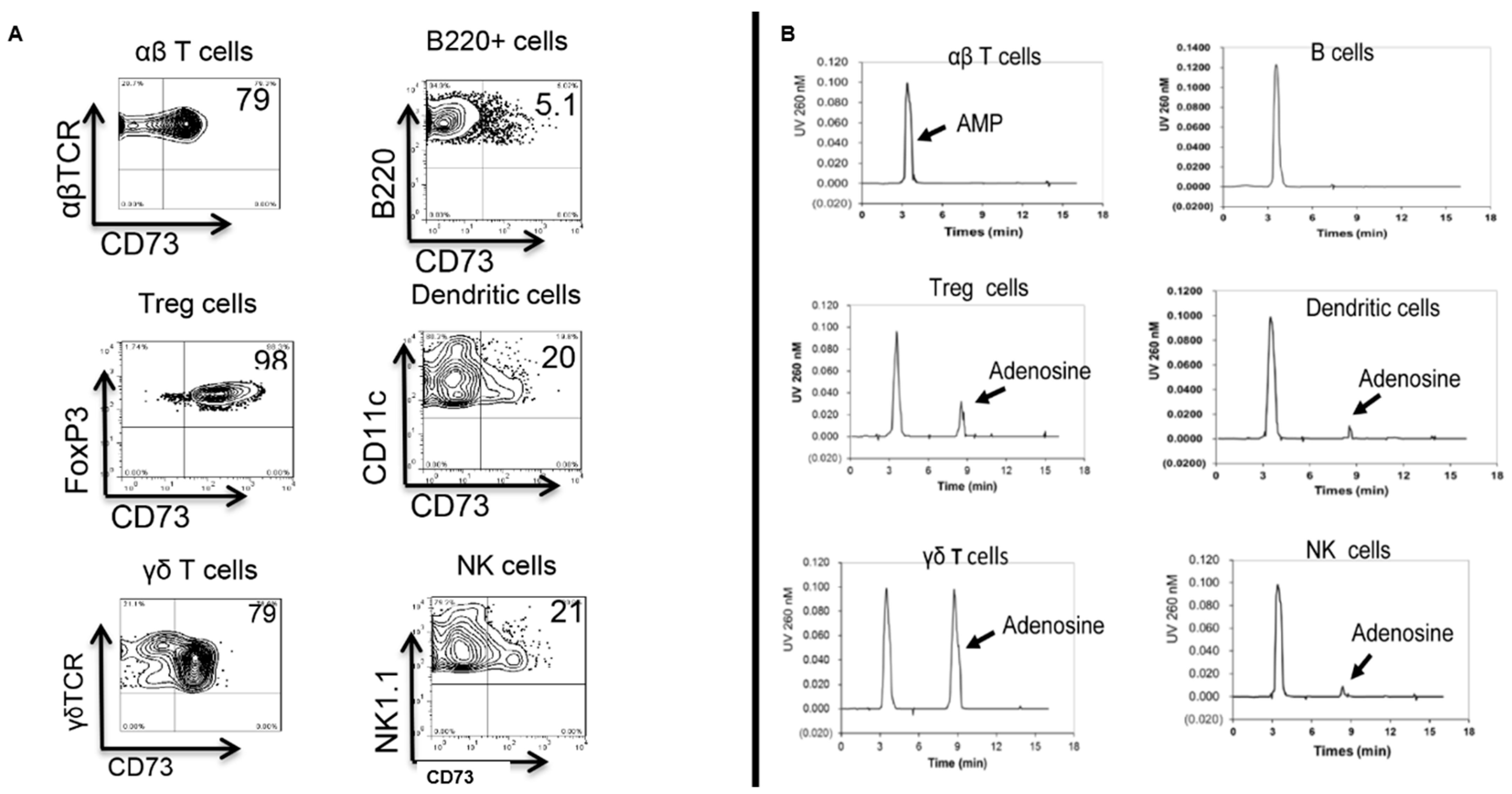
4. γδ T Cell Function Correlates to Their Expression of CD73
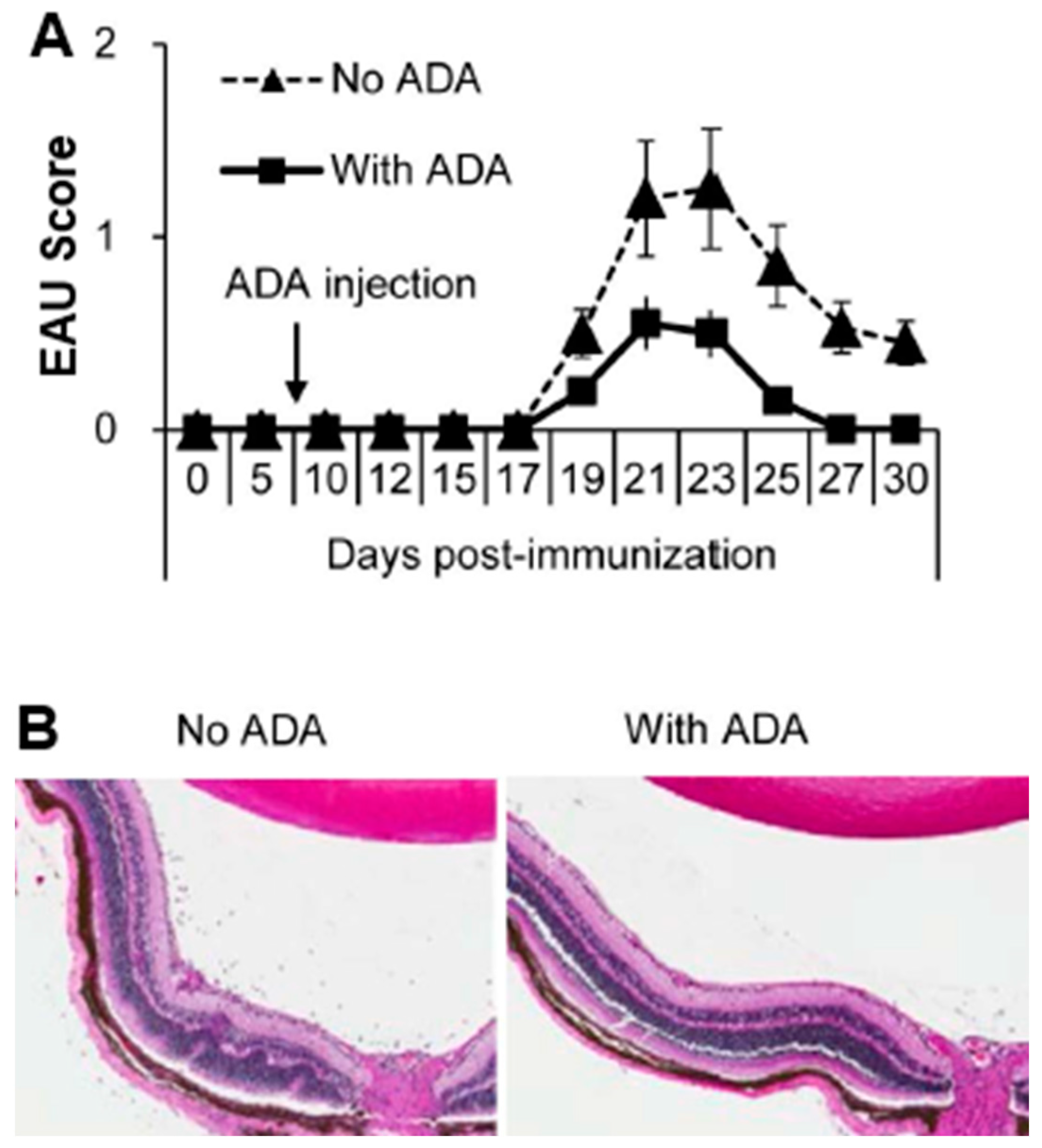
5. Role of Adenosine Deaminase (ADA) in γδ T Cell Regulation
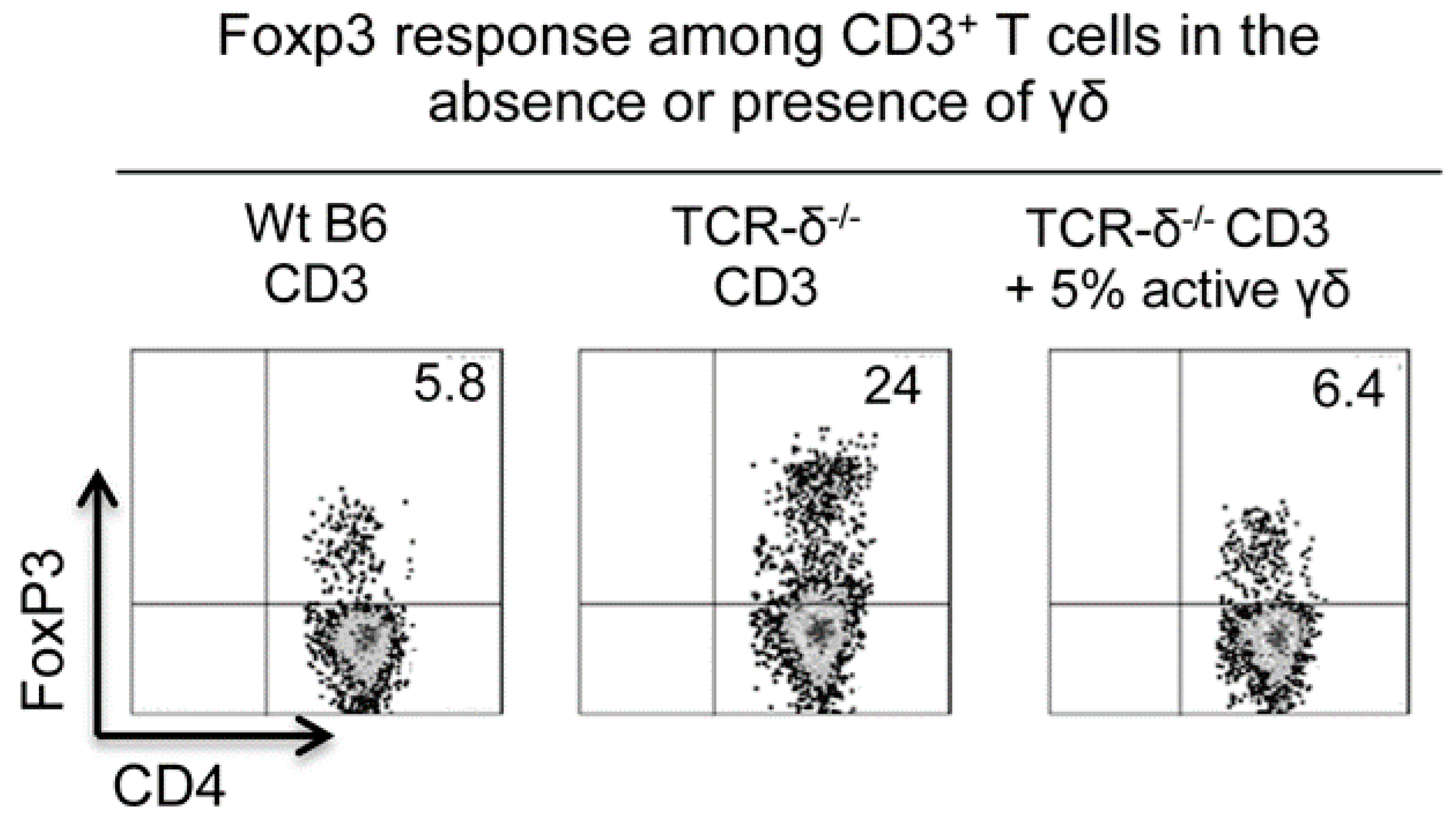
6. Regulatory Effect of γδ T Cells on Foxp3+ Treg Cell Response
7. Reciprocal Interactions between γδ T Cells and Adenosine Metabolism
8. Adenosine Effect on DCs
9. Unique Role of γδ T Cells in Adenosine-Mediated Immunoregulation
- Adenosine enhances Th17 responses by promoting γδ T-cell activation. Activated γδ T cells express an increased level of high-affinity adenosine receptors (A2AR), which can lead to an “adenosine sink.” The increased absorption of adenosine by γδ T cells enhances immune responses that are otherwise inhibited by adenosine, particularly Th17 responses;
- Adenosine induces a functionally unique DC subset that preferentially activates Th17 cells. The influence of adenosine affects DC differentiation by favoring DCs that are capable of supporting Th17+ pathogenic T cells and, therefore, promotes Th17 responses, whereas Th1 responses are suppressed;
- Adenosine-treated DCs produce more pro-Th17 cytokines and fewer Th1-promoting cytokines. Previous studies showed that adenosine-treated DCs have a reduced ability to produce IL-12, leading to reduced Th1 responses. However, we were able to demonstrate that while the adenosine-treated DCs produced decreased amounts of IL-12, their production of IL-23 significantly increased. As a result, Th17 responses were enhanced. Moreover, adenosine can enhance γδ T-cell activation, even though its direct effect on αβ T cells is inhibitory. Consistent activation of γδ T cells is an important step leading to higher Th17 responses;
- Activated γδ T cells have the strongest ability to bind adenosine, and their competition with αβ T cells for adenosine diminishes the effect of adenosine on αβ T cells.
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sun, D.; Coleclough, C.; Hu, X.Z. Heterogeneity of rat encephalitogenic T cells elicited by variants of the myelin basic protein(68-88) peptide. Eur. J. Immunol. 1995, 25, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Myers, L.K.; Stuart, J.M.; Kang, A.H. A CD4 cell is capable of transferring suppression of collagen- induced arthritis. J. Immunol. 1989, 143, 3976–3980. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, K.; Karlsson, M.; Andersson, L.; Holmdahl, R. Structures on the I-A molecule predisposing for susceptibility to type II collagen-induced autoimmune arthritis. Eur. J. Immunol. 1990, 20, 2127–2131. [Google Scholar] [CrossRef]
- Caspi, R.R.; Roberge, F.G.; McAllister, C.G.; El-Saied, M.; Kuwabara, T.; Gery, I.; Hanna, E.; Nussenblatt, R.B. T cell lines mediating experimental autoimmune uveoretinitis (EAU) in the rat. J. Immunol. 1986, 136, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Bettelli, E.; Carrier, Y.; Gao, W.; Korn, T.; Strom, T.B.; Oukka, M.; Weiner, H.L.; Kuchroo, V.K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006, 441, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Dong, C. Diversification of T-helper-cell lineages: Finding the family root of IL-17-producing cells. Nat. Rev. Immunol. 2006, 6, 329–334. [Google Scholar] [CrossRef]
- madi-Obi, A.; Yu, C.R.; Liu, X.; Mahdi, R.M.; Clarke, G.L.; Nussenblatt, R.B.; Gery, I.; Lee, Y.S.; Egwuagu, C.E. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat. Med. 2007, 13, 711–718. [Google Scholar] [CrossRef]
- Fredholm, B.B.; IJzerman, A.P.; Jacobson, K.A.; Linden, J.; Müller, C.E. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and Classification of Adenosine Receptors—An Update. Pharmacol. Rev. 2011, 63, 1–34. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Gao, Z.-G. Adenosine receptors as therapeutic targets. Nat. Rev. Drug. Discov. 2006, 5, 247–264. [Google Scholar] [CrossRef]
- Haskó, G.; Linden, J.; Cronstein, B.; Pacher, P. Adenosine receptors: Therapeutic aspects for inflammatory and immune diseases. Nat. Rev. Drug. Discov. 2008, 7, 759–770. [Google Scholar] [CrossRef]
- Sauer, A.V.; Brigida, I.; Carriglio, N.; Aiuti, A. Autoimmune dysregulation and purine metabolism in adenosine deaminase (ADA)-deficiency. Front. Immunol. 2012, 3, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Lappas, C.M.; Rieger, J.M.; Linden, J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J. Immunol. 2005, 174, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Fan, J.; Wang, L.; Thompson, L.F.; Liu, A.; Daniel, B.J.; Shin, T.; Curiel, T.J.; Zhang, B. CD73 on Tumor Cells Impairs Antitumor T-Cell Responses: A Novel Mechanism of Tumor-Induced Immune Suppression. Cancer Res. 2010, 70, 2245–2255. [Google Scholar] [CrossRef]
- Zarek, P.E.; Huang, C.-T.; Lutz, E.R.; Kowalski, J.; Horton, M.R.; Linden, J.; Drake, C.G.; Powell, J.D. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood 2008, 111, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Ehrentraut, H.; Westrich, J.A.; Eltzschig, H.K.; Clambey, E.T. Adora2b Adenosine Receptor Engagement Enhances Regulatory T Cell Abundance during Endotoxin-Induced Pulmonary Inflammation. PLoS ONE 2012, 7, e32416. [Google Scholar] [CrossRef] [PubMed]
- Naganuma, M.; Wiznerowicz, E.B.; Lappas, C.M.; Linden, J.; Worthington, M.T.; Ernst, P.B. Cutting Edge: Critical Role for A2A Adenosine Receptors in the T Cell-Mediated Regulation of Colitis. J. Immunol. 2006, 177, 2765–2769. [Google Scholar] [CrossRef] [PubMed]
- Barankiewicz, J.; Ronlov, G.; Jimenez, R.; Gruber, H.E. Selective adenosine release from human B but not T lymphoid cell line. J. Biol. Chem. 1990, 265, 15738–15743. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Ibla, J.C.; Furuta, G.T.; Leonard, M.O.; Jacobson, K.A.; Enjyoji, K.; Robson, S.C.; Colgan, S.P. Coordinated Adenine Nucleotide Phosphohydrolysis and Nucleoside Signaling in Posthypoxic Endothelium. J. Exp. Med. 2003, 198, 783–796. [Google Scholar] [CrossRef] [PubMed]
- Haskó, G.; Csoka, B.; Németh, Z.H.; Vizi, E.S.; Pacher, P. A2B adenosine receptors in immunity and inflammation. Trends. Immunol. 2009, 30, 263–270. [Google Scholar] [CrossRef]
- Cronstein, B.N.; Levin, R.I.; Belanoff, J.; Weissmann, G.; Hirschhorn, R. Adenosine: An endogenous inhibitor of neutrophil-mediated injury to endothelial cells. J. Clin. Investig. 1986, 78, 760–770. [Google Scholar] [CrossRef]
- Sitkovsky, M.V.; Lukashev, D.; Apasov, S.; Kojima, H.; Koshiba, M.; Caldwell, C.; Ohta, A.; Thiel, M. Physiological Control of Immune Response and Inflammatory Tissue Damage by Hypoxia-Inducible Factors and Adenosine A2A Receptors. Annu. Rev. Immunol. 2004, 22, 657–682. [Google Scholar] [CrossRef] [PubMed]
- Idzko, M.; Ferrari, D.; Eltzschig, H.K. Nucleotide signalling during inflammation. Nature 2014, 509, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Sitkovsky, M.V.; Ohta, A. The ‘danger’ sensors that STOP the immune response: The A2 adenosine receptors? Trends. Immunol. 2005, 26, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Beigi, R.D.; Kertesy, S.B.; Aquilina, G.; Dubyak, G.R. Oxidized ATP (oATP) attenuates proinflammatory signaling via P2 receptor-independent mechanisms. Br. J. Pharmacol. 2003, 140, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Canaday, D.H.; Beigi, R.; Silver, R.F.; Harding, C.V.; Boom, W.H.; Dubyak, G.R. ATP and Control of Intracellular Growth of Mycobacteria by T Cells. Infect. Immun. 2002, 70, 6456–6459. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, L.; Pacher, P.; Vizi, E.S.; Haskó, G. CD39 and CD73 in immunity and inflammation. Trend. Mol. Med. 2013, 19, 251–257. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Carmeliet, P. Hypoxia and Inflammation. N. Engl. J. Med. 2011, 364, 656–665. [Google Scholar] [CrossRef]
- Cronstein, B.N.; Eberle, M.A.; Gruber, H.E.; Levin, R.I. Methotrexate inhibits neutrophil function by stimulating adenosine release from connective tissue cells. Proc. Natl. Acad. Sci. USA 1991, 88, 2441–2445. [Google Scholar] [CrossRef]
- Ramlackhansingh, A.F.; Bose, S.K.; Ahmed, I.; Turkheimer, F.E.; Pavese, N.; Brooks, D.J. Adenosine 2A receptor availability in dyskinetic and nondyskinetic patients with Parkinson disease. Neurology 2011, 76, 1811–1816. [Google Scholar] [CrossRef]
- Cui, Y.; Shao, H.; Lan, C.; Nian, H.; O’Brien, R.L.; Born, W.K.; Kaplan, H.J.; Sun, D. Major Role of gd T Cells in the Generation of IL-17+ Uveitogenic T Cells. J. Immunol. 2009, 183, 560–567. [Google Scholar] [CrossRef]
- Nian, H.; Shao, H.; O’Brien, R.L.; Born, W.K.; Kaplan, H.J.; Sun, D. Activated gd cells promote the activation of uveitogenic T cells and exacerbate EAU development. Invest. Ophthalmol. Vis. Sci. 2011, 52, 5920–5927. [Google Scholar] [CrossRef]
- Liang, D.; Zuo, A.; Shao, H.; Chen, M.; Kaplan, H.J.; Sun, D. Roles of the Adenosine Receptor and CD73 in the Regulatory Effect of γδ T Cells. PLoS ONE 2014, 9, e108932. [Google Scholar] [CrossRef]
- Sun, D.; Chan, N.; Shao, H.; Born, W.K.; Kaplan, H.J. γδ T Cells Activated in Different Inflammatory Environments Are Functionally Distinct. J. Immunol. 2022, 208, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Zuo, A.; Shao, H.; Chen, M.; Kaplan, H.J.; Sun, D. Anti-inflammatory or pro-inflammatory effect of an adenosine receptor agonist on the Th17 autoimmune response is inflammatory environment-dependent. J. Immunol. 2014, 193, 5498–5505. [Google Scholar] [CrossRef] [PubMed]
- Barbi, J.; Pardoll, D.; Pan, F. Treg functional stability and its responsiveness to the microenvironment. Imm. Rev. 2014, 259, 115–139. [Google Scholar] [CrossRef] [PubMed]
- Bluestone, J.A.; Bour-Jordan, H.; Cheng, M.; Anderson, M. T cells in the control of organ-specific autoimmunity. J. Clin. Investig. 2015, 125, 2250–2260. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T Cells and Immune Tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef]
- Peng, G.; Wang, H.Y.; Peng, W.; Kiniwa, Y.; Seo, K.H.; Wang, R.F. Tumor-Infiltrating gd T Cells Suppress T and Dendritic Cell Function via Mechanisms Controlled by a Unique Toll-like Receptor Signaling Pathway. Immunity 2007, 27, 334–348. [Google Scholar] [CrossRef]
- Spencer, C.T.; Abate, G.; Blazevic, A.; Hoft, D.F. Only a Subset of Phosphoantigen-Responsive g9d2 T Cells Mediate Protective Tuberculosis Immunity. J. Immunol. 2008, 181, 4471–4484. [Google Scholar] [CrossRef]
- Chen, M.; Liang, D.; Zuo, A.; Shao, H.; Kaplan, H.J.; Sun, D. An A2B Adenosine Receptor Agonist Promotes Th17 Autoimmune Responses in Experimental Autoimmune Uveitis (EAU) via Dendritic Cell Activation. PLoS ONE 2015, 10, e0132348. [Google Scholar] [CrossRef]
- Liang, D.; Zuo, A.; Zhao, R.; Shao, H.; Born, W.K.; O’Brien, R.L.; Kaplan, H.J.; Sun, D. CD73 expressed on gd T cells shapes their regulatory effect in experimental autoimmune uveitis. PLoS ONE 2016, 11, e0150078. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Kaplan, H.J.; Sun, D. Bidirectional Effect of IFN-γ on Th17 Responses in Experimental Autoimmune Uveitis. Front. Ophthalmol. 2022, 2, 831084. [Google Scholar] [CrossRef] [PubMed]
- Nian, H.; Shao, H.; Zhang, G.; Born, W.K.; O’Brien, R.; Kaplan, H.J.; Sun, D. Regulatory effect of gd T cells on IL-17+ uveitogenic T cells. Invest. Ophthalmol. Vis. Sci. 2010, 51, 4661–4667. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khakh, B.S.; Burnstock, G. The Double Life of ATP. Sci.Am. 2009, 301, 84–92. [Google Scholar] [CrossRef]
- Junger, W.G. Immune cell regulation by autocrine purinergic signalling. Nat. Rev. Immunol. 2011, 11, 201–212. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Sitkovsky, M.V.; Robson, S.C. Purinergic Signaling during Inflammation. N. Engl. J. Med. 2012, 367, 2322–2333. [Google Scholar] [CrossRef]
- Nian, H.; Liang, D.; Zuo, A.; Wei, R.; Shao, H.; Born, W.K.; Kaplan, H.J.; Sun, D. Characterization of autoreactive and bystander IL-17+ T cells induced in immunized C57BL/6 mice. Invest. Ophthalmol. Vis. Sci. 2012, 53, 897–905. [Google Scholar] [CrossRef]
- Liang, D.; Zuo, A.; Shao, H.; Born, W.K.; O’Brien, R.L.; Kaplan, H.J.; Sun, D. Retinoic Acid Inhibits CD25+ Dendritic Cell Expansion and γδ T-Cell Activation in Experimental Autoimmune Uveitis. Invest. Ophthalmol. Vis. Sci. 2013, 54, 3493–3503. [Google Scholar] [CrossRef]
- Liang, D.; Zuo, A.; Shao, H.; Born, W.K.; O’Brien, R.L.; Kaplan, H.J.; Sun, D. IL-23 receptor expression on γδ T cells correlates with their enhancing or suppressive effects on autoreactive T cells in experimental autoimmune uveitis. J. Immunol. 2013, 191, 1118–1125. [Google Scholar] [CrossRef]
- Liang, D.; Shao, H.; Born, W.K.; O’Brien, R.L.; Kaplan, H.J.; Sun, D. High level expression of A2ARs is required for the enhancing function, but not for the inhibiting function, of gammadelta T cells in the autoimmune responses of EAU. PLoS ONE 2018, 13, e0199601. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; Gabrilovich, D.; Sotomayor, E.M. Immunosuppressive Strategies that are Mediated by Tumor Cells. Annu. Rev. Immunol. 2007, 25, 267–296. [Google Scholar] [CrossRef] [PubMed]
- Beavis, P.A.; Stagg, J.; Darcy, P.K.; Smyth, M.J. CD73: A potent suppressor of antitumor immune responses. Trends. Immunol. 2012, 33, 231–237. [Google Scholar] [CrossRef]
- Ohta, A.; Gorelik, E.; Prasad, S.J.; Ronchese, F.; Lukashev, D.; Wong, M.K.K.; Huang, X.; Caldwell, S.; Liu, K.; Smith, P.; et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc. Natl. Acad. Sci. USA 2006, 103, 13132–13137. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liang, D.; Sun, D. Blockade of Extracellular ATP Effect by Oxidized ATP Effectively Mitigated Induced Mouse Experimental Autoimmune Uveitis (EAU). PLoS ONE 2016, 11, e0155953. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Valenzuela, A.; Lluis, C.; Blanco, J. Enzymatic and extraenzymatic role of ecto-adenosine deaminase in lymphocytes. Immunol. Rev. 1998, 161, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Hershfield, M.S.; Buckley, R.H.; Greenberg, M.L.; Melton, A.L.; Schiff, R.; Hatem, C.; Kurtzberg, J.; Markert, M.L.; Kobayashi, R.H.; Kobayashi, A.L.; et al. Treatment of Adenosine Deaminase Deficiency with Polyethylene Glycol–Modified Adenosine Deaminase. N. Engl. J. Med. 1987, 316, 589–596. [Google Scholar] [CrossRef]
- Ozsahin, H.; Arredondo-Vega, F.X.; Santisteban, I.; Fuhrer, H.; Tuchschmid, P.; Jochum, W.; Aguzzi, A.; Lederman, H.M.; Fleischman, A.; Winkelstein, J.A.; et al. Adenosine Deaminase Deficiency in Adults. Blood 1997, 89, 2849–2855. [Google Scholar] [CrossRef]
- Blackburn, M.R. Too much of a good thing: Adenosine overload in adenosine-deaminase-deficient mice. Trends. Pharmacol. Sci. 2003, 24, 66–70. [Google Scholar] [CrossRef]
- Ghaemi Oskouie, F.; Shameli, A.; Yang, A.; Desrosiers, M.D.; Mucsi, A.D.; Blackburn, M.R.; Yang, Y.; Santamaria, P.; Shi, Y. High Levels of Adenosine Deaminase on Dendritic Cells Promote Autoreactive T Cell Activation and Diabetes in Nonobese Diabetic Mice. J. Immunol. 2011, 186, 6798–6806. [Google Scholar] [CrossRef]
- de Araújo Junqueira, A.F.T.; Dias, A.A.M.; Vale, M.L.; Spilborghs, G.M.G.T.; Bossa, A.S.; Lima, B.B.; Carvalho, A.F.; Guerrant, R.L.; Ribeiro, R.A.; Brito, G.A. Adenosine Deaminase Inhibition Prevents Clostridium difficile Toxin A-Induced Enteritis in Mice. Infect. Immun. 2011, 79, 653–662. [Google Scholar] [CrossRef]
- Desrosiers, M.D.; Cembrola, K.M.; Fakir, M.J.; Stephens, L.A.; Jama, F.M.; Shameli, A.; Mehal, W.Z.; Santamaria, P.; Shi, Y. Adenosine Deamination Sustains Dendritic Cell Activation in Inflammation. J. Immunol. 2007, 179, 1884–1892. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Zuo, A.; Zhao, R.; Shao, H.; Kaplan, H.J.; Sun, D. Regulation of Adenosine Deaminase on Induced Mouse Experimental Autoimmune Uveitis. J. Immunol. 2016, 196, 2646–2654. [Google Scholar] [CrossRef] [PubMed]
- Petermann, F.; Rothhammer, V.; Claussen, M.C.; Haas, J.D.; Blanco, L.R.; Heink, S.; Prinz, I.; Hemmer, B.; Kuchroo, V.K.; Oukka, M.; et al. gd T cells enhance autoimmunity by restraining regulatory T cell responses via an Interleukin-23-dependent mechanism. Immunity 2010, 33, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Visperas, A.; Shen, B.; Min, B. γδ T cells restrain extrathymic development of Foxp3+-inducible regulatory T cells via IFN-γ. Eur. J. Immunol. 2014, 44, 2448–2456. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, Z.; Huang, C.; McGowan, J.; Casper, T.; Sun, D.; Born, W.K.; O’Brien, R.L. γδ T Cell–Dependent Regulatory T Cells Prevent the Development of Autoimmune Keratitis. J. Immunol. 2015, 195, 5572–5581. [Google Scholar] [CrossRef] [PubMed]
- Do, J.s.; Visperas, A.; O’Brien, R.L.; Min, B. CD4 T cells play important roles in maintaining IL-17-producing gd T-cell subsets in naive animals. Immunol. Cell Biol. 2012, 90, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Woo, J.-I.; Shao, H.; Born, W.K.; O’Brien, R.L.; Kaplan, H.J.; Sun, D. Ability of γδ T cells to modulate the Foxp3 T cell response is dependent on adenosine. PLoS ONE 2018, 13, e0197189. [Google Scholar] [CrossRef] [PubMed]
- Haskó, G.; Pacher, P. Regulation of macrophage function by adenosine. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Novitskiy, S.V.; Ryzhov, S.; Zaynagetdinov, R.; Goldstein, A.E.; Huang, Y.; Tikhomirov, O.Y.; Blackburn, M.R.; Biaggioni, I.; Carbone, D.P.; Feoktistov, I.; et al. Adenosine receptors in regulation of dendritic cell differentiation and function. Blood 2008, 112, 1822–1831. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, A.A.; Gao, Z.-G.; Jung, U.; Foley, J.; Borenstein, T.; Jacobson, K.A.; Fowler, D.H. Activation of Th1 and Tc1 cell adenosine A2A receptors directly inhibits IL-2 secretion in vitro and IL-2-driven expansion in vivo. Blood 2005, 105, 4707–4714. [Google Scholar] [CrossRef]
- Liang, D.; Zuo, A.; Shao, H.; Chen, M.; Kaplan, H.J.; Sun, D. A2B adenosine receptor activation switches differentiation of bone marrow cells to a CD11c+Gr-1+ dendritic cell subset that promotes the Th17 response. Immun. Inflamm. Dis. 2015, 3, 360–373. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, H.; Kaplan, H.J.; Sun, D. The Role of Adenosine in γδ T-Cell Regulation of Th17 Responses in Experimental Autoimmune Uveitis. Biomolecules 2023, 13, 1432. https://doi.org/10.3390/biom13101432
Shao H, Kaplan HJ, Sun D. The Role of Adenosine in γδ T-Cell Regulation of Th17 Responses in Experimental Autoimmune Uveitis. Biomolecules. 2023; 13(10):1432. https://doi.org/10.3390/biom13101432
Chicago/Turabian StyleShao, Hui, Henry J. Kaplan, and Deming Sun. 2023. "The Role of Adenosine in γδ T-Cell Regulation of Th17 Responses in Experimental Autoimmune Uveitis" Biomolecules 13, no. 10: 1432. https://doi.org/10.3390/biom13101432
APA StyleShao, H., Kaplan, H. J., & Sun, D. (2023). The Role of Adenosine in γδ T-Cell Regulation of Th17 Responses in Experimental Autoimmune Uveitis. Biomolecules, 13(10), 1432. https://doi.org/10.3390/biom13101432





