Upcycling Fish By-Products into Bioactive Fish Oil: The Suitability of Microwave-Assisted Extraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards, Reagents, and Biological Material
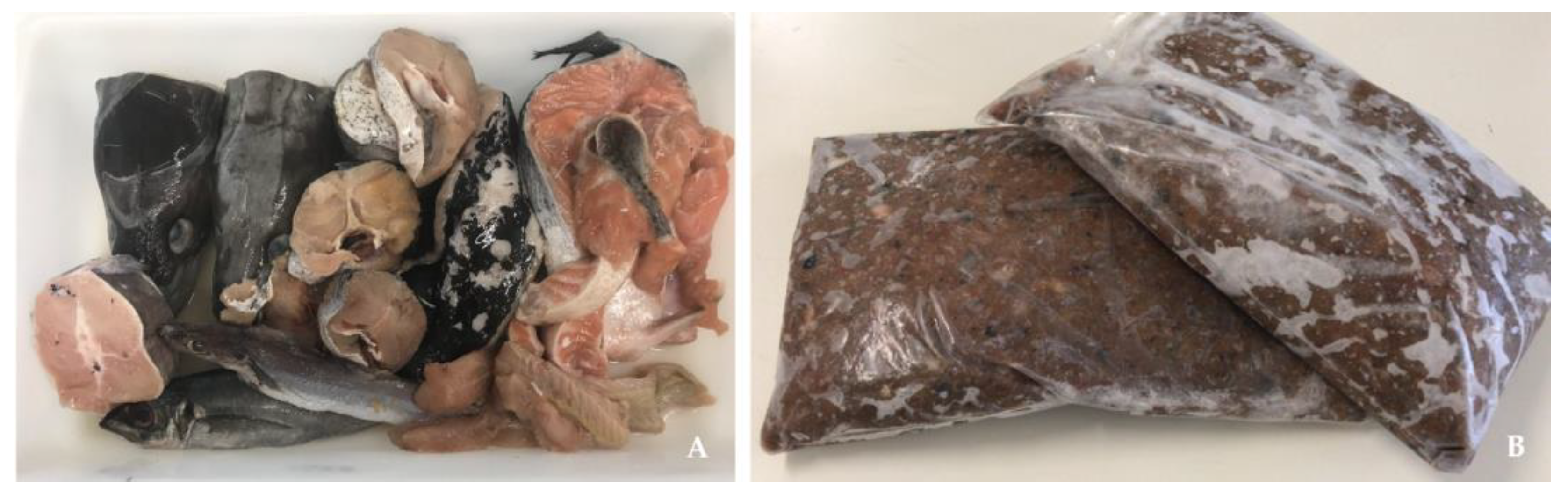
2.2. Fish By-Product and Sample Preparation
2.3. Experimental Design for MAE Optimization
2.4. Extraction Methods
2.4.1. Microwave-Assisted Extraction (MAE)
2.4.2. Soxhlet Extraction (SE)
2.5. Oil Yield Determination
2.6. MAE Optimization Using RSM
2.7. Experimental Validation of the Predictive Models
2.8. Evaluation of the Nutritional Properties of the Fish By-Product Oils
2.8.1. Lipid Profile
2.8.2. Lipid Quality Indices
2.9. Evaluation of Bioactive Properties of Fish By-Product Oils
2.9.1. Antimicrobial Activity
2.9.2. Cellular Antioxidant Activity (CAA)
2.9.3. Anti-Inflammatory Activity
2.9.4. Cytotoxic Activity
2.10. Statistical Analysis
3. Results and Discussion
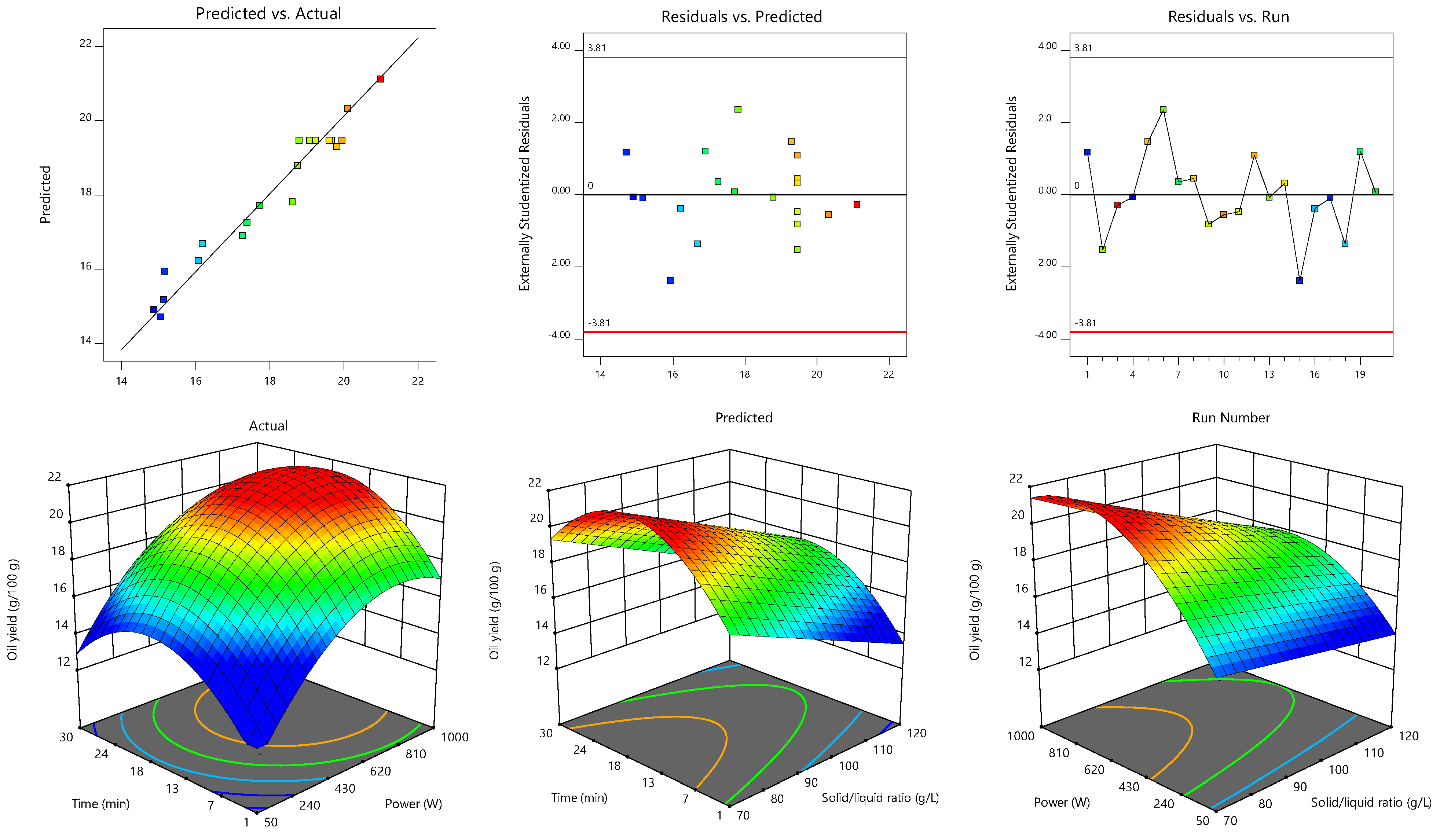
3.1. MAE Process Optimization
3.2. Extraction Yields of Fish By-Product Oils Obtained through MAE and SE
3.3. Fatty Acid Profile of the Fish By-Product Oils Obtained through MAE and SE
3.4. Biological Activities of the Fish By-Product Oils Obtained through MAE and SE
3.4.1. Antimicrobial Activity
3.4.2. Antioxidant and Anti-Inflammatory Activities
3.4.3. Cytotoxic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sadiq, M.B.; Singh, M.; Anal, A.K. Application of Food By-Products in Medical and Pharmaceutical Industries. In Food Processing By-Products and their Utilization; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2017; pp. 89–110. [Google Scholar] [CrossRef]
- Mirzapour-Kouhdasht, A.; Moosavi-Nasab, M.; Yousefi, R.; Eun, J.B. Bio/Multi-Functional Peptides Derived from Fish Gelatin Hydrolysates: Technological and Functional Properties. Biocatal. Agric. Biotechnol. 2021, 36, 102152. [Google Scholar] [CrossRef]
- Nawaz, A.; Li, E.; Irshad, S.; Xiong, Z.; Xiong, H.; Shahbaz, H.M.; Siddique, F. Valorization of Fisheries By-Products: Challenges and Technical Concerns to Food Industry. Trends Food Sci. Technol. 2020, 99, 34–43. [Google Scholar] [CrossRef]
- Al Khawli, F.; Pateiro, M.; Domínguez, R.; Lorenzo, J.M.; Gullón, P.; Kousoulaki, K.; Ferrer, E.; Berrada, H.; Barba, F.J. Innovative Green Technologies of Intensification for Valorization of Seafood and Their By-Products. Mar. Drugs 2019, 17, 689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamshidi, A.; Cao, H.; Xiao, J.; Simal-Gandara, J. Advantages of Techniques to Fortify Food Products with the Benefits of Fish Oil. Food Res. Int. 2020, 137, 109353. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 Fatty Acids EPA and DHA: Health Benefits Throughout Life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Innes, J.K.; Calder, P.C. The Differential Effects of Eicosapentaenoic Acid and Docosahexaenoic Acid on Cardiometabolic Risk Factors: A Systematic Review. Int. J. Mol. Sci. 2018, 19, 532. [Google Scholar] [CrossRef] [Green Version]
- Sahena, F.; Zaidul, I.S.M.; Jinap, S.; Saari, N.; Jahurul, H.A.; Abbas, K.A.; Norulaini, N.A. PUFAs in Fish: Extraction, Fractionation, Importance in Health. Compr. Rev. Food Sci. Food Saf. 2009, 8, 59–74. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions: Closing the Loop—An EU Action Plan for the Circular Economy; European Commission: Brussels, Belgium, 2015. [Google Scholar]
- Carvajal, A.K.; Mozuraityte, R. Fish Oils: Production and Properties. In Encyclopedia of Food and Health; Caballero, B., Finglas, P., Toldrá, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 693–698. [Google Scholar] [CrossRef]
- Liu, Y.; Ramakrishnan, V.V.; Dave, D. Lipid Class and Fatty Acid Composition of Oil Extracted from Atlantic Salmon By-Products under Different Optimization Parameters of Enzymatic Hydrolysis. Biocatal. Agric. Biotechnol. 2020, 30, 101866. [Google Scholar] [CrossRef]
- Bruno, S.F.; Ekorong, F.J.A.A.; Karkal, S.S.; Cathrine, M.S.B.; Kudre, T.G. Green and Innovative Techniques for Recovery of Valuable Compounds from Seafood By-Products and Discards: A Review. Trends Food Sci. Technol. 2019, 85, 10–22. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [Green Version]
- Marsol-Vall, A.; Aitta, E.; Guo, Z.; Yang, B. Green Technologies for Production of Oils Rich in n-3 Polyunsaturated Fatty Acids from Aquatic Sources. Crit. Rev. Food Sci. Nutr. 2021, 62, 2942–2962. [Google Scholar] [CrossRef]
- Alfio, V.G.; Manzo, C.; Micillo, R. From Fish Waste to Value: An Overview of the Sustainable Recovery of Omega-3 for Food Supplements. Molecules 2021, 26, 1002. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, B.; Pinela, J.; Mandim, F.; Heleno, S.A.; Ferreira, I.C.F.R.; Barba, F.J.; Berrada, H.; Caleja, C.; Barros, L. Nutritional and Bioactive Oils from Salmon (Salmo salar) Side Streams Obtained by Soxhlet and Optimized Microwave-Assisted Extraction. Food Chem. 2022, 386, 132778. [Google Scholar] [CrossRef] [PubMed]
- Ivanovs, K.; Blumberga, D. Extraction of Fish Oil Using Green Extraction Methods: A Short Review. Energy Procedia 2017, 128, 477–483. [Google Scholar] [CrossRef]
- Reis, F.S.; Barros, L.; Martins, A.; Ferreira, I.C.F.R. Chemical Composition and Nutritional Value of the Most Widely Appreciated Cultivated Mushrooms: An Inter-Species Comparative Study. Food Chem. Toxicol. 2012, 50, 191–197. [Google Scholar] [CrossRef] [Green Version]
- FAO. Fats and Fatty Acids in Human Nutrition: Report of an Expert Consultation (FAO Food and Nutrition Papers); Food and Agriculture Organization of the United Nations: Rome, Italy, 2010; ISBN 978-92-5-106733-8. [Google Scholar]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary Heart Disease: Seven Dietary Factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Mierliță, D. Effects of Diets Containing Hemp Seeds or Hemp Cake on Fatty Acid Composition and Oxidative Stability of Sheep Milk. S. Afr. J. Anim. Sci. 2018, 48, 504–515. [Google Scholar] [CrossRef]
- Sobral, F.; Sampaio, A.; Falcão, S.; Queiroz, M.J.R.P.; Calhelha, R.C.; Vilas-Boas, M.; Ferreira, I.C.F.R. Chemical Characterization, Antioxidant, Anti-Inflammatory and Cytotoxic Properties of Bee Venom Collected in Northeast Portugal. Food Chem. Toxicol. 2016, 94, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, M.A.; Omar, R.; Ethaib, S.; Siti Mazlina, M.K.; Awang Biak, D.R.; Nor Aisyah, R. Microwave-Assisted Extraction of Lipid from Fish Waste. IOP Conf. Ser. Mater. Sci. Eng. 2017, 206, 012096. [Google Scholar] [CrossRef]
- Ebora, D.; Santos, D.; Ancio Costa, V.; Bragagnolo, N. Development and Validation of a Novel Microwave Assisted Extraction Method for Fish Lipids. Eur. J. Lipid Sci. Technol. 2017, 119, 1600108. [Google Scholar] [CrossRef]
- Arsic, A. Oleic Acid and Implications for the Mediterranean Diet. In The Mediterranean Diet (Second Edition); Preedy, V.R., Watson, R.R., Eds.; Academic Press: Cambridge, UK, 2020; pp. 267–274. [Google Scholar] [CrossRef]
- de la Fuente, B.; Pinela, J.; Calhelha, R.C.; Heleno, S.A.; Ferreira, I.C.F.R.; Barba, F.J.; Berrada, H.; Caleja, C.; Barros, L. Sea Bass (Dicentrarchus labrax) and Sea Bream (Sparus aurata) Head Oils Recovered by Microwave-Assisted Extraction: Nutritional Quality and Biological Properties. Food Bioprod. Process. 2022, 136, 97–105. [Google Scholar] [CrossRef]
- Moovendhan, M.; Kavisri, M.; Vairamani, S.; Shanmugam, A. Valorization of Cephalopod Liver Viscera for Oil Production: Chemical Characteristics, Nutritional Profile and Pharmacological Activities. Biomass. Convers. Biorefin. 2021, 1, 3. [Google Scholar] [CrossRef]
- Rincón-Cervera, M.Á.; Villarreal-Rubio, M.B.; Valenzuela, R.; Valenzuela, A. Comparison of Fatty Acid Profiles of Dried and Raw By-Products from Cultured and Wild Fishes. Eur. J. Lipid Sci. Technol. 2017, 119, 1600516. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee on Nutrition, Trauma, and the Brain; Erdman, J.; Oria, M.; Pillsbury, L. Eicosapentaenoic Acid (EPA) and Docosahexaenoic Acid (DHA). In Nutrition and Traumatic Brain Injury: Improving Acute and Subacute Health Outcomes in Military Personnel; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar]
- Commission Regulation (EU) No 116/2010 of 9 February 2010 Amending Regulation (EC) No 1924/2006 of the European Parliament and of the Council with Regard to the List of Nutrition Claims. Off. J. Eur. Union 2010, 10, 16–18.
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef] [PubMed]
- Som, S.C.R.; Radhakrishnan, C.K. Antibacterial Activities of Polyunsaturated Fatty Acid Extracts from Sardinella longiceps and Sardinella fimbriata. Indian J. Mar. Sci. 2011, 40, 710–716. [Google Scholar]
- Shin, S.Y.; Bajpai, V.K.; Kim, H.R.; Kang, S.C. Antibacterial Activity of Eicosapentaenoic Acid (EPA) against Foodborne and Food Spoilage Microorganisms. LWT—Food Sci. Technol. 2007, 40, 1515–1519. [Google Scholar] [CrossRef]
- Ahmad, T.B.; Rudd, D.; Kotiw, M.; Liu, L.; Benkendorff, K. Correlation between Fatty Acid Profile and Anti-Inflammatory Activity in Common Australian Seafood By-Products. Mar. Drugs 2019, 17, 155. [Google Scholar] [CrossRef] [Green Version]
- To, N.B.; Nguyen, Y.T.K.; Moon, J.Y.; Ediriweera, M.K.; Cho, S.K. Pentadecanoic Acid, an Odd-Chain Fatty Acid, Suppresses the Stemness of MCF-7/SC Human Breast Cancer Stem-Like Cells through JAK2/STAT3 Signaling. Nutrients 2020, 12, 1663. [Google Scholar] [CrossRef]
- Weylandt, K.H.; Serini, S.; Chen, Y.Q.; Su, H.M.; Lim, K.; Cittadini, A.; Calviello, G. Omega-3 Polyunsaturated Fatty Acids: The Way Forward in Times of Mixed Evidence. Biomed. Res. Int. 2015, 2015, 143109. [Google Scholar] [CrossRef]


| Coded Values | Natural Values | ||
|---|---|---|---|
| X1: t (min) | X2: P (W) | X3: R (g/L) | |
| −1.68 | 1 | 50 | 70 |
| −1 | 7 | 243 | 80 |
| 0 | 15.5 | 525 | 95 |
| +1 | 24 | 807 | 110 |
| +1.68 | 30 | 1000 | 120 |
| Run | Experimental Domain | Experimental Response (Oil Yield) | |||
|---|---|---|---|---|---|
| X1: t (min) | X2: P (W) | X3: R (g/L) | S1 (g/100 g dw) | S2 (g/100 g dw) | |
| 1 | 7 | 243 | 80 | 15.29 | 16.08 |
| 2 | 24 | 243 | 80 | 18.04 | 17.39 |
| 3 | 7 | 807 | 80 | 14.98 | 19.82 |
| 4 | 24 | 807 | 80 | 16.75 | 20.11 |
| 5 | 7 | 243 | 110 | 12.57 | 14.88 |
| 6 | 24 | 243 | 110 | 13.56 | 15.17 |
| 7 | 7 | 807 | 110 | 13.22 | 16.19 |
| 8 | 24 | 807 | 110 | 14.23 | 17.74 |
| 9 | 1 | 525 | 95 | 13.05 | 15.14 |
| 10 | 30 | 525 | 95 | 15.47 | 17.27 |
| 11 | 15.5 | 50 | 95 | 14.26 | 15.07 |
| 12 | 15.5 | 1000 | 95 | 14.74 | 18.76 |
| 13 | 15.5 | 525 | 70 | 18.26 | 20.99 |
| 14 | 15.5 | 525 | 120 | 12.93 | 18.62 |
| 15 | 15.5 | 525 | 95 | 14.06 | 19.95 |
| 16 | 15.5 | 525 | 95 | 14.18 | 19.67 |
| 17 | 15.5 | 525 | 95 | 14.16 | 18.80 |
| 18 | 15.5 | 525 | 95 | 14.81 | 19.61 |
| 19 | 15.5 | 525 | 95 | 13.80 | 19.08 |
| 20 | 15.5 | 525 | 95 | 14.97 | 19.24 |
| Statistical Criteria | S1 (Equation (6)) | S2 (Equation (7)) |
|---|---|---|
| Model F-value | 60.79 | 49.35 |
| Lack of Fit | 0.9307 | 0.3477 |
| R2 | 0.9656 | 0.9579 |
| R2adj | 0.9497 | 0.9385 |
| Adequate Precision | 27.83 | 22.32 |
| Coefficient of Variation | 2.36 | 2.70 |
| SE | MAE | ||||
|---|---|---|---|---|---|
| S1 Oil | S2 Oil | S1 Oil | S2 Oil | ||
| Oil yield (g/100 g dw) | 18 ± 1 b | 34 ± 1 a | 17.9 ± 0.8 b | 20.6 ± 0.9 b | |
| Fatty acids (%) | |||||
| Myristic acid | C14:0 | 2.80 ± 0.07 a | 2.77 ± 0.05 a | 2.77 ± 0.07 a | 2.75 ± 0.06 a |
| Pentadecanoic acid | C15:0 | 0.272 ± 0.06 a | 0.279 ± 0.007 a | 0.267 ± 0.007 a | 0.278 ± 0.006 a |
| Palmitic acid | C16:0 | 12.3 ± 0.3 b | 14.2 ± 0.4 a | 12.2 ± 0.4 b | 14.1 ± 0.3 a |
| Palmitoleic acid | C16:1 | 3.8 ± 0.1 a | 3.8 ± 0.1 a | 3.66 ± 0.04 a | 3.7 ± 0.1 a |
| Heptadecanoic acid | C17:0 | 0.187 ± 0.004 | nd | 0.181 ± 0.005 | nd |
| Heptadecenoic acid | C17:1 | 0.218 ± 0.006 | nd | 0.192 ± 0.005 | nd |
| Stearic acid | C18:0 | 2.97 ± 0.04 b | 3.87 ± 0.09 a | 2.99 ± 0.06 b | 3.96 ± 0.08 a |
| Oleic acid | C18:1n9 | 36.0 ± 0.9 a | 34.9 ± 0.8 a | 36 ± 1 a | 34.8 ± 0.8 a |
| Linoleic acid | C18:2n6 | 9.9 ± 0.1 a | 10.1 ± 0.3 a | 10.3 ± 0.3 a | 10.1 ± 0.3 a |
| γ-Linoleic acid | C18:3n6 | 0.15 ± 0.01 c | 0.193 ± 0.005 a | 0.103 ± 0.002 d | 0.170 ± 0.003 b |
| α-Linolenic acid | C18:3n3 | 4.9 ± 0.1 a | 4.35 ± 0.05 b | 4.76 ± 0.07 a | 4.3 ± 0.1 b |
| Eicosenoic acid | C20:1 | 3.12 ± 0.06 a | 3.04 ± 0.08 a | 3.06 ± 0.07 a | 3.03 ± 0.09 a |
| Eicosadienoic acid | C20:2n6 | 0.95 ± 0.02 a | 0.77 ± 0.02 c | 0.86 ± 0.02 b | 0.76 ± 0.02 c |
| Dihomo-γ-linolenic acid | C20:3n6 | 0.194 ± 0.006 b | 0.225 ± 0.006 a | 0.193 ± 0.005 b | 0.23 ± 0.01 a |
| Arachidonic acid | C20:4n6 | 0.659 ± 0.008 b | 0.87 ± 0.02 a | 0.680 ± 0.007 b | 0.90 ± 0.02 a |
| Eicosatrienoic acid | C20:3n3 | 0.50 ± 0.01 b | 0.408 ± 0.008 c | 0.53 ± 0.02 a | 0.39 ± 0.01 c |
| Eicosapentaenoic acid | C20:5n3 | 6.3 ± 0.2 b | 7.0 ± 0.2 a | 6.7 ± 0.2 a,b | 7.0 ± 0.2 a |
| Nervonic acid | C24:1 | 1.86 ± 0.05 a | 1.89 ± 0.05 a | 1.9 ± 0.1 a | 1.94 ± 0.07 a |
| Docosahexaenoic acid | C22:6n3 | 12.9 ± 0.3 a | 11.3 ± 0.3 b | 12.8 ± 0.4 a | 11.5 ± 0.3 b |
| Fatty acid class | |||||
| Saturated fatty acids | SFA | 18.5 ± 0.4 b | 21.1 ± 0.3 a | 18.4 ± 0.5 b | 21.1 ± 0.4 a |
| Monounsaturated fatty acids | MUFA | 51 ± 1 a | 50.6 ± 0.9 a | 51.4 ± 0.9 a | 50.5 ± 0.4 a |
| Polyunsaturated fatty acids | PUFA | 30.1 ± 0.5 a | 28.3 ± 0.7 b | 30.22 ± 0.02 a | 28.4 ± 0.2 b |
| n3 | 18.3 ± 0.6 a | 16.1 ± 0.5 b | 18.1 ± 0.5 a | 16.2 ± 0.3 b | |
| n6 | 11.4 ± 0.6 a | 11.8 ± 0.1 a | 12 ± 1 a | 11.8 ± 0.1 a | |
| n9 | 36 ± 1 a | 35 ± 1 a | 36 ± 1 a | 35 ± 1 a | |
| Lipid quality indices | |||||
| n6/n3 PUFA | 0.62 ± 0.05 b | 0.73 ± 0.03 a | 0.65 ± 0.08 a,b | 0.73 ± 0.01 a | |
| Atherogenicity index | AI | 0.29 ± 0.01 b | 0.32 ± 0.01 a | 0.29 ± 0.002 b | 0.32 ± 0.01 a |
| Thrombogenicity index | TI | 0.21 ± 0.01 b | 0.26 ± 0.01 a | 0.21 ± 0.01 b | 0.26 ± 0.01 a |
| Hypocholesterolemic index | HI | 4.4 ± 0.1 a | 3.7 ± 0.1 b | 4.4 ± 0.3 a | 3.7 ± 0.1 b |
| SE | MAE | Positive Controls | ||||
|---|---|---|---|---|---|---|
| S1 Oil | S2 Oil | S1 Oil | S2 Oil | Streptomycin | Ampicillin | |
| Gram-negative bacteria | ||||||
| Enterobacter cloacae | 50 | 50 | 12.5 | 3.125 | 0.007 | 0.15 |
| Escherichia coli | 50 | 50 | 25 | 12.5 | 0.01 | 0.15 |
| Pseudomonas aeruginosa | >50 | 50 | 50 | 50 | 0.06 | 0.63 |
| Salmonella enterocolitica | 50 | 25 | 25 | 6.25 | 0.007 | 0.15 |
| Yersinia enterocolitica | 50 | 50 | 3.25 | 3.25 | 0.007 | 0.15 |
| Gram-positive bacteria | ||||||
| Bacillus cereus | 50 | 50 | 12.5 | 12.5 | 0.007 | nt |
| Listeria monocytogenes | 50 | 50 | 50 | 6.25 | 0.007 | 0.15 |
| Staphylococcus aureus | 25 | 50 | 6.25 | 3.125 | 0.007 | 0.15 |
| Ketoconazole | ||||||
| Aspergillus brasiliensis | 10 | >10 | 10 | 5 | 0.06 | - |
| Aspergillus fumigatus | >10 | 10 | 5 | >10 | 0.5 | - |
| SE | MAE | Positive Control * | |||
|---|---|---|---|---|---|
| S1 Oil | S2 Oil | S1 Oil | S2 Oil | ||
| NO production inhibition | 14 ± 1 c | 12 ± 1 b,c | 11 ± 1 b | 20.0 ± 0.4 d | 6.3 ± 0.4 a |
| Tumor cell lines | |||||
| AGS | 274 ± 9 d | 137 ± 8 b | 173 ± 2 b,c | 201 ± 15 c | 1.23 ± 0.03 a |
| CaCo-2 | 316 ± 26 b | 302 ± 27 b | 302 ± 4 b | 316 ± 26 b | 1.21 ± 0.02 a |
| MCF-7 | 233 ± 22 c | 56 ± 4 b,c | 218 ± 5 c | 67 ± 1 a,b | 1.02 ± 0.02 a |
| NCI-H460 | 252 ± 12 c | 241 ± 17 b,c | 209 ± 14 b | 226 ± 16 b,c | 1.01 ± 0.01 a |
| Nontumor cell lines | |||||
| PLP2 | 267 ± 21 c | 118 ± 7 b | 190 ± 12 b | 201 ± 11 b,c | 1.41 ± 0.06 a |
| VERO | 150 ± 13 b | 125 ± 6 b | 164 ± 13 b | 180 ± 8 b | 1.41 ± 0.06 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinela, J.; Fuente, B.d.l.; Rodrigues, M.; Pires, T.C.S.P.; Mandim, F.; Almeida, A.; Dias, M.I.; Caleja, C.; Barros, L. Upcycling Fish By-Products into Bioactive Fish Oil: The Suitability of Microwave-Assisted Extraction. Biomolecules 2023, 13, 1. https://doi.org/10.3390/biom13010001
Pinela J, Fuente Bdl, Rodrigues M, Pires TCSP, Mandim F, Almeida A, Dias MI, Caleja C, Barros L. Upcycling Fish By-Products into Bioactive Fish Oil: The Suitability of Microwave-Assisted Extraction. Biomolecules. 2023; 13(1):1. https://doi.org/10.3390/biom13010001
Chicago/Turabian StylePinela, José, Beatriz de la Fuente, Matilde Rodrigues, Tânia C. S. P. Pires, Filipa Mandim, André Almeida, Maria Inês Dias, Cristina Caleja, and Lillian Barros. 2023. "Upcycling Fish By-Products into Bioactive Fish Oil: The Suitability of Microwave-Assisted Extraction" Biomolecules 13, no. 1: 1. https://doi.org/10.3390/biom13010001
APA StylePinela, J., Fuente, B. d. l., Rodrigues, M., Pires, T. C. S. P., Mandim, F., Almeida, A., Dias, M. I., Caleja, C., & Barros, L. (2023). Upcycling Fish By-Products into Bioactive Fish Oil: The Suitability of Microwave-Assisted Extraction. Biomolecules, 13(1), 1. https://doi.org/10.3390/biom13010001












