Effects of Aging and Disease Conditions in Brain of Tumor-Bearing Mice: Evaluation of Purine DNA Damages and Fatty Acid Pool Changes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animal Studies and Xenografts Construction
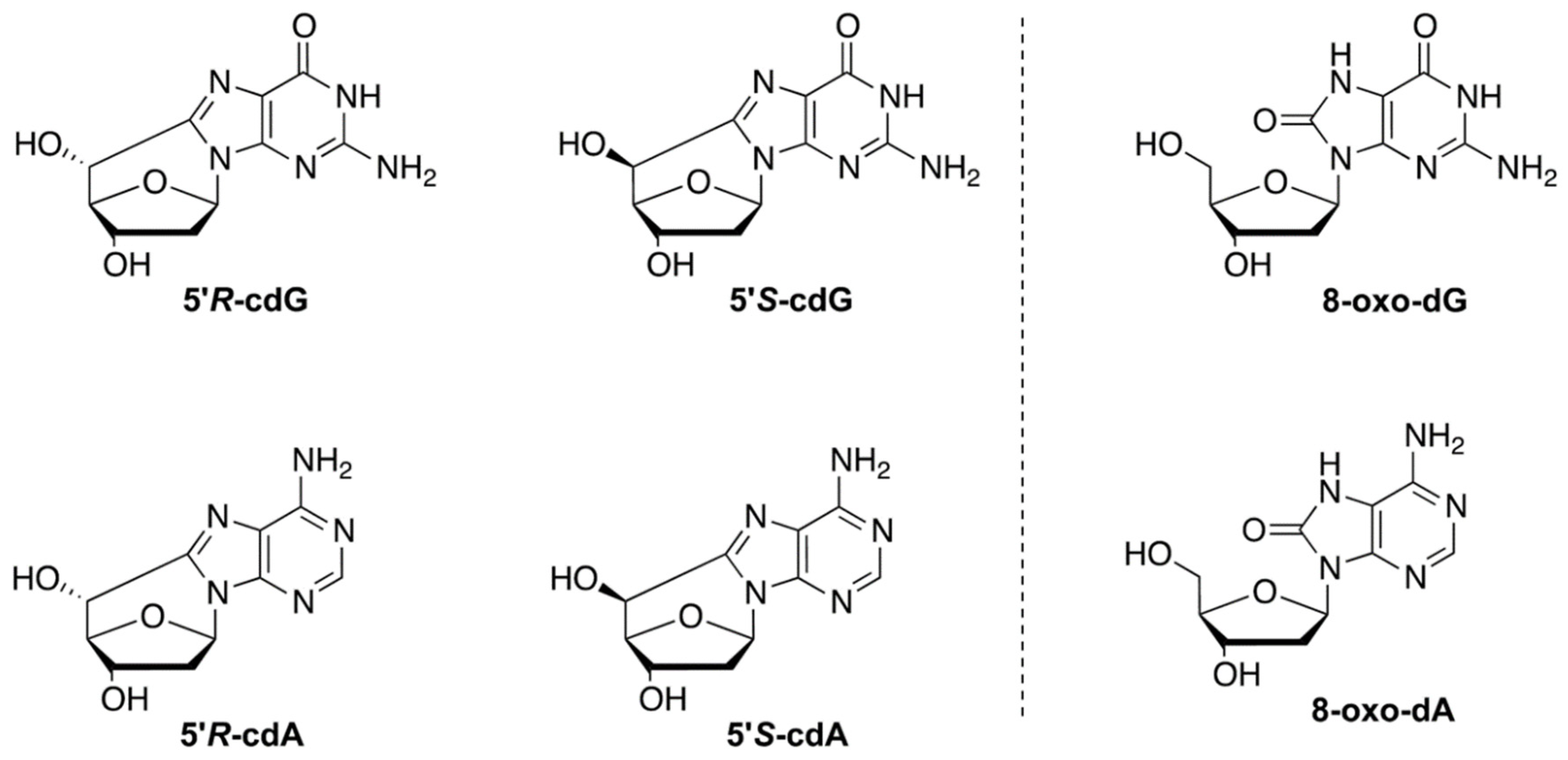
2.3. DNA Isolation and Quantification of Modified Nucleosides by Stable Isotope Dilution LC-MS/MS
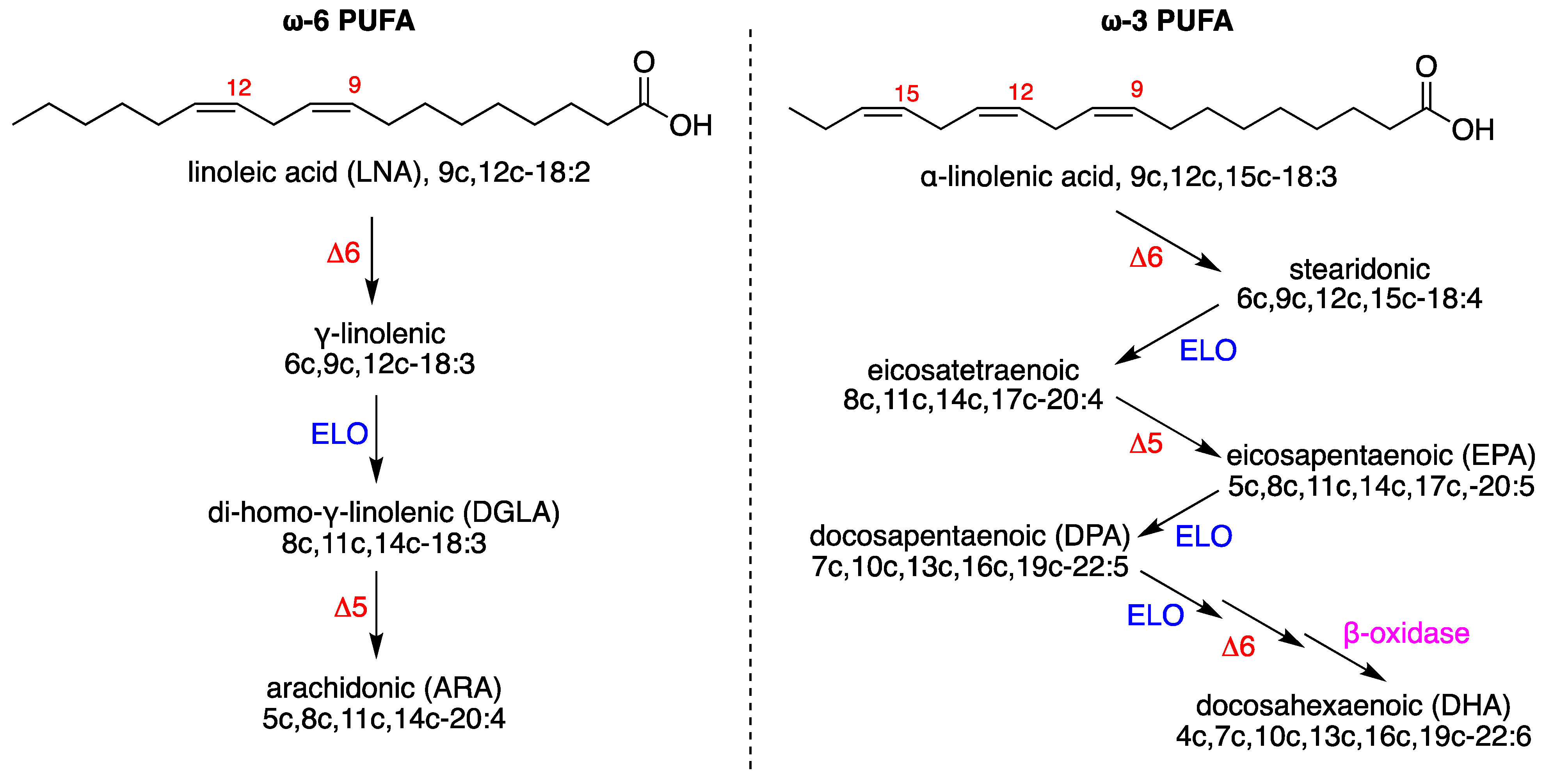
2.4. Fatty Acid Analysis of Brain Tissues
2.5. Statistical Analysis
3. Results
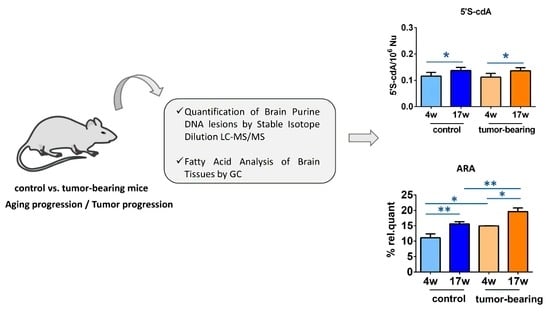
3.1. Protocol Outline
3.2. Evaluation of Purine DNA Lesions
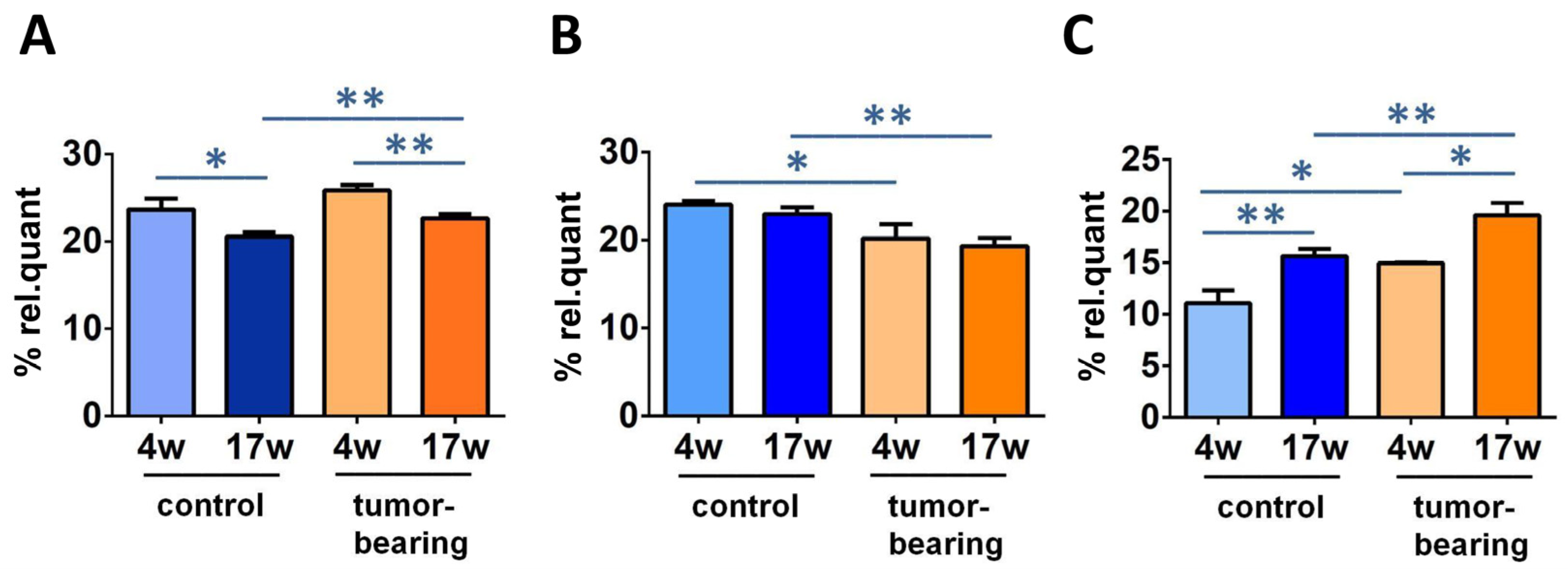
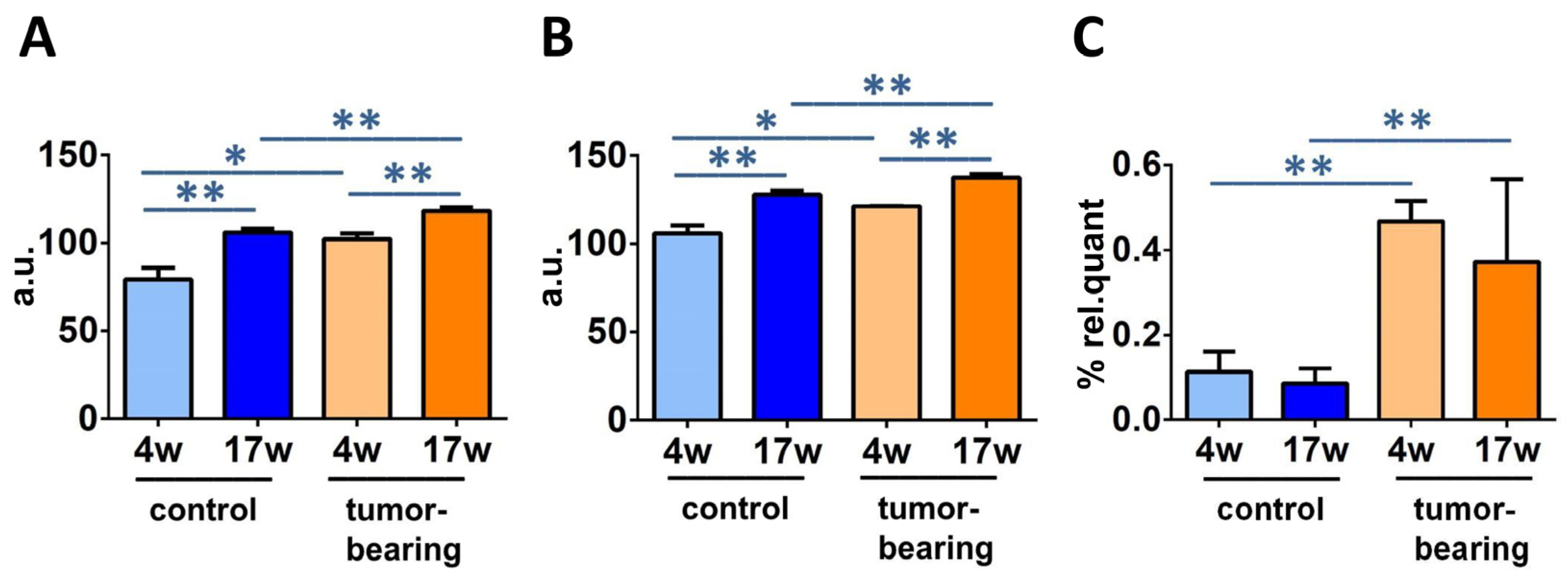
3.3. Evaluation of Brain Fatty Acid Pool
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Maynard, S.; Fang, E.F.; Scheibye-Knudsen, M.; Croteau, D.L.; Bohr, V.A. DNA Damage, DNA Repair, Aging, and Neurodegeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a025130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatgilialoglu, C.; Ferreri, C.; Geacintov, N.E.; Krokidis, M.G.; Liu, Y.; Masi, A.; Shafirovich, N.; Terzidis, M.A.; Tsegay, P.S. 5′,8-Cyclopurine lesions in DNA damage: Chemical, analytical, biological and diagnostic significance. Cells 2019, 8, 513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatgilialoglu, C.; Ferreri, C.; Krokidis, M.G.; Masi, A.; Terzidis, M. On the relevance of hydroxyl radical to purine DNA damage. Free Radic. Res. 2021, 55, 384–404. [Google Scholar] [CrossRef] [PubMed]
- Jaruga, P.; Dizdaroglu, M. 8,5′-Cyclopurine-2′-deoxynucleosides in DNA: Mechanisms of formation, measurement, repair and biological effects. DNA Repair 2008, 7, 1413–1425. [Google Scholar] [CrossRef] [PubMed]
- Kropachev, K.; Ding, S.; Terzidis, M.A.; Masi, A.; Liu, Z.; Cai, Y.; Kolbanovskiy, M.; Chatgilialoglu, C.; Broyde, S.; Geancitov, N.E.; et al. Structural basis for the recognition of diastereomeric 5′,8-cyclo-2′-deoxypurine lesions by the human nucleotide excision repair system. Nucl. Acids Res. 2014, 42, 5020–5032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuraoka, I.; Bender, C.; Romieu, A.; Cadet, J.; Wood, R.D.; Lindahl, T. Removal of oxygen free-radical-induced 5′,8-purine cyclodeoxynucleosides from DNA by the nucleotide excision-repair pathway in human cells. Proc. Natl. Acad. Sci. USA 2000, 97, 3832–3837. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Wang, P.; Cui, Y.; Wang, Y. Chemical analysis of DNA damage. Anal. Chem. 2018, 90, 556–576. [Google Scholar] [CrossRef] [PubMed]
- Chatgilialoglu, C.; Eriksson, L.A.; Krokidis, M.G.; Masi, A.; Wang, S.-D.; Zhang, R. Oxygen dependent purine lesions in double-stranded oligodeoxynucleotides: Kinetic and computational studies highlight the mechanism for 5′,8-cyplopurine formation. J. Am. Chem. Soc. 2020, 142, 5825–5833. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; Krokidis, M.G.; Masi, A.; Barata-Vallejo, S.; Ferreri, C.; Terzidis, M.A.; Szreder, T.; Bobrowski, K. New insights into the reaction paths of hydroxyl radicals with purine moieties in DNA and double-stranded oligonucleotides. Molecules 2019, 24, 3860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krokidis, M.G.; Parlanti, E.; D’Errico, M.; Pascucci, B.; Pino, A.; Alimonti, A.; Pietraforte, D.; Masi, A.; Ferreri, C.; Chatgilialoglu, C. Purine DNA lesions at different oxygen concentration in DNA repair-impaired human cells (EUE-siXPA). Cells 2019, 8, 1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krokidis, M.G.; D’Errico, M.; Pascucci, B.; Parlanti, E.; Masi, A.; Ferreri, C.; Chatgilialoglu, C. Oxygen-Dependent Accumulation of Purine DNA Lesions in Cockayne Syndrome Cells. Cells 2020, 9, 1671. [Google Scholar] [CrossRef]
- A Floyd, R.; Hensley, K. Oxidative stress in brain aging: Implications for therapeutics of neurodegenerative diseases. Neurobiol. Aging 2002, 23, 795–807. [Google Scholar] [CrossRef]
- Mori, T.; Nakane, H.; Iwamoto, T.; Krokidis, M.G.; Chatgilialoglu, C.; Tanaka, K.; Kaidoh, T.; Hasegawa, M.; Sugiura, S. High levels of oxidatively generated DNA damage 8,5′-cyclo-2′-deoxyadenosine accumulate in the brain tissues of xeroderma pigmentosum group A gene-knockout mice. DNA Repair 2019, 80, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Malard, E.; Valable, S.; Bernaudin, M.; Pérès, E.; Chatre, L. The reactive species interactome in the brain. Antioxid. Redox Signal. 2021, 35, 1176–1206. [Google Scholar] [CrossRef]
- Bozek, K.; Wei, Y.; Yan, Z.; Liu, X.; Xiong, J.; Sugimoto, M.; Tomita, M.; Pääbo, S.; Sherwood, C.C.; Hof, P.R.; et al. Organization and evolution of brain lipidome revealed by large-scale analysis of human, chimpanzee, macaque, and mouse tissues. Neuron 2015, 85, 695–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, W.; Chen, J.; Gong, L.; Liu, F.; Zhao, H.; Mu, J. Alteration of Glycerophospholipid Metabolism in Hippocampus of Post-stroke Depression Rats. J. Neurochem. Res. 2022, 47, 2052–2063. [Google Scholar] [CrossRef]
- Castellanos, D.B.; Martín-Jiménez, C.A.; Rojas-Rodríguez, F.; Barreto, G.E.; González, J. Brain lipidomics as a rising field in neurodegenerative contexts: Perspectives with Machine Learning approaches. Front. Neuroendocrin. 2021, 61, 100899. [Google Scholar] [CrossRef]
- Bazinet, R.P.; Layé, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef]
- Contreras, M.A.; Rapoport, S.I. Recent studies on interactions between n-3 and n-6 polyunsaturated fatty acids in brain and other tissues. Curr. Opin. Lipidol. 2002, 13, 267–272. [Google Scholar] [CrossRef]
- Farooqui, A.A.; Horrocks, L.A.; Farooqui, T. Modulation of inflammation in brain: A matter of fat. J. Neurochem. 2007, 101, 577–599. [Google Scholar] [CrossRef] [PubMed]
- Darios, F.; Davletov, B. ω-3 and ω-6 fatty acids stimulate cell membrane expansion by acting on syntaxin 3. Nature 2006, 440, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Innis, S.M. Dietary ω 3 fatty acids and the developing brain. Brain Res. 2008, 1237, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Calon, F.; Lim, G.P.; Yang, F.; Morihara, T.; Teter, B.; Ubeda, O.; Rostaing, P.; Triller, A.; Salem, N., Jr.; Ashe, K.H.; et al. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer’s disease mouse model. Neuron 2004, 43, 633–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robson, L.G.; Dyall, S.; Sidloff, D.; Michael-Titus, A.T. Omega-3 polyunsaturated fatty acids increase the neurite outgrowth of rat sensory neurones throughout development and in aged animals. Neurobiol. Aging 2010, 31, 678–687. [Google Scholar] [CrossRef]
- Bousquet, M.; Calon, F.; Cicchetti, F. Impact of ω-3 fatty acids in Parkinson’s disease. Ageing Res. Rev. 2011, 10, 453–463. [Google Scholar] [CrossRef]
- Hernando, S.; Requejo, C.; Herran, E.; Ruiz-Ortega, J.A.; Morera-Herreras, T.; Lafuente, J.V.; Ugedo, L.; Gainza, E.; Pedraz, J.L.; Igartua, M.; et al. Beneficial effects of n-3 polyunsaturated fatty acids administration in a partial lesion model of Parkinson’s disease: The role of glia and NRf2 regulation. Neurobiol. Dis. 2019, 121, 252–262. [Google Scholar] [CrossRef]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef]
- Gumusyayla, S.; Vural, G.; Bektas, H.; Deniz, O.; Neselioglu, S.; Erel, O. A novel oxidative stress marker in patients with Alzheimer’s disease: Dynamic thiol–disulphide homeostasis. Acta Neuropsychiatr. 2016, 28, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Zanganehnejad, Z.; Setorki, M. Effect of Biarum carduchrum extract on brain tissue thiol level in rat model of 6-hydroxydopamine-induced Parkinson’s disease. J. Herbmed. Pharmacol. 2018, 7, 136–140. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; Ferreri, C. Trans lipids: The free radical path. Acc. Chem. Res. 2005, 38, 441–448. [Google Scholar] [CrossRef]
- Ferreri, C.; Chatgilialoglu, C. Geometrical trans lipid isomers: A new target for lipidomics. ChemBioChem 2005, 6, 1722–1734. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, C.; Chatgilialoglu, C. Role of fatty acid-based functional lipidomics in the development of molecular diagnostic tools. Expert Rev. Mol. Diagn. 2012, 12, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Pamplona, R. Membrane phospholipids, lipoxidative damage and molecular integrity: A causal role in aging and longevity. Biochim. Biophys. Acta-Bioenergy 2008, 1777, 1249–1262. [Google Scholar] [CrossRef] [Green Version]
- Ferreri, C.; Masi, A.; Sansone, A.; Giacometti, G.; Larocca, A.; Menounou, G.; Scanferlato, R.; Tortorella, S.; Rota, D.; Chatgilialoglu, C. Fatty acids in Membranes as Homeostatic, Metabolic and Nutritional Biomarkers: Recent Advancements in Analytics and Diagnostics. Diagnostics 2017, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Krokidis, M.G.; Louka, M.; Efthimiadou, E.K.; Zervou, S.-K.; Papadopoulos, K.; Hiskia, A.; Ferreri, C.; Chatgilialoglu, C. Membrane lipidome reorganization and accumulation of tissue DNA lesions in tumor-bearing mice: An exploratory study. Cancers 2019, 11, 480. [Google Scholar] [CrossRef] [Green Version]
- Terzidis, M.A.; Chatgilialoglu, C. An ameliorative protocol for the quantification of purine 5′,8-cyclo-2′-deoxynucleosides in oxidized DNA. Front. Chem. 2015, 3, 47. [Google Scholar] [CrossRef] [Green Version]
- Chatgilialoglu, C. Cyclopurine (cPu) lesions: What, how and why? Free Radic. Res. 2019, 53, 941–943. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Prasinou, P.; Dafnis, I.; Giacometti, G.; Ferreri, C.; Chroni, A.; Chatgilialoglu, C. Fatty acid-based lipidomics and membrane remodeling induced by apoE3 and apoE4 in human neuroblastoma cells. Biochim. Biophys. Acta-Biomembr. 2017, 1859, 1967–1973. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, C.; Sansone, A.; Krokidis, M.G.; Masi, A.; Pascucci, B.; D’Errico, M.; Chatgilialoglu, C. Effects of Oxygen Tension for Membrane Lipidome Remodeling of Cockayne Syndrome Cell Models. Cells 2022, 11, 1286. [Google Scholar] [CrossRef] [PubMed]
- Morton, C.L.; Houghton, P.J. Establishment of human tumor xenografts in immunodeficient mice. Nat. Protoc. 2007, 2, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Redon, C.E.; Dickey, J.S.; Nakamura, A.J.; Kareva, I.G.; Naf, D.; Nowsheen, S.; Kryston, T.B.; Bonner, W.M.; Georgakilas, A.G.; Sedelnikova, O.A. Tumors induce complex DNA damage in distant proliferative tissues in vivo. Proc. Natl. Acad. Sci. USA 2010, 107, 17992–17997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hulbert, A.J. Explaining longevity of different animals: Is membrane fatty acid composition the missing link? Age 2008, 30, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Puca, A.A.; Andrew, P.; Novelli, V.; Viviani, C.; Somalvico, F.; Cirillo, N.A.; Chatgilialoglu, C.; Ferreri, C. Fatty acids profile of erythrocyte membranes as possible biomarker of longevity. Rejuvenation Res. 2008, 11, 63–72. [Google Scholar] [CrossRef]
- Hung, W.-L.; Whang, L.S.; Shahidi, F.; Pan, M.-H. Endogenous formation of trans fatty acids: Health implications and potential dietary intervention. J. Funct. Foods 2014, 25, 14–24. [Google Scholar] [CrossRef]
- Narciso, L.; Parlanti, E.; Racaniello, M.; Simonelli, V.; Cardinale, A.; Merlo, D.; Dogliotti, E. The Response to Oxidative DNA Damage in Neurons: Mechanisms and Disease. Neural Plast. 2016, 2016, 3619274. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.; Alavi, M.V.; Kim, K.Y.; Kang, T.; Scott, R.T.; Noh, Y.H.; Lindsey, J.D.; Wissinger, B.; Ellisman, M.H.; Weinreb, R.N.; et al. A new vicious cycle involving glutamate excitotoxicity, oxidative stress and mitochondrial dynamics. Cell Death Dis. 2011, 2, e240. [Google Scholar] [CrossRef]
- Jurk, D.; Wang, C.; Miwa, S.; Maddick, M.; Korolchuk, V.; Tsolou, A.; Gonos, E.S.; Thrasivoulou, C.; Saffrey, M.J.; Cameron, K.; et al. Postmitotic neurons develop a p21-dependent senescence-like phenotype driven by a DNA damage response. Aging Cell. 2012, 11, 996–1004. [Google Scholar] [CrossRef] [Green Version]
- Shanbhag, N.M.; Evans, M.D.; Mao, W.; Nana, A.L.; Seeley, W.W.; Adame, A.; Rissman, R.A.; Masliah, E.; Mucke, L. Early neuronal accumulation of DNA double strand breaks in Alzheimer’s disease. Acta Neuropathol. Commun. 2019, 7, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canugovi, C.; Misiak, M.; Ferrarelli, L.K.; Croteau, D.L.; Bohr, V.A. The role of DNA repair in brain related disease pathology. DNA Repair 2013, 12, 578–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borgesius, N.Z.; de Waard, M.C.; van der Pluijm, I.; Omrani, A.; Zondag, G.C.; van der Horst, G.T.; Melton, D.W.; Hoeijmakers, J.H.; Jaarsma, D.; Elgersma, Y. Accelerated age-related cognitive decline and neurodegeneration, caused by deficient DNA repair. J. Neurosci. 2011, 31, 12543–12553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flyunt, R.; Bazzanini, R.; Chatgilialoglu, C.; Mulazzani, Q.G. Fate of the 2′-Deoxyadenosin-5′-yl Radical under Anaerobic Conditions. J. Am. Chem. Soc. 2000, 122, 4225–4226. [Google Scholar] [CrossRef]
- Cui, L.; Ye, W.; Prestwich, E.G.; Wishnok, J.S.; Taghizadeh, K.; Dedon, P.C.; Tannenbaum, S.R. Comparative analysis of four oxidized guanine lesions from reactions of DNA with peroxynitrite, single oxygen, and γ-radiation. Chem. Res. Toxicol. 2013, 26, 195–202. [Google Scholar] [CrossRef]
- Chatgilialoglu, C. Biomimetic radical chemistry and applications. Molecules 2022, 27, 2042. [Google Scholar] [CrossRef]
- Wang, J.; Clauson, C.L.; Robbins, P.D.; Niedernhofer, L.J.; Wang, Y. The oxidative DNA lesions 8,5′-cyclopurines accumulate with aging in a tissue-specific manner. Aging Cell 2012, 11, 714–716. [Google Scholar] [CrossRef] [Green Version]
- Iwamoto, T.; Brooks, P.J.; Nishiwaki, T.; Nishimura, K.; Kobayashi, N.; Sugiura, S.; Mori, T. Quantitative and in situ detection of oxidatively generated DNA damage 8,5′-cyclo-2′-deoxyadenosine using an immunoassay with a novel monoclonal antibody. Photochem. Photobiol. 2014, 90, 829–836. [Google Scholar]
- Cooke, M.S.; Olinski, R.; Loft, S. European standards committee on urinary (DNA) lesion analysis. Cancer Epidemiol. Biomark. Prev. 2008, 17, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Chung, M.K.; Riby, J.; Li, H.; Iavarone, A.T.; Williams, E.R.; Zheng, Y.; Rappaport, S.M. A sandwich enzyme-linked immunosorbent assay for adducts of polycyclic aromatic hydrocarbons with human serum albumin. Anal. Biochem. 2010, 400, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Okahashi, Y.T.; Iwamoto, N.; Suzuki, S.; Shibutani, S.; Sugiura, S.; Itoh, T.; Nishiwaki, S.; Ueno, T.; Mori, T. Quantitative detection of 4-hydroxyequilenin-DNA adducts in mammalian cells using an immunoassay with a novel monoclonal antibody. Nucl. Acids Res. 2010, 38, e133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Wang, Y. Mass spectrometry for the assessment of the occurrence and biological consequences of DNA adducts. Chem. Soc. Rev. 2015, 44, 7829–7854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dizdaroglu, M.; Coskun, E.; Jaruga, P. Measurement of oxidatively induced DNA damage and its repair, by mass spectrometric techniques. Free. Radic. Res. 2015, 49, 525–548. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, B.; Guerrero, C.; Bahde, R.; Gupta, S.; Wang, Y. Quantification of oxidative DNA lesions in tissues of Long-Evans Cinnamon rats by capillary high-performance liquid chromatography-tandem mass spectrometry coupled with stable isotope dilution method. Anal. Chem. 2011, 83, 2201–2209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egler, R.A.; Fernandes, E.; Rothermund, K.; Sereika, S.; de Souza-Pinto, N.; Jaruga, P.; Dizdaroglu, M.; Prochownik, E.V. Regulation of reactive oxygen species, DNA damage, and c-Myc function by peroxiredoxin1. Oncogene 2005, 24, 8038–8050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirkali, G.; de Souza-Pinto, N.C.; Jaruga, P.; Bohr, V.A.; Dizdaroglu, M. Accumulation of (5′S)-8,5′-cyclo-2′-deoxyadenosine in organs of Cockayne syndrome complementation group B gene knockout mice. DNA Repair 2009, 8, 274–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schumacher, B.; Pothof, J.; Vijg, J.; Hoeijmakers, J.H.J. The central role of DNA damage in the ageing process. Nature 2021, 592, 695–703. [Google Scholar] [CrossRef]
- Kaluzny, M.A.; Duncan, L.A.; Merritt, M.V.; Epps, D.E. Rapid separation of lipid classes in high yield and purity using bonded phase columns. J. Lipid Res. 1985, 26, 135–140. [Google Scholar] [CrossRef]
- Fragopoulou, A.F.; Polyzos, A.; Papadopoulou, M.D.; Sansone, A.; Manta, A.K.; Balafas, E.; Kostomitsopoulos, N.; Skouroliakou, A.; Chatgilialoglu, C.; Georgakilas, A.; et al. Hippocampal lipidome and transcriptome profile alterations triggered by acute exposure of mice to GSM 1800 MHz mobile phone radiation: An exploratory study. Brain Behav. 2018, 8, e01001. [Google Scholar] [CrossRef]
- Pakiet, A.; Jakubiak, A.; Czumaj, A.; Sledzinski, T.; Mika, A. The effect of western diet on mice brain lipid composition. Nutr. Metab. 2019, 16, 81. [Google Scholar] [CrossRef]
- Chan, R.B.; Oliveira, T.G.; Cortes, E.P.; Honig, L.S.; Duff, K.E.; Small, S.A.; Wenk, M.R.; Shui, G.; Di Paolo, G. Comparative Lipidomic Analysis of Mouse and Human Brain with Alzheimer Disease. J. Biol. Chem. 2012, 287, 2678–2688. [Google Scholar] [CrossRef] [Green Version]
- Ferreri, C.; Samadi, A.; Sassatelli, F.; Landi, L.; Chatgilialoglu, C. Regioselective Cis-Trans Isomerization of Arachidonic Double Bonds by Thiyl Radicals: The Influence of Phospholipid Supramolecular Organization. J. Am. Chem. Soc. 2004, 126, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- van Diepen, J.A.; Berbée, J.F.; Havekes, L.M.; Rensen, P.C. Interactions between inflammation and lipid metabolism: Relevance for efficacy of anti-inflammatory drugs in the treatment of atherosclerosis. Atherosclerosis 2013, 228, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, S.; Shan, H.; Zhang, M. Biologic Effect of Hydrogen Sulfide and Its Role in Traumatic Brain Injury. Oxid. Med. Cell. Longev. 2020, 2020, 7301615. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Michaelis, E.K. Selective neuronal vulnerability to oxidative stress in the brain. Front. Aging Neurosci. 2010, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Else, P.L.; Hulbert, A.J. Membranes as metabolic pacemakers. Clin. Exp. Pharmacol. Physiol. 2003, 30, 559–564. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Huang, X. Lipid metabolism at membrane contacts: Dynamics and functions beyond lipid homeostasis. Front. Cell Dev. Biol. 2020, 8, 615856. [Google Scholar] [CrossRef]
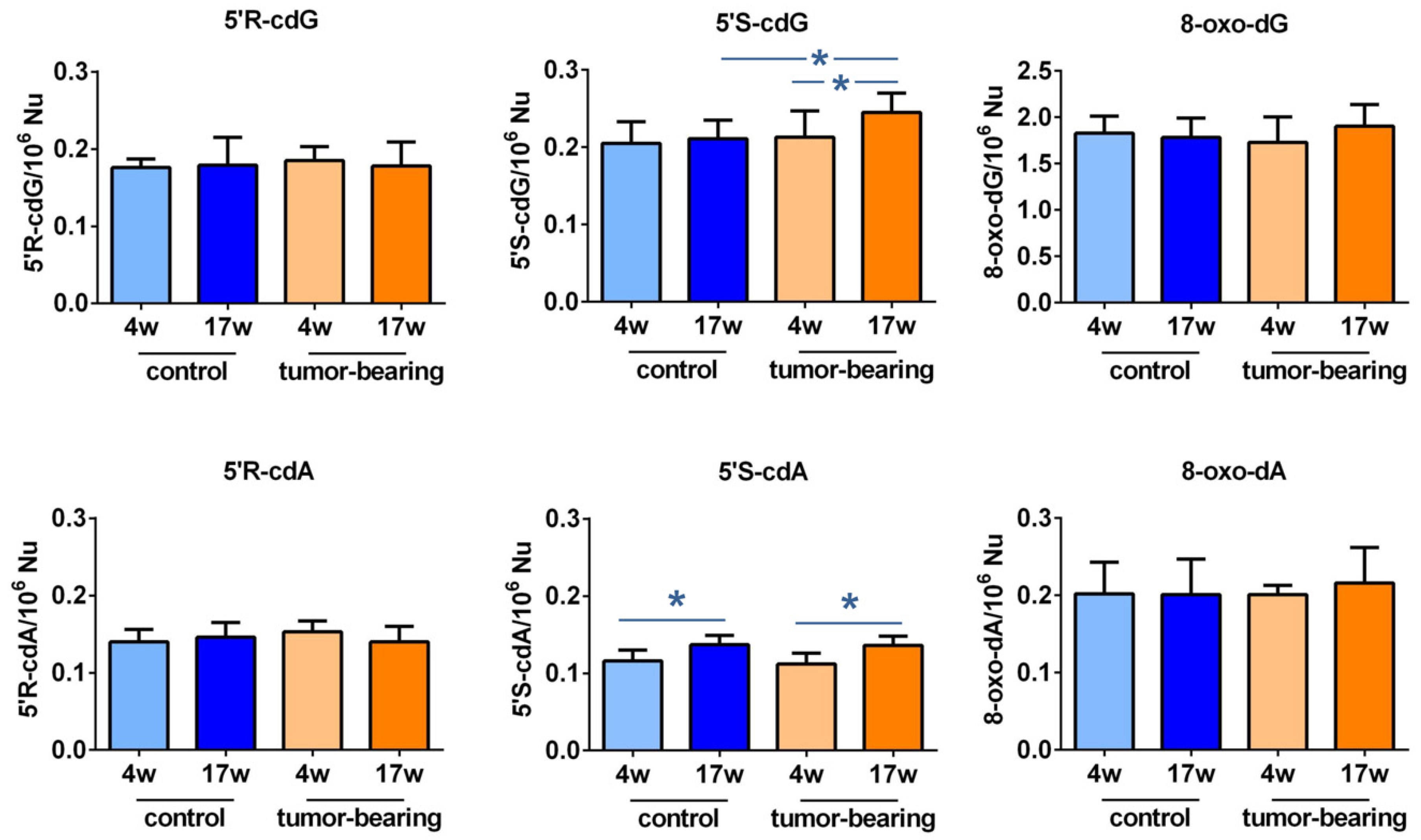
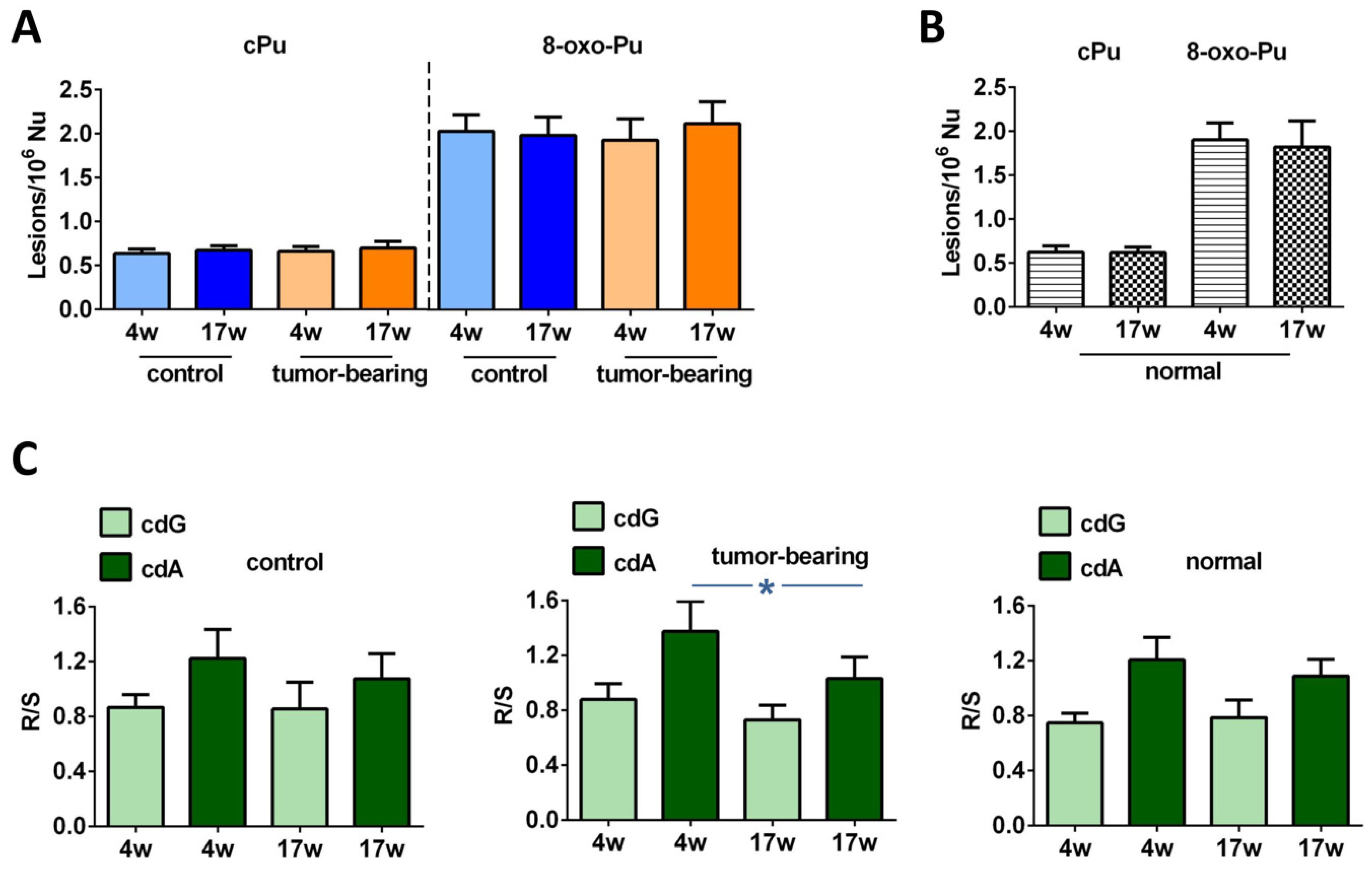
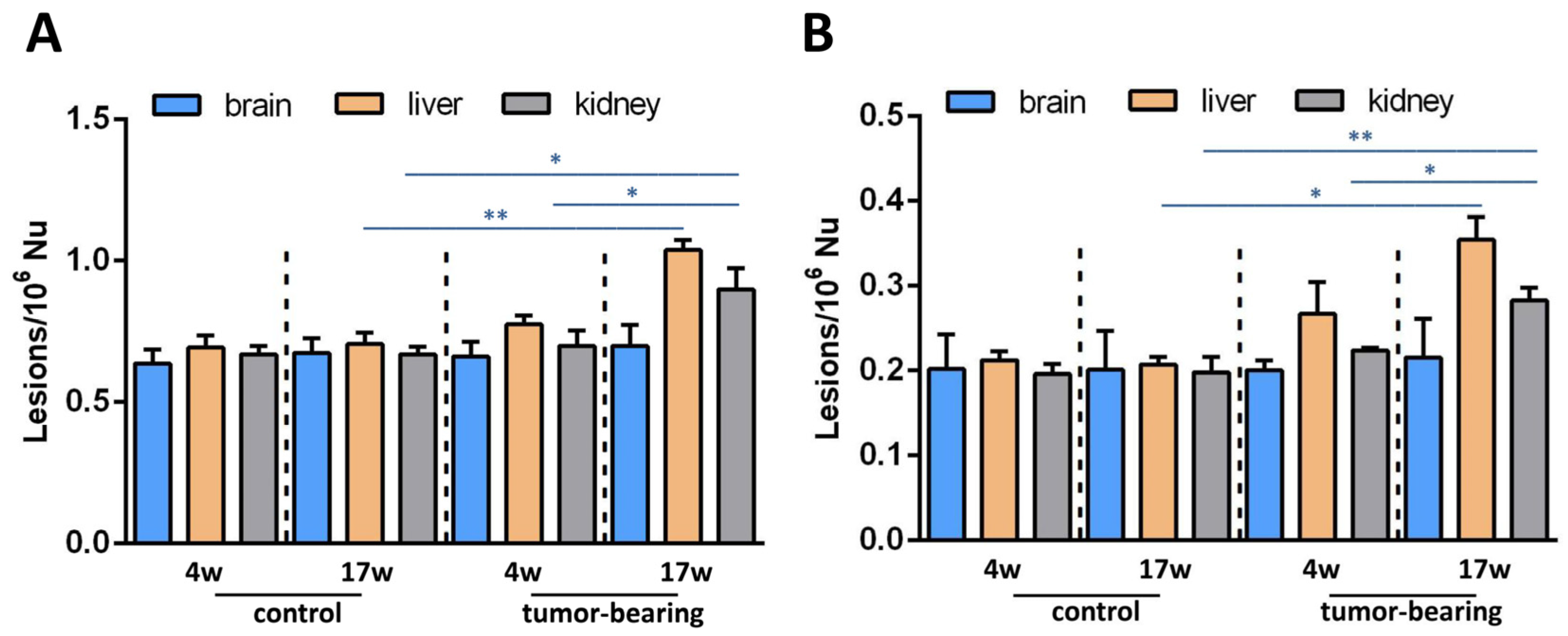
| 5′R-cdG | 5′R-cdA | 5′S-cdG | 5′S-cdA | 8-oxo-dG | 8-oxo-dA | |
|---|---|---|---|---|---|---|
| control 4w | 0.176 ± 0.011 | 0.140 ± 0.016 | 0.205 ± 0.028 | 0.116 ± 0.014 | 1.828 ± 0.183 | 0.202 ± 0.041 |
| control 17w | 0.179 ± 0.036 | 0.146 ± 0.019 | 0.211 ± 0.024 1,* | 0.137 ± 0.012 2,* | 1.783 ± 0.208 | 0.201 ± 0.046 |
| tumor-bearing 4w | 0.185 ± 0.018 | 0.153 ± 0.014 | 0.213 ± 0.034 | 0.112 ± 0.014 | 1.730 ± 0.276 | 0.201 ± 0.012 |
| tumor-bearing 17w | 0.178 ± 0.031 | 0.140 ± 0.020 | 0.245 ± 0.025 3,* | 0.136 ± 0.012 3,* | 1.903 ± 0.234 | 0.216 ± 0.046 |
| FAME | FA Family | Control (4w) | Control (17w) | Tumor- Bearing (4w) | Tumor- Bearing (17w) |
|---|---|---|---|---|---|
| Palmitic acid (16:0) | SFA | 23.67 ± 1.27 * | 20.53 ± 0.54 ** | 25.87 ± 0.60 | 22.67 ± 0.51 ** |
| Stearic acid (18:0) | 26.20 ± 1.77 | 26.24 ± 0.40 | 23.58 ± 0.04 | 24.22 ± 0.88 * | |
| Palmitoleic acid (9c-16:1) | MUFA | 0.70 ± 0.25 | 0.79 ± 0.22 | 0.86 ± 0.01 | 0.67 ± 0.14 |
| Oleic acid (9c-18:1) | 24.02 ± 0.41 | 22.96 ± 0.75 | 20.19 ± 1.60 * | 19.32 ± 0.91 ** | |
| Vaccenic acid (11c-18:1) | 8.01 ± 1.31 | 6.76 ± 0.83 | 6.12 ± 0.83 | 6.09 ± 0.09 | |
| LNA (18:2-ω6) | 1.02 ± 0.16 | 0.95 ± 0.12 | 0.92 ± 0.02 | 1.14 ± 0.15 | |
| DGLA (20:3-ω6) | PUFA ω6 | 1.18 ± 0.07 | 0.87 ± 0.21 | 0.82 ± 0.07 | 1.04 ± 0.12 |
| ARA (20:4-ω6) | 11.06 ± 1.26 ** | 15.63 ± 0.71 * | 14.96 ± 0.07 * | 19.62 ± 1.18 ** | |
| EPA (20:5-ω3) | PUFA ω3 | 0.57 ± 0.50 | 0.53 ± 0.18 | 1.13 ± 0.29 | 1.08 ± 0.61 |
| DHA (22:6-ω3) | 3.40 ± 0.16 ** | 4.60 ± 0.20 | 4.04 ± 0.18 * | 3.67 ± 0.65 | |
| 9t-18:1 | TFA | 0.03 ± 0.02 | 0.06 ± 0.04 | 0.06 ± 0.03 | 0.10 ± 0.05 |
| mt-ARA 20:4 | 0.11 ± 0.05 | 0.09 ± 0.03 | 0.47 ± 0.05 ** | 0.37 ± 0.19 * |
| FA Family | Index | Control (4w) | Control (17w) | Tumor- Bearing (4w) | Tumor- Bearing (17w) |
|---|---|---|---|---|---|
| SFA | 49.87 ± 0.50 ** | 46.77 ± 0.83 * | 49.45 ± 0.56 | 46.89 ± 0.47 | |
| MUFA | 32.74 ± 1.68 | 30.51 ± 0.48 | 27.16 ± 2.44 | 26.09 ± 0.83 ** | |
| PUFA ω6 | 13.26 ± 1.16 ** | 17.45 ± 0.61 * | 16.70 ± 0.01 * | 21.79 ± 1.36 ** | |
| PUFA ω3 | 3.98 ± 0.66 | 5.13 ± 0.33 | 5.17 ± 0.46 | 4.76 ± 0.42 | |
| PUFA 1 | 17.24 ± 1.36 ** | 22.58 ± 0.51 ** | 21.87 ± 0.45 * | 26.55 ± 0.94 ** | |
| TFA | 0.15 ± 0.04 | 0.14 ± 0.04 | 0.52 ± 0.01 ** | 0.47 ± 0.23 ** | |
| SFA/MUFA | 1.53 ± 0.09 | 1.53 ± 0.05 | 1.83 ± 0.19 | 1.80 ± 0.05 ** | |
| SFA/PUFA | 2.90 ± 0.22 ** | 2.07 ± 0.08 ** | 2.26 ± 0.02 * | 1.77 ± 0.08 ** | |
| ω6/ω3 | 3.39 ± 0.58 | 3.41 ± 0.29 | 3.25 ± 0.29 | 4.62 ± 0.72 | |
| Unsaturation Index (UI) | 105.87 ± 4.48 ** | 127.78 ± 2.38 ** | 121.17 ± 0.11 * | 137.40 ± 2.14 ** | |
| Peroxidation Index (PI) | 79.13 ± 6.76 ** | 105.94 ± 2.22 ** | 102.15 ± 3.22 * | 118.22 ± 1.88 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krokidis, M.G.; Prasinou, P.; Efthimiadou, E.K.; Boari, A.; Ferreri, C.; Chatgilialoglu, C. Effects of Aging and Disease Conditions in Brain of Tumor-Bearing Mice: Evaluation of Purine DNA Damages and Fatty Acid Pool Changes. Biomolecules 2022, 12, 1075. https://doi.org/10.3390/biom12081075
Krokidis MG, Prasinou P, Efthimiadou EK, Boari A, Ferreri C, Chatgilialoglu C. Effects of Aging and Disease Conditions in Brain of Tumor-Bearing Mice: Evaluation of Purine DNA Damages and Fatty Acid Pool Changes. Biomolecules. 2022; 12(8):1075. https://doi.org/10.3390/biom12081075
Chicago/Turabian StyleKrokidis, Marios G., Paraskevi Prasinou, Eleni K. Efthimiadou, Andrea Boari, Carla Ferreri, and Chryssostomos Chatgilialoglu. 2022. "Effects of Aging and Disease Conditions in Brain of Tumor-Bearing Mice: Evaluation of Purine DNA Damages and Fatty Acid Pool Changes" Biomolecules 12, no. 8: 1075. https://doi.org/10.3390/biom12081075
APA StyleKrokidis, M. G., Prasinou, P., Efthimiadou, E. K., Boari, A., Ferreri, C., & Chatgilialoglu, C. (2022). Effects of Aging and Disease Conditions in Brain of Tumor-Bearing Mice: Evaluation of Purine DNA Damages and Fatty Acid Pool Changes. Biomolecules, 12(8), 1075. https://doi.org/10.3390/biom12081075