SARS-CoV-2 and Skin: New Insights and Perspectives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Procedure
2.2. Immunohistochemistry Score
2.3. Statistical Analysis
2.4. Review of Literature
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Synowiec, A.; Szczepański, A.; Barreto-Duran, E.; Lie, L.K.; Pyrc, K. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): A Systemic Infection. Clin. Microbiol. Rev. 2021, 34, e00133-20. [Google Scholar] [CrossRef] [PubMed]
- Harrison, A.G.; Lin, T.; Wang, P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020, 41, 1100–1115. [Google Scholar] [CrossRef]
- WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19–11 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 21 November 2021).
- PLOS Medicine Editors. Pandemic preparedness and responses: WHO to turn to in a crisis? PLoS Med. 2020, 17, e1003167. [Google Scholar]
- WHO Statements. Available online: https://covid19.who.int/dashboard (accessed on 31 July 2022).
- WHO Statements. Available online: https://covid19.who.int/region/euro/country/it (accessed on 31 July 2022).
- Garduño-Soto, M.; Choreño-Parra, J.A.; Cazarin-Barrientos, J. Dermatological aspects of SARS-CoV-2 infection: Mechanisms and manifestations. Arch. Dermatol. Res. 2021, 313, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Bouaziz, J.D.; Duong, T.; Jachiet, M.; Velter, C.; Lestang, P.; Cassius, C.; Arsouze, A.; Domergue Than Trong, E.; Bagot, M.; Begon, E.; et al. Vascular skin symptoms in COVID-19: A French observational study. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e451–e452. [Google Scholar] [CrossRef] [PubMed]
- Burgueño, J.F.; Abreu, M.T. Reply to the Letter to the Editor: The Incidence and Outcomes of COVID-19 in Patients With IBD: A Rapid Review and Meta-Analysis. Inflamm. Bowel. Dis. 2020, 26, e127. [Google Scholar] [CrossRef] [PubMed]
- Foo, J.N.; Chew, E.G.Y.; Chung, S.J.; Peng, R.; Blauwendraat, C.; Nalls, M.A.; Mok, K.Y.; Satake, W.; Toda, T.; Chao, Y.; et al. Identification of Risk Loci for Parkinson Disease in Asians and Comparison of Risk Between Asians and Europeans: A Genome-Wide Association Study. JAMA Neurol. 2020, 77, 746–754. [Google Scholar] [CrossRef]
- Moriguchi, T.; Harii, N.; Goto, J.; Harada, D.; Sugawara, H.; Takamino, J.; Ueno, M.; Sakata, H.; Kondo, K.; Myose, N.; et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020, 94, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Hosier, H.; Farhadian, S.F.; Morotti, R.A.; Deshmukh, U.; Lu-Culligan, A.; Campbell, K.H.; Yasumoto, Y.; Vogels, C.B.; Casanovas-Massana, A.; Vijayakumar, P.; et al. SARS-CoV-2 infection of the placenta. J. Clin. Investig. 2020, 130, 4947–4953. [Google Scholar] [CrossRef]
- Resta, L.; Vimercati, A.; Cazzato, G.; Mazzia, G.; Cicinelli, E.; Colagrande, A.; Fanelli, M.; Scarcella, S.V.; Ceci, O.; Rossi, R. SARS-CoV-2 and Placenta: New Insights and Perspectives. Viruses 2021, 13, 723. [Google Scholar] [CrossRef]
- Garg, S.; Garg, M.; Prabhakar, N.; Malhotra, P.; Agarwal, R. Unraveling the mystery of COVID-19 cytokine storm: From skin to organ systems. Dermatol. Ther. 2020, 33, e13859. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, Y.; Zhou, L. Cutaneous manifestations in children with SARS-CoV-2 infection and/or COVID-19: What do we know after 10 months under this pandemic? Int. J. Dermatol. 2021, 16, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Recalcati, S. Cutaneous manifestations in COVID-19: A first perspective. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e212–e213. [Google Scholar] [CrossRef]
- Català, A.; Galván Casas, C.; Carretero Hernández, G.; García-Doval, I. Vesicular eruption in COVID-19—To exclude varicella: Reply from the authors. Br. J. Dermatol. 2020, 183, 791. [Google Scholar] [CrossRef] [PubMed]
- Recalcati, S.; Barbagallo, T.; Frasin, L.A.; Prestinari, F.; Cogliardi, A.; Provero, M.C.; Dainese, E.; Vanzati, A.; Fantini, F. Acral cutaneous lesions in the time of COVID-19. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 346–347. [Google Scholar] [CrossRef]
- Gunawan, C.; Angela, A.; Widysanto, A. Urticarial eruption in coronavirus disease 2019 infection: A case report in Tangerang, Indonesia. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e372–e373. [Google Scholar] [CrossRef] [PubMed]
- Damme, C.; Berlingin, E.; Saussez, S.; Accaputo, O. Acute urticaria with pyrexia as the first manifestations of a COVID-19 infection. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e300. [Google Scholar] [CrossRef]
- Falkenhain-López, D.; Sánchez-Velázquez, A.; López-Valle, A.; Ortiz-Frutos, F.J. SARS-Coronavirus-2 and acute urticaria. Int. J. Dermatol. 2020, 59, 867. [Google Scholar] [CrossRef]
- Cazzato, G.; Foti, C.; Colagrande, A.; Cimmino, A.; Scarcella, S.; Cicco, G.; Sablone, S.; Arezzo, F.; Romita, P.; Lettini, T.; et al. Skin Manifestation of SARS-CoV-2: The Italian Experience. J. Clin. Med. 2021, 10, 1566. [Google Scholar] [CrossRef]
- Cazzato, G.; Mazzia, G.; Cimmino, A.; Colagrande, A.; Sablone, S.; Lettini, T.; Rossi, R.; Santarella, N.; Elia, R.; Nacchiero, E.; et al. SARS-CoV-2 and Skin: The Pathologist’s Point of View. Biomolecules 2021, 11, 838. [Google Scholar] [CrossRef]
- Alramthan, A.; Aldaraji, W. Two cases of COVID-19 presenting with a clinical picture resembling chilblains: First report from the Middle East. Clin. Exp. Dermatol. 2020, 45, 746. [Google Scholar] [CrossRef] [PubMed]
- Trager, M.H.; Farmer, K.; Ulrich, C.; Basset-Seguin, N.; Herms, F.; Geskin, L.J.; Bouaziz, J.D.; Lebbé, C.; de Masson, A.; Bagot, M.; et al. Actinic cheilitis: A systematic review of treatment options. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Ingravallo, G.; Mazzotta, F.; Resta, L.; Sablone, S.; Cazzato, G.; Cimmino, A.; Rossi, R.; Colagrande, A.; Ferrante, B.; Troccoli, T.; et al. Inflammatory skin lesions in three SARS-CoV-2 swab-negative adolescents: A possible COVID-19 sneaky manifestation? Pediatr. Rep. 2021, 13, 181. [Google Scholar] [CrossRef] [PubMed]
- Manalo, I.F.; Smith, M.K.; Cheeley, J.; Jacobs, R. Reply to:“Reply: A dermatologic manifestation of COVID-19: Transient livedo reticularis”. J. Am. Acad. Dermatol. 2020, 83, e157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, W.; Xiao, M.; Li, Y.J.; Yang, Y.; Zhao, J.; Zhou, X.; Jiang, W.; Zhao, Y.Q.; Zhang, S.Y.; et al. Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia. Zhonghua Xue Ye Xue Za Zhi = Zhonghua Xueyexue Zazhi 2020, 41, 302–307. [Google Scholar]
- Jose, R.J.; Manuel, A. COVID-19 cytokine storm: The interplay between inflammation and coagulation. Lancet Respir. Med. 2020, 8, e46. [Google Scholar] [CrossRef]
- Spiezia, L.; Boscolo, A.; Correale, C.; Sella, N.; Pesenti, E.; Beghetto, L.; Campello, E.; Poletto, F.; Cerruti, L.; Cola, M.; et al. Different hypercoagulable profiles in patients with COVID-19 admitted to the internal medicine ward and the intensive care unit. Thromb. Haemost. 2020, 120, 1474. [Google Scholar] [CrossRef]
- Nie, D.; Zhang, J.; Xiong, M.; Wang, F.; Cao, P.; Zhang, Y.; Chen, X.; Chen, J.; Ma, X.; Zhou, X.; et al. Complete remission of refractory juvenile acute myeloid leukaemia with RUNX1-PRDM16 in Bloom syndrome after haematopoietic stem cell transplantation. Br. J. Haematol. 2020, 190, e166–e169. [Google Scholar] [CrossRef]
- Fernandez-Nieto, D.; Ortega-Quijano, D.; Suarez-Valle, A.; Jimenez-Cauhe, J.; Jaen-Olasolo, P.; Fernandez-Guarino, M. Facial rash and ocular pain. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 375. [Google Scholar] [CrossRef]
- Ehsani, A.H.; Nasimi, M.; Bigdelo, Z. Pityriasis rosea as a cutaneous manifestation of COVID-19 infection. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e436–e437. [Google Scholar] [CrossRef]
- Janah, H.; Zinebi, A.; Elbenaye, J. Atypical erythema multiforme palmar plaques lesions due to SARS-Cov-2. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e373–e375. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, A.G.; Robustelli Test, E.; Vezzoli, P.; Carugno, A.; Moggio, E.; Consonni, L.; Gianatti, A.; Sena, P. Acute generalized exanthematous pustulosis with erythema multiforme-like lesions induced by Hydroxychloroquine in a woman with coronavirus disease 2019 (COVID-19). J. Eur. Acad. Dermatol. Venereol. 2020, 34, e457–e459. [Google Scholar]
- Sachdeva, M.; Gianotti, R.; Shah, M.; Bradanini, L.; Tosi, D.; Veraldi, S.; Ziv, M.; Leshem, E.; Dodiuk-Gad, R.P. Cutaneous manifestations of COVID-19: Report of three cases and a review of literature. J. Dermatol. Sci. 2020, 98, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Estébanez, A.; Pérez-Santiago, L.; Silva, E.; Guillen-Climent, S.; García-Vázquez, A.; Ramón, M.D. Cutaneous manifestations in COVID-19: A new contribution. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e250–e251. [Google Scholar] [CrossRef]
- Castelnovo, L.; Capelli, F.; Tamburello, A.; Faggioli, P.M.; Mazzone, A. Symmetric cutaneous vasculitis in COVID-19 pneumonia. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 127. [Google Scholar] [CrossRef]
- De Medeiros, V.L.S.; Silva, L.F.T. Follow-up of skin lesions during the evolution of COVID-19: A case report. Arch. Dermatol. Res. 2020, 313, 603. [Google Scholar] [CrossRef]
- Loffredo, L.; Pacella, F.; Pacella, E.; Tiscione, G.; Oliva, A.; Violi, F. Conjunctivitis and COVID-19: A metanalysis. J. Med. Virol. 2020, 92, 1413. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Abdulle, A.E.; Timens, W.; Hillebrands, J.; Navis, G.J.; Gordijn, S.J.; Bolling, M.C.; Dijkstra, G.; Voors, A.A.; Osterhaus, A.D.; et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J. Pathol. 2020, 251, 228. [Google Scholar] [CrossRef]
- Conti, F. Fisiologia Medica (Vol. 1–2), 3rd ed.; Edi-Ermes: Milano, Italy, 2010; pp. 154–196. [Google Scholar]
- Albini, A.; Di Guardo, G.; Noonan, D.M.; Lombardo, M. The SARS-CoV-2 receptor, ACE-2, is expressed on many different cell types: Implications for ACE-inhibitor- and angiotensin II receptor blocker-based cardiovascular therapies. Intern. Emerg. Med. 2020, 15, 759. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; Mcguire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 183, 1735. [Google Scholar] [CrossRef]
- Glowacka, I.; Bertram, S.; Muller, M.A.; Allen, P.; Soilleux, E.; Pfefferle, S.; Steffen, I.; Tsegaye, T.S.; He, Y.; Gnirss, K.; et al. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J. Virol. 2011, 85, 4122. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, C.G.K.; Allon, S.J.; Nyquist, S.K.; Mbano, I.M.; Miao, V.N.; Tzouanas, C.N.; Cao, Y.; Yousif, A.S.; Bals, J.; Hauser, B.M.; et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell 2020, 181, 1016–1035.e19. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Mi, Z.; Wang, Z.; Pang, Z.; Liu, H.; Zhang, F. High Expression of ACE2 on Keratinocytes Reveals Skin as a Potential Target for SARS-CoV-2. J. Investig. Dermatol. 2021, 141, 206–209.e1. [Google Scholar] [CrossRef] [PubMed]
- Salamanna, F.; Maglio, M.; Landini, M.P.; Fini, M. Body Localization of ACE-2: On the Trail of the Keyhole of SARS-CoV-2. Front. Med. 2020, 7, 594495. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention (CDC) CDC 2019—Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel. 2020. Available online: https://www.fda.gov/media/134922/download (accessed on 31 July 2022).
- Cepheid Xpert® Xpress SARS-CoV-2: Instructions for Use for Labs. Available online: https://www.fda.gov/media/136314/download (accessed on 10 May 2021).
- Visikol Datasheet. Available online: https://visikol.com/services/digipath/cell-counting (accessed on 31 July 2022).
- Oxford Centre for Evidence-Based Medicine 2011 Levels of Evidence. Available online: http://www.cebm.net/wp-content/uploads/2014/06/CEBM-Levels-of-Evidence-2.1.pdf (accessed on 31 July 2022).
- Gepia Software. Available online: http://gepia2.cancer-pku.cn/#general (accessed on 19 October 2021).
- Maayanlab. Available online: https://maayanlab.cloud/archs4/ (accessed on 20 October 2021).
- Protein Human Atlas. Available online: https://www.proteinatlas.org/ENSG00000159640-ACE/tissue/skin (accessed on 20 October 2021).
- Novak, N.; Peng, W.; Naegeli, M.C.; Galvan, C.; Kolm-Djamei, I.; Brüggen, C.; Cabanillas, B.; Schmid-Grendelmeier, P.; Catala, A. SARS-CoV-2, COVID-19, skin and immunology—What do we know so far? Allergy 2021, 76, 698–713. [Google Scholar] [CrossRef]
- Dong, M.; Zhang, J.; Ma, X.; Tan, J.; Chen, L.; Liu, S.; Xin, Y.; Zhuang, L. ACE2, TMPRSS2 distribution and extrapulmonary organ injury in patients with COVID-19. Biomed. Pharmacother. 2020, 131, 110678. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Petitjean, S.J.L.; Koehler, M.; Zhang, Q.; Dumitru, A.C.; Chen, W.; Derclaye, S.; Vincent, S.P.; Soumillion, P.; Alsteens, D. Molecular interaction and inhibition of SARS-CoV-2 binding to the ACE2 receptor. Nat. Commun. 2020, 11, 4541. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Chen, L.; Zhao, X.; Wang, X.; Hu, M.; Le, Y.; Xue, F.; Li, X.; Zheng, J. SARS-CoV-2 might transmit through the skin while the skin barrier function could be the mediator. Med. Hypotheses. 2022, 159, 110752. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.L.; Lely, A.T.; Navis, G.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, Q.; Xia, X.; Liu, K.; Yu, Z.; Tao, W.; Gong, W.; Han, J.J. Individual variation of the SARS-CoV-2 receptor ACE2 gene expression and regulation. Aging Cell 2020, 19, e13168. [Google Scholar] [CrossRef]
- McGonagle, D.; Sharif, K.; O’Regan, A.; Bridgewood, C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun. Rev. 2020, 19, 102537. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Cauhe, J.; Ortega-Quijano, D.; Prieto-Barrios, M.; Moreno-Arrones, O.M.; Fernandez-Nieto, D. Reply to “COVID-19 can present with a rash and be mistaken for dengue”: Petechial rash in a patient with COVID-19 infection. J. Am. Acad. Dermatol. 2020, 83, e141–e142. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Q.; Guo, D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 2020, 92, 2249. [Google Scholar] [CrossRef] [PubMed]
- Prompetchara, E.; Ketloy, C.; Palaga, T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020, 38, 1–9. [Google Scholar]
- Wirestam, L.; Arve, S.; Linge, P.; Bengtsson, A.A. Neutrophils-Important Communicators in Systemic Lupus Erythematosus and Antiphospholipid Syndrome. Front. Immunol. 2019, 10, 2734. [Google Scholar] [CrossRef]
- Sun, N.Z.; Brezinski, E.A.; Berliner, J.; Haemel, A.; Connolly, M.K.; Gensler, L.; McCalmont, T.H.; Shinkai, K. Updates in adult-onset Still disease: Atypical cutaneous manifestations and associations with delayed malignancy. J. Am. Acad. Dermatol. 2015, 73, 294–303. [Google Scholar] [CrossRef]
- Soler, M.J.; Batlle, M.; Riera, M.; Campos, B.; Ortiz-Perez, J.T.; Anguiano, L.; Roca-Ho, H.; Farrero, M.; Mont, L.; Pascual, J.; et al. ACE2 and ACE in acute and chronic rejection after human heart transplantation. Int. J. Cardiol. 2019, 15, 59–64. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef]
- Puelles, V.G.; Lütgehetmann, M.; Lindenmeyer, M.T.; Sperhake, J.P.; Wong, M.N.; Allweiss, L.; Chilla, S.; Heinemann, A.; Wanner, N.; Liu, S.; et al. Multiorgan and renal tropism of SARS-CoV-2. N. Engl. J. Med. 2020, 383, 590–592. [Google Scholar] [CrossRef]
- Gisondi, P.; PIaserico, S.; Bordin, C.; Alaibac, M.; Girolomoni, G.; Naldi, L. Cutaneous manifestations of SARS-CoV-2 infection: A clinical update. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2499–2504. [Google Scholar] [CrossRef]
- Tembhre, M.K.; Parihar, A.S.; Sharma, V.K.; Imran, S.; Bhari, N.; Lakshmy, R.; Bhalla, A. Enhanced expression of angiotensin-converting enzyme 2 in psoriatic skin and its upregulation in keratinocytes by interferon-γ: Implication of inflammatory milieu in skin tropism of SARS-CoV-2. Br. J. Dermatol. 2021, 184, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Seque, C.A.; Enokihara, M.M.S.E.S.; Porro, A.M.; Tomimori, J. Skin manifestations associated with COVID-19. An. Bras. Dermatol. 2022, 97, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Y.; Li, L.; Zhang, Y.; Wang, X.S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis. Poverty 2020, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Fang, X.; Pang, Z.; Zhang, B.; Liu, H.; Zhang, F. COVID-19 and cutaneous manifestations: A systematic review. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2505–2510. [Google Scholar] [CrossRef]
- Tabary, M.; Khanmohammadi, S.; Araghi, F.; Dadkhahfar, S.; Tavangar, S.M. Pathologic features of COVID-19: A concise review. Pathol. Res. Pract. 2020, 216, 153097. [Google Scholar] [CrossRef]
- Criado, P.R.; Abdalla, B.M.Z.; de Assis, I.C.; van Blarcum de Graaff Mello, C.; Caputo, G.C.; Vieira, I.C. Are the cutaneous manifestations during or due to SARS-CoV-2 infection/COVID-19 frequent or not? Revision of possible pathophysiologic mechanisms. Inflamm. Res. 2020, 69, 745–756. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, R.; Zhang, H.; Rong, L.; Zhou, W.; Liang, Y.; Li, Q. Skin is a potential host of SARS-CoV-2: A clinical, single-cell transcriptome-profiling and histologic study. J. Am. Acad. Dermatol. 2020, 83, 1755–1757. [Google Scholar] [CrossRef] [PubMed]
- Curran, C.S.; Rivera, D.R.; Kopp, J.B. COVID-19 Usurps Host Regulatory Networks. Front. Pharmacol. 2020, 11, 1278. [Google Scholar] [CrossRef] [PubMed]
- Garduño-Soto, M.; Choreño-Parra, J.A. Cutaneous susceptibility to SARS-CoV-2 infection according to the expression of viral entry factors in the skin. Gac Med. Mex. 2020, 156, 354–357. [Google Scholar] [CrossRef]
- Ricardo Criado, P.; Pincelli, T.P.H.; Criado, R.F.J.; Abdalla, B.M.Z.; Belda Junior, W. Potential interactions of SARS-CoV-2 with human cell receptors in the skin: Understanding the enigma for a lower frequency of skin lesions compared to other tissues. Exp. Dermatol. 2020, 29, 936–944. [Google Scholar] [CrossRef]
- Magro, C.M.; Mulvey, J.; Kubiak, J.; Mikhail, S.; Suster, D.; Crowson, A.N.; Laurence, J.; Nuovo, G. Severe COVID-19: A multifaceted viral vasculopathy syndrome. Ann. Diagn. Pathol. 2021, 50, 151645. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Kang, J.; Li, G.; Ge, J.; Gu, J. Analysis of 2019-nCoV receptor ACE2 expression in different tissues and its significance study. Ann. Transl. Med. 2020, 8, 1077. [Google Scholar] [CrossRef] [PubMed]
- Sollena, P.; Cappilli, S.; Piccerillo, A.; Chiricozzi, A.; Peris, K. COVID-19 hygiene measures: Hand eczema and insights into ACE2 and integrins as key molecules for SARS-CoV-2 cutaneous transmission. Int. J. Dermatol. 2020, 59, 1409–1410. [Google Scholar] [CrossRef] [PubMed]
- Al-Benna, S. Gene Expression of Angiotensin-Converting Enzyme 2 Receptor in Skin and the Implications for COVID-19. Adv. Ski. Wound Care 2021, 34, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Cappel, M.A.; Cappel, J.A.; Wetter, D.A. Pernio (Chilblains), SARS-CoV-2, and COVID Toes Unified Through Cutaneous and Systemic Mechanisms. Mayo Clin. Proc. 2021, 96, 989–1005. [Google Scholar] [CrossRef]
- Bickler, S.W.; Cauvi, D.M.; Fisch, K.M.; Prieto, J.M.; Sykes, A.G.; Thangarajah, H.; Lazar, D.A.; Ignacio, R.C.; Gerstmann, D.R.; Ryan, A.F.; et al. Extremes of age are associated with differences in the expression of selected pattern recognition receptor genes and ACE2, the receptor for SARS-CoV-2: Implications for the epidemiology of COVID-19 disease. BMC Med. Genom. 2021, 14, 138. [Google Scholar] [CrossRef]
- Birlutiu, V.; Feiereisz, A.I.; Oprinca, G.; Dobritoiu, S.; Rotaru, M.; Birlutiu, R.M.; Iancu, G.M. Cutaneous manifestations associated with anosmia, ageusia and enteritis in SARS-CoV-2 infection—A possible pattern? Observational study and review of the literature. Int. J. Infect. Dis. 2021, 107, 72–77. [Google Scholar] [CrossRef]
- Baughn, L.B.; Sharma, N.; Elhaik, E.; Sekulic, A.; Bryce, A.H.; Fonseca, R. Targeting TMPRSS2 in SARS-CoV-2 Infection. Mayo Clin. Proc. 2020, 95, 1989–1999. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Liu, L.; Hu, X.; Wang, X.; Hu, H.; Hu, Z.; Zhou, Y.; Wang, M. Infection of human sweat glands by SARS-CoV-2. Cell Discov. 2020, 6, 84. [Google Scholar] [CrossRef]
- Bayram, Y.E.; Yildiz-Sevgi, D.; Yavuz, A.; Cancetin, M.; Gurler, M.Y. Management skin manifestation of multisystem inflammatory syndrome associated with SARS-CoV-2. Virol. J. 2022, 19, 1–8. [Google Scholar] [CrossRef]
- Lin, E.C.; Hong, C.H. IL-33 Enhances ACE2 Expression on Epidermal Keratinocytes in Atopic Dermatitis: A Plausible Issue for SARS-CoV-2 Transmission in Inflamed Atopic Skin. Biomedicines 2022, 10, 1183. [Google Scholar] [CrossRef] [PubMed]
- Ganier, C.; Harun, N.; Peplow, I.; Du-Harpur, X.; Arthurs, C.; Watt, F.M.; Lynch, M.D. Angiotensin-Converting Enzyme 2 Expression Is Detectable in Keratinocytes, Cutaneous Appendages, and Blood Vessels by Multiplex RNA In Situ Hybridization. Adv. Skin Wound Care 2022, 35, 219–223. [Google Scholar] [CrossRef] [PubMed]
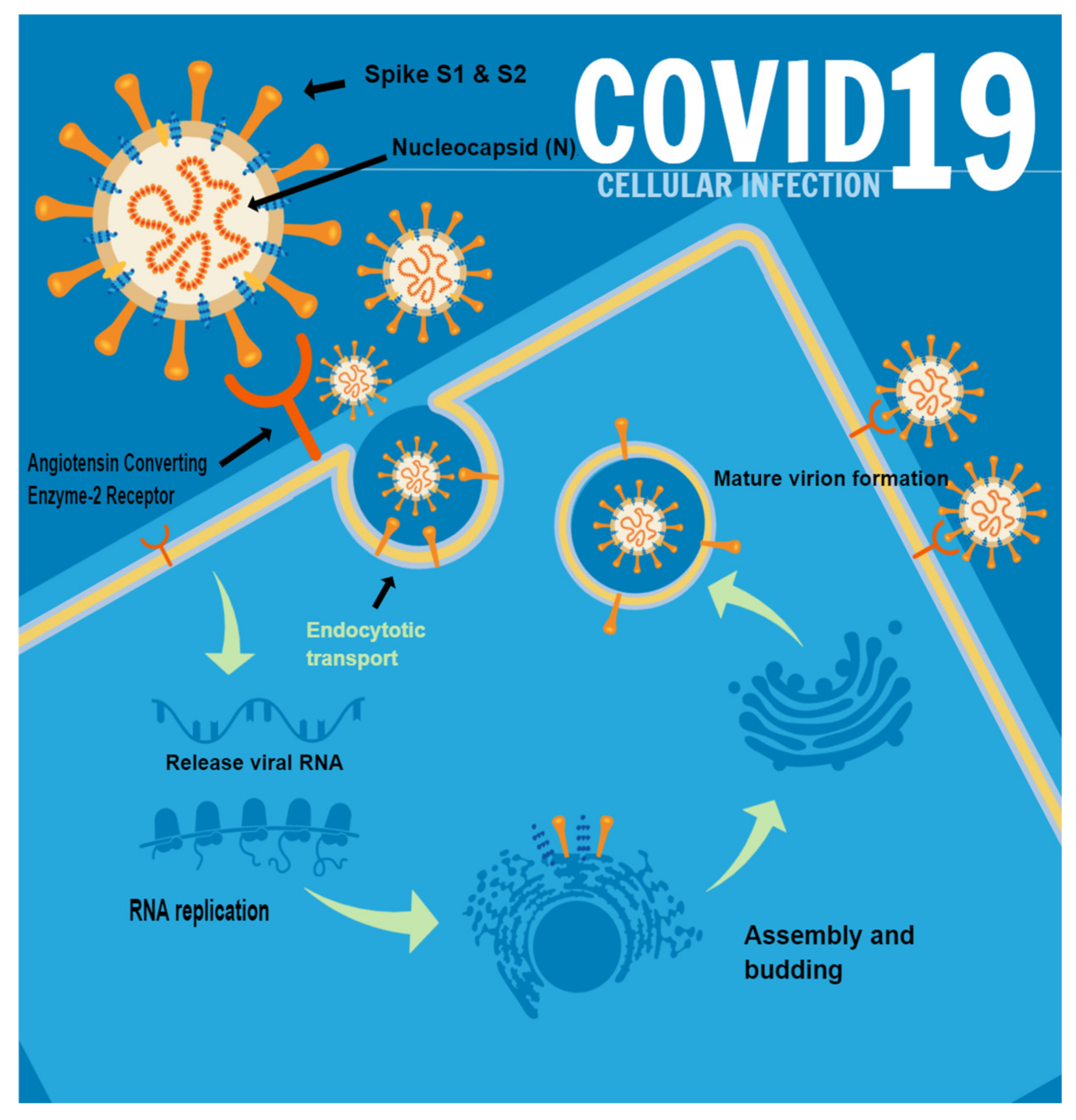
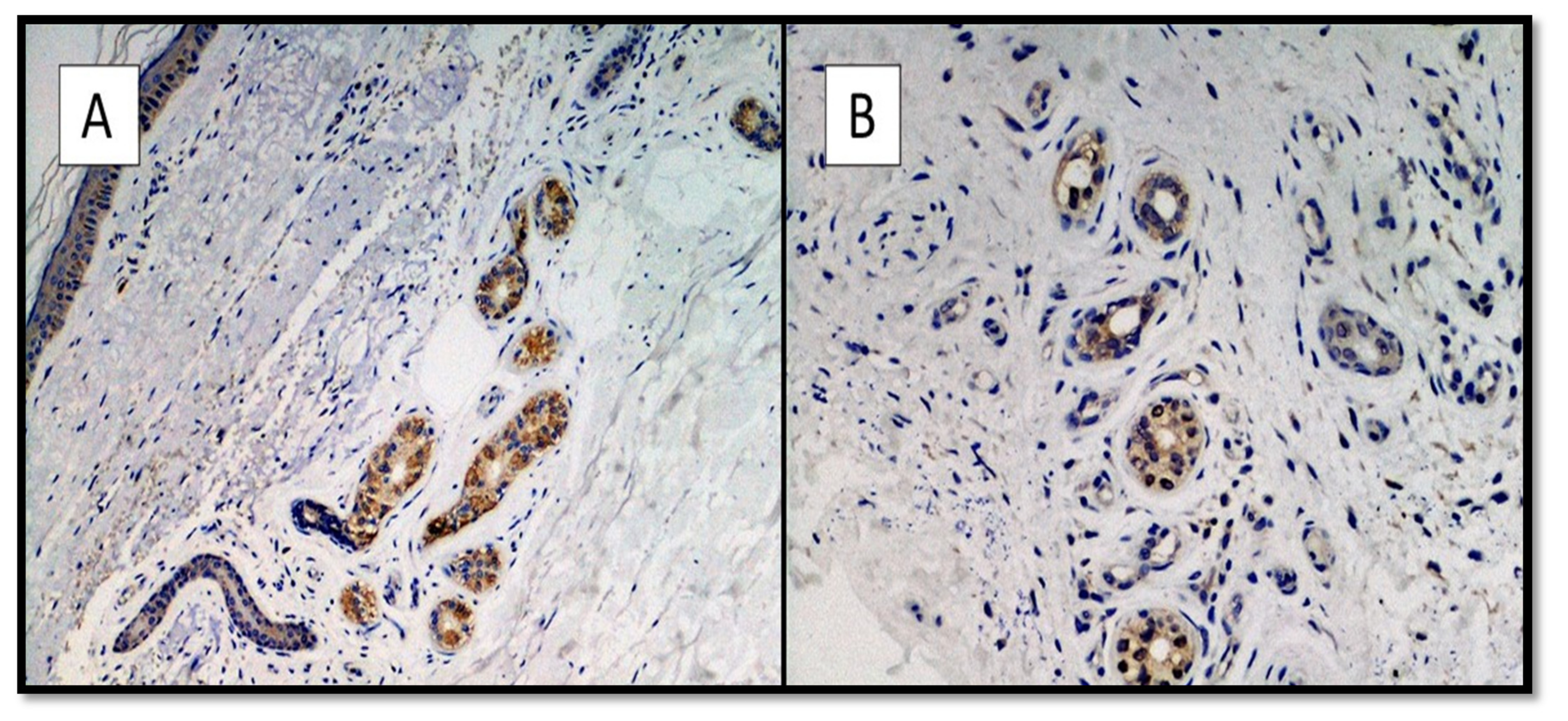


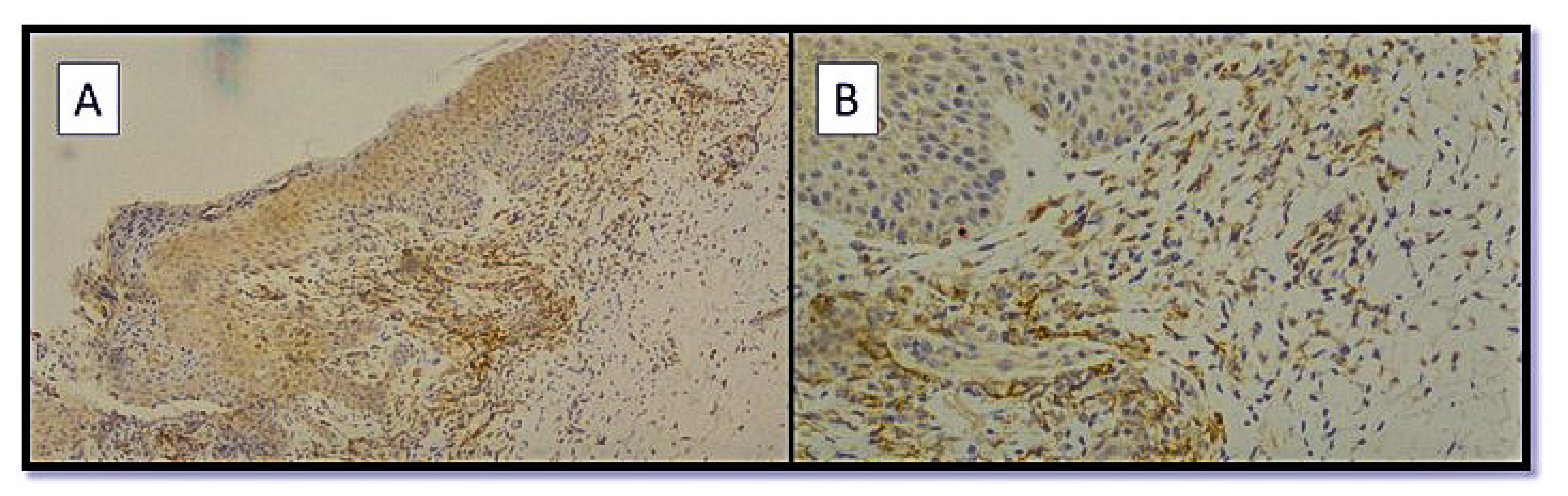
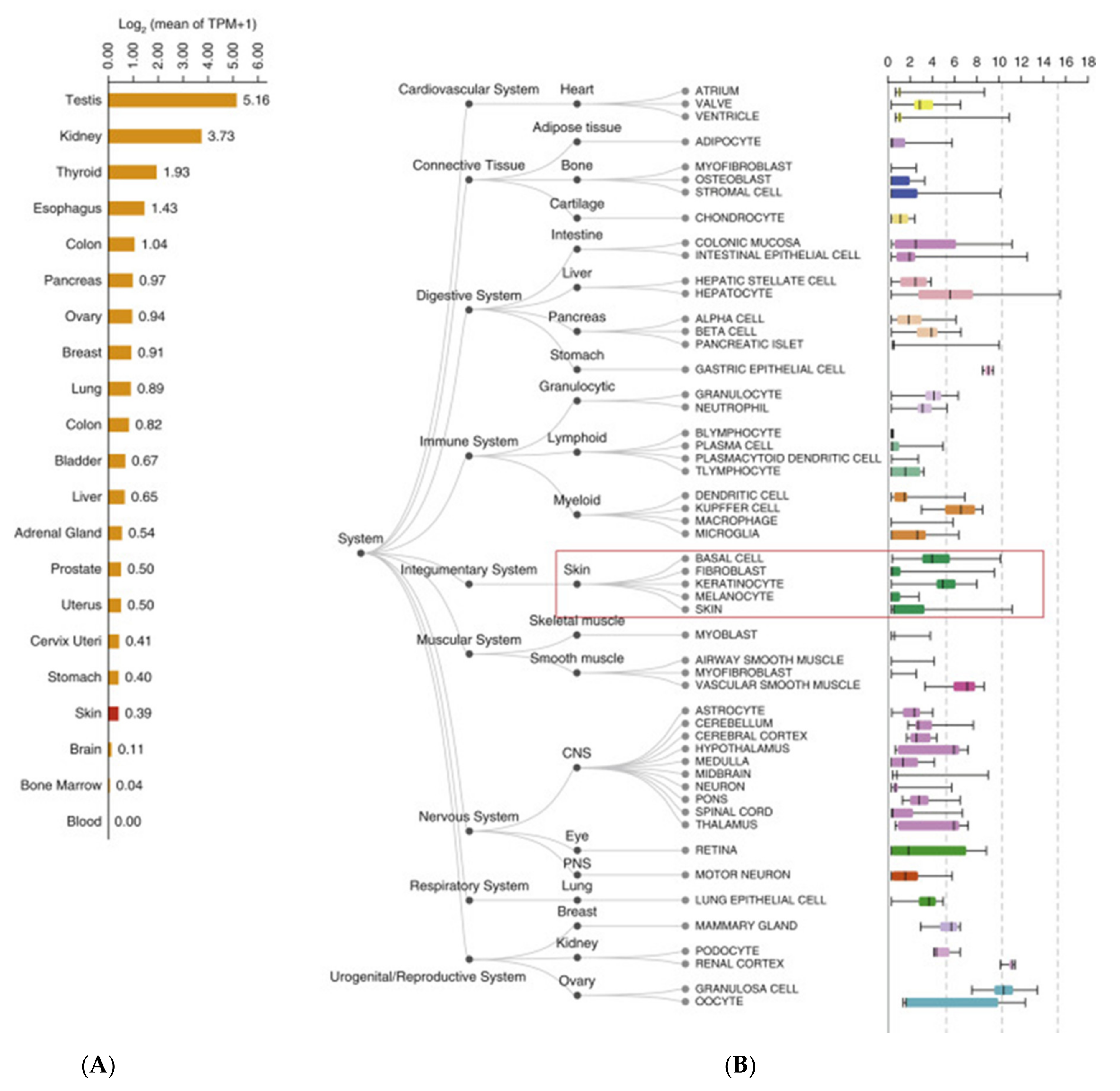


| Number of Patient | Age | Gender | Type of Dermatological Manifestation | Severity of COVID-19 |
|---|---|---|---|---|
| 1 | 62 | M | Erithema Pernio | Mild symptoms |
| 2 | 14 | M | Erithema Pernio | Mild/asymptomatic |
| 3 | 11 | M | Erithema Pernio | Mild/asymptomatic |
| 4 | 41 | M | Folliculitis-like rash | Moderate symptoms |
| 5 | 17 | F | Erithema Pernio | Mild symptoms |
| 6 | 29 | M | Urticarial rash | Mild symptoms |
| 7 | 52 | F | Varicelliform-like rash | Severe symptoms |
| 8 | 68 | F | Urticarial rash | Moderate symptoms |
| 9 | 16 | M | Erithema Pernio | Asymptomatic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cazzato, G.; Cascardi, E.; Colagrande, A.; Foti, C.; Stellacci, A.; Marrone, M.; Ingravallo, G.; Arezzo, F.; Loizzi, V.; Solimando, A.G.; et al. SARS-CoV-2 and Skin: New Insights and Perspectives. Biomolecules 2022, 12, 1212. https://doi.org/10.3390/biom12091212
Cazzato G, Cascardi E, Colagrande A, Foti C, Stellacci A, Marrone M, Ingravallo G, Arezzo F, Loizzi V, Solimando AG, et al. SARS-CoV-2 and Skin: New Insights and Perspectives. Biomolecules. 2022; 12(9):1212. https://doi.org/10.3390/biom12091212
Chicago/Turabian StyleCazzato, Gerardo, Eliano Cascardi, Anna Colagrande, Caterina Foti, Alessandra Stellacci, Maricla Marrone, Giuseppe Ingravallo, Francesca Arezzo, Vera Loizzi, Antonio Giovanni Solimando, and et al. 2022. "SARS-CoV-2 and Skin: New Insights and Perspectives" Biomolecules 12, no. 9: 1212. https://doi.org/10.3390/biom12091212
APA StyleCazzato, G., Cascardi, E., Colagrande, A., Foti, C., Stellacci, A., Marrone, M., Ingravallo, G., Arezzo, F., Loizzi, V., Solimando, A. G., Parente, P., Maiorano, E., Cormio, G., Vacca, A., & Resta, L. (2022). SARS-CoV-2 and Skin: New Insights and Perspectives. Biomolecules, 12(9), 1212. https://doi.org/10.3390/biom12091212















