Plasma Clearance of Intravenously Infused Adrenomedullin in Rats with Acute Renal Failure
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Renal Dysfunction Rats with Mercury Chloride Treatment
2.4. Renal Dysfunction Model Rats with Bilateral Renal Blood Flow Blockage
2.5. Human Adrenomedullin Infusion Study in Rats
2.6. Human Adrenomedullin Intraperitoneal Administration Study in E-RAMP2 KO
2.7. Measurement of AM in Plasma
2.8. Pharmacokinetic Analysis
2.9. mRNA Expression Analysis by Quantitative RT-PCR
2.10. Statistical Analysis
3. Results
3.1. Plasma Concentration of Exogenous AM Is Elevated in Rats with Mercury Chloride-Induced Renal Dysfunction Due to Decreased Total Systemic Clearance of AM
3.2. Expression of AM Receptors in Tissues Is Altered in Rats with Mercury Chloride-Induced Renal Dysfunction
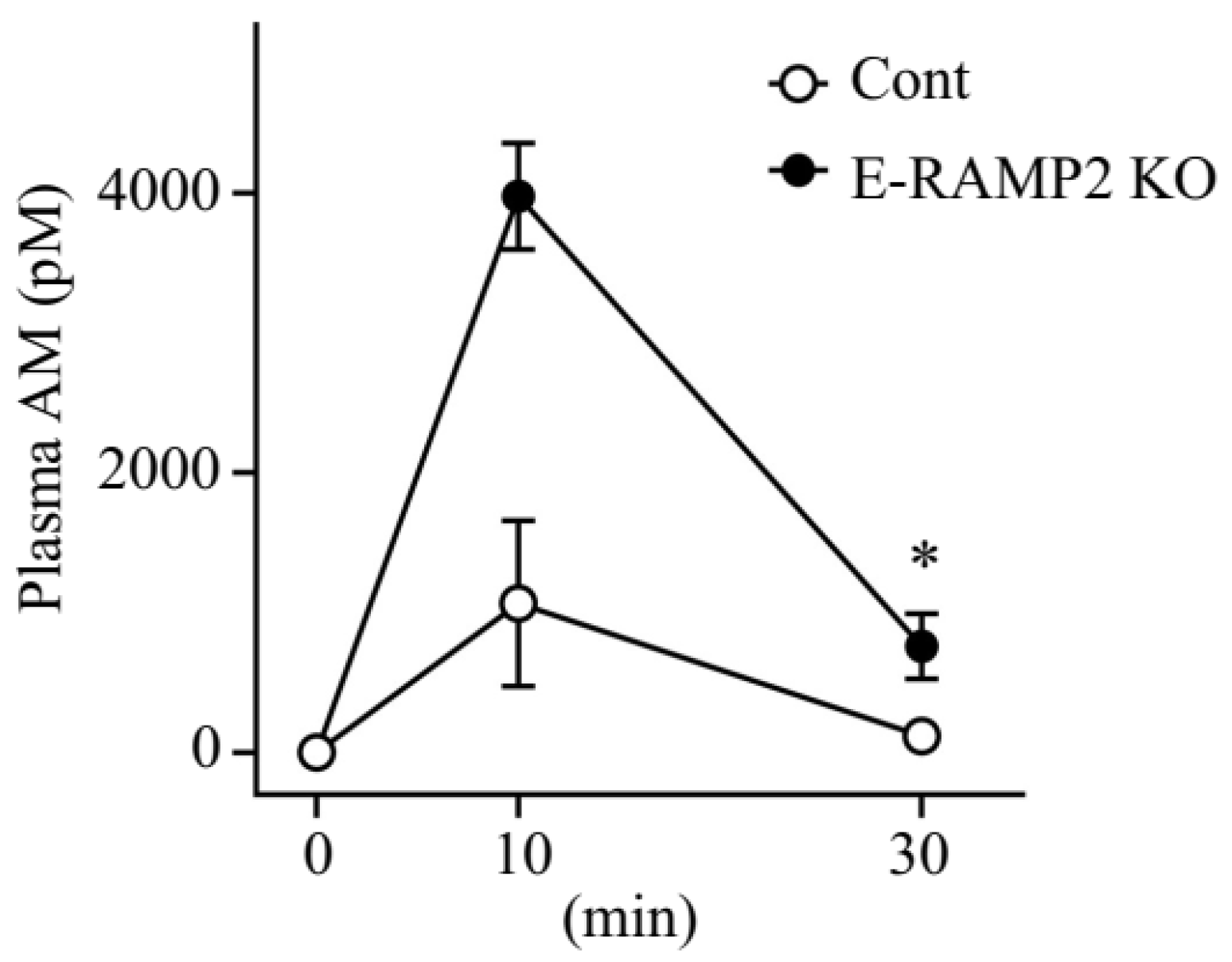
3.3. Plasma Concentrations of Exogenous AM Are Augmented in Vascular Endothelium-Specific Ramp2-Deficient Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kitamura, K.; Kangawa, K.; Kawamoto, M.; Ichiki, Y.; Nakamura, S.; Matsuo, H.; Eto, T. Adrenomedullin: A novel hypotensive peptide isolated from human pheochromocytoma. Biochem. Biophys. Res. Commun. 1993, 192, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, Y.; Kitamura, K.; Ichiki, Y.; Nakamura, S.; Kida, O.; Kangawa, K.; Eto, T. Hemodynamic effects of a novel hypotensive peptide, human adrenomedullin, in rats. Eur. J. Pharmacol. 1993, 241, 271–273. [Google Scholar] [CrossRef]
- Nuki, C.; Kawasaki, H.; Kitamura, K.; Takenaga, M.; Kangawa, K.; Eto, T.; Wada, A. Vasodilator effect of adrenomedullin and calcitonin gene-related peptide receptors in rat mesenteric vascular beds. Biochem. Biophys. Res. Commun. 1993, 196, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, K.; Sakata, J.; Kangawa, K.; Kojima, M.; Matsuo, H.; Eto, T. Cloning and characterization of cDNA encoding a precursor for human adrenomedullin. Biochem. Biophys. Res. Commun. 1993, 194, 720–725. [Google Scholar] [CrossRef]
- Kitamura, K.; Ichiki, Y.; Tanaka, M.; Kawamoto, M.; Emura, J.; Sakakibara, S.; Kangawa, K.; Matsuo, H.; Eto, T. Immunoreactive adrenomedullin in human plasma. FEBS Lett. 1994, 341, 288–290. [Google Scholar] [CrossRef]
- Shindo, T.; Kurihara, Y.; Nishimatsu, H.; Moriyama, N.; Kakoki, M.; Wang, Y.; Imai, Y.; Ebihara, A.; Kuwaki, T.; Ju, K.H.; et al. Vascular abnormalities and elevated blood pressure in mice lacking adrenomedullin gene. Circulation 2001, 104, 1964–1971. [Google Scholar] [CrossRef]
- Fritz-Six, K.L.; Dunworth, W.P.; Li, M.; Caron, K.M. Adrenomedullin signaling is necessary for murine lymphatic vascular development. J. Clin. Invest. 2008, 118, 40–50. [Google Scholar] [CrossRef]
- Niu, P.; Shindo, T.; Iwata, H.; Ebihara, A.; Suematsu, Y.; Zhang, Y.; Takeda, N.; Iimuro, S.; Hirata, Y.; Nagai, R. Accelerated cardiac hypertrophy and renal damage induced by angiotensin II in adrenomedullin knockout mice. Hypertens. Res. 2003, 26, 731–736. [Google Scholar] [CrossRef]
- Niu, P.; Shindo, T.; Iwata, H.; Iimuro, S.; Takeda, N.; Zhang, Y.; Ebihara, A.; Suematsu, Y.; Kangawa, K.; Hirata, Y.; et al. Protective effects of endogenous adrenomedullin on cardiac hypertrophy, fibrosis, and renal damage. Circulation 2004, 109, 1789–1794. [Google Scholar] [CrossRef]
- Nishimatsu, H.; Hirata, Y.; Shindo, T.; Kurihara, H.; Kakoki, M.; Nagata, D.; Hayakawa, H.; Satonaka, H.; Sata, M.; Tojo, A.; et al. Role of endogenous adrenomedullin in the regulation of vascular tone and ischemic renal injury: Studies on transgenic/knockout mice of adrenomedullin gene. Circ. Res. 2002, 90, 657–663. [Google Scholar] [CrossRef]
- McLatchie, L.M.; Fraser, N.J.; Main, M.J.; Wise, A.; Brown, J.; Thompson, N.; Solari, R.; Lee, M.G.; Foord, S.M. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 1998, 393, 333–339. [Google Scholar] [CrossRef]
- Fischer, J.A.; Muff, R.; Born, W. Functional relevance of G-protein-coupled-receptor-associated proteins, exemplified by receptor-activity-modifying proteins (RAMPs). Biochem. Soc. Trans. 2002, 30, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Hay, D.L.; Garelja, M.L.; Poyner, D.R.; Walker, C.S. Update on the pharmacology of calcitonin/CGRP family of peptides: IUPHAR Review 25. Br. J. Pharmacol. 2018, 175, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Sakurai, T.; Kamiyoshi, A.; Ichikawa-Shindo, Y.; Kawate, H.; Shindo, T. Adrenomedullin-RAMP2 System in Vascular Endothelial Cells. J. Atheroscler. Thromb. 2015, 22, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Shindo, T.; Tanaka, M.; Kamiyoshi, A.; Ichikawa-Shindo, Y.; Kawate, H.; Yamauchi, A.; Sakurai, T. Regulation of cardiovascular development and homeostasis by the adrenomedullin-RAMP system. Peptides 2019, 111, 55–61. [Google Scholar] [CrossRef]
- Majid, D.S.; Kadowitz, P.J.; Coy, D.H.; Navar, L.G. Renal responses to intra-arterial administration of adrenomedullin in dogs. Am. J. Physiol. 1996, 270, F200–F205. [Google Scholar] [CrossRef]
- Inal, S.; Koc, E.; Ulusal-Okyay, G.; Pasaoglu, O.T.; Isik-Gönül, I.; Oz-Oyar, E.; Pasaoglu, H.; Güz, G. Protective effect of adrenomedullin on contrast induced nephropathy in rats. Nefrologia 2014, 34, 724–731. [Google Scholar]
- Nagae, T.; Mori, K.; Mukoyama, M.; Kasahara, M.; Yokoi, H.; Suganami, T.; Sawai, K.; Yoshioka, T.; Koshikawa, M.; Saito, Y.; et al. Adrenomedullin inhibits connective tissue growth factor expression, extracellular signal-regulated kinase activation and renal fibrosis. Kidney Int. 2008, 74, 70–80. [Google Scholar] [CrossRef]
- Segawa, K.; Minami, K.; Sata, T.; Kuroiwa, A.; Shigematsu, A. Inhibitory effect of adrenomedullin on rat mesangial cell mitogenesis. Nephron 1996, 74, 577–579. [Google Scholar] [CrossRef]
- Ishimitsu, T.; Nishikimi, T.; Saito, Y.; Kitamura, K.; Eto, T.; Kangawa, K.; Matsuo, H.; Omae, T.; Matsuoka, H. Plasma levels of adrenomedullin, a newly identified hypotensive peptide, in patients with hypertension and renal failure. J. Clin. Investig. 1994, 94, 2158–2161. [Google Scholar] [CrossRef]
- McGregor, D.O.; Troughton, R.W.; Frampton, C.; Lynn, K.L.; Yandle, T.; Richards, A.M.; Nicholls, M.G. Hypotensive and natriuretic actions of adrenomedullin in subjects with chronic renal impairment. Hypertension 2001, 37, 1279–1284. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lainchbury, J.G.; Troughton, R.W.; Lewis, L.K.; Yandle, T.G.; Richards, A.M.; Nicholls, M.G. Hemodynamic, hormonal, and renal effects of short-term adrenomedullin infusion in healthy volunteers. J. Clin. Endocrinol. Metab. 2000, 85, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Ochoa-Callejero, L.; Sakurai, T.; Kamiyoshi, A.; Ichikawa-Shindo, Y.; Iinuma, N.; Arai, T.; Yoshizawa, T.; Iesato, Y.; Lei, Y.; et al. Vascular endothelial adrenomedullin-RAMP2 system is essential for vascular integrity and organ homeostasis. Circulation 2013, 127, 842–853. [Google Scholar] [CrossRef]
- Trifillis, A.L.; Kahng, M.W.; Trump, B.F. Metabolic studies of HgCl2-induced acute renal failure in the rat. Exp. Mol. Pathol. 1981, 35, 14–24. [Google Scholar] [CrossRef]
- Kawano, S.; Kawagoe, Y.; Kuwasako, K.; Shimamoto, S.; Igarashi, K.; Tokashiki, M.; Kitamura, K.; Kato, J. Gender-related alterations in plasma adrenomedullin level and its correlation with body weight gain. Endocr. Connect. 2015, 4, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Ohta, H.; Tsuji, T.; Asai, S.; Sasakura, K.; Teraoka, H.; Kitamura, K.; Kangawa, K. One-step direct assay for mature-type adrenomedullin with monoclonal antibodies. Clin. Chem. 1999, 45, 244–251. [Google Scholar] [CrossRef]
- Srisawasdi, P.; Vanavanan, S.; Charoenpanichkit, C.; Kroll, M.H. The effect of renal dysfunction on BNP, NT-proBNP, and their ratio. Am. J. Clin. Pathol. 2010, 133, 14–23. [Google Scholar] [CrossRef]
- Haagsma, B.H.; Pound, A.W. Mercuric chloride-induced renal tubular necrosis in the rat. Br. J. Exp. Pathol. 1979, 60, 341–352. [Google Scholar]
- Zalups, R.K. Molecular interactions with mercury in the kidney. Pharmacol. Rev. 2000, 52, 113–143. [Google Scholar]
- Nishikimi, T.; Kitamura, K.; Saito, Y.; Shimada, K.; Ishimitsu, T.; Takamiya, M.; Kangawa, K.; Matsuo, H.; Eto, T.; Omae, T.; et al. Clinical studies on the sites of production and clearance of circulating adrenomedullin in human subjects. Hypertension 1994, 24, 600–604. [Google Scholar] [CrossRef]
- Ishiyama, Y.; Kitamura, K.; Ichiki, Y.; Sakata, J.; Kida, O.; Kangawa, K.; Eto, T. Haemodynamic responses to rat adrenomedullin in anaesthetized spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. 1995, 22, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Ornan, D.A.; Chaudry, I.H.; Wang, P. Saturation of adrenomedullin receptors plays an important role in reducing pulmonary clearance of adrenomedullin during the late stage of sepsis. Biochim. Biophys. Acta 2002, 1586, 299–306. [Google Scholar] [CrossRef]
- Coppock, H.A.; Owji, A.A.; Austin, C.; Upton, P.D.; Jackson, M.L.; Gardiner, J.V.; Ghatei, M.A.; Bloom, S.R.; Smith, D.M. Rat-2 fibroblasts express specific adrenomedullin receptors, but not calcitonin-gene-related-peptide receptors, which mediate increased intracellular cAMP and inhibit mitogen-activated protein kinase activity. Biochem. J. 1999, 338 Pt 1, 15–22. [Google Scholar] [CrossRef]
- Juaneda, C.; Dumont, Y.; Chabot, J.G.; Fournier, A.; Quirion, R. Adrenomedullin receptor binding sites in rat brain and peripheral tissues. Eur. J. Pharmacol. 2003, 474, 165–174. [Google Scholar] [CrossRef]
- Flahaut, M.; Rossier, B.C.; Firsov, D. Respective roles of calcitonin receptor-like receptor (CRLR) and receptor activity-modifying proteins (RAMP) in cell surface expression of CRLR/RAMP heterodimeric receptors. J. Biol. Chem. 2002, 277, 14731–14737. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, H.M.; Notoya, M.V.; Inazawa, K.M.; Kitamura, K.K.; Eto, T.; Kangawa, K. Autoradiographic Studies on the Binding Sites of 125I-Adrenomedullin in Rat Tissues. Acta Histochemica Cytochemica 1998, 31, 335–343. [Google Scholar] [CrossRef]
- Ono, Y.; Okano, I.; Kojima, M.; Okada, K.; Kangawa, K. Decreased gene expression of adrenomedullin receptor in mouse lungs during sepsis. Biochem. Biophys. Res. Commun. 2000, 271, 197–202. [Google Scholar] [CrossRef]
- Hiragushi, K.; Wada, J.; Eguchi, J.; Matsuoka, T.; Yasuhara, A.; Hashimoto, I.; Yamashita, T.; Hida, K.; Nakamura, Y.; Shikata, K.; et al. The role of adrenomedullin and receptors in glomerular hyperfiltration in streptozotocin-induced diabetic rats. Kidney Int. 2004, 65, 540–550. [Google Scholar] [CrossRef][Green Version]
- Merabet, N.; Nsaibia, M.J.; Nguyen, Q.T.; Shi, Y.F.; Letourneau, M.; Fournier, A.; Tardif, J.C.; Harel, F.; Dupuis, J. PulmoBind Imaging Measures Reduction of Vascular Adrenomedullin Receptor Activity with Lack of effect of Sildenafil in Pulmonary Hypertension. Sci. Rep. 2019, 9, 6609. [Google Scholar] [CrossRef]
- Lee, S.A.; Cozzi, M.; Bush, E.L.; Rabb, H. Distant Organ Dysfunction in Acute Kidney Injury: A Review. Am. J. Kidney Dis. 2018, 72, 846–856. [Google Scholar] [CrossRef]
- Reiss, A.B.; Miyawaki, N.; Moon, J.; Kasselman, L.J.; Voloshyna, I.; D’Avino, R., Jr.; De Leon, J. CKD, arterial calcification, atherosclerosis and bone health: Inter-relationships and controversies. Atherosclerosis 2018, 278, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Okusa, M.D. Crosstalk between the nervous system and the kidney. Kidney Int. 2020, 97, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Musgrove, J.; Wolf, M. Regulation and Effects of FGF23 in Chronic Kidney Disease. Annu. Rev. Physiol. 2020, 82, 365–390. [Google Scholar] [CrossRef] [PubMed]
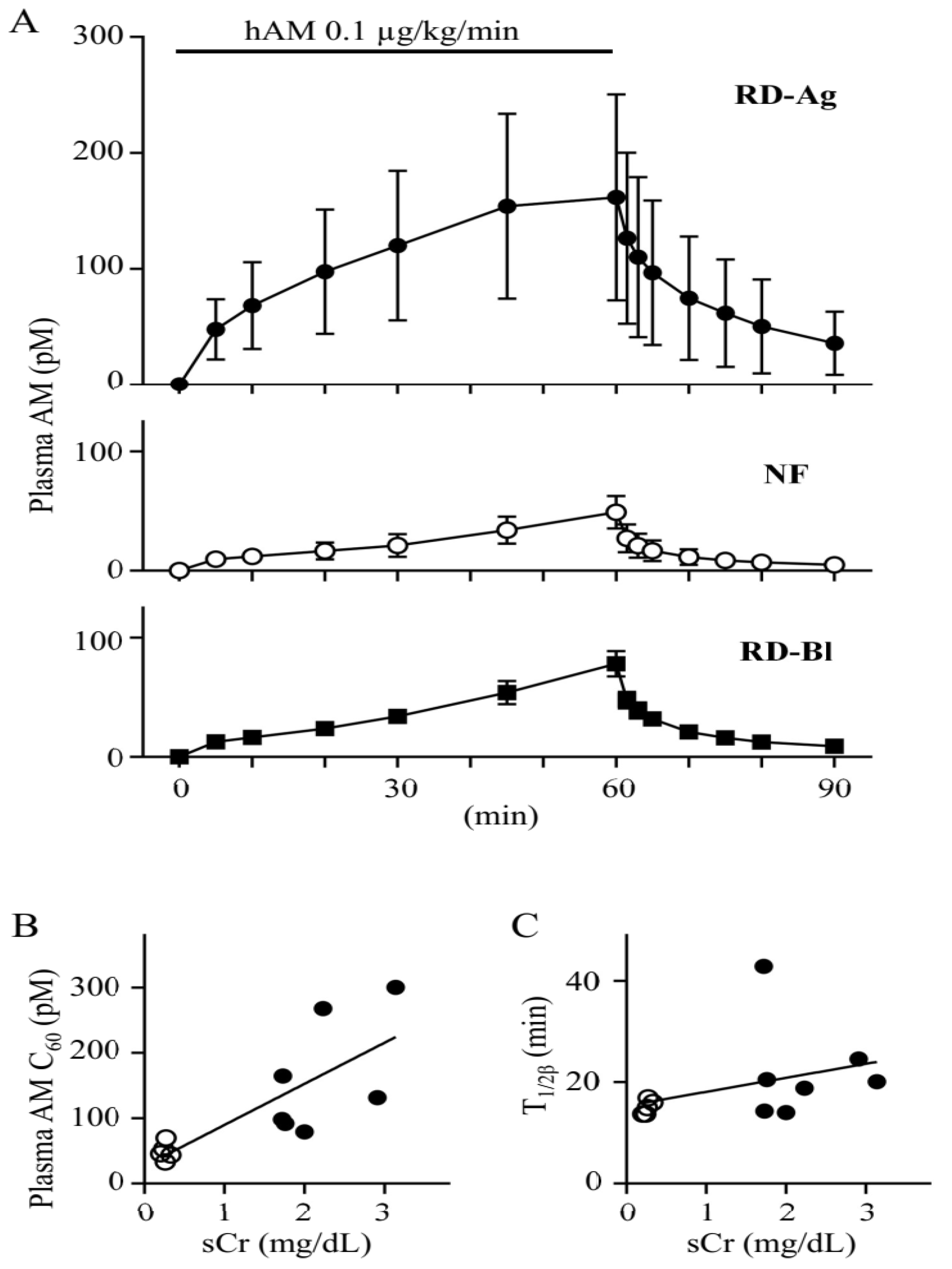

| NF (n = 5) | RD-Ag (n = 7) | RD-Bl (n = 3) | |
|---|---|---|---|
| AUC0–60 (pg·min/mL) | 8.7 ± 3.1 | 40.0 ± 21.1 ** | 13.5 ± 1.9 |
| T1/2β (min) | 15.0 ± 1.4 | 22.2 ± 9.8 | 16.7 ± 1.8 |
| CLtotal (mL/min/kg) | 752.3 ± 230.2 | 191.7 ± 101.7 *** | 450.1 ± 68.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosoda, H.; Nakamura, T.; Yoshihara, F. Plasma Clearance of Intravenously Infused Adrenomedullin in Rats with Acute Renal Failure. Biomolecules 2022, 12, 1281. https://doi.org/10.3390/biom12091281
Hosoda H, Nakamura T, Yoshihara F. Plasma Clearance of Intravenously Infused Adrenomedullin in Rats with Acute Renal Failure. Biomolecules. 2022; 12(9):1281. https://doi.org/10.3390/biom12091281
Chicago/Turabian StyleHosoda, Hiroshi, Tsutomu Nakamura, and Fumiki Yoshihara. 2022. "Plasma Clearance of Intravenously Infused Adrenomedullin in Rats with Acute Renal Failure" Biomolecules 12, no. 9: 1281. https://doi.org/10.3390/biom12091281
APA StyleHosoda, H., Nakamura, T., & Yoshihara, F. (2022). Plasma Clearance of Intravenously Infused Adrenomedullin in Rats with Acute Renal Failure. Biomolecules, 12(9), 1281. https://doi.org/10.3390/biom12091281





