Abstract
Wilms’ tumor 1-associating protein (WTAP) is required for N6-methyladenosine (m6A) RNA methylation modifications, which regulate biological processes such as RNA splicing, cell proliferation, cell cycle, and embryonic development. m6A is the predominant form of mRNA modification in eukaryotes. WTAP exerts m6A modification by binding to methyltransferase-like 3 (METTL3) in the nucleus to form the METTL3-methyltransferase-like 14 (METTL14)-WTAP (MMW) complex, a core component of the methyltransferase complex (MTC), and localizing to the nuclear patches. Studies have demonstrated that WTAP plays a critical role in various cancers, both dependent and independent of its role in m6A modification of methyltransferases. Here, we describe the recent findings on the structural features of WTAP, the mechanisms by which WTAP regulates the biological functions, and the molecular mechanisms of its functions in various cancers. By summarizing the latest WTAP research, we expect to provide new directions and insights for oncology research and discover new targets for cancer treatment.
1. Introduction
Post-transcriptional modifications (PTMs) are an important part of epigenetics that can occur on all types of biological macromolecules, such as RNA, DNA, and proteins, and most PTMs have important effects on physiological processes [1,2]. Currently, more than 170 different RNA chemical modifications have been identified in living organisms [3], including N6-methyladenosine (m6A), pseudouridine (Ψ), N4-acetylcytidine (ac4C), 5-methylcytidine (m5C), N1-methyladenosine (m1A), and N7-methylguanosine (m7G) modifications [2,4]. First discovered in the 1970s [5], m6A modification has subsequently been demonstrated to be the most abundant form of internal modification of long noncoding RNAs (lncRNAs) and messenger RNAs (mRNAs) in many eukaryotes, accounting for nearly 80% of RNA methylation modifications [6]. It is estimated that 0.1–0.4% of the total adenine nucleotide content of mammalian transcripts is methylated, particularly at the start of the 3′-UTR, near the translation stop codon, usually embedded in the consensus sequence 5′-RRACH-3′ [7,8,9]. Studies have shown that m6A participates in most steps of RNA metabolism, including mRNA transcription, translation, splicing, folding, degradation, and export [9,10]. The completion of these modification processes is dependent on methyltransferases (writers), demethylases (erasers), and methylation-reading proteins (readers). Moreover, the modification process is dynamic and reversible [11,12] (Figure 1). Studies have demonstrated that the m6A methylation modifications are involved in the development of various diseases, such as malignant tumors [13,14,15], cardiovascular diseases [16,17], autoimmune diseases [18], and psychiatric diseases [19].
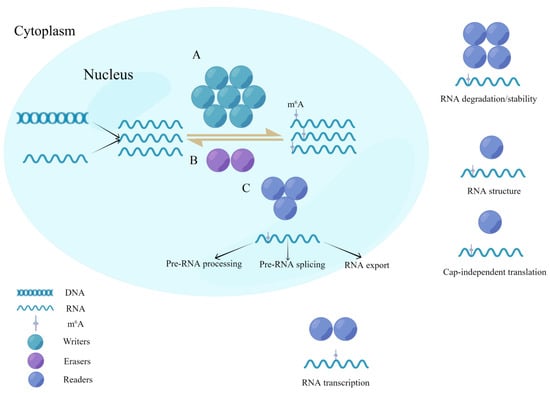
Figure 1.
Dynamic reversible process for writers, readers, and erasers in m6A modifications. (A) Methyltransferases (writers) are a class of catalytic enzymes that catalyze m6A methylation modification of bases in substrate RNA by forming a complex. (B) Demethylases (erasers) have the opposite function of writers and are capable of demethylating RNA in the nucleus. (C) Methylated reading proteins (readers) are specific RNA-binding proteins that undergo m6A modifications to produce specific biological functions.
Wilms’ tumor 1-associating protein (WTAP) is a WT-associating protein whose transcription is regulated by WT-1. WTAP is a ubiquitously expressed nuclear protein, and the protein is localized throughout the nucleoplasm as well as in spots, and partially clustered with splicing factor [20]. Studies have demonstrated that WTAP plays an important role in a variety of diseases, such as malignancies [14], cardiovascular diseases [21,22,23], lumbar disk diseases [24], and autoimmune diseases [25]. WTAP regulates the development of these diseases in two forms: one is m6A modification dependent on methyltransferase activity and the other is targeted modification independent of m6A methyltransferase, of which m6A modification dependent on methyltransferase activity is the most widely studied. In addition, WTAP has been demonstrated to catalyze substrate RNAs by forming the methyltransferase-like 3 (METTL3)- methyltransferase-like 14 (METTL14) -WTAP (MMW) complex as a core component of the methyltransferase complex (MTC) [26]. The role of METTL3 and METTL14 as core components of the MTC in tumors has been described in detail [27,28]. In this article, the latest regulatory mechanisms of WTAP in tumors are elaborated to provide researchers with new ideas for tumor treatment.
2. Methyltransferase
Methyltransferases, also called ‘writers’, catalyze the m6A methylation modification of bases in mRNA [29]. The first methyltransferase was purified as a protein complex by Bokar et al. in 1994 [30]. Further studies revealed an m6A MTC with METTL3-METTL14 heterodimer at the core and consisting of other components such as WTAP, vir-like m6A methyltransferase associated (VIRMA), zinc finger CCCH-type-containing 13 (ZC3H13), RNA-binding motif protein 15 (RBM15), and RNA-binding motif protein 15B (RBM15B) [31,32,33,34,35,36] (Figure 2). METTL3, the first m6A methyltransferase [37], is not only a subunit of the core component of methyltransferases but also a catalytic subunit that uses S-adenosylmethionine (SAM) as a methyl donor [38]. METTL14 was confirmed to be a homologue of METTL3 with 43% homology to METTL3 [39], which forms a stable heterodimer with METTL3 in a 1:1 ratio [31]. METTL3 in the heterodimer plays the catalytic role. Its internal SAM-binding structural domain catalyzes the transfer of methyl groups from SAM to adenine bases in RNA to produce S-adenosyl homocysteine (SAH). In contrast, METTL14 serves mainly to stabilize the structure of MTC and to identify specific RNA sequences 5′-RRACH-3′ as catalytic substrates [32,40,41]. In MTC, WTAP is not catalytically active; however, it promotes m6A methylation by binding to METTL3 in the nucleus to form the MMW complex and localizing to the nuclear speckles [32,42]. VIRMA recruits the MMW complex to direct regioselective methylation and mediates preferential mRNA methylation at the 3′UTR, near the stop codon [33]. Very few studies have looked at the role of RBM15 and RBM15B concerning methylation. RBM15 and RBM15B are shown to recruit the MTC to their target transcripts by binding directly to U-rich sequences on mRNA [35]. Further studies showed that ZC3H13 could bind RBM15 to WTAP to catalyze m6A methylation [36].
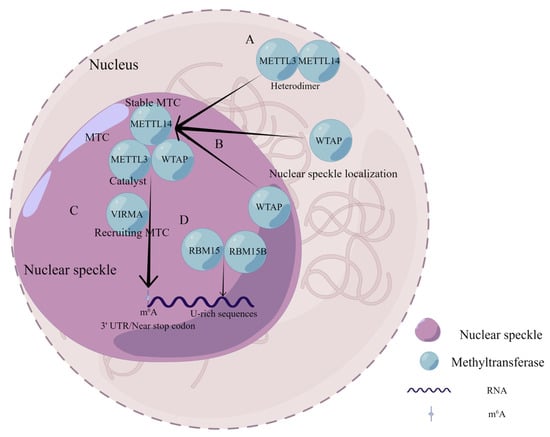
Figure 2.
Composition and functional characteristics of MTC in m6A modification. (A) METTL3 and METTL14 form a stable heterodimer in a 1 to 1 ratio. (B) WTAP binds to METTL3 in the nucleus to form the MMW complex and assists in the localization of the complex to the nuclear speckles. (C) VIRMA recruits the MMW complex to direct regioselective methylation and mediates preferential mRNA methylation at the 3′UTR, near stop codon. (D) RBM15 and RBM15B recruit MTC to their target transcripts by binding directly to U-rich sequences on RNA.
Subsequently, methyltransferase-like 16 (METTL16) [43], methyltransferase-like 5 (METTL5) [44], and CCHC zinc finger-containing protein (ZCCHC4) [45] were found to function as methyltransferases. The N-terminal structural domain of METTL16 with m6A methyltransferase activity is responsible for the m6A of A43 of the U6 small nuclear RNA (snRNA). The A43 is located in a highly conserved ‘ACAGAGA’ sequence of U6, which is base-paired to 50 splice sites of the pre-mRNA, suggesting an essential role in METTL16 splicing regulation [43,46]. In addition, METTL16 methylation of the ‘UACAGAGA’ sequence in the 3′UTR hairpin of SAM synthase methionine adenosyltransferase 2A (MAT2A), inducing MAT2A splicing to regulate SAM homeostasis [47,48,49]. Recent studies have identified METTL5 as an 18S ribosomal RNA (rRNA) m6A-modifying enzyme, and ZCCHC4 was identified as a eukaryotic 28S rRNA m6A-modifying enzyme [44,45,50,51].
3. WTAP Functions and Its Structural Characteristics
Little et al. first isolated the WTAP protein containing 396 amino acids by a yeast two-hybrid system [20]. Fluorescein in situ hybridization revealed that the WTAP gene is located on human chromosome 6q25–27 and is widely expressed in various human tissues [20]. The structural model of the WTAP protein was generated through the AlphaFold protein structure online website (https://alphafold.ebi.ac.uk/ (accessed on 20 May 2022), Figure 3). Studies have demonstrated that WTAP is involved in m6A RNA methylation modification [32,42], cell proliferation [52,53], cell-cycle regulation [34,54,55], RNA splicing [56,57], and embryonic development [34,58,59]. As previously described, WTAP exerts m6A modification by binding to METTL3 and METTL14 to form the MMW complex [32]. High-throughput sequencing indicated enriched binding of ‘GGAC’ sequences of METTL3 and METTL14 and ‘GACU’ sequences of WTAP [31]. Interestingly, WTAP did not affect the in vitro activity of the METTL3-METTL14 complex but affected its activity in vivo, independent of WT-1 [42]. Studies have revealed that WTAP consists of an extended N-terminal coiled-coil region followed by a rather unstructured C-terminal [32]. The interaction of WTAP with METTL3 or METTL14 depends on its highly conserved N-terminus [42]. In addition, immunofluorescence microscopy uncovered that METTL3 and METTL14 were present in the nucleus, while WTAP regulated the accumulation of METTL3 and METTL14 in the nuclear speckle [42]. Identification of potential nuclear localization signal (NLS) on METTL3, METTL14 and WTAP revealed how the MMW complex reaches inside the nucleus [32]. WTAP has a potential NLS at its N-terminal end, and METTL3 has a potential NLS in the helical domain between the N-terminal pilot helix and methyltransferase domain (MTD). The NLS of METTL3 and WTAP have nuclear import functionality [32]. NLS in METTL14 is located within its terminal structural domain; however, the NLS in METTL14 does not have the function of transporting METTL14 into the nucleus. Further studies demonstrated that METTL14 localizes to the nucleus by binding to METTL3, forming a heterodimer [32]. However, how WTAP regulates the accumulation of METTL3 and METTL14 in nuclear speckles is yet to be uncovered. mRNA is the major RNA species bound by METTL3 and WTAP. Most binding sites are identified in the coding sequence (CDS), and UTR regions of mRNA and motifs ‘AGGACU’ and ‘UGUGGACU’ are enriched in METTL3 and WTAP-binding clusters, respectively [42]. Additionally, there is a significant spatial correlation with 69% METTL3 and 74% WTAP-binding clusters in UTR and CDS regions overlapping with m6A sites [42].
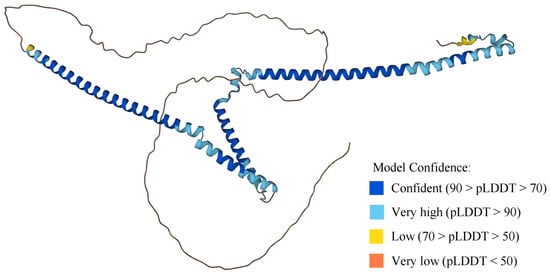
Figure 3.
Structural model of WTAP protein in AlphaFold protein structure website. The AlphaFold protein structure website generates a per-residue confidence score (pLDDT) ranging from 0 to 100.
WTAP also acts as a splicing regulator, driving protein diversity and complexity [60]. WTAP is also a component of the interchromatin granule cluster corresponding to nuclear speckles, many of which interact with proteins involved in gene expression, such as pre-mRNA splicing factors and transcription factors [20,61]. A proteomics study identified splicing factors that interact with WTAP, including the proteins thyroid hormone receptor associated protein 3 (THRAP3), BCL2 associated transcription factor 1 (BCLAF1), serine- and arginine-rich splicing factor 1 (SRSF1) and serine- and arginine-rich splicing factor 3 (SRSF3) containing arginine-/serine-rich structural domains and heterogeneous nuclear ribonucleoprotein (hnRNPs), as well as the fragile X mental retardation 1 (FMR1), G-quadruplex-binding proteins, fragile X mental retardation syndrome-related protein 1 (FXR1), and fragile X mental retardation syndrome-related protein 2 (FXR2) [56,57]. In addition, studies have demonstrated that WTAP localizes to nuclear speckles through interactions with THRAP3 and BCLAF1 and colocalizes with splicing factors [20,57,62]. Ping et al. proposed that the MMW complex functions in alternative splicing [42]. However, Horiuchi et al. identified another protein complex including WTAP, VIRMA, Cbl proto-oncogene like 1 (CBLL1), and ZC3H13 in GC-rich sequences, forming G-quadruplexes to inhibit the regulation of alternative splicing and selective polyadenylation [56]. In addition, studies have revealed that WTAP regulates G2/M transition through stabilization of cyclin A2 mRNA [55]. WTAP also recruits METTL3 and METTL14 to RNA and actively controls adipogenesis by promoting the mitotic cell-cycle transition. Knockdown of any of the three proteins inhibits the upregulation of cyclin A2 during mitosis, leading to impaired adipogenesis [54]. WTAP knockdown has been shown to promote apoptosis in porcine embryonic cells and affect embryonic development; however, the mechanism remains unclear [59].
4. Regulation of WTAP Expression
WTAP is widely expressed in a variety of human tissues (Figure 4) and is dysregulated in cancer expression through different mechanisms (Table 1). Numerous studies have explained the role of different noncoding RNAs (ncRNAs), including microRNAs (miRNAs), lncRNAs, and piwi-interacting RNAs (piRNAs) in tumor development. They have been extensively studied in tumor screening, diagnosis, treatment, prognosis, and drug response [63,64,65]. PiRNA-30473 in diffuse large B-cell lymphoma (DLBCL) reduces WTAP mRNA decline and enhances WTAP mRNA stability by binding to the 3′UTR of WTAP [66]. In renal cancer, miR-501-3p upregulates WTAP expression by targeting binding to WTAP [67]. MiR-139-5p negatively upregulates WTAP expression in hepatocellular carcinoma by targeting the 3′-UTR of WTAP [68]. In addition, the discovery of competing endogenous RNA (ceRNA) regulatory networks further enriched the link between ncRNAs and tumorigenesis [69,70,71,72]. LncRNA PCGEM1 can serve as a ceRNA to compete for miR-433-3 to upregulate WTAP expression in non-small-cell lung cancer (NSCLC) [73]. In hepatocellular carcinoma, LINC00839 upregulates WTAP expression by acting as a ceRNA contending to bind miR-144-3p [74]. Under the hypoxic regime, lncRNA EMS forms a ceRNA to upregulate WTAP expression in esophageal cancer by competing with WTAP to bind miR-758-3p. In addition, overexpression of lncRNA EMS and WTAP in this ceRNA is associated with worse overall survival (OS) and disease-free survival (DFS). In contrast, low expression of miR-758-3p is associated with a poorer DFS and OS. Therefore, ceRNA regulatory network can be used as an indicator for prognosis [75]. LncRNA SNHG10 competes for binding miR-141-3p by acting as a ceRNA to upregulate WTAP expression in osteosarcoma [76]. In pancreatic cancer, lncRNA DUXAP8 competitively binds miR-448 to upregulate WTAP expression [77]. Interestingly, WTAP promotes the stability of lncRNA DLGAP1-AS1 through m6A modification, and lncRNA DLGAP1-AS1 upregulates WTAP expression in breast cancer through 3′-UTR binding to miR-299-3p. However, whether lncRNA DLGAP1-AS1 competes with WTAP to bind miR-299-3p remains to be understood [78].
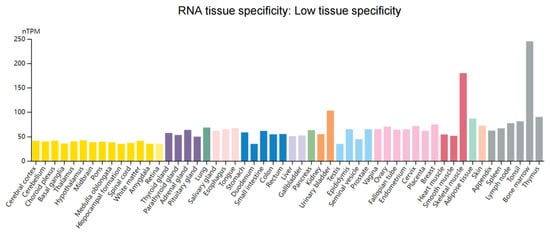
Figure 4.
Overview of RNA expression of WTAP in human tissues. Normalized expression (nTPM) of 55 tissue types through the Human Protein Atlas online database (https://www.proteinatlas.org/ (accessed on 25 May 2022)). Data in the database are integrated from RNA-seq dataset of the Human Protein Atlas and RNA-seq dataset of the Genotype-Tissue Expression. Each color corresponds to a tissue with common functional characteristics.
There have been studies identifying ncRNAs independent mechanisms for regulation of WTAP expression. Heat shock protein 90 (Hsp90) can stabilize WTAP protein expression by inhibiting the ubiquitin–proteasome pathway [79]. In pancreatic cancer, overexpression of pseudogene Wilms tumor 1-associated protein pseudogene 1 (WTAPP1) enhances the recruitment of more translation initiation factor–eukaryotic translation initiation factor 3 (EIF3) complex (EIF3A to EIF3M) by EIF3B and promotes WTAP translation [80]. Inhibitor of growth family member 2 (ING2) was shown to negatively regulate WTAP expression in NSCLC [81]. In colorectal cancer, increased expression of arrestin beta 2 (ARRB2) could upregulate WTAP expression; however, the specific regulation mechanism is unclear [82]. In breast cancer, extracellular signal-regulated kinase 1 (ERK1) and extracellular signal-regulated kinase 2 (ERK2) phosphorylate WTAP at serine 341 and stabilize WTAP, thus upregulating WTAP protein levels [83]. In nasopharyngeal carcinoma, WTAP is upregulated by CREB-binding protein (KAT3A)-mediated acetylation of H3K27 [84]. He et al. analyzed glioma single-nucleotide polymorphisms in the Chinese child population and found that WTAP rs7766006 increased the risk of glioma by upregulating WTAP mRNA expression; however, these findings were preliminary and lacked reliable experimental support [85]. In Epstein–Barr virus-associated gastric carcinoma (EBVaGC), EBV-encoded small RNA1 (EBER1) can downregulate WTAP expression by activating the nuclear factor kappa-B (NF-κB) signaling pathway [86]. In addition, the mechanistic target of rapamycin complex 1 (mTORC1) signaling has also been shown to increase WTAP protein abundance [87]. Subsequently, Cho et al. revealed that mTORC1 via its kinase S6K enhanced the targeting of 5′UTR of eIF4A/4B to WTAP mRNA, leading to the translation of WTAP [52]. Interestingly, METTL3, one of the methyltransferase complex members, is crucial for WTAP homeostasis, and both its knockdown and overexpression can lead to the upregulation of WTAP protein. On the one hand, increased METTL3 levels can lead to higher WTAP protein levels, independent of the METTL3 catalytic activity but by increasing WTAP mRNA translation and protein stability. On the other hand, decreased METTL3 levels lead to increased WTAP mRNA levels, ultimately leading to increased WTAP protein levels. However, in the absence of functional METTL3, upregulation of WTAP was not sufficient to promote cell growth [88].

Table 1.
Regulation of WTAP expression.
Table 1.
Regulation of WTAP expression.
| Type | Moleculars/Signals | Mechanism | Expression | Tumor Types | Reference |
|---|---|---|---|---|---|
| piRNA | piRNA-30473 | Targeted WTAP | Enhancing the stability of WTAP mRNA | DLBCL | [66] |
| miRNAs | miR-501-3p | Targeted WTAP | Upregulation of WTAP expression | Renal cancer | [67] |
| miR-139-5p | Targeted WTAP | Upregulation of WTAP expression | Hepatocellular carcinoma | [68] | |
| lncRNAs | lncRNA PCGEM1 | lncRNA PCGEM1/miR-433-3/WTAP | Upregulation of WTAP expression | NSCLC | [73] |
| LINC00839 | LINC00839/miR-144-3p/WTAP | Upregulation of WTAP expression | Hepatocellular carcinoma | [74] | |
| lncRNA EMS | lncRNA EMS/miR-758-3p/WTAP | Upregulation of WTAP expression | Esophageal cancer | [75] | |
| lncRNA SNHG10 | lncRNA SNHG10/miR-141-3p/WTAP | Upregulation of WTAP expression | Osteosarcoma | [76] | |
| lncRNA DUXAP8 | lncRNA DUXAP8/miR-448/WTAP | Upregulation of WTAP expression | Pancreatic cancer | [77] | |
| lncRNA DLGAP1-AS1 | lncRNA DLGAP1-AS1/miR-299-3p/WTAP | Upregulation of WTAP expression | Breast cancer | [78] | |
| Genes | Hsp90 | Inhibiting the ubiquitin–proteasome pathway | Stabilization of WTAP expression | DLBCL | [79] |
| WTAPP1 | Recruiting more translation initiation factor EIF3 complex to promote WTAP translation | Upregulation of WTAP expression | Pancreatic cancer | [80] | |
| ING2 | Negative regulation of WTAP expression | Upregulation of WTAP expression | NSCLC | [81] | |
| ARRB2 | Interaction with WTAP | Upregulation of WTAP expression | Colorectal cancer | [82] | |
| ERK1/ERK2 | Phosphorylation of WTAP at serine 341 and stabilization of WTAP | Upregulation of WTAP expression | Breast cancer | [83] | |
| KAT3A | KAT3A-mediated acetylation of H3K27 | Upregulation of WTAP expression | Nasopharyngeal carcinoma | [84] | |
| EBER1 | Activation of NF-κB signaling pathway | Downregulation of WTAP expression | EBVaGC | [86] | |
| METTL3 | Increased METTL3 levels promote translation of WTAP mRNA and protein stabilization | Upregulation of WTAP expression | / | [88] | |
| Decreased METTL3 levels lead to increased WTAP mRNA levels | Upregulation of WTAP expression | / | [88] | ||
| Other forms | rs7766006 | single-nucleotide polymorphisms | Upregulation of WTAP expression | Glioma in Chinese children | [85] |
| mTORC1-S6K pathway | Enhanced eIF4A/4B-targeted WTAP translation | Upregulation of WTAP expression | / | [52] |
piRNAs: piwi-interacting RNAs; miRNAs: microRNAs; lncRNAs: long noncoding RNAs; DLBCL: diffuse large B-cell lymphoma; NSCLC: non-small-cell lung cancer; EBVaGC: Epstein–Barr virus-associated gastric carcinoma.
5. Role of WTAP in Carcinogenesis
With these critical biological and pathological functions, WTAP has been increasingly studied for its crucial role in various tumors, both dependent and independent of its methyltransferase activity (Table 2).
5.1. Blood System
5.1.1. Acute Myeloid Leukemia
Acute myeloid leukemia (AML) is one of the most common malignancies of the hematopoietic system [89]. Previous studies indicated that WTAP is overexpressed in AML patients and can be an independent risk prognostic factor for AML [90]. In vitro studies demonstrated that the knockdown of WTAP reduces the proliferative capacity of AML cells, shifts the cell cycle to the G1/S phase, and makes the cells more susceptible to apoptosis [90]. In addition, WTAP overexpression leads to chemotherapeutic agents (Rifamycin) resistance in AML cells [90]. Further studies have demonstrated that WTAP knockdown in AML prolongs the half-life of MYC mRNA by decreasing the level of m6A methylation, and thereby upregulates MYC expression to activate the phosphatidylinositol 3-kinase (PI3K)/protein serine-threonine kinase (AKT) signaling pathway [90]. However, the consequences of PI3K/AKT signaling regulation by WTAP on cell behaviors in AML are yet to be characterized [90].
MiR-550-1 expression is downregulated in AML and predicts poor prognosis [91]. Hu et al. used a public dataset and in silico analysis to infer that WTAP is a direct target gene downstream of miR-550-1 [91]. Further analysis suggested that miR-550-1 may mediate a reduction in m6A levels by targeting WTAP, which further reduces WW domain containing transcription regulator 1 (WWTR1) stability and mediates, at least in part, its antileukemic activity [91].
5.1.2. Lymphoma
Lymphoma is a hematologic disorder manifested by the clonal proliferation of lymphocytes. DLBCL is the most common pathological subtype of lymphoma, accounting for approximately 25% to 30% of patients with lymphoma [92]. WTAP is overexpressed in DLBCL and can promote cell proliferation and inhibit apoptosis [79]. Treatment with the antitumor drug (Etoposide) in WTAP-silenced cells induced an increased apoptosis rate. The researchers speculated that DLBCL patients with high WTAP expression levels might experience ineffective treatment with chemotherapy [79]. Previous studies have indicated that Hsp90 stabilizes many tumor-promoting proteins [93]. Hsp90 has also been shown to stabilize WTAP expression by inhibiting the ubiquitin–proteasome pathway [79]. In addition, B-cell lymphoma 6 (BCL6), a transcriptional repressor, a vital tumor protein in DLBCL that has been evaluated as a therapeutic target, forms a complex with Hsp90 and WTAP [94]. However, whether the WTAP-Hsp90-BCL6 complex is involved in the transcriptional activity or hinders other cellular functions has not been further explored [79].
WTAP expression was enhanced in human NK/T-cell lymphoma (NKTCL) cells. In vitro experiments showed that WTAP deletion inhibited proliferation and antitumor drug (cisplatin) resistance of NKTCL cells and promoted apoptosis of tumor cells. Mechanistically, WTAP stabilizes dual-specificity phosphatase 6 (DUSP6) mRNA in an m6A-dependent manner and targets it for mRNA methylation to confer NKTCL chemoresistance [95].
5.2. Digestive System
5.2.1. Esophageal Cancer
Esophageal cancer often has poor prognosis due to its aggressive nature, and is reported to be the sixth leading cause of cancer-related death worldwide [96]. WTAP, an oncogene, is overexpressed in esophageal cancer cells [75,97]. In addition, WTAP upregulation in esophageal cancer is associated with poor prognosis [97]. Higher WTAP expression was observed in esophageal squamous cell carcinoma compared to esophageal adenocarcinoma [97]. Studies have exhibited that hypoxia generates intratumoral oxygen gradients that contribute to tumor plasticity and heterogeneity and promote tumor cell invasion, metastasis, and drug resistance [98]. WTAP overexpression under normoxic and hypoxic conditions promoted cell proliferation, inhibited apoptosis, and induced resistance to cisplatin [75]. Mechanistically, lncRNA EMS is overexpressed in hypoxic environment and can act as ceRNA to compete for binding miR-758-3p to upregulate WTAP expression to promote cisplatin resistance in esophageal tumor cells [75].
5.2.2. Gastric Cancer
Gastric cancer is the fifth most common cancer and the third most common cause of cancer death globally [99]. WTAP was overexpressed in gastric cancer and is associated with poor prognosis [100,101,102], and accelerates glucose uptake and promotes lactate production in gastric cancer cells [100]. WTAP overexpression also accelerates the extracellular acidification rate of gastric cancer cells [100]. These studies suggest that WTAP promotes the Warburg effect in gastric cancer cells [100]. Furthermore, silencing WTAP leads to the inhibition of proliferation in gastric cancer cells [100]. Mechanistically, WTAP promotes the stability of hexokinase 2 (HK2) mRNA, a core molecule that regulates glucose metabolism, thus increasing glucose uptake in gastric cancer cells [100]. In addition, the bioinformatic analysis showed that high expression of WTAP is associated with RNA methylation, while low expression of WTAP is associated with high T-cell-related immune responses [101].
5.2.3. Liver Cancer
Liver cancer is the sixth leading primary cancer and the fourth leading cause of cancer-related deaths worldwide [103]. WTAP is overexpressed in hepatocellular carcinoma and predicts poor prognosis [53]. Cellular and bioinformatics studies showed that the growth and proliferation of cancer cells are inhibited after WTAP knockdown [53,104]. Mechanistically, WTAP inhibits the expression of ETS proto-oncogene 1 (ETS1), a tumor suppressor gene, in an m6A HuR-mediated manner. WTAP knockdown led to a significant decrease in m6A levels, while the expression levels of ETS1 were increased. ETS1 silencing rescued the inhibitory effect of WTAP knockdown on the growth and viability of hepatocellular carcinoma cells. Interestingly, further studies established that WTAP inhibits ETS1 expression by interfering with the link between HuR protein and ETS1 mRNA [53]. In addition, WTAP knockdown led to a G2/M phase block in hepatocellular carcinoma via the ETS1-p21/p27 axis, promoting apoptosis in tumor cells [53]. WTAP knockdown inhibited hepatocellular carcinoma growth and metastasis in hepatocellular carcinoma by promoting autophagosome production [105]. Further mechanistic studies revealed that liver kinase B1 (LKB1), the upstream kinase of Adenosine 5′-monophosphate activated protein kinas (AMPK), is regulated by WTAP and mediates AMPK phosphorylation in an m6A-dependent manner, thereby promoting hepatocellular carcinoma autophagy [105,106,107].
Several studies showed that ncRNAs interact with WTAP to influence hepatocellular carcinoma progression. LINC00839 upregulates WTAP expression and enhances the malignant features of hepatocellular carcinoma by regulating the miR-144-3p/WTAP axis. The proliferation of hepatocellular carcinoma cells was inhibited when LINC00839 was knocked down, and overexpression of WTAP expression eliminated this functional inhibition [74]. WTAP overexpression in hepatocellular carcinoma maintained the malignant phenotype of hepatocellular carcinoma. Further studies demonstrated that miR-139-5p targets the 3′-UTR of WTAP to promote the proliferation and invasive ability of hepatocellular carcinoma by regulating epithelial–mesenchymal transition (EMT) signaling [68].
5.2.4. Pancreatic Cancer
Pancreatic cancer is the most malignant tumor, with a 5-year survival rate of about only 10% [108]. Overexpression of WTAP promoted migration, invasion, and gemcitabine resistance in pancreatic cancer cells [109]. Further studies uncovered that WTAP directly bound focal adhesion kinase (Fak) mRNA and stabilized it, activating the Fak signaling pathway and promoting the malignant characteristics of pancreatic cancer cells. A small-molecule Fak inhibitor (GSK2256098) was able to reverse the cancer-promoting effect of WTAP on pancreatic cancer [109]. LncRNA DUXAP8 can regulate the Fak signaling pathway through miR-448/WTAP axis to promote migration and invasion of pancreatic cancer cells [77]. WTAPP1 was significantly elevated in pancreatic ductal adenocarcinoma and is associated with a poor patient prognosis, and promotes the proliferation and invasive ability of the cells. Mechanistically, increased WTAPP1 mRNA levels in pancreatic ductal adenocarcinoma are caused by m6A modification, which stabilizes WTAPP1 mRNA by recruiting the RNA stabilizer HuR by CCHC-type zinc finger nucleic-acid-binding protein (CNBP). Overexpressed WTAPP1 mRNA binds WTAP mRNA and enhances the recruitment of the transcription factor EIF3 complex, and further promotes WTAP translation [80]. Ultimately, the increase in WTAP protein enhanced the activation of the Wnt signaling and stimulated the malignancy of pancreatic ductal cells [80].
5.2.5. Colorectal Cancer
Colorectal cancer (CRC) is the fourth most common malignancy globally. Many studies have shown its close association with epigenetic dysregulation [110]. WTAP has significantly higher expression in CRC than in colorectal adenomas and normal tissue. However, survival analysis revealed that there is no significant difference between high and low WTAP expression in CRC [111]. However, in contrast to Dong et al., Wang et al. demonstrated that high WTAP expression predicted poor prognosis from the perspective of tumor differentiation [112]. The degree of tumor differentiation refers to the degree of cell maturation. Highly differentiated tumor cells are relatively less malignant, while undifferentiated or poorly differentiated tumor cells are relatively more malignant. At the transcriptional and translational levels, WTAP, METTL3, and METTL14 were highly expressed in hypodifferentiated tumor tissues, and the total m6A expression level was generally higher in general hypodifferentiated tissues. This suggested that a high total m6A level and higher expression of WTAP, METTL3, and METTL14 may be a malignant feature [112]. In addition, bioinformatics analysis inferred that WTAP expression was significantly higher in colon adenocarcinoma than in normal tissue, while no significant difference was found in rectal adenocarcinoma [113].
Carbonic anhydrase IV (CA4) is downregulated in CRC, and upregulating CA4 in in vitro experiments inhibits CRC cell proliferation, induces apoptosis, and stalls the cell cycle in G1 phase [114]. Mechanistically, CA4 interacts directly with WTAP, and the tumor suppressor function of CA4 is dependent on WTAP modification. WTAP knockdown eliminates the inhibitory effect of CA4 on CRC cell viability and colony-forming ability. In addition, CA4 can inhibit CRC progression by inducing WTAP degradation through polyubiquitination, and CA4 also inhibits the Wnt/β-linked protein signaling pathway by activating the WTAP degradation product WT-1 [114]. Urothelial cancer associated 1 (UCA1) was also upregulated in CRC and promoted tumor cell proliferation. It was later discovered that METTL3 and WTAP could m6A modify UCA1, and METTL3 and WTAP knockdown reduced UCA1 expression and inhibited CRC cell proliferation [115]. In addition, overexpression of ARRB2 led to the upregulation of WTAP, which promoted CRC cell proliferation and migration. However, the specific regulatory mechanism remains to be understood [82].
5.2.6. Cholangiocarcinoma
Cholangiocarcinoma is a highly lethal epithelial malignancy that is highly aggressive and heterogeneous [116]. Immunohistochemistry showed that WTAP was overexpressed in cholangiocarcinoma tissues and promoted the proliferation, invasion, and migration of cholangiocarcinoma cells. In addition, WTAP overexpression in cholangiocarcinoma induced the expression of metastasis-related genes such as cathepsin H, matrix metallopeptidase 7 (MMP7), matrix metallopeptidase 28 (MMP28), and mucin 1 (Muc1); however, the specific regulatory mechanisms have not been investigated [117].
5.3. Reproductive System
5.3.1. Breast Cancer
Breast cancer is the most prevalent malignancy in women and is the leading cause of most cancer-related deaths among women [118]. C5aR1+ neutrophil overexpression in breast cancer is associated with poor survival. C5aR1+ also induces breast cancer glycolysis. Further studies inferred that tumor necrosis factor-α (TNFα) and interleukin-1β (IL1β) secreted by C5aR1+ neutrophils synergistically activated the ERK1/2 signaling to phosphorylate WTAP at serine 341, thereby stabilizing WTAP, which further promotes RNA m6A modification of enolase 1 (ENO1) and leads to upregulation of ENO1, thus affecting the glycolytic activity of breast cancer cells [83]. LncRNA DLGAP1-AS1 is highly expressed in Adriamycin-resistant breast cancer cells and promotes the proliferation of drug-resistant cells. Mechanistically, WTAP promotes the stability of lncRNA DLGAP1-AS1 in breast cancer through m6A modifications. Interestingly, overexpression of lncRNA DLGAP1-AS1 3′-UTR targeted miR-299-3p to upregulate WTAP expression. However, further exploration is required to understand the lncRNA DLGAP1-AS1/miR-299-3p/WTAP regulatory axis in breast cancer [78].
5.3.2. Endometrial Cancer
Endometrial cancer is the most common gynecological cancer in developed countries, and its incidence is increasing worldwide [119]. WTAP is overexpressed in endometrial cancer. In vitro experiments revealed that WTAP deletion enhanced cisplatin-induced apoptosis and induced cell-cycle arrest. Further analysis revealed that WTAP deletion led to increased expression of the proapoptotic proteins BCL2-associated X (BAX) and cleaved poly ADP-ribose polymerase (PARP) and decreased expression of the antiapoptotic protein myeloid cell leukemin-1 (Mcl-1), and induced cell cycle G2/M phase arrest [120]. Mechanistic analysis indicated that WTAP induced resistance to cisplatin by promoting glycogen synthase kinase 3β (GSK3β) phosphorylation at GSK-3β (Ser9 site) and by activating the Wnt/β-catenin pathway by promoting β-catenin translocation to the nucleus [120]. A high WTAP expression in endometrial cancer is closely associated with its poor prognosis, and it promotes endometrial cancer cell proliferation, migration, and invasion and inhibits apoptosis [121]. Mechanistic studies showed that WTAP increased the m6A modification of caveolin 1 (CAV-1) mRNA 3′-UTR and downregulated CAV-1 expression, thereby activating the NF-κB signaling pathway in endometrial cancer to maintain the malignant characteristics of tumor cells [121].
5.3.3. Ovarian Cancer
Ovarian cancer is the third most common gynecological malignancy globally, but has the highest mortality rate among gynecological tumors [122]. WTAP overexpression in ovarian cancer is associated with poor prognosis. In vitro experiments revealed that proliferation, invasion, and migration of tumor cells were inhibited in WTAP knockdown [123]. Furthermore, HBS1-like translational GTPase (HBS1L) and family with sequence similarity 76 member A (FAM76A) were identified as two WTAP-related hub genes by the weighted gene coexpression network analysis method, and WTAP-mediated m6A modifications may regulate them. However, this in silico conclusion needs further experimental validation [123]. WTAP was also overexpressed in high-grade serous ovarian cancer and correlated with poor prognosis. WTAP knockdown led to the inhibition of tumor cell proliferation and promotion of apoptosis. Moreover, WTAP downregulation increased E-calmodulin expression and decreased waveform protein expression, thus inhibiting migration [124]. Mechanistically, WTAP maintains the high-grade serous ovarian cancer phenotype by associating with MAPK and AKT signaling pathways; however, further experimental validation is required [124].
5.4. Urinary System
5.4.1. Renal Cell Carcinoma
Renal cell carcinoma accounts for more than 400,000 new patients worldwide each year, with the majority of patients being elderly men over 60 years of age [125]. WTAP is upregulated in renal cell carcinoma and is associated with poor prognosis. In vitro experiments implied that high WTAP expression could promote the migration and proliferation of renal cell carcinoma [126]. Further studies revealed that WTAP enhanced cyclin dependent kinase 2 (CDK2) mRNA stability by directly targeting to the 3′-UTR of CDK2 transcript, thus upregulating CDK2 expression and promoting the proliferation of renal cell carcinoma cells [126]. Downregulation of miR-501-3p expression in renal cell carcinoma promotes renal cancer cell proliferation [67]. Mechanistically, miR-501-3p negatively regulates WTAP expression in renal cell carcinoma by targeting the 3′-UTR of WTAP [67]. In flow cytology experiments, it was observed that WTAP knockdown resulted in many G1/S phase blocks, suggesting that WTAP downregulation inhibits the proliferation of kidney cancer cells [67]. Additionally, miR-501-3p overexpression or WTAP knockdown altered the overall RNA m6A levels in renal cancer cells. However, the effect on renal cancer cells remains to be explored [67].
5.4.2. Bladder Cancer
Bladder cancer is a common malignancy in women and is the fourth most common malignancy in men [127]. WTAP expression was significantly increased at the transcriptional and translational levels than in normal tissues. Its overexpression is associated with poor prognosis [128,129]. Moreover, knockdown of WTAP in bladder cancer cells significantly accelerated tumor cell apoptosis and attenuated cell viability and the ability of anchored non-dependent growth [129]. Further studies indicated that circ0008399 and WTAP colocalized and interacted in cells, but their expression did not affect each other’s expression [129]. Mechanistically, circ0008399 promotes the formation of MMW complex to promote the overall level of m6A modification in bladder cancer cells. circ0008399/WTAP promotes TNFAIP3 expression by increasing m6A-dependent TNF alpha induced protein 3 (TNFAIP3) mRNA stability in bladder cancer cells, which in turn inhibits bladder cancer cell apoptosis and mediates reduced bladder cancer chemosensitivity to cisplatin [129]. Additionally, WTAP overexpression could impair the promotion of bladder cancer cell apoptosis induced by circ0008399 knockdown, as well as reverse the reduction in viability and anchorage-independent growth ability [129].
5.5. Respiratory System
5.5.1. Nasopharyngeal Cancer
Nasopharyngeal cancer has a distinct geographic distribution and is particularly prevalent in East and Southeast Asia, closely related to lifestyle and environment [130]. In nasopharyngeal carcinoma, KAT3A upregulates WTAP expression by mediating H3K27 acetylation, and WTAP overexpression predicts poor prognosis and promotes tumor cell growth and metastasis [84]. Mechanistic studies have indicated that lncRNA DIAPH1-AS1 was identified as an m6A target of WTAP. WTAP enhances its stability by mediating m6A modification of lncRNA DIAPH1-AS1 through synergistic interaction with insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2) [84]. Furthermore, lncRNA DIAPH1-AS1 acts as a molecular adapter to promote the formation of lncRNA MTDH-LASP1 complex and upregulate LIM and SH3 protein 1 (LASP1) expression, ultimately promoting the growth and metastasis of nasopharyngeal carcinoma cells [84].
5.5.2. Lung Cancer
Lung cancer has the highest mortality rate of all cancers, with an estimated 2 million new cases and 1.76 million deaths per year [131]. ING2 is downregulated in NSCLC tissues. Cell function analysis indicated that overexpressing ING2 inhibited the growth, infiltration, and metastasis, and blocked the apoptosis of NSCLC cells through EMT signaling. WTAP expression is inhibited by overexpressed ING2. Rescue experiments show that WTAP downregulation and overexpression partially mitigate the effects of ING2 knockdown and overexpression on tumor cell proliferation, respectively [81]. However, further analysis of the specific regulatory mechanisms between WTAP and EMT is yet to be unraveled [81]. Weng et al. found that the lncRNA PCGEM1 was overexpressed in NSCLC cells and combined with miR-433-3p to upregulate WTAP expression, promoting the NSCLC malignant phenotype [73]. Furthermore, WTAP overexpression reversed the inhibitory effects of lncRNA PCGEM1 silencing on tumor cell proliferation, migration, and invasion [73]. Using bioinformatics, WTAP can be used as an independent prognostic favorable factor for squamous lung cancer; however, validated clinical follow-up data are lacking to support this view [132].
5.6. Other Tumors
5.6.1. Osteosarcoma
Osteosarcoma is one of the most common orthopedic malignancies, with a 5-year survival rate of less than 20% after developing metastases [133]. Elevated WTAP in osteosarcoma is associated with poor prognosis and is an independent prognostic risk factor for patients with osteosarcoma [134]. In vitro and in vivo experiments have demonstrated that WTAP acts as an oncogene in osteosarcoma, promoting cell proliferation, invasion, and migration [134]. Further studies have indicated that WTAP inhibits homeobox-containing 1 (HMBOX1) expression by regulating HMBOX1 m6A modification at its 3′ UTR in an m6A-dependent manner [134]. In osteosarcoma, low HMBOX1 expression predicts poor overall survival and is involved in WTAP-mediated proliferation and metastasis of osteosarcoma. The maintenance of this malignant feature is mediated in part by WTAP/HMBOX1 through the PI3K/AKT signaling pathway [134]. Ren et al. revealed that lncRNA FOXD2-AS1 is overexpressed in osteosarcoma and is associated with poor prognosis. Functional studies have shown that lncRNA FOXD2-AS1 promotes tumor migration, proliferation, and growth in vivo and in vitro [135]. Mechanistically WTAP enhances the stability of lncRNA FOXD2-AS1 through m6A modification and upregulates its expression. Overexpression of lncRNA FOXD2-AS1 promotes the progression of osteosarcoma by targeting forkhead box M1 (FOXM1) [135]. Additionally, lncRNA SNHG10 is overexpressed in osteosarcoma, and silencing lncRNA SNHG10 decreases proliferation, migration, and invasion and accelerates apoptosis of osteosarcoma cells. Mechanistically, lncRNA SNHG10 competes as ceRNA to bind miR-141-3p and upregulates WTAP expression to maintain the malignant features of osteosarcoma. lncRNA SNHG10 silencing leads to the inhibition of cell proliferation, migration, and invasion and the promotion of apoptosis. These phenotypes were rescued upon WTAP overexpression [76].
5.6.2. Glioma
The most common malignant primary brain tumor in adults is glioma [136]. WTAP was shown to be overexpressed in glioblastoma [137,138]. In addition, significantly higher WTAP expression is present in high-grade glioma tissues (grades III-IV) than in low-grade glioma tissues (grades I-II). Prognostic analysis showed significantly lower survival in the WTAP high-expression group than in the WTAP low-expression group in low-grade glioma subtypes and high-grade glioma subtypes [138]. Further studies revealed that WTAP overexpression promoted the proliferation, invasion, and migration of glioblastoma cells. Mechanistically, WTAP overexpression enhanced epidermal growth factor signaling-induced EGFR phosphorylation to promote the migration and invasion of glioblastoma cells, whereas WTAP knockdown or overexpression had no significant effect on total EGFR [137]. In addition, dysregulation of WTAP affected the expression of many hub genes involved in cancer cell migration, such as matrix metallopeptidase 3 (MMP3), thrombospondin 1 (THBS1), C-C motif chemokine ligand 2 (CCL2), C-C motif chemokine ligand 3 (CCL3), lysyl oxidase like 1 (LOXL1), and hyaluronan synthase 1 (HAS1) [137].

Table 2.
Molecular regulatory mechanisms of WTAP in tumors.
Table 2.
Molecular regulatory mechanisms of WTAP in tumors.
| Cancer Type | Upstream Regulators | Downstream Targets | Mechanism | m6A | Target Pathways | Cellular Phenotypes | Reference | |
|---|---|---|---|---|---|---|---|---|
| Blood system | AML | / | MYC | Affecting the half-life of MYC mRNA | Yes | PI3K/AKT signaling pathway | Proliferation, apoptosis, and drug resistance | [90] |
| miR-550-1 | WWTR1 | Increased WWTR1 stability | Yes | / | Proliferation and apoptosis | [91] | ||
| DLBCL | Hsp90 | / | Stabilized WTAP expression | No | / | Proliferation, apoptosis, and drug resistance | [79] | |
| NKTCL | / | DUSP6 | Increased DUSP6 stability | Yes | / | Proliferation, apoptosis, and drug resistance | [95] | |
| Digestive System | Esophageal cancer | lncRNA EMS/miR-758-3p | / | Upregulated WTAP expression | No | / | Invasion, metastasis, and drug resistance | [75] |
| Gastric cancer | / | HK2 | Increased HK2 stability | Yes | / | Glucose metabolism | [100] | |
| Liver cancer | / | ETS1 | Decreased ETS1 stability | Yes | / | Proliferation and apoptosis | [53] | |
| / | LKB1 | Affecting LKB1 stability | Yes | AMPK signaling pathway | Autophagy | [105] | ||
| LINC00839/miR-144-3p | / | Upregulated WTAP expression | No | / | Proliferation | [74] | ||
| miR-139-5p | / | Upregulated WTAP expression | No | EMT signaling | Proliferation and invasion | [68] | ||
| Pancreatic cancer | / | Fak | Stabilized Fak expression | No | Fak signaling pathway | Migration, invasion, and drug resistance | [109] | |
| lncRNA DUXAP8/miR-448 | / | Upregulated WTAP expression | No | Fak signaling pathway | Proliferation and invasion | [77] | ||
| WTAPP1 | / | Increased WTAP stability | Yes | Wnt signaling pathway | Proliferation and invasion | [80] | ||
| CRC | / | CA4 | Affecting CA4 stability | No | / | Proliferation and apoptosis | [114] | |
| CA4 | / | Polyubiquitination inhibits WTAP protein degradation | No | Wnt/β-linked signaling pathway | Tumor progression | [114] | ||
| / | UCA1 | Affecting UCA1 stability | Yes | / | Proliferation | [115] | ||
| ARRB2 | / | / | / | / | Proliferation and migration | [82] | ||
| Reproductive system | Breast cancer | IL1β/TNFα activates ERK1/2 signaling | ENO1 | Upregulated ENO1 expression | Yes | / | Glycolysis | [83] |
| / | lncRNA DLGAP1-AS1 | Increased lncRNA DLGAP1-AS1 stability | Yes | / | Proliferation and drug resistance | [78] | ||
| lncRNA DLGAP1-AS1/miR-299-3p | / | Upregulated WTAP expression | No | / | / | [78] | ||
| Endometrial cancer | / | GSK3β | Promoting GSK3β phosphorylation | No | Wnt/β-catenin | Apoptosis and drug resistance | [120] | |
| / | CAV-1 | Decreased CAV-1 stability | Yes | NF-κB signaling pathway | Proliferation, migration, and invasion | [121] | ||
| Urinary system | Renal cell carcinoma | / | CDK2 | Increased CDK2 stability | No | / | Proliferation and invasion | [126] |
| miR-501-3p | / | Upregulated WTAP expression | No | / | Proliferation | [67] | ||
| Bladder cancer | circ0008399 | TNFAIP3 | Increased TNFAIP3 stability | Yes | / | Apoptosis and drug resistance | [129] | |
| Respiratory system | Nasopharyngeal cancer | KAT3A mediates the acetylation of H3K27 | lncRNA DIAPH1-AS1 | Increased lncRNA DIAPH1-AS1 stability | Yes | / | Growth and metastasis | [84] |
| Lung cancer | ING2 | / | Affecting ING2 expression | / | EMT signaling | Proliferation and apoptosis | [81] | |
| lncRNA PCGEM1/miR-433-3p | / | Upregulated WTAP expression | No | / | Proliferation, migration, and invasion | [73] | ||
| Other tumors | Osteosarcoma | / | HMBOX1 | Inhibition of HMBOX1 expression | Yes | PI3K/AKT signaling pathway | Proliferation, migration, and invasion | [134] |
| / | LncRNA FOXD2-AS1 | Increased lncRNA FOXD2-AS1 stability | Yes | / | Proliferation and invasion | [135] | ||
| lncRNA SNHG10/miR-141-3p | / | Upregulated WTAP expression | No | / | Proliferation, migration, and invasion and apoptosis | [76] | ||
| Glioma | / | / | Enhanced EGFR phosphorylation | No | / | Proliferation, migration, and invasion | [137] | |
AML: Acute myeloid leukemia; DLBCL: diffuse large B-cell lymphoma; NKTCL: NK/T-cell lymphoma; CRC: colorectal cancer; PI3K: phosphatidylinositol 3-kinase; AKT: protein serine-threonine kinase; AMPK: Adenosine 5′-monophosphate activated protein kinas; EMT: epithelial–mesenchymal transition.
6. Targeting WTAP for Potential Clinical Prospects
m6A modifications are commonly found in eukaryotic transcriptional regulation and are involved in the progression of many cancers, and the use of m6A regulators as tumor therapeutic targets provides new ideas for tumor treatment [6,139,140]. mTOR signaling regulates various biological processes such as cell metabolism, immunity, growth, and autophagy [141], and its dysfunction plays an important role in a variety of cancers; thus, mTOR is considered as one of the potential targets for tumor therapy [142,143]. Research has demonstrated that mTOR can regulate overall m6A modification levels, and WTAP is required for mTOR-dependent m6A modifications [52,87]. Mechanistically, as presented in Table 1, mTORC1 enhances WTAP translation through kinase S6K and regulates cMyc repressor, MAX dimerization protein 2 (MXD2), to inhibit cMyc signaling [52]. As we all know, cMyc is one of the central hubs of tumor progression [144,145]. MXD protein inhibits cMyc activity by competing with the cMyc transcriptional coactivator MAX [146]. Specifically, WTAP inhibits MXD2 expression by m6A modification, leading to increased binding of cMyc to MAX, which promotes cMyc transcriptional activity and mTORC1-activated cancer cell proliferation [52]. Recent studies have revealed that METTL3 inhibitors can effectively inhibit the progression of AML, representing the beginning of a new era in cancer treatment [147]. Thus, WTAP inhibitors can increase MXD2 expression, leading to reduced cMyc binding to MAX, thereby inhibiting mTORC1-activated cancer cell proliferation. In summary, the WTAP-MXD2-cMyc axis is a potential therapeutic target for mTORC1-driven cancers (Figure 5).
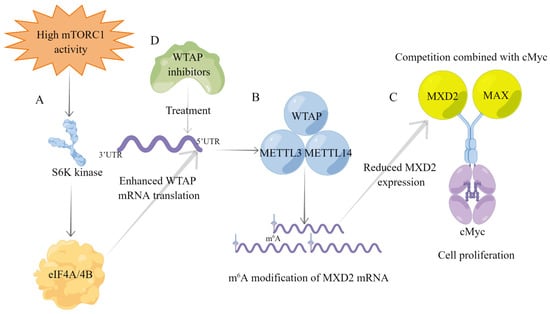
Figure 5.
Overview of the mTORC1-WTAP-MXD2-cMyc axis for cancer progression. (A) Upregulation of mTORC1 signaling in tumors enhances WTAP mRNA translation via downstream S6K kinase. (B) The MMW complex reduces MXD2 expression by m6A modification of MXD2 mRNA. (C) MXD2 competes with MAX to bind cMyc. The decreased MXD2 expression leads to increased MAX binding to cMyc, thereby promoting tumor cell proliferation. (D) WTAP inhibitors inhibit WTAP expression and reduce MXD2 m6A modification, thereby inhibiting mTORC1 signaling to activate cancer cell proliferation.
Chemotherapy and targeted and immune therapies have achieved remarkable results in the clinical treatment of tumors. However, the development of drug resistance has greatly limited the clinical benefits of these treatments [148,149,150]. In recent years, the involvement of m6A in tumor regulation has received wide attention, and its induction of tumor drug resistance also provides new directions for tumor therapy [151,152,153]. WTAP enhances the stability of DUSP6 mRNA in an m6A-dependent manner, thereby promoting the development of resistance to cisplatin in NKTCL cells [95]. Circ0008399 interacts with WTAP in bladder cancer to increase the stability of TNFAIP3 mRNA in an m6A-dependent manner, thereby inducing tumor cell resistance to cisplatin [129]. A growing number of studies suggest that ncRNAs play a key role in tumor drug resistance [154,155,156]. WTAP increases the stability of lncRNA DLGAP1-AS1 in an m6A-dependent manner and induces resistance to Adriamycin in breast cancer cells. Meanwhile, upregulated lncRNA DLGAP1-AS1 enhances tumor cell drug resistance through miR-299-3p/WTAP axis feedback [78]. Under hypoxic conditions, the lncRNA-EMS/miR-758-3p/WTAP regulatory axis can induce cisplatin resistance in esophageal cancer cells [75]. In addition, WTAP can directly regulate the formation of tumor drug resistance. WTAP promotes cisplatin resistance in endometrial cancer by promoting GSK3β phosphorylation at Ser9 to activate the Wnt/β-linked protein pathway [120]. In pancreatic cancer, WTAP promotes chemoresistance to gemcitabine by stabilizing Fak mRNA through direct binding to Fak mRNA [109]. In conclusion, WTAP can mediate tumor resistance formation, both dependent and independent of its role in m6A modification of methyltransferases. Therefore, WTAP has high clinical therapeutic promise in tumor therapy.
7. Conclusions and Perspectives
Malignant tumors have eight biological characteristics, which are resistance to cell death, maintenance of proliferative signals, achievement of replicative immortality, evasion of growth inhibitors, activation of invasion and metastasis, induction/entry into the vasculature, acquiring the ability to avoid immune destruction, and reprogramming of cellular metabolism [157]. Gene epigenetics regulates gene expression mainly at DNA modification, chromatin reorganization, noncoding RNA regulation, and histone modification, which play an essential role in maintaining oncological hallmarks [2,64,158]. With research on enzymes that regulate protein and DNA modifications, it has been discovered that many of these modifying enzymes can be used as targets for cancer therapy [2,139]. m6A is the most abundant form of modification on coding and noncoding RNA in eukaryotes [6]. Methyltransferases, such as METTL3 and METTL14, have received much attention because they can catalyze substrate RNA modification, thus participating in tumor development and providing potential targets for tumor therapy [27,28]. WTAP, as one of the core components of MTC, is involved in the development of multiple cancers and is therefore expected to be a potential therapeutic target for tumors.
In this article, we describe the structural characteristics of WTAP and its involvement in regulating the development of various tumors. On the one hand, WTAP acts as a methyltransferase adaptor to catalyze the substrate RNA and affects the stability of RNA, thus participating in the regulation of tumor development; on the other hand, WTAP is involved in tumor development through upstream regulation or targeting of downstream targets. Therefore, WTAP can be considered a core target for tumor treatment. However, there are no reports yet on the regulatory mechanisms of WTAP in tumor immunity, one of the tumor hallmarks. Studies have revealed that the tumor microenvironment of patients can be classified into ‘immunoinflammatory’, ‘immune evasion’, and ‘immunodesert’ based on m6A-regulated gene expression by unsupervised clustering analysis [159,160,161]. Different immune-system phenotypes have different immunotherapeutic effects, and choosing the appropriate treatment plan according to different phenotypes is in line with the modern concept of precision therapy. Therefore, it is a good direction for follow-up studies to focus on the role of WTAP in tumor microenvironment.
Additionally, the tumor microenvironment is a complex system, and epigenomics is one of the factors influencing its complexity [157,162]. Studies have established that genes can act as both cancer suppressors and cancer promoters. P53 is an oncogene with a broad and powerful function, and more than half of tumor patients carry P53 mutations, making it a popular target for oncology treatment. However, recent studies have revealed that p53 also possesses a cancer-promoting role [163,164]. Previous articles also advocated that the methyltransferases METTL3 and METTL14 possess both cancer-promoting and cancer-inhibiting effects [27,28]. WTAP, as the main component of methyltransferase complex, has a strong correlation with METTL3 and METTL14; however, we have not found any articles on the role of WTAP as a cancer suppressor, which may be a new direction for future research.
In conclusion, WTAP plays an essential role in various tumors, either dependent or independent of its methyltransferase activity. Research on WTAP is expected to bring new hope for tumor treatment. However, WTAP has been less studied in terms of antitumor immunity, tumor microenvironment, and oncogenic effects, and future studies targeting these aspects would be a good direction.
Author Contributions
Y.F.: conceptualization, writing—original draft preparation; X.L. and H.S.: data curation, visualization; Z.G. and Z.Z.: writing—review and editing; K.Y.: supervision, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research project was supported by the following funds: Changzhou Sci & Tech Program (Grant number: CZ20220025); “333 Project” of Jiangsu Province (Grant number: BRA2020157); 333 High-Level Talent Training Project (Grant number: 2022-2); Supported by Changzhou Sci & Tech Program (Grant number: CJ20210060); “Six One Project”, Research Projects of High-level Medical Personnel of Jiangsu Province (Grant number: LGY2019025); High-level Talent Selection and Training Project of the 16th Batch of “Six Talent Peak” in Jiangsu Province (Grant number: WSN-245); Medical Scientific Research Foundation of Jiangsu Commission of Health (Grant number: H2018083); Jiangsu Provincial Medical Youth Talent (Jiangsu Health Scientific Education 2017 no.3); High-Level Medical Talents Training Project (Grant number: 2016CZBJ042).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that there is no conflict of interest in this study.
Abbreviations
WTAP: Wilms’ tumor 1-associating protein; SAH: S-adenosyl homocysteine; METTL16: methyltransferase-like 16; SAM: S-adenosylmethionine; snRNA: small nuclear RNA; MAT2A: methionine adenosyltransferase 2A; VIRMA: vir-like m6A methyltransferase associated; nTPM: normalized expression; lncRNAs: long noncoding RNAs; RBM15: RNA-binding motif protein 15; m6A: N6-methyladenosine; mRNAs: messenger RNAs; METTL3: methyltransferase-like 3; PI3K: phosphatidylinositol 3-kinase; ZC3H13: zinc finger CCCH-type-containing 13; mRNAs: messenger RNAs; MMW: METTL3-METTL14-WTAP; MTC: methyltransferase complex; PTMs: post-transcriptional modifications; METTL14: methyltransferase-like 14; m1A: N 1-methyladenosine; ac4C: N 4-acetylcytidine; Ψ: pseudouridine; m5C: 5-methylcytidine; m7G: N 7-methylguanosine; RBM15B: RNA-binding motif protein 15B; LASP1: LIM and SH3 protein 1; MMP3: matrix metallopeptidase 3; HMBOX1: homeobox-containing 1; ZCCHC4: CCHC zinc finger-containing protein; rRNA: ribosomal RNA; SRSF3: serine- and arginine-rich splicing factor 3; NLS: nuclear localization signals; METTL5: methyltransferase-like 5; MTD: methyltransferase domain; CDS: coding sequence; FMR1: fragile X mental retardation 1; SRSF1: serine- and arginine-rich splicing factor 1; BCLAF1: BCL2-associated transcription factor 1; hnRNPs: heterogeneous nuclear ribonucleoprotein; ncRNAs: noncoding RNAs; CBLL1: Cbl proto-oncogene like 1; FXR2: fragile X mental retardation syndrome-related protein 2; miRNAs: microRNAs; DLBCL: diffuse large B-cell lymphoma; ceRNA: competing endogenous RNA; piRNAs: piwi-interacting RNAs; Hsp90: heat shock protein 90; DFS: disease-free survival; OS: overall survival; WTAPP1: Wilms tumor 1-associated protein pseudogene 1; ERK1: Extracellular signal-regulated kinase 1; ARBB2: arrestin beta 2; EBVaGC: Epstein–Barr virus-associated gastric carcinoma; ERK2: extracellular signal-regulated kinase 2; KAT3A: CREB-binding protein; EBER1: EBV-encoded small RNA1; NF-κB: nuclear factor kappa-B; WWTR1: WW domain-containing transcription regulator 1; mTORC1: mechanistic target of rapamycin complex 1; AML: acute myeloid leukemia; ING2: inhibitor of growth family member 2; AKT: protein serine-threonine kinase; BCL6: B-cell lymphoma 6; AMPK: adenosine 5‘-monophosphate-activated protein kinase; DUSP6: dual-specificity phosphatase 6; HK2: hexokinase 2; ETS1: ETS proto-oncogene 1; NKTCL: NK/T-cell lymphoma; LKB1: liver kinase B1; Fak: focal adhesion kinase; CNBP: zinc finger nucleic-acid-binding protein; CRC: colorectal cancer; CA4: carbonic anhydrase IV; UCA1: urothelial cancer-associated 1; Mcl-1: myeloid cell leukemin-1; MMP7: matrix metallopeptidase 7; MMP28: matrix metallopeptidase 28; Muc1: mucin 1; IL1β: interleukin-1β; TNFα: tumor necrosis factor-α; ENO1: enolase 1; BAX: BCL2-associated X; Ser9: GSK-3β; CAV-1: caveolin 1; HBS1L: HBS1-like translational GTPase; IGF2BP2: insulin-like growth factor 2 mRNA-binding protein 2; FAM76A: family with sequence similarity 76 member A; CDK2: cyclin-dependent kinase 2; CCL2: C-C motif chemokine ligand 2; TNFAIP3: TNF alpha-induced protein 3; FOXM1: forkhead box M1; CCL3: C-C motif chemokine ligand 3; HAS1: hyaluronan synthase 1; NSCLC: non-small-cell lung cancer; LOXL1: lysyl oxidase-like 1; MXD2: MAX dimerization protein 2; THBS1: thrombospondin 1; FXR1: fragile X mental retardation syndrome-related protein 1; GSK3β: glycogen synthase kinase 3β; pLDDT: per-residue confidence score; THRAP3: thyroid hormone receptor associated protein 3; EIF3: eukaryotic translation initiation factor 3; PARP: poly ADP-ribose polymerase; EMT: epithelial–mesenchymal transition.
References
- Wiener, D.; Schwartz, S. The epitranscriptome beyond m6A. Nat. Rev. Genet. 2021, 22, 119–131. [Google Scholar] [CrossRef]
- Barbieri, I.; Kouzarides, T. Role of RNA modifications in cancer. Nat. Rev. Cancer 2020, 20, 303–322. [Google Scholar] [CrossRef]
- Boccaletto, P.; Stefaniak, F.; Ray, A.; Cappannini, A.; Mukherjee, S.; Purta, E.; Kurkowska, M.; Shirvanizadeh, N.; Destefanis, E.; Groza, P.; et al. MODOMICS: A database of RNA modification pathways. 2021 update. Nucleic Acids Res. 2022, 50, D231–D235. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Song, J.; Liu, Y.; Song, C.X.; Yi, C. Mapping the epigenetic modifications of DNA and RNA. Protein Cell 2020, 11, 792–808. [Google Scholar] [CrossRef]
- Desrosiers, R.; Friderici, K.; Rottman, F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc. Natl. Acad. Sci. USA 1974, 71, 3971–3975. [Google Scholar] [CrossRef]
- Zheng, H.X.; Zhang, X.S.; Sui, N. Advances in the profiling of N(6)-methyladenosine (m6A) modifications. Biotechnol. Adv. 2020, 45, 107656. [Google Scholar] [CrossRef]
- Meyer, K.D.; Saletore, Y.; Zumbo, P.; Elemento, O.; Mason, C.E.; Jaffrey, S.R. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell 2012, 149, 1635–1646. [Google Scholar] [CrossRef]
- Dominissini, D.; Moshitch-Moshkovitz, S.; Schwartz, S.; Salmon-Divon, M.; Ungar, L.; Osenberg, S.; Cesarkas, K.; Jacob-Hirsch, J.; Amariglio, N.; Kupiec, M.; et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 2012, 485, 201–206. [Google Scholar] [CrossRef]
- An, Y.; Duan, H. The role of m6A RNA methylation in cancer metabolism. Mol. Cancer 2022, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Gregory, R.I. RNAmod: An integrated system for the annotation of mRNA modifications. Nucleic Acids Res. 2019, 47, W548–W555. [Google Scholar] [CrossRef] [Green Version]
- Zaccara, S.; Ries, R.J.; Jaffrey, S.R. Reading, writing and erasing mRNA methylation. Nat. Rev. Mol. Cell Biol. 2019, 20, 608–624. [Google Scholar] [CrossRef]
- Yang, Y.; Hsu, P.J.; Chen, Y.S.; Yang, Y.G. Dynamic transcriptomic m6A decoration: Writers, erasers, readers and functions in RNA metabolism. Cell Res. 2018, 28, 616–624. [Google Scholar] [CrossRef]
- He, L.; Li, H.; Wu, A.; Peng, Y.; Shu, G.; Yin, G. Functions of N6-methyladenosine and its role in cancer. Mol. Cancer 2019, 18, 176. [Google Scholar] [CrossRef]
- Wang, T.; Kong, S.; Tao, M.; Ju, S. The potential role of RNA N6-methyladenosine in Cancer progression. Mol. Cancer 2020, 19, 88. [Google Scholar] [CrossRef]
- Zhou, Z.; Lv, J.; Yu, H.; Han, J.; Yang, X.; Feng, D.; Wu, Q.; Yuan, B.; Lu, Q.; Yang, H. Mechanism of RNA modification N6-methyladenosine in human cancer. Mol. Cancer 2020, 19, 104. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, S.; Wu, X.; Zhou, X. m6A RNA Methylation in Cardiovascular Diseases. Mol. Ther. 2020, 28, 2111–2119. [Google Scholar] [CrossRef]
- Zhang, B.; Jiang, H.; Dong, Z.; Sun, A.; Ge, J. The critical roles of m6A modification in metabolic abnormality and cardiovascular diseases. Genes Dis. 2021, 8, 746–758. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Li, J.; Zhao, B.; Huang, G.; Li, X.; Xie, Z.; Zhou, Z. The Emerging Role of m6A Modification in Regulating the Immune System and Autoimmune Diseases. Front. Cell Dev. Biol. 2021, 9, 755691. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, B.; Nie, Z.; Duan, L.; Xiong, Q.; Jin, Z.; Yang, C.; Chen, Y. The role of m6A modification in the biological functions and diseases. Signal Transduct. Target. Ther. 2021, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Little, N.A.; Hastie, N.D.; Davies, R.C. Identification of WTAP, a novel Wilms’ tumour 1-associating protein. Hum. Mol. Genet. 2000, 9, 2231–2239. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.; Gong, Y.; Shen, L.; Li, J.; Han, J.; Song, B.; Hu, L.; Wang, Q.; Wang, Z. Total Panax notoginseng saponin inhibits vascular smooth muscle cell proliferation and migration and intimal hyperplasia by regulating WTAP/p16 signals via m6A modulation. Biomed. Pharmacother. 2020, 124, 109935. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Ma, Y.; Zeng, Y.; Lu, C.; Yang, F.; Jiang, N.; Zhang, X.; Wang, Y.; Xu, Y.; et al. WTAP promotes myocardial ischemia/reperfusion injury by increasing endoplasmic reticulum stress via regulating m6A modification of ATF4 mRNA. Aging 2021, 13, 11135–11149. [Google Scholar] [CrossRef]
- Wang, L.J.; Xue, Y.; Li, H.; Huo, R.; Yan, Z.; Wang, J.; Xu, H.; Wang, J.; Cao, Y.; Zhao, J.Z. Wilms’ tumour 1-associating protein inhibits endothelial cell angiogenesis by m6A-dependent epigenetic silencing of desmoplakin in brain arteriovenous malformation. J. Cell Mol. Med. 2020, 24, 4981–4991. [Google Scholar] [CrossRef]
- Li, G.; Ma, L.; He, S.; Luo, R.; Wang, B.; Zhang, W.; Song, Y.; Liao, Z.; Ke, W.; Xiang, Q.; et al. WTAP-mediated m6A modification of lncRNA NORAD promotes intervertebral disc degeneration. Nat. Commun. 2022, 13, 1469. [Google Scholar] [CrossRef]
- Kong, Y.; Wu, R.; Zhang, S.; Zhao, M.; Wu, H.; Lu, Q.; Fu, S.; Su, Y. Wilms’ tumor 1-associating protein contributes to psoriasis by promoting keratinocytes proliferation via regulating cyclinA2 and CDK2. Int. Immunopharmacol. 2020, 88, 106918. [Google Scholar] [CrossRef]
- Huang, W.; Chen, T.Q.; Fang, K.; Zeng, Z.C.; Ye, H.; Chen, Y.Q. N6-methyladenosine methyltransferases: Functions, regulation, and clinical potential. J. Hematol. Oncol. 2021, 14, 117. [Google Scholar] [CrossRef]
- Zhou, H.; Yin, K.; Zhang, Y.; Tian, J.; Wang, S. The RNA m6A writer METTL14 in cancers: Roles, structures, and applications. Biochim. Biophys. Acta. Rev. Cancer 2021, 1876, 188609. [Google Scholar] [CrossRef]
- Zeng, C.; Huang, W.; Li, Y.; Weng, H. Roles of METTL3 in cancer: Mechanisms and therapeutic targeting. J. Hematol. Oncol. 2020, 13, 117. [Google Scholar] [CrossRef]
- Oerum, S.; Meynier, V.; Catala, M.; Tisné, C. A comprehensive review of m6A/m6Am RNA methyltransferase structures. Nucleic Acids Res. 2021, 49, 7239–7255. [Google Scholar] [CrossRef]
- Bokar, J.A.; Rath-Shambaugh, M.E.; Ludwiczak, R.; Narayan, P.; Rottman, F. Characterization and partial purification of mRNA N6-adenosine methyltransferase from HeLa cell nuclei. Internal mRNA methylation requires a multisubunit complex. J. Biol. Chem. 1994, 269, 17697–17704. [Google Scholar]
- Liu, J.; Yue, Y.; Han, D.; Wang, X.; Fu, Y.; Zhang, L.; Jia, G.; Yu, M.; Lu, Z.; Deng, X.; et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat. Chem. Biol. 2014, 10, 93–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schöller, E.; Weichmann, F.; Treiber, T.; Ringle, S.; Treiber, N.; Flatley, A.; Feederle, R.; Bruckmann, A.; Meister, G. Interactions, localization, and phosphorylation of the m6A generating METTL3-METTL14-WTAP complex. Rna 2018, 24, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Liu, J.; Cui, X.; Cao, J.; Luo, G.; Zhang, Z.; Cheng, T.; Gao, M.; Shu, X.; Ma, H.; et al. VIRMA mediates preferential m6A mRNA methylation in 3′UTR and near stop codon and associates with alternative polyadenylation. Cell Discov. 2018, 4, 10. [Google Scholar] [CrossRef]
- Wen, J.; Lv, R.; Ma, H.; Shen, H.; He, C.; Wang, J.; Jiao, F.; Liu, H.; Yang, P.; Tan, L.; et al. Zc3h13 Regulates Nuclear RNA m6A Methylation and Mouse Embryonic Stem Cell Self-Renewal. Mol. Cell 2018, 69, 1028–1038.e1026. [Google Scholar] [CrossRef] [PubMed]
- Patil, D.P.; Chen, C.K.; Pickering, B.F.; Chow, A.; Jackson, C.; Guttman, M.; Jaffrey, S.R. m6A RNA methylation promotes XIST-mediated transcriptional repression. Nature 2016, 537, 369–373. [Google Scholar] [CrossRef]
- Knuckles, P.; Lence, T.; Haussmann, I.U.; Jacob, D.; Kreim, N.; Carl, S.H.; Masiello, I.; Hares, T.; Villaseñor, R.; Hess, D.; et al. Zc3h13/Flacc is required for adenosine methylation by bridging the mRNA-binding factor Rbm15/Spenito to the m6A machinery component Wtap/Fl(2)d. Genes Dev. 2018, 32, 415–429. [Google Scholar] [CrossRef]
- Bokar, J.A.; Shambaugh, M.E.; Polayes, D.; Matera, A.G.; Rottman, F.M. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. Rna 1997, 3, 1233–1247. [Google Scholar]
- Wang, P.; Doxtader, K.A.; Nam, Y. Structural Basis for Cooperative Function of Mettl3 and Mettl14 Methyltransferases. Mol. Cell 2016, 63, 306–317. [Google Scholar] [CrossRef]
- Bujnicki, J.M.; Feder, M.; Radlinska, M.; Blumenthal, R.M. Structure prediction and phylogenetic analysis of a functionally diverse family of proteins homologous to the MT-A70 subunit of the human mRNA:m6A methyltransferase. J. Mol. Evol. 2002, 55, 431–444. [Google Scholar] [CrossRef]
- Wang, X.; Huang, J.; Zou, T.; Yin, P. Human m6A writers: Two subunits, 2 roles. RNA Biol. 2017, 14, 300–304. [Google Scholar] [CrossRef]
- Zhou, K.I.; Pan, T. Structures of the m6A Methyltransferase Complex: Two Subunits with Distinct but Coordinated Roles. Mol. Cell 2016, 63, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Ping, X.L.; Sun, B.F.; Wang, L.; Xiao, W.; Yang, X.; Wang, W.J.; Adhikari, S.; Shi, Y.; Lv, Y.; Chen, Y.S.; et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014, 24, 177–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoyama, T.; Yamashita, S.; Tomita, K. Mechanistic insights into m6A modification of U6 snRNA by human METTL16. Nucleic Acids Res. 2020, 48, 5157–5168. [Google Scholar] [CrossRef]
- van Tran, N.; Ernst, F.G.M.; Hawley, B.R.; Zorbas, C.; Ulryck, N.; Hackert, P.; Bohnsack, K.E.; Bohnsack, M.T.; Jaffrey, S.R.; Graille, M.; et al. The human 18S rRNA m6A methyltransferase METTL5 is stabilized by TRMT112. Nucleic Acids Res. 2019, 47, 7719–7733. [Google Scholar] [CrossRef]
- Ma, H.; Wang, X.; Cai, J.; Dai, Q.; Natchiar, S.K.; Lv, R.; Chen, K.; Lu, Z.; Chen, H.; Shi, Y.G.; et al. N(6-)Methyladenosine methyltransferase ZCCHC4 mediates ribosomal RNA methylation. Nat. Chem. Biol. 2019, 15, 88–94. [Google Scholar] [CrossRef]
- Warda, A.S.; Kretschmer, J.; Hackert, P.; Lenz, C.; Urlaub, H.; Höbartner, C.; Sloan, K.E.; Bohnsack, M.T. Human METTL16 is a N(6)-methyladenosine (m6A) methyltransferase that targets pre-mRNAs and various non-coding RNAs. EMBO Rep. 2017, 18, 2004–2014. [Google Scholar] [CrossRef]
- Pendleton, K.E.; Chen, B.; Liu, K.; Hunter, O.V.; Xie, Y.; Tu, B.P.; Conrad, N.K. The U6 snRNA m6A Methyltransferase METTL16 Regulates SAM Synthetase Intron Retention. Cell 2017, 169, 824–835.e814. [Google Scholar] [CrossRef]
- Watabe, E.; Togo-Ohno, M.; Ishigami, Y.; Wani, S.; Hirota, K.; Kimura-Asami, M.; Hasan, S.; Takei, S.; Fukamizu, A.; Suzuki, Y.; et al. m(6) A-mediated alternative splicing coupled with nonsense-mediated mRNA decay regulates SAM synthetase homeostasis. EMBO J. 2021, 40, e106434. [Google Scholar] [CrossRef]
- Doxtader, K.A.; Wang, P.; Scarborough, A.M.; Seo, D.; Conrad, N.K.; Nam, Y. Structural Basis for Regulation of METTL16, an S-Adenosylmethionine Homeostasis Factor. Mol. Cell 2018, 71, 1001–1011.e1004. [Google Scholar] [CrossRef]
- Pinto, R.; Vågbø, C.B.; Jakobsson, M.E.; Kim, Y.; Baltissen, M.P.; O’Donohue, M.F.; Guzmán, U.H.; Małecki, J.M.; Wu, J.; Kirpekar, F.; et al. The human methyltransferase ZCCHC4 catalyses N6-methyladenosine modification of 28S ribosomal RNA. Nucleic Acids Res. 2020, 48, 830–846. [Google Scholar] [CrossRef]
- Ren, W.; Lu, J.; Huang, M.; Gao, L.; Li, D.; Wang, G.G.; Song, J. Structure and regulation of ZCCHC4 in m6A-methylation of 28S rRNA. Nat. Commun. 2019, 10, 5042. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Lee, G.; Pickering, B.F.; Jang, C.; Park, J.H.; He, L.; Mathur, L.; Kim, S.S.; Jung, S.; Tang, H.W.; et al. mTORC1 promotes cell growth via m6A-dependent mRNA degradation. Mol. Cell 2021, 81, 2064–2075.e2068. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Peng, C.; Chen, J.; Chen, D.; Yang, B.; He, B.; Hu, W.; Zhang, Y.; Liu, H.; Dai, L.; et al. WTAP facilitates progression of hepatocellular carcinoma via m6A-HuR-dependent epigenetic silencing of ETS1. Mol. Cancer 2019, 18, 127. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Ohsugi, M.; Sasako, T.; Awazawa, M.; Umehara, T.; Iwane, A.; Kobayashi, N.; Okazaki, Y.; Kubota, N.; Suzuki, R.; et al. The RNA Methyltransferase Complex of WTAP, METTL3, and METTL14 Regulates Mitotic Clonal Expansion in Adipogenesis. Mol. Cell Biol. 2018, 38, e00116-18. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, K.; Umetani, M.; Minami, T.; Okayama, H.; Takada, S.; Yamamoto, M.; Aburatani, H.; Reid, P.C.; Housman, D.E.; Hamakubo, T.; et al. Wilms’ tumor 1-associating protein regulates G2/M transition through stabilization of cyclin A2 mRNA. Proc. Natl. Acad. Sci. USA 2006, 103, 17278–17283. [Google Scholar] [CrossRef]
- Horiuchi, K.; Kawamura, T.; Hamakubo, T. Wilms’ tumor 1-associating protein complex regulates alternative splicing and polyadenylation at potential G-quadruplex-forming splice site sequences. J. Biol. Chem. 2021, 297, 101248. [Google Scholar] [CrossRef]
- Horiuchi, K.; Kawamura, T.; Iwanari, H.; Ohashi, R.; Naito, M.; Kodama, T.; Hamakubo, T. Identification of Wilms’ tumor 1-associating protein complex and its role in alternative splicing and the cell cycle. J. Biol. Chem. 2013, 288, 33292–33302. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H.; Zhao, X.; Luo, Q.; Wang, Q.; Tan, K.; Wang, Z.; Jiang, J.; Cui, J.; Du, E.; et al. Arginine methylation of METTL14 promotes RNA N(6)-methyladenosine modification and endoderm differentiation of mouse embryonic stem cells. Nat. Commun. 2021, 12, 3780. [Google Scholar] [CrossRef]
- Hao, J.; Huang, S.; Wang, D.; Jin, Y.; Zhang, M.; Zhang, J.; Yu, X. Loss of WTAP Impairs Early Parthenogenetic Embryo Development. Animals 2021, 11, 1675. [Google Scholar] [CrossRef]
- Wang, E.T.; Sandberg, R.; Luo, S.; Khrebtukova, I.; Zhang, L.; Mayr, C.; Kingsmore, S.F.; Schroth, G.P.; Burge, C.B. Alternative isoform regulation in human tissue transcriptomes. Nature 2008, 456, 470–476. [Google Scholar] [CrossRef]
- Saitoh, N.; Spahr, C.S.; Patterson, S.D.; Bubulya, P.; Neuwald, A.F.; Spector, D.L. Proteomic analysis of interchromatin granule clusters. Mol. Biol. Cell 2004, 15, 3876–3890. [Google Scholar] [CrossRef] [PubMed]
- Lamond, A.I.; Spector, D.L. Nuclear speckles: A model for nuclear organelles. Nat. Rev. Mol. Cell Biol. 2003, 4, 605–612. [Google Scholar] [CrossRef]
- Anastasiadou, E.; Jacob, L.S.; Slack, F.J. Non-coding RNA networks in cancer. Nat. Rev. Cancer 2018, 18, 5–18. [Google Scholar] [CrossRef]
- Goodall, G.J.; Wickramasinghe, V.O. RNA in cancer. Nat. Rev. Cancer 2021, 21, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Xu, Z.Y.; Ruan, S.M.; Mo, S.; Qin, J.J.; Cheng, X.D. Long non-coding RNAs towards precision medicine in gastric cancer: Early diagnosis, treatment, and drug resistance. Mol. Cancer 2020, 19, 96. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Fan, G.; Song, S.; Jiang, Y.; Qian, C.; Zhang, W.; Su, Q.; Xue, X.; Zhuang, W.; Li, B. piRNA-30473 contributes to tumorigenesis and poor prognosis by regulating m6A RNA methylation in DLBCL. Blood 2021, 137, 1603–1614. [Google Scholar] [CrossRef]
- He, L.; Chen, S.; Ying, Y.; Xie, H.; Li, J.; Ma, X.; Wang, W.; Shen, H.; Wang, X.; Zheng, X.; et al. MicroRNA-501-3p inhibits the proliferation of kidney cancer cells by targeting WTAP. Cancer Med. 2021, 10, 7222–7232. [Google Scholar] [CrossRef]
- Liu, W.; Gao, X.; Chen, X.; Zhao, N.; Sun, Y.; Zou, Y.; Guan, Y.; Yang, L.; Pei, X.; Wang, G.; et al. miR-139-5p Loss-Mediated WTAP Activation Contributes to Hepatocellular Carcinoma Progression by Promoting the Epithelial to Mesenchymal Transition. Front. Oncol. 2021, 11, 611544. [Google Scholar] [CrossRef]
- Bai, Q.; Pan, Z.; Nabi, G.; Rashid, F.; Liu, Y.; Khan, S. Emerging role of competing endogenous RNA and associated noncoding RNAs in thyroid cancer. Am. J. Cancer Res. 2022, 12, 961–973. [Google Scholar]
- Wang, L.; Cho, K.B.; Li, Y.; Tao, G.; Xie, Z.; Guo, B. Long Noncoding RNA (lncRNA)-Mediated Competing Endogenous RNA Networks Provide Novel Potential Biomarkers and Therapeutic Targets for Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 5758. [Google Scholar] [CrossRef] [PubMed]
- Abdollahzadeh, R.; Daraei, A.; Mansoori, Y.; Sepahvand, M.; Amoli, M.M.; Tavakkoly-Bazzaz, J. Competing endogenous RNA (ceRNA) cross talk and language in ceRNA regulatory networks: A new look at hallmarks of breast cancer. J. Cell Physiol. 2019, 234, 10080–10100. [Google Scholar] [CrossRef]
- Du, H.; Chen, Y. Competing endogenous RNA networks in cervical cancer: Function, mechanism and perspective. J. Drug Target. 2019, 27, 709–723. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.; Qiu, K.; Gao, W.; Shi, C.; Shu, F. LncRNA PCGEM1 accelerates non-small cell lung cancer progression via sponging miR-433-3p to upregulate WTAP. BMC Pulm. Med. 2020, 20, 213. [Google Scholar] [CrossRef]
- Zhou, X.; Chang, Y.; Zhu, L.; Shen, C.; Qian, J.; Chang, R. LINC00839/miR-144-3p/WTAP (WT1 Associated protein) axis is involved in regulating hepatocellular carcinoma progression. Bioengineered 2021, 12, 10849–10861. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.J.; Pang, Y.; Jin, G.; Zhang, H.Y.; Wang, W.H.; Liu, J.W.; Tuo, G.X.; Wu, P.; Yang, Y.; Wang, Z.Q.; et al. Hypoxia induces chemoresistance of esophageal cancer cells to cisplatin through regulating the lncRNA-EMS/miR-758-3p/WTAP axis. Aging 2021, 13, 17155–17176. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Liu, M.; Zhang, Y.; Xie, L.; Shi, Z.; Wang, G. SNHG10/miR-141-3p/WTAP axis promotes osteosarcoma proliferation and migration. J. Biochem. Mol. Toxicol. 2022, 36, e23031. [Google Scholar] [CrossRef]
- Li, J.R.; Liu, L.; Luo, H.; Chen, Z.G.; Wang, J.H.; Li, N.F. Long Noncoding RNA DUXAP8 Promotes Pancreatic Carcinoma Cell Migration and Invasion Via Pathway by miR-448/WTAP/Fak Signaling Axis. Pancreas 2021, 50, 317–326. [Google Scholar] [CrossRef]
- Huang, T.; Cao, L.; Feng, N.; Xu, B.; Dong, Y.; Wang, M. N(6)-methyladenosine (m6A)-mediated lncRNA DLGAP1-AS1enhances breast canceradriamycin resistance through miR-299-3p/WTAP feedback loop. Bioengineered 2021, 12, 10935–10944. [Google Scholar] [CrossRef]
- Kuai, Y.; Gong, X.; Ding, L.; Li, F.; Lei, L.; Gong, Y.; Liu, Q.; Tan, H.; Zhang, X.; Liu, D.; et al. Wilms’ tumor 1-associating protein plays an aggressive role in diffuse large B-cell lymphoma and forms a complex with BCL6 via Hsp90. Cell Commun. Signal 2018, 16, 50. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, J.; Ye, Y.; Liu, K.; Zeng, L.; Huang, J.; Pan, L.; Li, M.; Bai, R.; Zhuang, L.; et al. N(6) -methyladenosine-Mediated Upregulation of WTAPP1 Promotes WTAP Translation and Wnt Signaling to Facilitate Pancreatic Cancer Progression. Cancer Res. 2021, 81, 5268–5283. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, S.J.; Li, Z.; Ma, Y.; Song, Y.R. ING2-WTAP is a potential therapeutic target in non-small cell lung cancer. Biochem. Biophys. Res. Commun. 2022, 605, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Lin, Z.; Ye, Y.; Luo, R.; Zeng, L. ARRB2 promotes colorectal cancer growth through triggering WTAP. Acta. Biochim. Biophys. Sin. 2021, 53, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Liu, Y.; Yang, X.; Xu, X.; Yan, Y.; Zhang, J. C5aR1-positive neutrophils promote breast cancer glycolysis through WTAP-dependent m6A methylation of ENO1. Cell Death Dis. 2021, 12, 737. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.X.; Zheng, Z.Q.; Yang, P.Y.; Lin, L.; Zhou, G.Q.; Lv, J.W.; Zhang, L.L.; Chen, F.; Li, Y.Q.; Wu, C.F.; et al. WTAP-mediated m6A modification of lncRNA DIAPH1-AS1 enhances its stability to facilitate nasopharyngeal carcinoma growth and metastasis. Cell Death Differ. 2022, 29, 1137–1151. [Google Scholar] [CrossRef]
- He, J.; Yuan, L.; Lin, H.; Lin, A.; Chen, H.; Luo, A.; Zhuo, Z.; Liu, X. Genetic variants in m6A modification core genes are associated with glioma risk in Chinese children. Mol. Ther. Oncolytics 2021, 20, 199–208. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, Y.; Sun, L.; Zhao, Z.; Liu, W.; Luo, B. EBV downregulates the m6A “writer” WTAP in EBV-associated gastric carcinoma. Virus Res. 2021, 304, 198510. [Google Scholar] [CrossRef]
- Villa, E.; Sahu, U.; O’Hara, B.P.; Ali, E.S.; Helmin, K.A.; Asara, J.M.; Gao, P.; Singer, B.D.; Ben-Sahra, I. mTORC1 stimulates cell growth through SAM synthesis and m6A mRNA-dependent control of protein synthesis. Mol. Cell 2021, 81, 2076–2093.e2079. [Google Scholar] [CrossRef]
- Sorci, M.; Ianniello, Z.; Cruciani, S.; Larivera, S.; Ginistrelli, L.C.; Capuano, E.; Marchioni, M.; Fazi, F.; Fatica, A. METTL3 regulates WTAP protein homeostasis. Cell Death Dis. 2018, 9, 796. [Google Scholar] [CrossRef]
- Newell, L.F.; Cook, R.J. Advances in acute myeloid leukemia. Bmj 2021, 375, n2026. [Google Scholar] [CrossRef]
- Naren, D.; Yan, T.; Gong, Y.; Huang, J.; Zhang, D.; Sang, L.; Zheng, X.; Li, Y. High Wilms’ tumor 1 associating protein expression predicts poor prognosis in acute myeloid leukemia and regulates m6A methylation of MYC mRNA. J. Cancer Res. Clin. Oncol. 2021, 147, 33–47. [Google Scholar] [CrossRef]
- Hu, C.; Yu, M.; Li, C.; Wang, Y.; Li, X.; Ulrich, B.; Su, R.; Dong, L.; Weng, H.; Huang, H.; et al. miR-550-1 functions as a tumor suppressor in acute myeloid leukemia via the hippo signaling pathway. Int. J. Biol. Sci. 2020, 16, 2853–2867. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, E.A.; Barraclough, A.; Sehn, L.H. Limited-stage diffuse large B-cell lymphoma. Blood 2022, 139, 822–834. [Google Scholar] [CrossRef] [PubMed]
- Whitesell, L.; Lindquist, S.L. HSP90 and the chaperoning of cancer. Nat. Rev. Cancer 2005, 5, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, M.G.; Oswald, E.; Yu, W.; Xue, F.; MacKerell, A.D., Jr.; Melnick, A.M. The Expanding Role of the BCL6 Oncoprotein as a Cancer Therapeutic Target. Clin. Cancer Res. 2017, 23, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Shen, L.; Yang, H.; Gong, H.; Du, X.; Li, J. m6A methyltransferase Wilms’ tumor 1-associated protein facilitates cell proliferation and cisplatin resistance in NK/T cell lymphoma by regulating dual-specificity phosphatases 6 expression via m6A RNA methylation. IUBMB Life 2021, 73, 108–117. [Google Scholar] [CrossRef]
- Smyth, E.C.; Lagergren, J.; Fitzgerald, R.C.; Lordick, F.; Shah, M.A.; Lagergren, P.; Cunningham, D. Oesophageal cancer. Nat. Rev. Dis. Primers 2017, 3, 17048. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, Y.; Xie, Y.; Zhang, L.; Gao, M.; Li, S.; Wang, F. m6A Regulators Is Differently Expressed and Correlated With Immune Response of Esophageal Cancer. Front. Cell Dev. Biol. 2021, 9, 650023. [Google Scholar] [CrossRef]
- Jing, X.; Yang, F.; Shao, C.; Wei, K.; Xie, M.; Shen, H.; Shu, Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 2019, 18, 157. [Google Scholar] [CrossRef]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Yu, H.; Zhao, K.; Zeng, H.; Li, Z.; Chen, K.; Zhang, Z.; Li, E.; Wu, Z. N(6)-methyladenosine (m6A) methyltransferase WTAP accelerates the Warburg effect of gastric cancer through regulating HK2 stability. Biomed. Pharmacother. 2021, 133, 111075. [Google Scholar] [CrossRef]
- Li, H.; Su, Q.; Li, B.; Lan, L.; Wang, C.; Li, W.; Wang, G.; Chen, W.; He, Y.; Zhang, C. High expression of WTAP leads to poor prognosis of gastric cancer by influencing tumour-associated T lymphocyte infiltration. J. Cell Mol. Med. 2020, 24, 4452–4465. [Google Scholar] [CrossRef] [PubMed]
- Guan, K.; Liu, X.; Li, J.; Ding, Y.; Li, J.; Cui, G.; Cui, X.; Sun, R. Expression Status And Prognostic Value Of m6A-associated Genes in Gastric Cancer. J. Cancer 2020, 11, 3027–3040. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ramadori, P.; Pfister, D.; Seehawer, M.; Zender, L.; Heikenwalder, M. The immunological and metabolic landscape in primary and metastatic liver cancer. Nat. Rev. Cancer 2021, 21, 541–557. [Google Scholar] [CrossRef]
- Liang, L.; Xu, H.; Dong, Q.; Qiu, L.; Lu, L.; Yang, Q.; Zhao, W.; Li, Y. WTAP Is Correlated With Unfavorable Prognosis, Tumor Cell Proliferation, and Immune Infiltration in Hepatocellular Carcinoma. Front. Oncol. 2022, 12, 852000. [Google Scholar] [CrossRef]
- Li, G.; Deng, L.; Huang, N.; Cui, Z.; Wu, Q.; Ma, J.; Pan, Q.; Sun, F. m6A mRNA Methylation Regulates LKB1 to Promote Autophagy of Hepatoblastoma Cells through Upregulated Phosphorylation of AMPK. Genes 2021, 12, 1747. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Y. AMPK and Autophagy. Adv. Exp. Med. Biol. 2019, 1206, 85–108. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Shu, S.; Cai, J.; Tang, C.; Dong, Z. AMPK/mTOR Signaling in Autophagy Regulation During Cisplatin-Induced Acute Kidney Injury. Front. Physiol. 2020, 11, 619730. [Google Scholar] [CrossRef]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef]
- Li, B.Q.; Liang, Z.Y.; Seery, S.; Liu, Q.F.; You, L.; Zhang, T.P.; Guo, J.C.; Zhao, Y.P. WT1 associated protein promotes metastasis and chemo-resistance to gemcitabine by stabilizing Fak mRNA in pancreatic cancer. Cancer Lett. 2019, 451, 48–57. [Google Scholar] [CrossRef]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Dong, X.F.; Wang, Y.; Tang, C.H.; Huang, B.F.; Du, Z.; Wang, Q.; Shao, J.K.; Lu, H.J.; Wang, C.Q. Upregulated WTAP expression in colorectal cancer correlates with tumor site and differentiation. PLoS ONE 2022, 17, e0263749. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Fan, X.; Zhu, J.; Xu, D.; Li, R.; Chen, R.; Hu, J.; Shen, Y.; Hao, J.; Wang, K.; et al. The differentiation of colorectal cancer is closely relevant to m6A modification. Biochem. Biophys. Res. Commun. 2021, 546, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, L.; Dong, Z.; Li, J.; Yu, Y.; Chen, X.; Ren, F.; Cui, G.; Sun, R. Expression patterns and prognostic value of m6A-related genes in colorectal cancer. Am. J. Transl. Res. 2019, 11, 3972–3991. [Google Scholar] [PubMed]
- Zhang, J.; Tsoi, H.; Li, X.; Wang, H.; Gao, J.; Wang, K.; Go, M.Y.; Ng, S.C.; Chan, F.K.; Sung, J.J.; et al. Carbonic anhydrase IV inhibits colon cancer development by inhibiting the Wnt signalling pathway through targeting the WTAP-WT1-TBL1 axis. Gut 2016, 65, 1482–1493. [Google Scholar] [CrossRef] [PubMed]
- He, R.Z.; Jiang, J.; Hu, X.; Lei, M.; Li, J.; Luo, W.; Duan, L.; Hu, Z.; Mo, Y.Y.; Luo, D.X.; et al. Stabilization of UCA1 by N6-methyladenosine RNA methylation modification promotes colorectal cancer progression. Cancer Cell Int. 2021, 21, 616. [Google Scholar] [CrossRef]
- Brindley, P.J.; Bachini, M.; Ilyas, S.I.; Khan, S.A.; Loukas, A.; Sirica, A.E.; Teh, B.T.; Wongkham, S.; Gores, G.J. Cholangiocarcinoma. Nat. Rev. Dis. Primers. 2021, 7, 65. [Google Scholar] [CrossRef]
- Jo, H.J.; Shim, H.E.; Han, M.E.; Kim, H.J.; Kim, K.S.; Baek, S.; Choi, K.U.; Hur, G.Y.; Oh, S.O. WTAP regulates migration and invasion of cholangiocarcinoma cells. J. Gastroenterol. 2013, 48, 1271–1282. [Google Scholar] [CrossRef]
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast cancer. Lancet 2021, 397, 1750–1769. [Google Scholar] [CrossRef]
- Crosbie, E.J.; Kitson, S.J.; McAlpine, J.N.; Mukhopadhyay, A.; Powell, M.E.; Singh, N. Endometrial cancer. Lancet 2022, 399, 1412–1428. [Google Scholar] [CrossRef]
- Xie, W.; Liu, N.; Wang, X.; Wei, L.; Xie, W.; Sheng, X. Wilms’ Tumor 1-Associated Protein Contributes to Chemo-Resistance to Cisplatin Through the Wnt/β-Catenin Pathway in Endometrial Cancer. Front. Oncol. 2021, 11, 598344. [Google Scholar] [CrossRef]
- Li, Q.; Wang, C.; Dong, W.; Su, Y.; Ma, Z. WTAP facilitates progression of endometrial cancer via CAV-1/NF-κB axis. Cell Biol. Int. 2021, 45, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Kuroki, L.; Guntupalli, S.R. Treatment of epithelial ovarian cancer. Bmj 2020, 371, m3773. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, J.; Li, K.; Huang, Y.; Dai, Y.; Xu, C.; Kang, Y. Identification of WTAP-related genes by weighted gene co-expression network analysis in ovarian cancer. J. Ovarian Res. 2020, 13, 119. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.L.; Ma, X.D.; Tong, J.F.; Li, J.Q.; Guan, X.J.; Yang, J.H. WTAP is a prognostic marker of high-grade serous ovarian cancer and regulates the progression of ovarian cancer cells. OncoTargets Ther. 2019, 12, 6191–6201. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Tang, J.; Wang, F.; Cheng, G.; Si, S.; Sun, X.; Han, J.; Yu, H.; Zhang, W.; Lv, Q.; Wei, J.F.; et al. Wilms’ tumor 1-associating protein promotes renal cell carcinoma proliferation by regulating CDK2 mRNA stability. J. Exp. Clin. Cancer Res. 2018, 37, 40. [Google Scholar] [CrossRef]
- Lenis, A.T.; Lec, P.M.; Chamie, K.; Mshs, M.D. Bladder Cancer: A Review. Jama 2020, 324, 1980–1991. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X. Relationship between the genetic expression of WTAP and bladder cancer and patient prognosis. Oncol. Lett. 2018, 16, 6966–6970. [Google Scholar] [CrossRef]
- Wei, W.; Sun, J.; Zhang, H.; Xiao, X.; Huang, C.; Wang, L.; Zhong, H.; Jiang, Y.; Zhang, X.; Jiang, G. Circ0008399 Interaction with WTAP Promotes Assembly and Activity of the m6A Methyltransferase Complex and Promotes Cisplatin Resistance in Bladder Cancer. Cancer Res. 2021, 81, 6142–6156. [Google Scholar] [CrossRef]
- Chen, Y.P.; Chan, A.T.C.; Le, Q.T.; Blanchard, P.; Sun, Y.; Ma, J. Nasopharyngeal carcinoma. Lancet 2019, 394, 64–80. [Google Scholar] [CrossRef]
- Thai, A.A.; Solomon, B.J.; Sequist, L.V.; Gainor, J.F.; Heist, R.S. Lung cancer. Lancet 2021, 398, 535–554. [Google Scholar] [CrossRef]
- Gu, C.; Shi, X.; Qiu, W.; Huang, Z.; Yu, Y.; Shen, F.; Chen, Y.; Pan, X. Comprehensive Analysis of the Prognostic Role and Mutational Characteristics of m6A-Related Genes in Lung Squamous Cell Carcinoma. Front. Cell Dev. Biol. 2021, 9, 661792. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, Z.; Fan, T.M.; Irudayaraj, J.M.K. Osteosarcoma mechanobiology and therapeutic targets. Br. J. Pharmacol. 2022, 179, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, Y.; Zhi, S.; Ding, Z.; Wang, W.; Peng, Y.; Huang, Y.; Zheng, R.; Yu, H.; Wang, J.; et al. WTAP promotes osteosarcoma tumorigenesis by repressing HMBOX1 expression in an m6A-dependent manner. Cell Death Dis. 2020, 11, 659. [Google Scholar] [CrossRef]
- Ren, Z.; Hu, Y.; Sun, J.; Kang, Y.; Li, G.; Zhao, H. N(6)-methyladenosine methyltransferase WTAP-stabilized FOXD2-AS1 promotes the osteosarcoma progression through m6A/FOXM1 axis. Bioengineered 2022, 13, 7963–7973. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wu, Z.; Zhang, H.; Zhang, N.; Wu, W.; Wang, Z.; Dai, Z.; Zhang, X.; Zhang, L.; Peng, Y.; et al. Glioma targeted therapy: Insight into future of molecular approaches. Mol. Cancer 2022, 21, 39. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.I.; Lee, S.W.; Han, M.E.; Kim, H.J.; Seo, S.A.; Hur, G.Y.; Jung, S.; Kim, B.S.; Oh, S.O. Expression and roles of Wilms’ tumor 1-associating protein in glioblastoma. Cancer Sci. 2012, 103, 2102–2109. [Google Scholar] [CrossRef]
- Xi, Z.; Xue, Y.; Zheng, J.; Liu, X.; Ma, J.; Liu, Y. WTAP Expression Predicts Poor Prognosis in Malignant Glioma Patients. J. Mol. Neurosci. 2016, 60, 131–136. [Google Scholar] [CrossRef]
- Huang, H.; Weng, H.; Chen, J. m6A Modification in Coding and Non-coding RNAs: Roles and Therapeutic Implications in Cancer. Cancer Cell 2020, 37, 270–288. [Google Scholar] [CrossRef]
- Deng, L.J.; Deng, W.Q.; Fan, S.R.; Chen, M.F.; Qi, M.; Lyu, W.Y.; Qi, Q.; Tiwari, A.K.; Chen, J.X.; Zhang, D.M.; et al. m6A modification: Recent advances, anticancer targeted drug discovery and beyond. Mol. Cancer 2022, 21, 52. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Kong, Q.; Zhang, H.; Wang, J.; Luo, T.; Jiang, Y. Targeting mTOR for cancer therapy. J. Hematol. Oncol. 2019, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Szwed, A.; Kim, E.; Jacinto, E. Regulation and metabolic functions of mTORC1 and mTORC2. Physiol. Rev. 2021, 101, 1371–1426. [Google Scholar] [CrossRef]
- Lourenco, C.; Resetca, D.; Redel, C.; Lin, P.; MacDonald, A.S.; Ciaccio, R.; Kenney, T.M.G.; Wei, Y.; Andrews, D.W.; Sunnerhagen, M.; et al. MYC protein interactors in gene transcription and cancer. Nat. Rev. Cancer 2021, 21, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Madden, S.K.; de Araujo, A.D.; Gerhardt, M.; Fairlie, D.P.; Mason, J.M. Taking the Myc out of cancer: Toward therapeutic strategies to directly inhibit c-Myc. Mol. Cancer 2021, 20, 3. [Google Scholar] [CrossRef]
- Mathsyaraja, H.; Freie, B.; Cheng, P.F.; Babaeva, E.; Catchpole, J.T.; Janssens, D.; Henikoff, S.; Eisenman, R.N. Max deletion destabilizes MYC protein and abrogates Eµ-Myc lymphomagenesis. Genes Dev. 2019, 33, 1252–1264. [Google Scholar] [CrossRef]
- Yankova, E.; Blackaby, W.; Albertella, M.; Rak, J.; De Braekeleer, E.; Tsagkogeorga, G.; Pilka, E.S.; Aspris, D.; Leggate, D.; Hendrick, A.G.; et al. Small-molecule inhibition of METTL3 as a strategy against myeloid leukaemia. Nature 2021, 593, 597–601. [Google Scholar] [CrossRef]
- Aldea, M.; Andre, F.; Marabelle, A.; Dogan, S.; Barlesi, F.; Soria, J.C. Overcoming Resistance to Tumor-Targeted and Immune-Targeted Therapies. Cancer Discov. 2021, 11, 874–899. [Google Scholar] [CrossRef]
- Chatterjee, N.; Bivona, T.G. Polytherapy and Targeted Cancer Drug Resistance. Trends Cancer 2019, 5, 170–182. [Google Scholar] [CrossRef]
- Wang, W.T.; Han, C.; Sun, Y.M.; Chen, T.Q.; Chen, Y.Q. Noncoding RNAs in cancer therapy resistance and targeted drug development. J. Hematol. Oncol. 2019, 12, 55. [Google Scholar] [CrossRef]
- Lan, Q.; Liu, P.Y.; Bell, J.L.; Wang, J.Y.; Hüttelmaier, S.; Zhang, X.D.; Zhang, L.; Liu, T. The Emerging Roles of RNA m6A Methylation and Demethylation as Critical Regulators of Tumorigenesis, Drug Sensitivity, and Resistance. Cancer Res. 2021, 81, 3431–3440. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Jiang, J.; Assaraf, Y.G.; Xiao, H.; Chen, Z.S.; Huang, C. Surmounting cancer drug resistance: New insights from the perspective of N(6)-methyladenosine RNA modification. Drug Resist. Updat. 2020, 53, 100720. [Google Scholar] [CrossRef]
- Jin, D.; Guo, J.; Wu, Y.; Du, J.; Yang, L.; Wang, X.; Di, W.; Hu, B.; An, J.; Kong, L.; et al. m6A mRNA methylation initiated by METTL3 directly promotes YAP translation and increases YAP activity by regulating the MALAT1-miR-1914-3p-YAP axis to induce NSCLC drug resistance and metastasis. J. Hematol. Oncol. 2019, 12, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Xie, K.; Zhou, H.; Wu, Y.; Li, C.; Liu, Y.; Liu, Z.; Xu, Q.; Liu, S.; Xiao, D.; et al. Role of non-coding RNAs and RNA modifiers in cancer therapy resistance. Mol. Cancer 2020, 19, 47. [Google Scholar] [CrossRef]
- Wei, L.; Sun, J.; Zhang, N.; Zheng, Y.; Wang, X.; Lv, L.; Liu, J.; Xu, Y.; Shen, Y.; Yang, M. Noncoding RNAs in gastric cancer: Implications for drug resistance. Mol. Cancer 2020, 19, 62. [Google Scholar] [CrossRef]
- Wei, L.; Wang, X.; Lv, L.; Liu, J.; Xing, H.; Song, Y.; Xie, M.; Lei, T.; Zhang, N.; Yang, M. The emerging role of microRNAs and long noncoding RNAs in drug resistance of hepatocellular carcinoma. Mol. Cancer 2019, 18, 147. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Dawson, M.A.; Kouzarides, T. Cancer epigenetics: From mechanism to therapy. Cell 2012, 150, 12–27. [Google Scholar] [CrossRef]
- Chong, W.; Shang, L.; Liu, J.; Fang, Z.; Du, F.; Wu, H.; Liu, Y.; Wang, Z.; Chen, Y.; Jia, S.; et al. m6A regulator-based methylation modification patterns characterized by distinct tumor microenvironment immune profiles in colon cancer. Theranostics 2021, 11, 2201–2217. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, Q.; Li, B.; Wang, D.; Wang, L.; Zhou, Y.L. m6A regulator-mediated methylation modification patterns and tumor microenvironment infiltration characterization in gastric cancer. Mol. Cancer 2020, 19, 53. [Google Scholar] [CrossRef]
- Fan, Y.; Zhou, Y.; Lou, M.; Li, X.; Zhu, X.; Yuan, K. m6A Regulator-Mediated Methylation Modification Patterns and Characterisation of Tumour Microenvironment Infiltration in Non-Small Cell Lung Cancer. J. Inflamm. Res. 2022, 15, 1969–1989. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.E. Epigenetic Therapies for Cancer. N. Engl. J. Med. 2020, 383, 650–663. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Attardi, L.D. A Balancing Act: p53 Activity from Tumor Suppression to Pathology and Therapeutic Implications. Annu. Rev. Pathol. 2022, 17, 205–226. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Cao, J.; Topatana, W.; Juengpanich, S.; Li, S.; Zhang, B.; Shen, J.; Cai, L.; Cai, X.; Chen, M. Targeting mutant p53 for cancer therapy: Direct and indirect strategies. J. Hematol. Oncol. 2021, 14, 157. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).