The Double-Edged Role of Extracellular Vesicles in the Hallmarks of Aging
Abstract
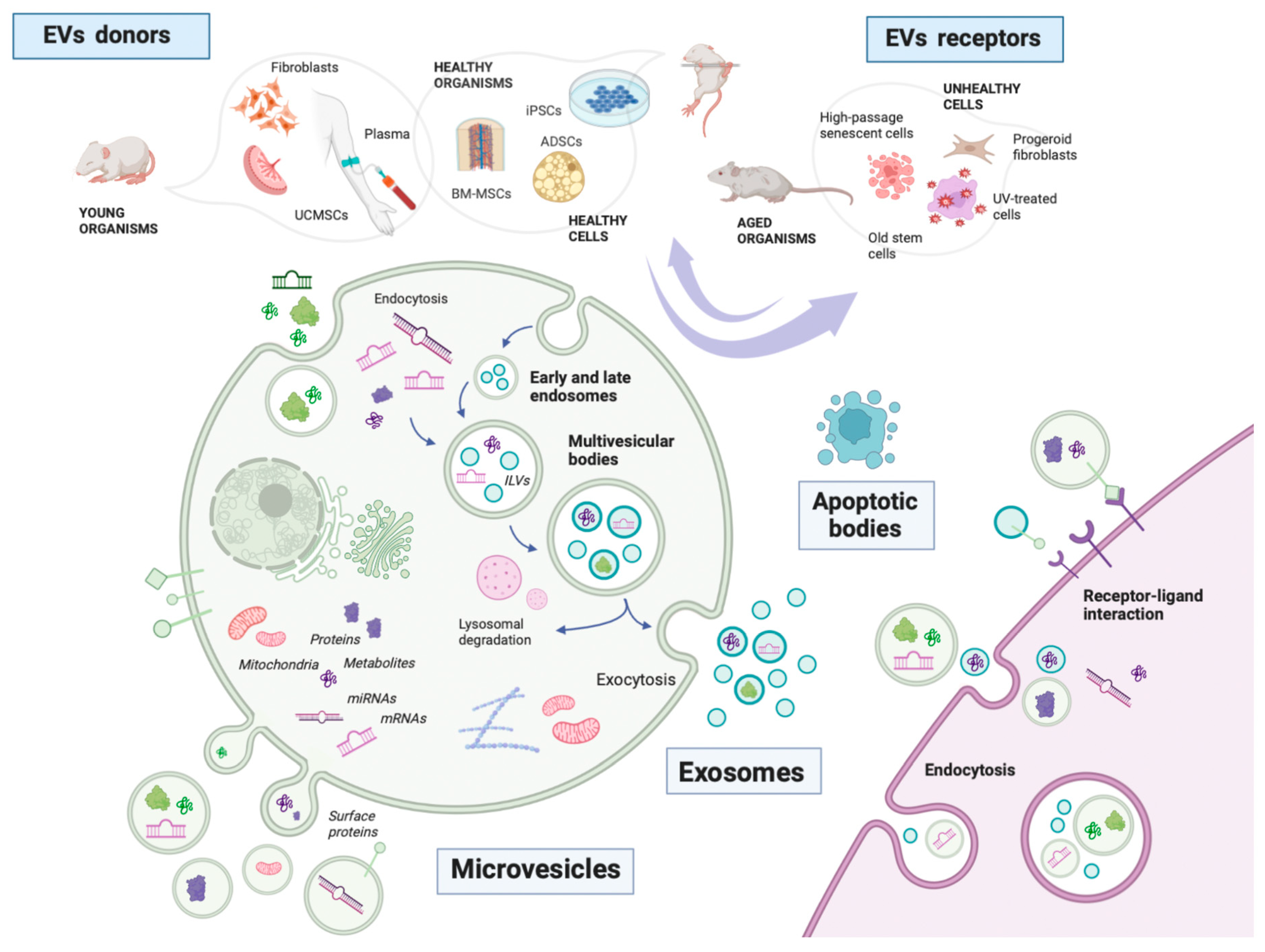
1. Extracellular Vesicles in Aging
1.1. What Are Extracellular Vesicles?
1.2. Where Do EVs Come from in Aging Studies?
2. EVs and the Hallmarks of Aging
2.1. What Are the Hallmarks of Aging?
2.2. Primary Hallmarks
2.2.1. EVs and Genomic Instability
2.2.2. EVs and Telomere Attrition
2.2.3. EVs and Epigenetic Alterations
2.2.4. EVs and Loss of Proteostasis
2.3. Antagonistic Hallmarks
2.3.1. EVs and Deregulated Nutrient Sensing
2.3.2. EVs and Mitochondrial Dysfunction
2.3.3. EVs and Cellular Senescence
2.4. Integrative Hallmarks
2.4.1. EVs and Stem Cell Exhaustion
2.4.2. EVs and Altered Intercellular Communication
3. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 2015, 65, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Chen, H.; Wang, Y.; Zhang, L.; Wang, X. Roles of extracellular vesicles in the aging microenvironment and age-related diseases. J. Extracell. Vesicles 2021, 10, e12154. [Google Scholar] [CrossRef]
- Lananna, B.V.; Ichiro, I.S. Friends and Foes: Extracellular Vesicles in Aging and Rejuvenation; FASEB BioAdvances; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2021; Volume 3, pp. 787–801. [Google Scholar]
- Margolis, L.; Sadovsky, Y. The biology of extracellular vesicles: The known unknowns. PLoS Biol. 2019, 17, e3000363. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Mark, M.T.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Hade, M.; Suire, C.; Suo, Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells 2021, 10, 1959. [Google Scholar] [CrossRef]
- Doyle, L.; Wang, M. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Iso-lation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.; Vader, P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J. B lymphocytes secrete anti-gen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef]
- Skotland, T.; Sagini, K.; Sandvig, K.; Llorente, A. An emerging focus on lipids in extracellular vesicles. Adv. Drug Deliv. Rev. 2020, 159, 308–321. [Google Scholar] [CrossRef]
- van der Koog, L.; Gandek, T.B.; Nagelkerke, A. Liposomes and Extracellular Vesicles as Drug Delivery Systems: A Comparison of Composition, Pharmacokinetics, and Functionalization. Adv. Healthc Mater. 2022, 11, 2100639. [Google Scholar] [CrossRef]
- Subra, C.; Laulagnier, K.; Perret, B.; Record, M. Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie 2007, 89, 205–212. [Google Scholar] [CrossRef]
- Ghanam, J.; Chetty, V.K.; Barthel, L.; Reinhardt, D.; Hoyer, P.-F.; Thakur, B.K. DNA in extracellular vesicles: From evolution to its current application in health and disease. Cell Biosci. 2022, 12, 37. [Google Scholar] [CrossRef]
- Dellar, E.R.; Hill, C.; Melling, G.E.; Carter, D.R.F.; Baena-Lopez, L.A. Unpacking extracellular vesicles: RNA cargo loading and fun-ction. J. Extracell. Biol. 2022, 1, e40. [Google Scholar] [CrossRef]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef]
- Thomou, T.; Mori, M.A.; Dreyfuss, J.M.; Konishi, M.; Sakaguchi, M.; Wolfrum, C. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 2017, 542, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Alberro, A.; Osorio-Querejeta, I.; Sepúlveda, L.; Fernández-Eulate, G.; Mateo-Abad, M.; Muñoz-Culla, M.; Carregal-Romero, S.; Matheu, A.; Vergara, I.; de Munain, A.L.; et al. T cells and immune functions of plasma extracellular vesicles are differentially modulated from adults to centenarians. Aging 2019, 11, 10723–10741. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shi, H.; Yin, S.; Ji, C.; Zhang, X.; Zhang, B.; Wu, P.; Shi, Y.; Mao, F.; Yan, Y.; et al. Human Mesenchymal Stem Cell Derived Exosomes Alleviate Type 2 Diabetes Mellitus by Reversing Peripheral Insulin Resistance and Relieving β-Cell Destruction. ACS Nano 2018, 12, 7613–7628. [Google Scholar] [CrossRef]
- Yoshida, M.; Satoh, A.; Lin, J.B.; Mills, K.F.; Sasaki, Y.; Rensing, N.; Wong, M.; Apte, R.S.; Imai, S.-I. Extracellular Vesicle-Contained eNAMPT Delays Aging and Extends Lifespan in Mice. Cell Metab. 2019, 30, 329–342.e5. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, L.; Ruan, L.; Oh, J.; Dong, X.; Zhuge, Q.; Su, D.-M. Extracellular vesicles extracted from young donor serum attenuate inflammaging via partially rejuvenating aged T-cell immunotolerance. FASEB J. 2018, 32, 5899–5912. [Google Scholar] [CrossRef] [PubMed]
- Gomes de Andrade, G.; Reck Cechinel, L.; Bertoldi, K.; Galvão, F.; Valdeci Worm, P.; Rodrigues Siqueira, I. The Aging Process Alters IL-1β and CD63 Levels Differently in Extracellular Vesicles Obtained from the Plasma and Cerebrospinal Fluid. Neuroimmunomodulation 2018, 25, 18–22. [Google Scholar] [CrossRef]
- Tietje, A.; Maron, K.N.; Wei, Y.; Feliciano, D. Cerebrospinal Fluid Extracellular Vesicles Undergo Age Dependent Declines and Contain Known and Novel Non-coding RNAs. PLoS ONE 2014, 9, e113116. [Google Scholar] [CrossRef]
- Machida, T.; Tomofuji, T.; Ekuni, D.; Maruyama, T.; Yoneda, T.; Kawabata, Y.; Mizuno, H.; Miyai, H.; Kunitomo, M.; Morita, M. MicroRNAs in Salivary Exosome as Potential Biomarkers of Aging. Int. J. Mol. Sci. 2015, 16, 21294–21309. [Google Scholar] [CrossRef]
- Galley, J.D.; Besner, G.E. The Therapeutic Potential of Breast Milk-Derived Extracellular Vesicles. Nutrients 2020, 12, 745. [Google Scholar] [CrossRef]
- Fafián-Labora, J.A.; Rodríguez-Navarro, J.A.; O’Loghlen, A. Small Extracellular Vesicles Have GST Activity and Ameliorate Senescence-Related Tissue Damage. Cell Metab. 2020, 32, 71–86.e5. [Google Scholar] [CrossRef]
- Xia, W.; Li, M.; Jiang, X.; Huang, X.; Gu, S.; Ye, J.; Zhu, L.; Hou, M.; Zan, T. Young fibroblast-derived exosomal microRNA-125b transfers beneficial effects on aged cutaneous wound healing. J. Nanobiotechnology 2022, 20, 144. [Google Scholar] [CrossRef]
- Storci, G.; De Carolis, S.; Papi, A.; Bacalini, M.G.; Gensous, N.; Marasco, E.; Tesei, A.; Fabbri, F.; Arienti, C.; Zanoni, M.; et al. Genomic stability, anti-inflammatory phenotype, and up-regulation of the RNAseH2 in cells from centenarians. Cell Death Differ. 2019, 26, 1845–1858. [Google Scholar] [CrossRef]
- lvarez-Viejo, M. Mesenchymal stem cells from different sources and their derived exosomes: A pre-clinical perspective. World J. Stem. Cells 2020, 12, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Mas-Bargues, C.; Borrás, C. Importance of stem cell culture conditions for their derived extracellular vesicles therapeutic effect. Free. Radic. Biol. Med. 2021, 168, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Mas-Bargues, C.; Sanz-Ros, J.; Román-Domínguez, A.; Gimeno-Mallench, L.; Inglés, M.; Viña, J.; Borrás, C. Extracellular Vesicles from Healthy Cells Improves Cell Function and Stemness in Premature Senescent Stem Cells by miR-302b and HIF-1α Activation. Biomolecules 2020, 10, 957. [Google Scholar] [CrossRef] [PubMed]
- Shelke, G.; Lässer, C.; Gho, Y.S.; Lötvall, J. Importance of exosome depletion protocols to eliminate functional and RNA-containing extracellular vesicles from fetal bovine serum. J. Extracell. Vesicles 2014, 3, 24783. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Chen, B.; Zhang, J.; Sun, Y.; Yuan, J.; Niu, X. Human ESC-sEVs alleviate age-related bone loss by rejuvenating senescent bone marrow-derived mesenchymal stem cells. J. Extracell Vesicles 2020, 9, 1800971. [Google Scholar] [CrossRef] [PubMed]
- Thabet, E.; Yusuf, A.; Abdelmonsif, D.A.; Nabil, I.; Mourad, G.; Mehanna, R.A. Extracellular vesicles miRNA-21: A potential thera-peutic tool in premature ovarian dysfunction. Mol. Hum. Reprod. 2020, 26, 906–919. [Google Scholar] [CrossRef]
- Zhang, Y.; Kim, M.S.; Jia, B.; Yan, J.; Zuniga-Hertz, J.P.; Han, C.; Cai, D. Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature 2017, 548, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.C.; Kim, T.K.; Noori, A.; Merrihew, G.E.; Robbins, J.E.; Golubeva, A.; Wang, K.; MacCoss, M.; Kaeberlein, M. Composition of Caenorhabditis elegans extrace-llular vesicles suggests roles in metabolism, immunity, and aging. Geroscience 2020, 42, 1133–1145. [Google Scholar] [CrossRef]
- Cho, E.G.; Choi, S.Y.; Kim, H.; Choi, E.J.; Lee, E.J.; Park, P.J.; Ko, J.; Kim, K.P.; Baek, H.S. Panax ginseng-Derived Extracellular Vesicles Facilitate An-ti-Senescence Effects in Human Skin Cells: An Eco-Friendly and Sustainable Way to Use Ginseng Substances. Cells 2021, 10, 486. [Google Scholar] [CrossRef]
- Oh, M.; Lee, J.; Kim, Y.J.; Rhee, W.J.; Park, J.H. Exosomes Derived from Human Induced Pluripotent Stem Cells Ameliorate the Aging of Skin Fibroblasts. Int. J. Mol. Sci. 2018, 19, 1715. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, J.; Yuan, H.; Xu, Z.; Li, Q.; Niu, X. Exosomes Secreted by Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Repair Critical-Sized Bone Defects through Enhanced Angiogenesis and Osteogenesis in Osteo-porotic Rats. Int. J. Biol. Sci. 2016, 12, 836–849. [Google Scholar] [CrossRef]
- Nishiga, M.; Guo, H.; Wu, J.C. Induced pluripotent stem cells as a biopharmaceutical factory for extracellular vesicles. Eur. Heart J. 2018, 39, 1848–1850. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Cha, H.; Park, J.H. Derivation of Cell-Engineered Nanovesicles from Human Induced Pluripotent Stem Cells and Their Protective Effect on the Senescence of Dermal Fibroblasts. Int. J. Mol. Sci. 2020, 21, 343. [Google Scholar] [CrossRef] [PubMed]
- Adamiak, M.; Cheng, G.; Bobis-Wozowicz, S.; Zhao, L.; Kedracka-Krok, S.; Samanta, A.; Karnas, E.; Xuan, Y.-T.; Skupien-Rabian, B.; Chen, X.; et al. Induced Pluripotent Stem Cell (iPSC)–Derived Extracellular Vesicles Are Safer and More Effective for Cardiac Repair Than iPSCs. Circ. Res. 2018, 122, 296–309. [Google Scholar] [CrossRef]
- Zhai, M.; Zhu, Y.; Yang, M.; Mao, C. Human Mesenchymal Stem Cell Derived Exosomes Enhance Cell-Free Bone Regeneration by Altering Their miRNAs Profiles. Adv. Sci. 2020, 7, 2001334. [Google Scholar] [CrossRef]
- Zhang, K.; Yu, L.; Li, F.R.; Li, X.; Wang, Z.; Zou, X.; Zhang, C.; Lv, K.; Zhou, B.; Mitragotri, S.; et al. Topical Application of Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells in Combination with Sponge Spicules for Treatment of Photoaging. Int. J. Nanomed. 2020, 15, 2859–2872. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.-W.; Li, F.-X.; Liu, Y.-W.; Rao, S.-S.; Yin, H.; Huang, J.; Chen, C.-Y.; Hu, Y.; Zhang, Y.; Tan, Y.-J.; et al. Aptamer-functionalized exosomes from bone marrow stromal cells target bone to promote bone regeneration. Nanoscale 2019, 11, 20884–20892. [Google Scholar] [CrossRef]
- de Jong, O.G.; van Balkom, B.W.M.; Schiffelers, R.M.; Bouten, C.V.C.; Verhaar, M.C. Extracellular vesicles: Potential roles in regene-rative medicine. Front. Immunol. 2014, 5, 608. [Google Scholar] [CrossRef]
- Samuel, M.; Fonseka, P.; Sanwlani, R.; Gangoda, L.; Chee, S.H.; Keerthikumar, S.; Spurling, A.; Chitti, S.V.; Zanker, D.; Ang, C.-S.; et al. Oral administration of bovine milk-derived extracellular vesicles induces senescence in the primary tumor but accelerates cancer metastasis. Nat. Commun. 2021, 12, 3950. [Google Scholar] [CrossRef]
- Han, C.; Zhou, J.; Liu, B.; Liang, C.; Pan, X.; Zhang, Y.; Zhang, Y.; Wang, Y.; Shao, L.; Zhu, B.; et al. Delivery of miR-675 by stem cell-derived exosomes encapsulated in silk fibroin hydrogel prevents aging-induced vascular dysfunction in mouse hindlimb. Mater. Sci. Eng. C 2019, 99, 322–332. [Google Scholar] [CrossRef]
- Sidhom, K.; Obi, P.O.; Saleem, A. A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option? Int. J. Mol. Sci. 2020, 21, 6466. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Schmauck-Medina, T.; Molière, A.; Lautrup, S.; Zhang, J.; Chlopicki, S.; Madsen, H.B. New hallmarks of ageing: A 2022 Co-penhagen ageing meeting summary. Aging 2022, 14, 6829–6839. [Google Scholar] [CrossRef] [PubMed]
- Moskalev, A.A.; Shaposhnikov, M.V.; Plyusnina, E.N.; Zhavoronkov, A.; Budovsky, A.; Yanai, H.; Fraifeld, V.E. The role of DNA damage and repair in aging through the prism of Koch-like criteria. Ageing Res. Rev. 2013, 12, 661–684. [Google Scholar] [CrossRef]
- Hoeijmakers, J.H.J. DNA damage, aging, and cancer. N. Engl. J. Med. 2009, 361, 1475–1485. [Google Scholar] [CrossRef]
- Lord, C.J.; Ashworth, A. The DNA damage response and cancer therapy. Nature 2012, 481, 287–294. [Google Scholar] [CrossRef]
- Ganem, N.J.; Godinho, S.A.; Pellman, D. A mechanism linking extra centrosomes to chromosomal instability. Nature 2009, 460, 278–282. [Google Scholar] [CrossRef]
- Chennakrishnaiah, S.; Tsering, T.; Gregory, C.; Tawil, N.; Spinelli, C.; Montermini, L.; Karatzas, N.; Aprikian, S.; Choi, D.; Klewes, L.; et al. Extracellular vesicles from genetically unstable, oncogene-driven cancer cells trigger micronuclei formation in endothelial cells. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Zhu, X.; You, Y.; Li, Q.; Zeng, C.; Fu, F.; Guo, A. BCR-ABL1–positive microvesicles transform normal hematopoietic trans-plants through genomic instability: Implications for donor cell leukemia. Leukemia 2014, 28, 1666–1675. [Google Scholar] [CrossRef]
- Zhu, F.; Wei, C.; Wu, H.; Shuai, B.; Yu, T.; Gao, F.; Yuan, Y.; Zuo, D.; Liu, X.; Zhang, L.; et al. Hypoxic mesenchymal stem cell-derived exosomes alleviate ulcerative colitis injury by limiting intestinal epithelial cells reactive oxygen species accumulation and DNA damage through HIF-1α. Int. Immunopharmacol. 2022, 113, 109426. [Google Scholar] [CrossRef]
- Wen, S.; Dooner, M.; Cheng, Y.; Papa, E.; Del Tatto, M.; Pereira, M.; Deng, Y.; Goldberg, L.; Aliotta, J.; Chatterjee, D.; et al. Mesenchymal stromal cell-derived extracellular vesicles rescue radiation damage to murine marrow hematopoietic cells. Leukemia 2016, 30, 2221–2231. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.M.; Luo, T.; von der Ohe, J.; de Juan Mora, B.; Schmitt, R.; Hass, R. Human MSC-Derived Exosomes Reduce Cellular Se-nescence in Renal Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 13562. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yang, Y.; Zhao, B.; Yang, Y.; Wang, J.; Shen, K.; Yang, X.; Hu, D.; Zheng, G.; Han, J. Exosomes Derived From Adipose-Derived Mesenchymal Stem Cells Ameliorate Radiation-Induced Brain Injury by Activating the SIRT1 Pathway. Front. Cell Dev. Biol. 2021, 9, 693782. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, W.; Li, X. Induced pluripotent stem cell-derived mesenchymal stem cells deliver exogenous miR-105-5p via small extracellular vesicles to rejuvenate senescent nucleus pulposus cells and attenuate intervertebral disc degeneration. Stem Cell Res. Ther. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Blackburn, E.H.; Greider, C.W.; Szostak, J.W. Telomeres and telomerase: The path from maize, Tetrahymena and yeast to human cancer and aging. Nat. Med. 2006, 12, 1133–1138. [Google Scholar] [CrossRef]
- Blasco, M.A. The epigenetic regulation of mammalian telomeres. Nat. Rev. Genet. 2007, 8, 299–309. [Google Scholar] [CrossRef]
- Armanios, M.; Blackburn, E.H. The telomere syndromes. Nat. Rev. Genet. 2012, 13, 693–704. [Google Scholar] [CrossRef]
- Boonekamp, J.J.; Simons, M.J.P.; Hemerik, L.; Verhulst, S. Telomere length behaves as biomarker of somatic redundancy rather than biological age. Aging Cell 2013, 12, 330–332. [Google Scholar] [CrossRef]
- Blasco, M.A.; Lee, H.-W.; Hande, M.P.; Samper, E.; Lansdorp, P.M.; DePinho, R.A.; Greider, C.W. Telomere Shortening and Tumor Formation by Mouse Cells Lacking Telomerase RNA. Cell 1997, 91, 25–34. [Google Scholar] [CrossRef]
- Tomás-Loba, A.; Flores, I.; Fernández-Marcos, P.J.; Cayuela, M.L.; Maraver, A.; Tejera, A.; Borrás, C.; Matheu, A.; Klatt, P.; Flores, J.M.; et al. Telomerase Reverse Transcriptase Delays Aging in Cancer-Resistant Mice. Cell 2008, 135, 609–622. [Google Scholar] [CrossRef]
- Bernardes de Jesus, B.; Vera, E.; Schneeberger, K.; Tejera, A.M.; Ayuso, E.; Bosch, F. Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. EMBO Mol. Med. 2012, 4, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Blasco, M.A. Telomere length, stem cells and aging. Nat Chem Biol. 2007, 3, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, D.; LaBella, K.A.; DePinho, R.A. Telomeres: History, health, and hallmarks of aging. Cell 2021, 184, 306–322. [Google Scholar] [CrossRef] [PubMed]
- Rossiello, F.; Jurk, D.; Passos, J.F.; di Fagagna, F.D. Telomere dysfunction in ageing and age-related diseases. Nature 2022, 24, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Cusanelli, E.; Romero, C.A.P.; Chartrand, P. Telomeric Noncoding RNA TERRA Is Induced by Telomere Shortening to Nucleate Telomerase Molecules at Short Telomeres. Mol. Cell 2013, 51, 780–791. [Google Scholar] [CrossRef]
- Wang, Z.; Lieberman, P.M. The crosstalk of telomere dysfunction and inflammation through cell-free TERRA containing exosomes. RNA Biol. 2016, 13, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Deng, Z.; Dahmane, N.; Tsai, K.; Wang, P.; Williams, D.R.; Kossenkov, A.V.; Showe, L.C.; Zhang, R.; Huang, Q.; et al. Telomeric repeat-containing RNA (TERRA) constitutes a nucleoprotein component of extracellular inflammatory exosomes. Proc. Natl. Acad. Sci. USA 2015, 112, E6293–E6300. [Google Scholar] [CrossRef]
- Al-Mayah, A.H.J.; Bright, S.J.; Bowler, D.A.; Slijepcevic, P.; Goodwin, E.; Kadhim, M.A. Exosome-Mediated Telomere Instability in Human Breast Epithelial Cancer Cells after X Irradiation. Radiat. Res. 2017, 187, 98–106. [Google Scholar] [CrossRef]
- Sonoda, S.; Murata, S.; Nishida, K.; Kato, H.; Uehara, N.; Kyumoto, Y.N.; Yamaza, H.; Takahashi, I.; Kukita, T.; Yamaza, T. Extracellular vesicles from deciduous pulp stem cells recover bone loss by regulating telomerase activity in an osteoporosis mouse model. Stem Cell Res. Ther. 2020, 11, 296. [Google Scholar] [CrossRef]
- Sonoda, S.; Murata, S.; Kato, H.; Zakaria, F.; Kyumoto-Nakamura, Y.; Uehara, N.; Yamaza, H.; Kukita, T.; Yamaza, T. Targeting of Deciduous Tooth Pulp Stem Cell–Derived Extracellular Vesicles on Telomerase-Mediated Stem Cell Niche and Immune Regulation in Systemic Lupus Erythematosus. J. Immunol. 2021, 206, 3053–3063. [Google Scholar] [CrossRef]
- Lee, B.-R.; Kim, J.-H.; Choi, E.-S.; Cho, J.H.; Kim, E. Effect of young exosomes injected in aged mice. Int. J. Nanomed. 2018, 13, 5335–5345. [Google Scholar] [CrossRef]
- Sanz-Ros, J.; Romero-García, N.; Mas-Bargues, C.; Monleón, D.; Gordevicius, J.; Brooke, R.T.; Dromant, M.; Díaz, A.; Derevyanko, A.; Guío-Carrión, A.; et al. Small extracellular vesicles from young adipose-derived stem cells prevent frailty, improve health span, and decrease epigenetic age in old mice. Sci. Adv. 2022, 8, eabq2226. [Google Scholar] [CrossRef]
- Lanna, A.; Vaz, B.; D’Ambra, C.; Valvo, S.; Vuotto, C.; Chiurchiù, V.; Devine, O.; Sanchez, M.; Borsellino, G.; Akbar, A.N.; et al. An intercellular transfer of telomeres rescues T cells from senescence and promotes long-term immunological memory. Nature 2022, 24, 1461–1474. [Google Scholar] [CrossRef]
- Likonen, D.; Pinchasi, M.; Beery, E.; Sarsor, Z.; Signorini, L.F.; Gervits, A.; Sharan, R.; Lahav, M.; Raanani, P.; Uziel, O. Exosomal telomerase transcripts reprogram the microRNA transcriptome profile of fibroblasts and partially contribute to CAF formation. Sci. Rep. 2022, 12, 16415. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Shah, P.P.; Nativio, R.; Berger, S.L. Epigenetic Mechanisms of Longevity and Aging. Cell 2016, 166, 822–839. [Google Scholar] [CrossRef]
- Chulpanova, D.S.; Pukhalskaia, T.V.; Rizvanov, A.A.; Solovyeva, V.V. Contribution of Tumor-Derived Extracellular Vesicles to Malignant Transformation of Normal Cells. Bioengineering 2022, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Mensà, E.; Guescini, M.; Giuliani, A.; Bacalini, M.G.; Ramini, D.; Corleone, G. Small extracellular vesicles deliver miR-21 and miR-217 as pro-senescence effectors to endothelial cells. J. Extracell. Vesicles 2020, 9, 1725285. [Google Scholar] [CrossRef]
- Koga, H.; Kaushik, S.; Cuervo, A.M. Protein homeostasis and aging: The importance of exquisite quality control. Ageing Res. Rev. 2011, 10, 205–215. [Google Scholar] [CrossRef]
- Barbosa, M.C.; Grosso, R.; Fader, C.M. Hallmarks of Aging: An Autophagic Perspective. Front. Endocrinol. 2019, 9, 790. [Google Scholar] [CrossRef]
- Powers, E.T.; Morimoto, R.I.; Dillin, A.; Kelly, J.W.; Balch, W.E. Biological and Chemical Approaches to Diseases of Proteostasis Deficiency. Annu. Rev. Biochem. 2009, 78, 959–991. [Google Scholar] [CrossRef]
- Rajendran, L.; Bali, J.; Barr, M.M.; Court, F.A.; Krämer-Albers, E.-M.; Picou, F.; Raposo, G.; Van Der Vos, K.E.; Van Niel, G.; Wang, J.; et al. Emerging roles of extracellular vesicles in the nervous system. J. Neurosci. 2014, 34, 15482. [Google Scholar] [CrossRef]
- Rajendran, L.; Honsho, M.; Zahn, T.R.; Keller, P.; Geiger, K.D.; Verkade, P. Alzheimer’s disease β-amyloid peptides are released in association with exosomes. Proc. Natl. Acad. Sci. USA 2006, 103, 11172. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, X.; Song, M.; He, Z.; Cui, G.; Peng, G.; Dieterich, C.; Antebi, A.; Jing, N.; Shen, Y. A secreted microRNA disrupts autophagy in distinct tissues of Caenorhabditis elegans upon ageing. Nat. Commun. 2019, 10, 4827. [Google Scholar] [CrossRef]
- Guo, H.; Chitiprolu, M.; Roncevic, L.; Javalet, C.; Hemming, F.J.; Trung, M.T.; Meng, L.; Latreille, E.; de Souza, C.T.; McCulloch, D.; et al. Atg5 Disassociates the V1V0-ATPase to Promote Exosome Production and Tumor Metastasis Independent of Canonical Macroautophagy. Dev. Cell 2017, 43, 716–730.e7. [Google Scholar] [CrossRef]
- Jin, J.; Shi, Y.; Gong, J.; Zhao, L.; Li, Y.; He, Q. Exosome secreted from adipose-derived stem cells attenuates diabetic neph-ropathy by promoting autophagy flux and inhibiting apoptosis in podocyte. Stem. Cell Res. Ther. 2019, 15, 10. [Google Scholar]
- Ebrahim, N.; Ahmed, I.A.; Hussien, N.I.; Dessouky, A.A.; Farid, A.S.; Elshazly, A.M.; Mostafa, O.; El Gazzar, W.B.; Sorour, S.M.; Seleem, Y.; et al. Mesenchymal Stem Cell-Derived Exosomes Ameliorated Diabetic Nephropathy by Autophagy Induction through the mTOR Signaling Pathway. Cells 2018, 7, 226. [Google Scholar] [CrossRef]
- Qu, Y.; Zhang, Q.; Cai, X.; Li, F.; Ma, Z.; Xu, M.; Lu, L. Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J. Cell. Mol. Med. 2017, 21, 2491–2502. [Google Scholar] [CrossRef]
- Liu, L.; Jin, X.; Hu, C.-F.; Li, R.; Zhou, Z.; Shen, C.-X. Exosomes Derived from Mesenchymal Stem Cells Rescue Myocardial Ischaemia/Reperfusion Injury by Inducing Cardiomyocyte Autophagy Via AMPK and Akt Pathways. Cell. Physiol. Biochem. 2017, 43, 52–68. [Google Scholar] [CrossRef]
- Chen, H.X.; Liang, F.C.; Gu, P.; Xu, B.L.; Xu, H.J.; Wang, W.T. Exosomes derived from mesenchymal stem cells repair a Par-kinson’s disease model by inducing autophagy. Cell Death Dis. 2020, 11, 1–17. [Google Scholar]
- Kuang, Y.; Zheng, X.; Zhang, L.; Ai, X.; Venkataramani, V.; Kilic, E. Adipose-derived mesenchymal stem cells reduce au-tophagy in stroke mice by extracellular vesicle transfer of miR-25. J. Extracell. Vesicles 2020, 10, e12024. [Google Scholar] [CrossRef]
- Junnila, R.K.; List, E.O.; Berryman, D.; Murrey, J.W.; Kopchick, J.J. The GH/IGF-1 axis in ageing and longevity. Nat. Rev. Endocrinol. 2013, 9, 366–376. [Google Scholar] [CrossRef]
- Barzilai, N.; Huffman, D.M.; Muzumdar, R.H.; Bartke, A. The Critical Role of Metabolic Pathways in Aging. Diabetes 2012, 61, 1315–1322. [Google Scholar] [CrossRef]
- Mair, W.; Morantte, I.; Rodrigues, A.P.C.; Manning, G.; Montminy, M.; Shaw, R.J.; Dillin, A. Lifespan extension induced by AMPK and calcineurin is mediated by CRTC-1 and CREB. Nature 2011, 470, 404–408. [Google Scholar] [CrossRef]
- Lamming, D.W.; Ye, L.; Katajisto, P.; Goncalves, M.D.; Saitoh, M.; Stevens, D.M.; Davis, J.G.; Salmon, A.B.; Richardson, A.; Ahima, R.S.; et al. Rapamycin-Induced Insulin Resistance Is Mediated by mTORC2 Loss and Uncoupled from Longevity. Science 2012, 335, 1638–1643. [Google Scholar] [CrossRef]
- Harrison, D.E.; Strong, R.; Sharp, Z.D.; Nelson, J.F.; Astle, C.M.; Flurkey, K. Rapamycin fed late in life extends lifespan in ge-netically heterogeneous mice. Nature 2009, 460, 392. [Google Scholar] [CrossRef]
- Han, M.; Cao, Y.; Guo, X.; Chu, X.; Li, T.; Xue, H.; Xin, D.; Yuan, L.; Ke, H.; Li, G.; et al. Mesenchymal stem cell-derived extracellular vesicles promote microglial M2 polarization after subarachnoid hemorrhage in rats and involve the AMPK/NF-κB signaling pathway. Biomed. Pharmacother. 2021, 133, 111048. [Google Scholar] [CrossRef]
- Labora, J.A.F.; Morente-López, M.; Sánchez-Dopico, M.J.; Arntz, O.J.; Van De Loo, F.A.J.; De Toro, J.; Arufe, M.C. Influence of mesenchymal stem cell-derived extracellular vesicles in vitro and their role in ageing. Stem Cell Res. Ther. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Verdin, E. NAD + in aging, metabolism, and neurodegeneration. Science 2015, 350, 1208–1213. [Google Scholar] [CrossRef]
- Yoon, M.J.; Yoshida, M.; Johnson, S.; Takikawa, A.; Usui, I.; Tobe, K.; Nakagawa, T.; Yoshino, J.; Imai, S.-I. SIRT1-Mediated eNAMPT Secretion from Adipose Tissue Regulates Hypothalamic NAD+ and Function in Mice. Cell Metab. 2015, 21, 706–717. [Google Scholar] [CrossRef]
- Green, D.R.; Galluzzi, L.; Kroemer, G. Mitochondria and the Autophagy–Inflammation–Cell Death Axis in Organismal Aging. Science 2011, 333, 1109–1112. [Google Scholar] [CrossRef]
- Trifunovic, A.; Wredenberg, A.; Falkenberg, M.; Spelbrink, J.N.; Rovio, A.T.; Bruder, C.E.; Bohlooly, Y.M.; Gidlöf, S.; Oldfors, A.; Wibom, R.; et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 2004, 429, 417–423. [Google Scholar] [CrossRef]
- Sahin, E.; DePinho, R.A. Axis of ageing: Telomeres, p53 and mitochondria. Nat. Rev. Mol. Cell Biol. 2012, 13, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Egan, D.F.; Shackelford, D.B.; Mihaylova, M.M.; Gelino, S.; Kohnz, R.A.; Mair, W.; Vasquez, D.S.; Joshi, A.; Gwinn, D.M.; Taylor, R.; et al. Phosphorylation of ULK1 (hATG1) by AMP-Activated Protein Kinase Connects Energy Sensing to Mitophagy. Science 2011, 331, 456–461. [Google Scholar] [CrossRef]
- Fang, E.F.; Waltz, T.B.; Kassahun, H.; Lu, Q.; Kerr, J.S.; Morevati, M.; Fivenson, E.M.; Wollman, B.N.; Marosi, K.; Wilson, M.A.; et al. Tomatidine enhances lifespan and healthspan in C. elegans through mitophagy induction via the SKN-1/Nrf2 pathway. Sci. Rep. 2017, 7, 46208. [Google Scholar] [CrossRef]
- Chen, G.; Kroemer, G.; Kepp, O. Mitophagy: An Emerging Role in Aging and Age-Associated Diseases. Front. Cell Dev. Biol. 2020, 8, 200. [Google Scholar] [CrossRef]
- Picca, A.; Guerra, F.; Calvani, R.; Coelho-Junior, H.J.; Bossola, M.; Landi, F.; Bernabei, R.; Bucci, C.; Marzetti, E. Generation and Release of Mitochondrial-Derived Vesicles in Health, Aging and Disease. J. Clin. Med. 2020, 9, 1440. [Google Scholar] [CrossRef] [PubMed]
- Choong, C.-J.; Okuno, T.; Ikenaka, K.; Baba, K.; Hayakawa, H.; Koike, M.; Yokota, M.; Doi, J.; Kakuda, K.; Takeuchi, T.; et al. Alternative mitochondrial quality control mediated by extracellular release. Autophagy 2020, 17, 2962–2974. [Google Scholar] [CrossRef]
- Picca, A.; Calvani, R.; Coelho-Junior, H.J.; Landi, F.; Bernabei, R.; Marzetti, E. Inter-Organelle Membrane Contact Sites and Mito-chondrial Quality Control during Aging: A Geroscience View. Cells 2020, 9, 598. [Google Scholar] [CrossRef]
- D’Acunzo, P.; Pérez-González, R.; Kim, Y.; Hargash, T.; Miller, C.; Alldred, M.J.; Erdjument-Bromage, H.; Penikalapati, S.C.; Pawlik, M.; Saito, M.; et al. Mitovesicles are a novel population of ex-tracellular vesicles of mitochondrial origin altered in Down syndrome. Sci. Adv. 2021, 7, eabe5085. [Google Scholar] [CrossRef]
- Lazo, S.; Hooten, N.N.; Green, J.; Eitan, E.; Mode, N.A.; Liu, Q.; Zonderman, A.B.; Ezike, N.; Mattson, M.P.; Ghosh, P.; et al. Mitochondrial DNA in extracellular vesicles declines with age. Aging Cell 2020, 20, e13283. [Google Scholar] [CrossRef]
- Kadota, T.; Yoshioka, Y.; Fujita, Y.; Araya, J.; Minagawa, S.; Hara, H. Extracellular Vesicles from Fibroblasts Induce Epithe-lial-Cell Senescence in Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2020, 63, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Clemens, Z.J.; Shinde, S.N.; Sivakumar, S.; Pius, A.; Bhatia, A.; Picciolini, S.; Carlomagno, C.; Gualerzi, A.; Bedoni, M.; et al. Regulation of aged skeletal muscle regeneration by circulating extracellular vesicles. Nat. Aging 2021, 1, 1148–1161. [Google Scholar] [CrossRef]
- Shuler, K.T.; Wilson, B.E.; Muñoz, E.R.; Mitchell, A.D.; Selsby, J.T.; Hudson, M.B. Muscle Stem Cell-Derived Extracellular Vesicles Reverse Hydrogen Peroxide-Induced Mitochondrial Dysfunction in Mouse Myotubes. Cells 2020, 9, 2544. [Google Scholar] [CrossRef] [PubMed]
- Jametti, L.P.; Bernstock, J.D.; Willis, C.; Manferrari, G.; Rogall, R.; Fernandez-Vizarra, E.; Williamson, J.; Braga, A.; Bosch, A.V.D.; Leonardi, T.; et al. Neural stem cells traffic functional mitochondria via extracellular vesicles. PLoS Biol. 2021, 19, e3001166. [Google Scholar] [CrossRef]
- Dutra Silva, J.; Su, Y.; Calfee, C.S.; Delucchi, K.L.; Weiss, D.; McAuley, D.F. Mesenchymal stromal cell extracellular vesicles rescue mitochondrial dysfunction and improve barrier integrity in clinically relevant models of ARDS. Eur. Respir. J. 2021, 58, 2002978. [Google Scholar] [CrossRef]
- Ikeda, G.; Santoso, M.R.; Tada, Y.; Li, A.M.; Vaskova, E.; Jung, J.-H.; O’Brien, C.; Egan, E.; Ye, J.; Yang, P.C. Mitochondria-Rich Extracellular Vesicles From Autologous Stem Cell–Derived Cardiomyocytes Restore Energetics of Ischemic Myocardium. J. Am. Coll. Cardiol. 2021, 77, 1073–1088. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Liu, S.; Wang, C.; Wang, Y.; Wan, M.; Liu, F. Mesenchymal Stem Cell-Derived Extracellular Vesicles Attenuate Mitochondrial Damage and Inflammation by Stabilizing Mitochondrial DNA. ACS Nano. 2021, 15, 1519–1538. [Google Scholar] [CrossRef]
- Xu, F.; Wu, Y.; Yang, Q.; Cheng, Y.; Xu, J.; Zhang, Y.; Dai, H.; Wang, B.; Ma, Q.; Chen, Y.; et al. Engineered Extracellular Vesicles with SHP2 High Expression Promote Mitophagy for Alzheimer’s Disease Treatment. Adv. Mater. 2022, 34, 2207107. [Google Scholar] [CrossRef]
- He, S.; Sharpless, N.E. Senescence in Health and Disease. Cell 2017, 169, 1000–1011. [Google Scholar] [CrossRef]
- Campisi, J.; d’Adda di Fagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–840. [Google Scholar] [CrossRef]
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018, 28, 436–453. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.; Blasco, M.A.; Serrano, M. Cellular Senescence in Cancer and Aging. Cell 2007, 130, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Espín, D.; Serrano, M. Cellular senescence: From physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014, 15, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jurk, D.; Maddick, M.; Nelson, G.; Martin-Ruiz, C.; Von Zglinicki, T. DNA damage response and cellular senescence in tissues of aging mice. Aging Cell 2009, 8, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Gluscevic, M.; Baker, D.J.; Laberge, R.-M.; Marquess, D.; Dananberg, J.; Van Deursen, J.M. Senescent cells: An emerging target for diseases of ageing. Nat. Rev. Drug Discov. 2017, 16, 718–735. [Google Scholar] [CrossRef]
- Baker, D.J.; Childs, B.G.; Durik, M.; Wijers, M.E.; Sieben, C.J.; Zhong, J.; Saltness, R.A.; Jeganathan, K.B.; Verzosa, G.C.; Pezeshki, A.; et al. Naturally occurring p16Ink4a-positive cells shorten healthy lifespan. Nature 2016, 530, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Pirtskhalava, T.; Farr, J.N.; Weigand, B.M.; Palmer, A.K.; Weivoda, M.M.; Inman, C.L.; Ogrodnik, M.B.; Hachfeld, C.M.; Fraser, D.G.; et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018, 24, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Martin-Montalvo, A.; Mercken, E.M.; Mitchell, S.J.; Palacios, H.H.; Mote, P.L.; Scheibye-Knudsen, M.; Gomes, A.P.; Ward, T.M.; Minor, R.K.; Blouin, M.-J.; et al. Metformin improves healthspan and lifespan in mice. Nat. Commun. 2013, 4, 2192. [Google Scholar] [CrossRef]
- Acosta, J.C.; Banito, A.; Wuestefeld, T.; Georgilis, A.; Janich, P.; Morton, J.P.; Athineos, D.; Kang, T.-W.; Lasitschka, F.; Andrulis, M.; et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nature 2013, 15, 978–990. [Google Scholar] [CrossRef]
- Demirci, D.; Dayanc, B.; Mazi, F.A.; Senturk, S. The Jekyll and Hyde of Cellular Senescence in Cancer. Cells 2021, 10, 208. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Paine, M.S.; Brooks, A.M.; McCubrey, J.A.; Renegar, R.H.; Wang, R.; Terrian, D.M. Senescence-Associated Exosome Release from Human Prostate Cancer Cells. Cancer Res 2008, 68, 7864–7871. [Google Scholar] [CrossRef]
- Yu, X.; Riley, T.; Levine, A.J. The regulation of the endosomal compartment by p53 the tumor suppressor gene. FEBS J. 2009, 276, 2201–2212. [Google Scholar] [CrossRef]
- Takahashi, A.; Okada, R.; Nagao, K.; Kawamata, Y.; Hanyu, A.; Yoshimoto, S. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat. Commun. 2017, 8, 15287. [Google Scholar] [CrossRef]
- Elbakrawy, E.; Bains, S.K.; Bright, S.; Al-Abedi, R.; Mayah, A.; Goodwin, E.; Kadhim, M. Radiation-Induced Senescence Bystander Effect: The Role of Exosomes. Biology 2020, 9, 191. [Google Scholar] [CrossRef] [PubMed]
- Weilner, S.; Schraml, E.; Wieser, M.; Messner, P.; Schneider, K.; Wassermann, K.; Micutkova, L.; Fortschegger, K.; Maier, A.B.; Westendorp, R.; et al. Secreted microvesicular miR-31 inhibits osteogenic differentiation of mesenchymal stem cells. Aging Cell 2016, 15, 744–754. [Google Scholar] [CrossRef]
- Fulzele, S.; Mendhe, B.; Khayrullin, A.; Johnson, M.; Kaiser, H.; Liu, Y. Muscle-derived miR-34a increases with age in circu-lating extracellular vesicles and induces senescence of bone marrow stem cells. Aging 2019, 11, 1791–1803. [Google Scholar] [CrossRef]
- Borghesan, M.; Fafián-Labora, J.; Eleftheriadou, O.; Carpintero-Fernández, P.; Paez-Ribes, M.; Vizcay-Barrena, G. Small Ex-tracellular Vesicles Are Key Regulators of Non-cell Autonomous Intercellular Communication in Senescence via the Inter-feron Protein IFITM3. Cell Rep. 2019, 27, 3956–3971.e6. [Google Scholar] [CrossRef]
- Fafián-Labora, J.A.; O’Loghlen, A. NF-κB/IKK activation by small extracellular vesicles within the SASP. Aging Cell 2021, 20, e13426. [Google Scholar] [CrossRef]
- Jiang, F.; Chen, Q.; Wang, W.; Ling, Y.; Yan, Y.; Xia, P. Hepatocyte-derived extracellular vesicles promote endothelial inflammation and atherogenesis via microRNA-1. J. Hepatol. 2019, 72, 156–166. [Google Scholar] [CrossRef]
- Liu, S.; Mahairaki, V.; Bai, H.; Ding, Z.; Li, J.; Witwer, K.W.; Cheng, L. Highly Purified Human Extracellular Vesicles Produced by Stem Cells Alleviate Aging Cellular Phenotypes of Senescent Human Cells. STEM CELLS 2019, 37, 779–790. [Google Scholar] [CrossRef]
- Kim, M.K.; Choi, Y.C.; Cho, S.H.; Choi, J.S.; Cho, Y.W. The Antioxidant Effect of Small Extracellular Vesicles Derived from Aloe vera Peels for Wound Healing. Tissue Eng. Regen. Med. 2021, 18, 561–571. [Google Scholar] [CrossRef]
- Dorronsoro, A.; Santiago, F.E.; Grassi, D.; Zhang, T.; Lai, R.C.; McGowan, S.J.; Angelini, L.; Lavasani, M.; Corbo, L.; Lu, A.; et al. Mesenchymal stem cell-derived extracellular vesicles reduce senescence and extend health span in mouse models of aging. Aging Cell 2021, 20, e13337. [Google Scholar] [CrossRef]
- Deng, M.; Yu, T.Z.; Li, D.; Wang, X.; Zhou, G.; Liu, W.; Cao, Y.; Xia, W.; Zhang, W.J. Human umbilical cord mesenchymal stem cell-derived and dermal fibroblast-derived extracellular vesicles protect dermal fibroblasts from ultraviolet radiation-induced photoaging in vitro. Photochem. Photobiol. Sci. 2020, 19, 406–414. [Google Scholar] [CrossRef]
- van Balkom, B.W.M.; de Jong, O.G.; Smits, M.; Brummelman, J.; den Ouden, K.; de Bree, P.M. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood 2013, 121, 3997–4006. [Google Scholar] [CrossRef] [PubMed]
- Sharpless, N.E.; DePinho, R.A. How stem cells age and why this makes us grow old. Nat. Rev. Mol. Cell Biol. 2007, 8, 703–713. [Google Scholar] [CrossRef]
- Rossi, D.J.; Bryder, D.; Seita, J.; Nussenzweig, A.; Hoeijmakers, J.; Weissman, I.L. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature 2007, 447, 725–729. [Google Scholar] [CrossRef]
- Molofsky, A.V.; Slutsky, S.G.; Joseph, N.M.; He, S.; Pardal, R.; Krishnamurthy, J. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature 2006, 443, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, H.; Kusumoto, D.; Hashimoto, H.; Yuasa, S. Stem Cell Aging in Skeletal Muscle Regeneration and Disease. Int. J. Mol. Sci. 2020, 21, 1830. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Lee, Y.D.; Wagers, A.J. Stem cell aging: Mechanisms, regulators and therapeutic opportunities. Nat. Med. 2014, 20, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Conboy, I.M.; Conboy, M.J.; Wagers, A.J.; Girma, E.R.; Weissman, I.L.; Rando, T.A. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 2005, 433, 760–764. [Google Scholar] [CrossRef]
- Villeda, S.A.; Luo, J.; Mosher, K.I.; Zou, B.; Britschgi, M.; Bieri, G.; Stan, T.M.; Fainberg, N.; Ding, Z.; Eggel, A.; et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 2011, 477, 90–94. [Google Scholar] [CrossRef]
- Lavasani, M.; Robinson, A.R.; Lu, A.; Song, M.; Feduska, J.M.; Ahani, B.; Tilstra, J.S.; Feldman, C.H.; Robbins, P.D.; Niedernhofer, L.J.; et al. Muscle-derived stem/progenitor cell dysfunction limits healthspan and lifespan in a murine progeria model. Nat. Commun. 2012, 3, 608. [Google Scholar] [CrossRef]
- Xiao, Y.-Z.; Yang, M.; Xiao, Y.; Guo, Q.; Huang, Y.; Li, C.-J.; Cai, D.; Luo, X.-H. Reducing Hypothalamic Stem Cell Senescence Protects against Aging-Associated Physiological Decline. Cell Metab. 2020, 31, 534–548.e5. [Google Scholar] [CrossRef]
- Hu, G.; Xia, Y.; Chen, B.; Zhang, J.; Gong, L.; Chen, Y. ESC-sEVs Rejuvenate Aging Hippocampal NSCs by Transferring SMADs to Regulate the MYT1-Egln3-Sirt1 Axis. Mol. Ther. 2021, 29, 103–120. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J. Frailty in Older Adults: Evidence for a Pheno-type. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Fafián-Labora, J.A.; O’Loghlen, A. Classical and Nonclassical Intercellular Communication in Senescence and Ageing. Trends Cell Biol. 2020, 30, 628–639. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. A Ser. Biol. Sci. Med. Sci. 2014, 69 (Suppl. 1), S4–S9. [Google Scholar] [CrossRef]
- Tsukamoto, H.; Kouwaki, T.; Oshiumi, H. Aging-Associated Extracellular Vesicles Contain Immune Regulatory microRNAs Alleviating Hyperinflammatory State and Immune Dysfunction in the Elderly. Iscience 2020, 23, 101520. [Google Scholar] [CrossRef]
- Deng, S.; Zhou, X.; Ge, Z.; Song, Y.; Wang, H.; Liu, X. Exosomes from adipose-derived mesenchymal stem cells ameliorate cardiac damage after myocardial infarction by activating S1P/SK1/S1PR1 signaling and promoting macrophage M2 polariza-tion. Int. J. Biochem. Cell Biol. 2019, 1, 114. [Google Scholar] [CrossRef]
- Tofiño-Vian, M.; Guillén, M.I.; Pérez Del Caz, M.D.; Castejón, M.A.; Alcaraz, M.J. Extracellular vesicles from adipose-derived mes-enchymal stem cells downregulate senescence features in osteoarthritic osteoblasts. Oxid. Med. Cell. Longev. 2017, 2017, 7197598. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Jeffery, I.B. Gut microbiota and aging. Science 2015, 350, 1214–1215. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.; Soffer, E.; Elinav, E. Transforming medicine with the microbiome. Sci. Transl. Med. 2019, 11, eaaw1815. [Google Scholar] [CrossRef] [PubMed]
- Bárcena, C.; Valdés-Mas, R.; Mayoral, P.; Garabaya, C.; Durand, S.; Rodríguez, F.; Fernández-García, M.T.; Salazar, N.; Nogacka, A.M.; Garatachea, N.; et al. Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice. Nat. Med. 2019, 25, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, C.; Liu, Z.; Luo, Z.; Rao, S.; Jin, L.; Wan, T.; Yue, T.; Tan, Y.; Yin, H.; et al. Extracellular Vesicles from Child Gut Microbiota Enter into Bone to Preserve Bone Mass and Strength. Adv. Sci. 2021, 8, 2004831. [Google Scholar] [CrossRef]
- Díez-Sainz, E.; Milagro, F.I.; Riezu-Boj, J.I.; Lorente-Cebrián, S. Effects of gut microbiota–derived extracellular vesicles on obesity and diabetes and their potential modulation through diet. J. Physiol. Biochem. 2021, 78, 485–499. [Google Scholar] [CrossRef]

| Hallmark | Pro-/Anti- | IVV/IVT | EVs’ Receptor | EVs’ Donor | EV Cargo | Cell-Level and Organismal-Level Effects | Ref. | |
|---|---|---|---|---|---|---|---|---|
| P R I M A R Y | Genomic instability | Pro- | IVT | Human mononuclear cells from healthy donors | K-562 cell line (lymphoblasts) | NA | Inhibited tumor suppressor genes P53 and RIZ1. Activated cytidine deaminase and ROS, leading to DNA breakage and recombination Treated cells exhibit a leukemia-like malignant phenotype | [55] |
| IVT | HUVECs | RAS-3 cell line (intestinal epithelial cell line containing c-HRAS oncogene) | BCR-ABL1 mRNA | Increased the level of DNA damage response markers, such as the phosphorylation of histone γH2AX Increased oncogenic transformation potential | [54] | |||
| Anti- | IVV | Intestinal epithelial cells | BM-MSCs | NA | Limited intestinal epithelial cells’ ROS accumulation and DNA damage Alleviated ulcerative colitis injury | [56] | ||
| IVT | FDC-P1 cell line (bone marrow hematopoietic cells) | BM-MSCs | miR106b-3, miR155-5p, and miR210-5p | Rescued radiation-associated DNA damage | [57] | |||
| IVT | Renal epithelial cells | h-UCMSCs (human umbilical cord MSCs) | NA | Decreased DNA damage foci and senescence | [52] | |||
| IVT | Microglia from adult rats (primary culture) | ADSCs | NA | Activation of SIRT1 Reduced levels of caspase-3, MDA, 8-OHdG, and TNF-α Promoted the recovery of SOD, catalase, and IL-10 Ameliorated radiation-induced brain injury | [58] | |||
| IVT | Nucleus pulposus cells | iMSCs | miR-105-5p | SIRT6 activation Rejuvenated senescent nucleus pulposus cells and attenuated intervertebral disc degeneration | [59] | |||
| Telomere attrition | Pro- | IVT | Peripheral blood mononuclear cells | TRF2-induced BJ-5ta fibroblasts | cfTERRA | Stimulated the transcription of inflammatory cytokine genes (TNFα, IL6, and C-X-C chemokine 10) | [71] | |
| IVT | Breast cancer cells | Irradiated breast cancer cells | Proteins and RNAs | Reduced telomere length Reduced telomerase activity | [73] | |||
| Anti- | IVT IVV | BM-MSCs Ovariectomized mice MRL/lpr mice (systemic lupus-erythrematosus-like) | Stem cells from human exfoliated deciduous teeth (SHED) | miR-346 | Increased Tert mRNA expression and telomerase activity Increased hematopoietic niche formation and osteoblast differentiation Recovered bone volume and alleviate osteoporosis | [74,75] | ||
| IVT IVV | T cells and T cells deficient in telomerase (TERT KO cells) | Autologous-antigen-presenting cells (polymorphonuclear cells) | Telomeric DNA | Increased telomere length by Rad51 recombination factor Increased T cell proliferation rates and delayed senescence, increase in central memory T cells Increased mice survival after viral infection | [78] | |||
| IVV | Old mice | Serum of young mice | Several miRNAs (miR-126b-5p) | Upregulated telomerase-complex-related genes (Men1 and Mre11a) in the liver and lungs | [76] | |||
| Epigenetic alterations | Pro- | IVT | Human mononuclear cells from healthy donors | K-562 cell line (lymphoblasts) | NA | Global DNA hypermethylation, including promoters of the tumor suppressor genes P53 and RIZ1, and the upregulation of methyltransferases | [55] | |
| IVT | HUVECs | Senescent HUVECs | miR-21-5p and miR-217 | Downregulation of DNA methyltransferase 1 and SIRT1 Expression of SASP molecules and cell cycle inhibitors | [82] | |||
| Anti- | IVV | Old mice | Young ADSCs | miRNAs | Reduced epigenetic clocks of the liver and kidney Improved renal function and increase healthspan | [77] | ||
| Loss of proteostasis | Pro- | IVT | N2a cells (neural-crest-derived cell line) | Amyloid beta peptide (Aβ) | Responsible for the release of Aβ into the extracellular milieu Involved in the pathogenesis of AD | [88] | ||
| Anti- | IVT IVV | Podocytes | ADSCs | miR-486 | Promoted autophagy Attenuated diabetic nephropathy | [89] | ||
| IVV | Renal tissue | BM-MSCs | NA | Autophagy induction through the mTOR signaling pathway Attenuated diabetic nephropathy | [90] | |||
| IVT IVV | Hepatic tissue and HSC-T6 cells | ADSCs | miRNA-181-5p | Autophagy activation Prevented liver fibrosis | [91] | |||
| IVT IVV | Cardiomyocytes | BM-MSCs | NA | Autophagy activation via AMPK/mTOR or Akt/mTOR Rescued myocardial ischemia/reperfusion injury | [92] | |||
| IVT | SH-SY5Y cell line (from neuroblastoma cell line) | h-UCMSCs | NA | Induced autophagy in neural tissue Improved Parkinson’s disease features | [93] | |||
| IVT IVV | Neurons under glucose–oxygen deprivation Mice exposed to cerebral ischemia | ADSCs | miR-25-3p | Reduced autophagy and cell death by modulating p53-BNIP3 signaling Reduced infarct size and improve neurological recovery | [94] | |||
| A N T A G O N I S T I C | Deregulated nutrient sensing | Pro- | IVT | Young MSCs | Old MSC-EVs | NA | Increased mTOR pathways | |
| Anti- | IVV | Old mice | Plasma from young mice | miR-126b-5p and miR-466c-5p | Reduced mTOR and IGF1R levels in the lungs and liver | [76] | ||
| IVV | Old mice | BM-MSCs | NA | Reduced mTOR and IGF1R levels in the parietal cortex Promoted microglial M2 polarization | [101] | |||
| IVT | Cardiomyocytes | BM-MSCs | NA | AMPK activation results in increased autophagy Rescued myocardial ischemia/reperfusion injury | [92] | |||
| IVT | Old MSCs | Young MSCs | miR-188-3p | Rictor targeting downregulates mTOR pathways and increases AMPK pathways. Improved pluripotency of MSCs | [27] | |||
| IVV | Old mice | Plasma from young mice | eNAMPT | Increased NAD+ bioavailability in tissues Increased lifespan and healthspan | [22] | |||
| Mitochondrial dysfunction | Pro- | IVT | Old mice | mtDNA | mtDNA in mitovesicles declines with age Cell-free mtDNA causes chronic inflammation | [113] | ||
| IVT | Lung epithelial cells | Idiopathic pulmonary fibrosis—lung fibroblasts | miR-23b-3p, miR-494-3p | Suppressed SIRT3 expression Increased mitochondrial ROS and mitochondrial damage | [114] | |||
| Pro- Anti- | IVT | HeLA cells | Plasma from young and old mice | NA | Improved (young EVs’) or worsened (old EVs’) oxygen consumption rates | [113] | ||
| Anti- | IVT | MDPSCs from old mice | Serum from young and old mice | α-Klotho transcripts | Increased basal oxygen consumption rates Improved mitochondrial ultrastructure | [115] | ||
| IVT | MDPSCs | Oxidatively injured myotubes | NA | Increased basal oxygen consumption rates and spare respiratory capacity | [116] | |||
| IVV IVT | mtDNA-deficient L929 Rho0 cells Mice with multiple sclerosis | NSCs | Functional mitochondria | EV-transferred mitochondria replaced the cell’s own, improving mitochondrial function Increased cell survival When taken up by immune cells, decreased inflammation Ameliorated clinical deficits | [117] | |||
| IVV IVT | Mice with ARDS-like damage Primary human airway epithelial and pulmonary endothelial cells | MSCs | Functional mitochondria | Restored cell barrier integrity and levels of oxidative phosphorylation Restored mitochondrial respiration in lung tissue Reduced lung injury in ARDS | [118] | |||
| IVV IVT | Hypoxia-injured iPSC-derived cardiomyocytes Mice with myocardial infarction | Autologous-stem-cell-derived cardiomyocytes | Functional mitochondria PGC-1α | Improved mitochondrial function Increased mitogenesis Significantly improved post-infarction cardiac function | [119] | |||
| IVV | Mice with acute kidney injury | MSCs | TFAM | Stabilized mtDNA via the formation of the TFAM–mtDNA complex Reversed mtDNA deletion and mitochondrial oxidative phosphorylation defects Attenuated renal damage | [120] | |||
| IVV | Mice with AD | MSCs, SHP2-enriched | 2 SHP | Selective induction of mitophagy in neural cells Decreased apoptosis and inflammation Decreased synaptic loss and cognitive decline are reduced | [121] | |||
| Cellular senescence | Pro- | IVT | MSCS | Senescent MSCs | miR-31 | Inhibited osteogenic differentiation via Frizzled-3 factor knockout Inhibited proliferation | [138] | |
| IVT IVV | BMSCs | Senescent muscle cells | miR-34a | Senescence induction and decreased SIRT1 expression | [139] | |||
| IVT | HFFF2 human foreskin primary fibroblasts | Senescent HFFF2 expressing an empty vector or oncogenic H-RAS (iC and iRAS cells) | IFITM3 | Senescence induction via paracrine transmission | [140] | |||
| IVV | HFFF2 human foreskin primary fibroblasts | Senescent HFFF2 expressing an empty vector or oncogenic H-RAS (iC and iRAS cells) | NA | Senescence induction through the NF-κB/IKK pathway | [141] | |||
| Anti- | IVT | Fibroblasts from old mice or progeroid mice | Fibroblasts from young mice | GSTM-2 | Senescence markers decreased (p16, p21, SA-ß-Gal and yH2AX) Reduced ROS and lipid peroxidation levels | [27] | ||
| IVT | Senescent MSCs | MSCs | Peroxiredoxins | Decreased senescence and ROS | [143] | |||
| IVV IVT | Ercc1−/− mice MSCs from old mice Progeroid MDPSCs (Zmpste24−/−) | hESC-derived BM-MSCs | miRNAs | Decreased senescence in vivo in kidney, liver, lung, and brain Decreased senescence in vitro (p16, p21, p53, PTEN, MYC, IL-1, and IL-6) through a possible senomorphic effect (downregulation of SASP factors) Increased the lifespan of Ercc1−/− mice | [144] | |||
| IVT | UV-radiated dermal fibroblasts | h-UCMSCs Dermal fibroblasts | NA | Decreased senescence Promoted the expressions of GPX-1 as well as Col-1 and decreased the expression of MMP-1 | [145] | |||
| IVV IVT | Old mice Senescent C2C12 myoblasts | Young ADSCs | miR-125b-5p, miR-let7c-5p, and miR-214-3p | Decreased senescence in vivo in kidney and muscle (laminB1, yH2AX) through a possible senomorphic effect (downregulation of SASP factors) Decreased senescence in vitro (SA-ß-Gal, SASP factors) with no selective increase in apoptosis | [77] | |||
| I N T E G R A T I V E | Stem cell exhaustion | Anti- | IVT | MSCs from old mice Progeroid MDPSCs | hESC-derived BM-MSCs | miRNAs | Decreased senescence of BM-MSCs Decreased senescence of MDPSCs and increased differentiation | [144] |
| IVV | Old mice | hESCs | Proteins modulating anti-aging genes | Promoted proliferation and osteogenic differentiation of BM-MSCs Alleviated age-related bone loss | [34] | |||
| IVV | Old mice | Hypothalamic NSCs from young mice | miRNA | Improved healthspan (physical tests, memory, and socialty) Rescued hypothalamic NSCs’ senescence | [36,155] | |||
| IVV | Old mice | ESCs | SMADs 4-5 | Activation of the MYT1–Egln3–Sirt1 axis Improved hNSCs’ stemness | [156] | |||
| IVV | Ovariectomized mice | SHED | miR-346 | Increased BM-MSCs’ proliferation and stemness Recovered bone volume and alleviate osteoporosis | [74] | |||
| IVV | Old mice | Young ADSCs | miRNAs | Increased proliferation and reduced fibrosis of renal tubules Improved renal function Higher cross-sectional area of muscle fibers, predominancy of type II fibers, and higher protein content Improved physical condition, decreased frailty scores, and increased healthspan | [77] | |||
| IVT | MDPSCs from old mice | Serum from young and old mice | α-Klotho transcripts | Increased myogenic differentiation potential | [115] | |||
| IVT | Senescent DP-MSCs (passage or hyperoxia pretreatment) | DP-MSCs | miR-302b | Increased expression of the pluripotency factors OCT4, SOX2, KLF4, and cMYC via HIF-1α activation A metabolic shift towards highly glycolytic and low oxidative profiles Recovered stemness | [32] | |||
| Altered intercellular communication | Anti- | IVT IVV | Cardiomyocytes, H9c2 myoblasts, cardiac fibroblasts, and HAPI cells | ADSCs | NA | Promoted macrophage M2 polarization and decreased LPS-induced inflammation Attenuated hypoxic damage | [163] | |
| IVT | Osteoarthritic osteoblasts | ADSCs | NA | Reduced the levels of inflammatory mediators (IL-6 and PGE2) | [164] | |||
| IVV | Intestinal epithelial cells | BM-MSCs | NA | Reduced IL-17A, RORγt, and IL-23 | [56] | |||
| IVV | Serum, CNS, and salivary glands | Young mice serum | NA | Reduced the levels of inflammatory mediators (IL-6, IL-1β, and TNF-a) Reduced CD4+IFN-γ+ T cells Reduced CNS-penetrating CD3+ T cells and macrophages, as well as MHC-II expression by microglia Reduced chronic autoimmune predisposition | [165] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-García, N.; Huete-Acevedo, J.; Mas-Bargues, C.; Sanz-Ros, J.; Dromant, M.; Borrás, C. The Double-Edged Role of Extracellular Vesicles in the Hallmarks of Aging. Biomolecules 2023, 13, 165. https://doi.org/10.3390/biom13010165
Romero-García N, Huete-Acevedo J, Mas-Bargues C, Sanz-Ros J, Dromant M, Borrás C. The Double-Edged Role of Extracellular Vesicles in the Hallmarks of Aging. Biomolecules. 2023; 13(1):165. https://doi.org/10.3390/biom13010165
Chicago/Turabian StyleRomero-García, Nekane, Javier Huete-Acevedo, Cristina Mas-Bargues, Jorge Sanz-Ros, Mar Dromant, and Consuelo Borrás. 2023. "The Double-Edged Role of Extracellular Vesicles in the Hallmarks of Aging" Biomolecules 13, no. 1: 165. https://doi.org/10.3390/biom13010165
APA StyleRomero-García, N., Huete-Acevedo, J., Mas-Bargues, C., Sanz-Ros, J., Dromant, M., & Borrás, C. (2023). The Double-Edged Role of Extracellular Vesicles in the Hallmarks of Aging. Biomolecules, 13(1), 165. https://doi.org/10.3390/biom13010165









