INTERCEPT Pathogen Reduction in Platelet Concentrates, in Contrast to Gamma Irradiation, Induces the Formation of trans-Arachidonic Acids and Affects Eicosanoid Release during Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Donors
2.3. Clinical Laboratory Analyses
2.3.1. Platelet Function and Activation by Light Transmission Aggregometry (LTA)
2.3.2. Thromboelastometry
2.3.3. Residual Leukocytes and Sterility Tests
2.3.4. Pathogen Inactivation of Platelets
2.3.5. Irradiation of Platelets
2.4. Sample Preparation for Eicosanoid and Fatty Acids Analyses
2.5. Synthesis of trans-Arachidonic Acids (trans-AAs)
2.6. LC–MS/MS Analysis
2.7. LC–MS/MS Data Processing
2.8. Statistical Analysis
3. Results
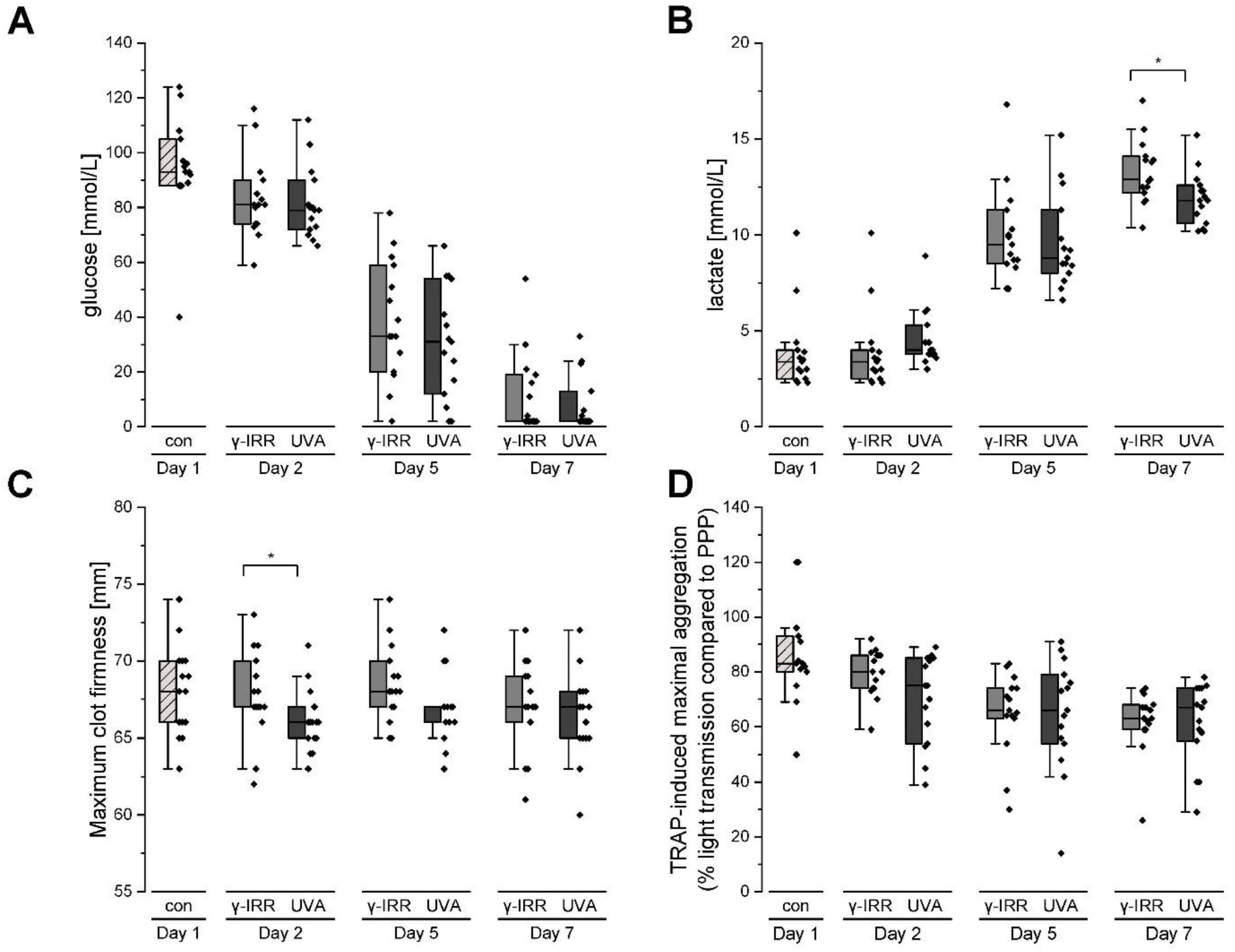
3.1. Platelet Metabolism and Function Are Hardly Affected Either by Gamma Irradiation or INTERCEPT Treatment
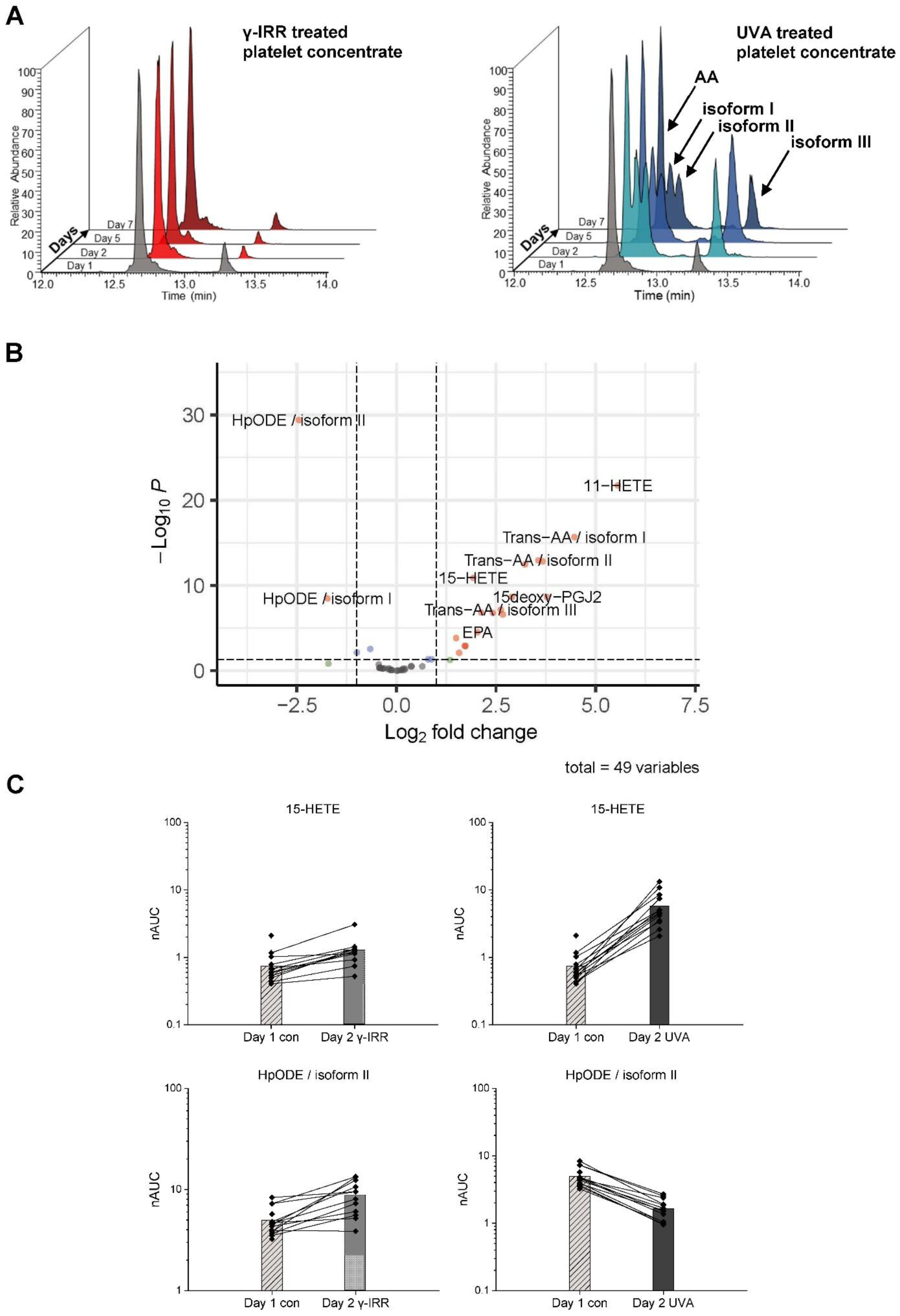
3.2. INTERCEPT Treatment for Pathogen Reduction Induces the Specific Formation of trans-Arachidonic Acids (trans-AAs) and Also Affects Other Eicosanoids
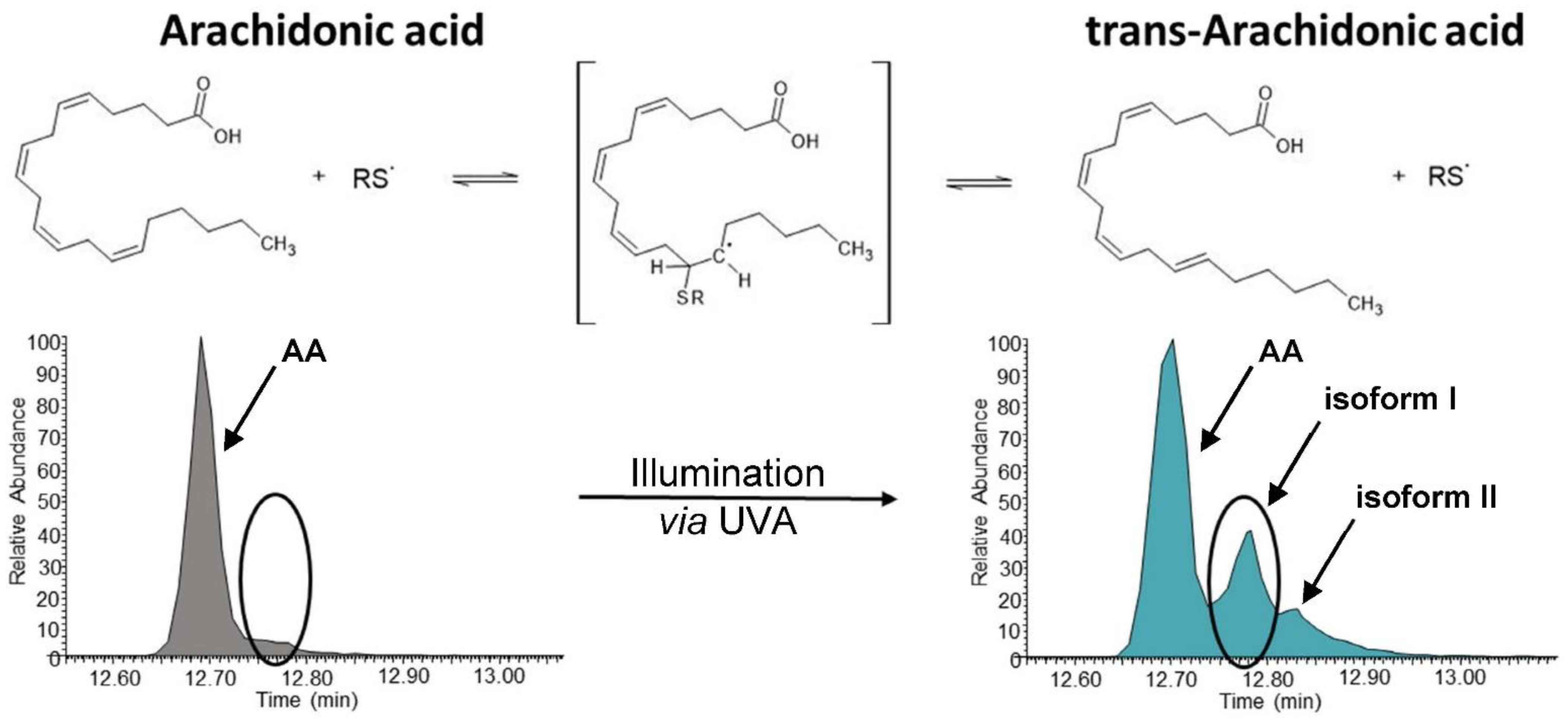
3.3. UVA Treatment Is Capable of Forming trans-Arachidonic Acids in the Presence of a Sulhydryl Donor
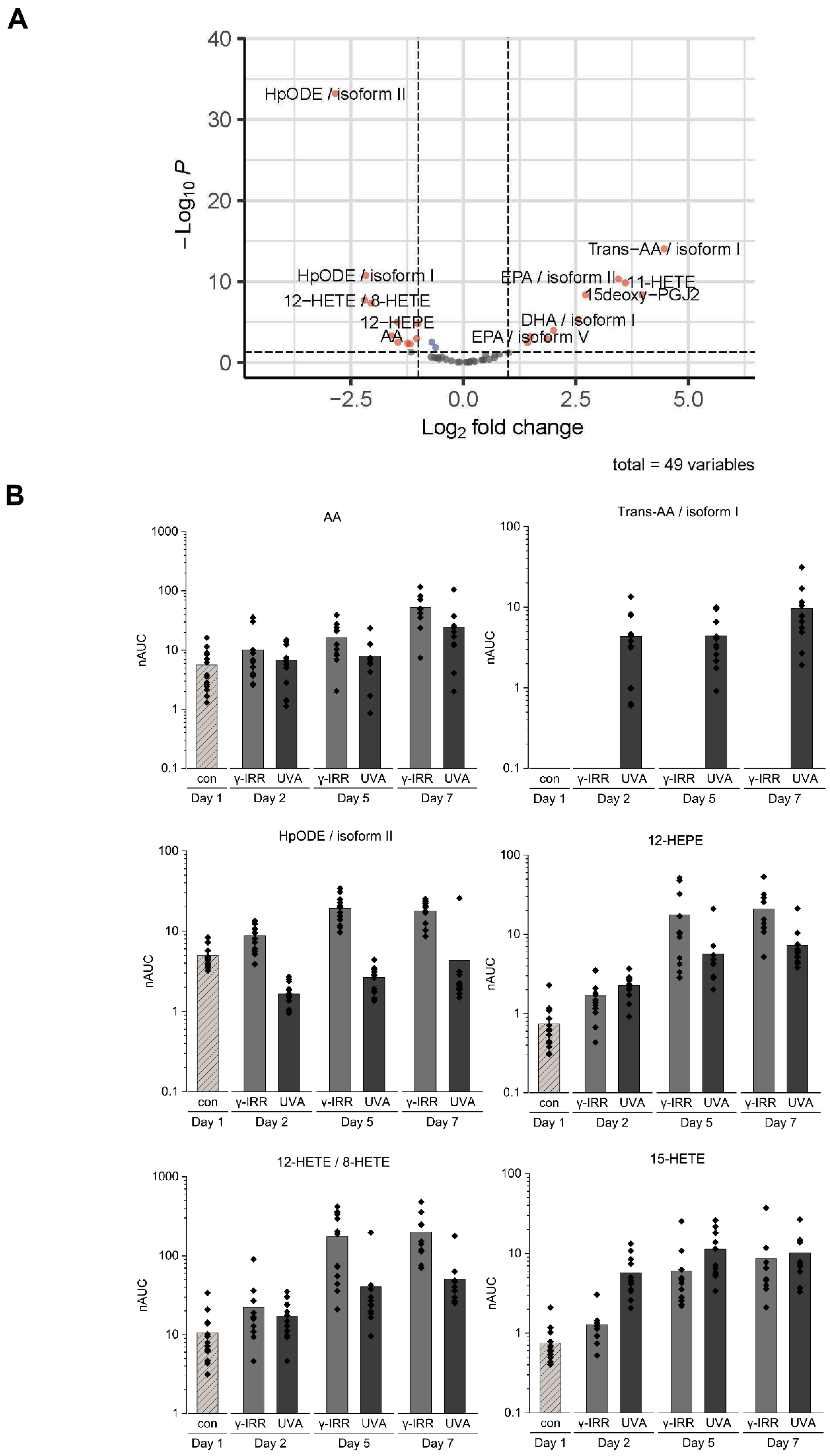
3.4. Stored Platelet Concentrates Still Show Significant Differences in Their Eicosanoid Profile
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Anderson, K.C.; Weinstein, H.J. Transfusion-associated graft-versus-host disease. N. Engl. J. Med. 1990, 323, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Burns, L.J.; Westberg, M.W.; Burns, C.P.; Klassen, L.W.; Goeken, N.E.; Ray, T.L.; Macfarlane, D.E. Acute graft-versus-host disease resulting from normal donor blood transfusions. Acta Haematol. 1984, 71, 270–276. [Google Scholar] [CrossRef]
- von Fliedner, V.; Higby, D.J.; Kim, U. Graft-versus-host reaction following blood product transfusion. Am. J. Med. 1982, 72, 951–961. [Google Scholar] [CrossRef]
- Ohto, H.; Yasuda, H.; Noguchi, M.; Abe, R. Risk of transfusion-associated graft-versus-host disease as a result of directed donations from relatives. Transfusion 1992, 32, 691–693. [Google Scholar] [CrossRef] [PubMed]
- Aoun, E.; Shamseddine, A.; Chehal, A.; Obeid, M.; Taher, A. Transfusion-associated GVHD: 10 years’ experience at the American University of Beirut-Medical Center. Transfusion 2003, 43, 1672–1676. [Google Scholar] [CrossRef] [PubMed]
- Serefhanoglu, K.; Turan, H.; Saba, T.; Ozer, I.; Tosun, E.; Arslan, H. Transfusion-associated graft-versus-host disease in an immunocompetent individual following cardiac surgery. J. Natl. Med. Assoc. 2005, 97, 418–420. [Google Scholar] [PubMed]
- Triulzi, D.J. The art of plasma transfusion therapy. Transfusion 2006, 46, 1268–1270. [Google Scholar] [CrossRef] [PubMed]
- Agbaht, K.; Altintas, N.D.; Topeli, A.; Gokoz, O.; Ozcebe, O. Transfusion-associated graft-versus-host disease in immunocompetent patients: Case series and review of the literature. Transfusion 2007, 47, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S. Guidelines for platelet transfusion. JAMA 1988, 259, 2453–2454. [Google Scholar] [CrossRef]
- Leitner, G.C.; Neuhauser, M.; Weigel, G.; Kurze, S.; Fischer, M.B.; Hocker, P. Altered intracellular purine nucleotides in gamma-irradiated red blood cell concentrates. Vox Sang. 2001, 81, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Garban, F.; Guyard, A.; Labussiere, H.; Bulabois, C.E.; Marchand, T.; Mounier, C.; Caillot, D.; Bay, J.O.; Coiteux, V.; Schmidt-Tanguy, A.; et al. Comparison of the Hemostatic Efficacy of Pathogen-Reduced Platelets vs Untreated Platelets in Patients with Thrombocytopenia and Malignant Hematologic Diseases: A Randomized Clinical Trial. JAMA Oncol. 2018, 4, 468–475. [Google Scholar] [CrossRef] [PubMed]
- van der Meer, P.F.; Ypma, P.F.; van Geloven, N.; van Hilten, J.A.; van Wordragen-Vlaswinkel, R.J.; Eissen, O.; Zwaginga, J.J.; Trus, M.; Beckers, E.A.M.; Te Boekhorst, P.; et al. Hemostatic efficacy of pathogen-inactivated vs untreated platelets: A randomized controlled trial. Blood 2018, 132, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Brixner, V.; Bug, G.; Pohler, P.; Kramer, D.; Metzner, B.; Voss, A.; Casper, J.; Ritter, U.; Klein, S.; Alakel, N.; et al. Efficacy of UVC-treated, pathogen-reduced platelets versus untreated platelets: A randomized controlled non-inferiority trial. Haematologica 2021, 106, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- Cid, J.; Lozano, M. Pathogen inactivation of platelets for transfusion. Platelets 2022, 33, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Estcourt, L.J.; Malouf, R.; Hopewell, S.; Trivella, M.; Doree, C.; Stanworth, S.J.; Murphy, M.F. Pathogen-reduced platelets for the prevention of bleeding. Cochrane Database Syst. Rev. 2017, 7, CD009072. [Google Scholar] [CrossRef]
- Marrocco, C.; D’Alessandro, A.; Girelli, G.; Zolla, L. Proteomic analysis of platelets treated with gamma irradiation versus a commercial photochemical pathogen reduction technology. Transfusion 2013, 53, 1808–1820. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.S.; Chan, H.F.; Leung, H.H.; Galano, J.M.; Oger, C.; Durand, T.; Lee, J.C. Short-time UVA exposure to human keratinocytes instigated polyunsaturated fatty acid without inducing lipid peroxidation. Free Radic. Res. 2017, 51, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Trostchansky, A.; Moore-Carrasco, R.; Fuentes, E. Oxidative pathways of arachidonic acid as targets for regulation of platelet activation. Prostaglandins Other Lipid Mediat. 2019, 145, 106382. [Google Scholar] [CrossRef] [PubMed]
- Tessaro, F.H.; Ayala, T.S.; Martins, J.O. Lipid mediators are critical in resolving inflammation: A review of the emerging roles of eicosanoids in diabetes mellitus. BioMed Res. Int. 2015, 2015, 568408. [Google Scholar] [CrossRef]
- Crescente, M.; Menke, L.; Chan, M.V.; Armstrong, P.C.; Warner, T.D. Eicosanoids in platelets and the effect of their modulation by aspirin in the cardiovascular system (and beyond). Br. J. Pharmacol. 2019, 176, 988–999. [Google Scholar] [CrossRef] [Green Version]
- Petrukhina, G.N.; Makarov, V.A. Natural eicosanoids in regulation of blood coagulation. Biochemistry 1998, 63, 93–101. [Google Scholar] [PubMed]
- Niederstaetter, L.; Neuditschko, B.; Brunmair, J.; Janker, L.; Bileck, A.; Del Favero, G.; Gerner, C. Eicosanoid Content in Fetal Calf Serum Accounts for Reproducibility Challenges in Cell Culture. Biomolecules 2021, 11, 113. [Google Scholar] [CrossRef] [PubMed]
- Leitner, G.C.; List, J.; Horvath, M.; Eichelberger, B.; Panzer, S.; Jilma-Stohlawetz, P. Additive solutions differentially affect metabolic and functional parameters of platelet concentrates. Vox Sang. 2016, 110, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Pietersz, R.N.; Reesink, H.W.; Panzer, S.; Gilbertson, M.P.; Borosak, M.E.; Wood, E.M.; Leitner, G.C.; Rabitsch, W.; Ay, C.; Lambermont, M.; et al. Prophylactic platelet transfusions. Vox Sang. 2012, 103, 159–176. [Google Scholar] [CrossRef]
- Curry, N.S.; Davenport, R.; Pavord, S.; Mallett, S.V.; Kitchen, D.; Klein, A.A.; Maybury, H.; Collins, P.W.; Laffan, M. The use of viscoelastic haemostatic assays in the management of major bleeding: A British Society for Haematology Guideline. Br. J. Haematol. 2018, 182, 789–806. [Google Scholar] [CrossRef]
- Muller, M.C.; Meijers, J.C.; Vroom, M.B.; Juffermans, N.P. Utility of thromboelastography and/or thromboelastometry in adults with sepsis: A systematic review. Crit. Care 2014, 18, R30. [Google Scholar] [CrossRef]
- Leitner, G.C.; Ho, M.; Tolios, A.; Hopfinger, G.; Rabitsch, W.; Wohlfarth, P. The assessment of platelet function by thromboelastometry as a point-of-care test to guide Intercept-treated platelet support in hemato-oncological patients and hematopoietic stem cell transplantation recipients. Transfusion 2020, 60, 1391–1399. [Google Scholar] [CrossRef]
- Ferreri, C.; Kratzsch, S.; Brede, O.; Marciniak, B.; Chatgilialoglu, C. Trans lipid formation induced by thiols in human monocytic leukemia cells. Free Radic. Biol. Med. 2005, 38, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Fahy, E.; Sud, M.; Cotter, D.; Subramaniam, S. LIPID MAPS online tools for lipid research. Nucleic Acids Res. 2007, 35, W606–W612. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R. RStudio, PBC, Boston, MA. 2020. Available online: http://www.rstudio.com/ (accessed on 9 June 2022).
- Lazar, C.; Burger, T. imputeLCMD: A Collection of Methods for Left-Censored Missing Data Imputation. R package version 2.1. 2022. Available online: https://CRAN.R-project.org/package=imputeLCMD (accessed on 19 July 2022).
- Blighe, K.R.S.; Lewis, M. EnhancedVolcano: Publication-Ready Volcano Plots with Enhanced Colouring and Labeling. R Package Version 1.14.0. 2022. Available online: https://github.com/kevinblighe/EnhancedVolcano (accessed on 24 June 2022).
- Thomas, C.P.; Morgan, L.T.; Maskrey, B.H.; Murphy, R.C.; Kuhn, H.; Hazen, S.L.; Goodall, A.H.; Hamali, H.A.; Collins, P.W.; O’Donnell, V.B. Phospholipid-esterified eicosanoids are generated in agonist-activated human platelets and enhance tissue factor-dependent thrombin generation. J. Biol. Chem. 2010, 285, 6891–6903. [Google Scholar] [CrossRef] [Green Version]
- Tahir, A.; Bileck, A.; Muqaku, B.; Niederstaetter, L.; Kreutz, D.; Mayer, R.L.; Wolrab, D.; Meier, S.M.; Slany, A.; Gerner, C. Combined Proteome and Eicosanoid Profiling Approach for Revealing Implications of Human Fibroblasts in Chronic Inflammation. Anal. Chem. 2017, 89, 1945–1954. [Google Scholar] [CrossRef] [PubMed]
- Muqaku, B.; Eisinger, M.; Meier, S.M.; Tahir, A.; Pukrop, T.; Haferkamp, S.; Slany, A.; Reichle, A.; Gerner, C. Multi-omics Analysis of Serum Samples Demonstrates Reprogramming of Organ Functions Via Systemic Calcium Mobilization and Platelet Activation in Metastatic Melanoma. Mol. Cell Proteom. 2017, 16, 86–99. [Google Scholar] [CrossRef]
- Muqaku, B.; Pils, D.; Mader, J.C.; Aust, S.; Mangold, A.; Muqaku, L.; Slany, A.; Del Favero, G.; Gerner, C. Neutrophil Extracellular Trap Formation Correlates with Favorable Overall Survival in High Grade Ovarian Cancer. Cancers 2020, 12, 505. [Google Scholar] [CrossRef]
- Jiang, H.; Kruger, N.; Lahiri, D.R.; Wang, D.; Vatele, J.M.; Balazy, M. Nitrogen dioxide induces cis-trans-isomerization of arachidonic acid within cellular phospholipids. Detection of trans-arachidonic acids in vivo. J. Biol. Chem. 1999, 274, 16235–16241. [Google Scholar] [CrossRef] [PubMed]
- Niforou, A.; Magriplis, E.; Klinaki, E.; Niforou, K.; Naska, A. On account of trans fatty acids and cardiovascular disease risk—There is still need to upgrade the knowledge and educate consumers. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1811–1818. [Google Scholar] [CrossRef]
- Souabni, H.; Thoma, V.; Bizouarn, T.; Chatgilialoglu, C.; Siafaka-Kapadai, A.; Baciou, L.; Ferreri, C.; Houee-Levin, C.; Ostuni, M.A. trans Arachidonic acid isomers inhibit NADPH-oxidase activity by direct interaction with enzyme components. Biochim. Biophys. Acta 2012, 1818, 2314–2324. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Jeon, B.; Jeon, M.S.; Lee, H.; Kim, T.Y. Reciprocal regulation of 12- and 15-lipoxygenases by UV-irradiation in human keratinocytes. FEBS Lett. 2008, 582, 3249–3253. [Google Scholar] [CrossRef]
- Turnbull, R.E.; Sander, K.N.; Turnbull, J.; Barrett, D.A.; Goodall, A.H. Profiling oxylipins released from human platelets activated through the GPVI collagen receptor. Prostaglandins Other Lipid Mediat. 2022, 158, 106607. [Google Scholar] [CrossRef] [PubMed]
- Imai, A.; Takahashi, M.; Nozawa, Y. Phospholipid metabolism in human platelets preserved at 22 degrees C: Differential effects of storage on phospholipase A2- and C-mediated reactions. Cryobiology 1984, 21, 255–259. [Google Scholar] [CrossRef]
| (a) | ||||
| γ-irradiated | INTERCEPT-treated | |||
| MCF, mm (mean/SD) | CFT, s (mean/SD) | MCF, mm (mean/SD) | CFT, s (mean/SD) | |
| Day 1 | 68 (3) | 152 (22) | 68 (3) | 152 (22) |
| Day 2 | 68 (3) | 162 (33) | 66 (1) | 156 (29) |
| Day 5 | 68 (3) | 152 (40) | 67 (3) | 142 (21) |
| Day 7 | 67 (3) | 157 (23) | 65 (2) | 173 (27) |
| (b) | ||||
| γ-irradiated | INTERCEPT-treated | |||
| Collagen, MA (mean/SD) | TRAP-6, MA (mean/SD) | Collagen, MA (mean/SD) | TRAP-6, MA (mean/SD) | |
| Day 1 | 88 (15) | 91 (18) | 88 (15) | 91 (18) |
| Day 2 | 81 (7) | 81 (6) | 81 (10) | 69 (17) |
| Day 5 | 74 (10) | 65 (15) | 78 (11) | 65 (24) |
| Day 7 | 68 (12) | 62 (12) | 69 (11) | 62 (14) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leitner, G.C.; Hagn, G.; Niederstaetter, L.; Bileck, A.; Plessl-Walder, K.; Horvath, M.; Kolovratova, V.; Tanzmann, A.; Tolios, A.; Rabitsch, W.; et al. INTERCEPT Pathogen Reduction in Platelet Concentrates, in Contrast to Gamma Irradiation, Induces the Formation of trans-Arachidonic Acids and Affects Eicosanoid Release during Storage. Biomolecules 2022, 12, 1258. https://doi.org/10.3390/biom12091258
Leitner GC, Hagn G, Niederstaetter L, Bileck A, Plessl-Walder K, Horvath M, Kolovratova V, Tanzmann A, Tolios A, Rabitsch W, et al. INTERCEPT Pathogen Reduction in Platelet Concentrates, in Contrast to Gamma Irradiation, Induces the Formation of trans-Arachidonic Acids and Affects Eicosanoid Release during Storage. Biomolecules. 2022; 12(9):1258. https://doi.org/10.3390/biom12091258
Chicago/Turabian StyleLeitner, Gerda C., Gerhard Hagn, Laura Niederstaetter, Andrea Bileck, Kerstin Plessl-Walder, Michaela Horvath, Vera Kolovratova, Andreas Tanzmann, Alexander Tolios, Werner Rabitsch, and et al. 2022. "INTERCEPT Pathogen Reduction in Platelet Concentrates, in Contrast to Gamma Irradiation, Induces the Formation of trans-Arachidonic Acids and Affects Eicosanoid Release during Storage" Biomolecules 12, no. 9: 1258. https://doi.org/10.3390/biom12091258
APA StyleLeitner, G. C., Hagn, G., Niederstaetter, L., Bileck, A., Plessl-Walder, K., Horvath, M., Kolovratova, V., Tanzmann, A., Tolios, A., Rabitsch, W., Wohlfarth, P., & Gerner, C. (2022). INTERCEPT Pathogen Reduction in Platelet Concentrates, in Contrast to Gamma Irradiation, Induces the Formation of trans-Arachidonic Acids and Affects Eicosanoid Release during Storage. Biomolecules, 12(9), 1258. https://doi.org/10.3390/biom12091258








