Detergent-Free Isolation of Membrane Proteins and Strategies to Study Them in a Near-Native Membrane Environment
Abstract
1. Introduction
2. Traditional Approaches to Express, Purify and Reconstitute Membrane Proteins
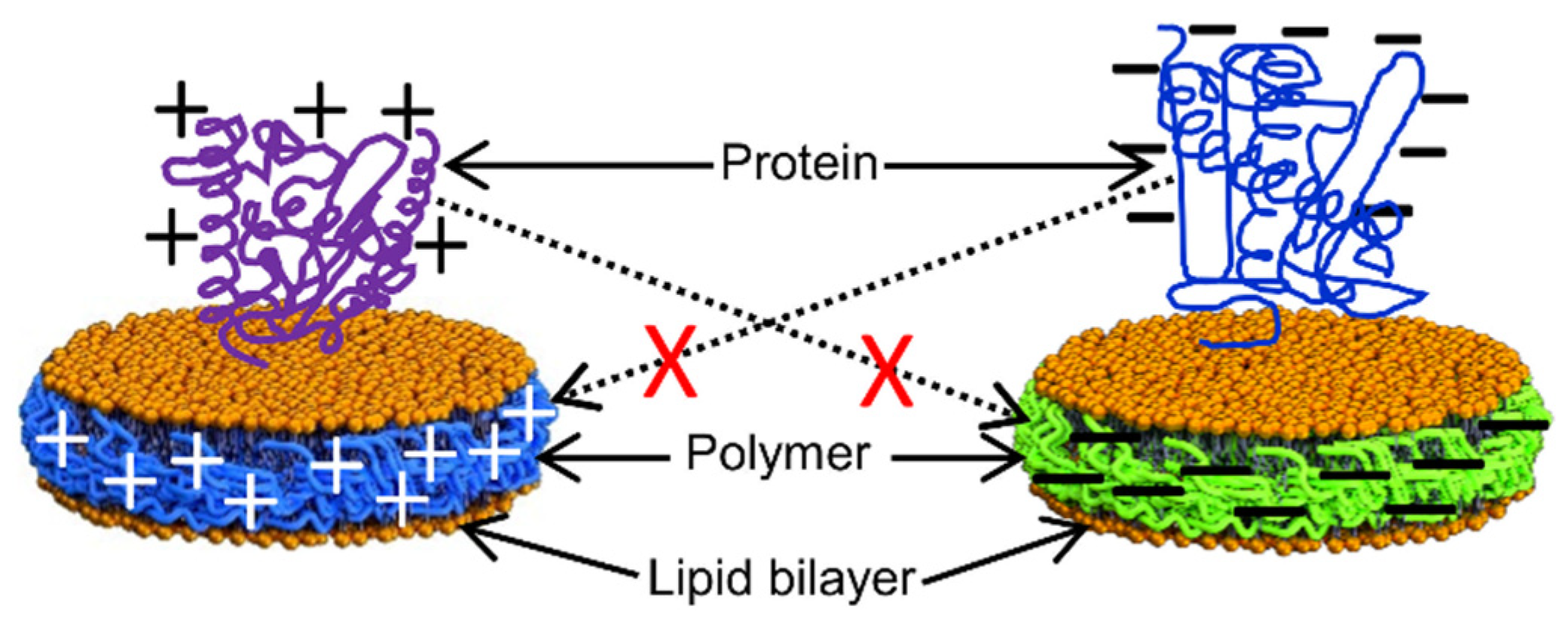
3. Challenges with the Purification and Reconstitution of Membrane Proteins
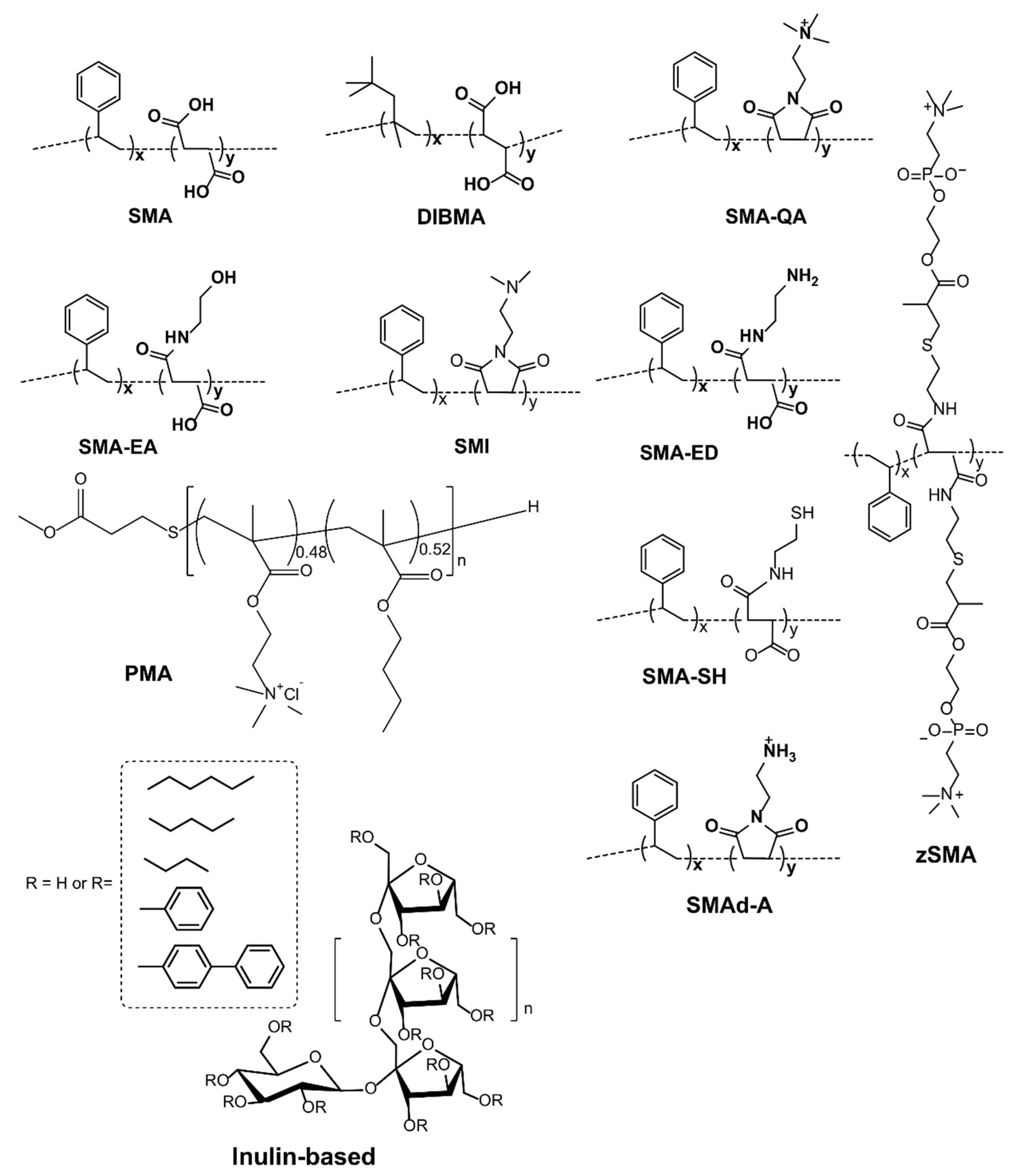
4. Detergent-Free Isolation of Membrane Proteins Using Amphipathic Polymers
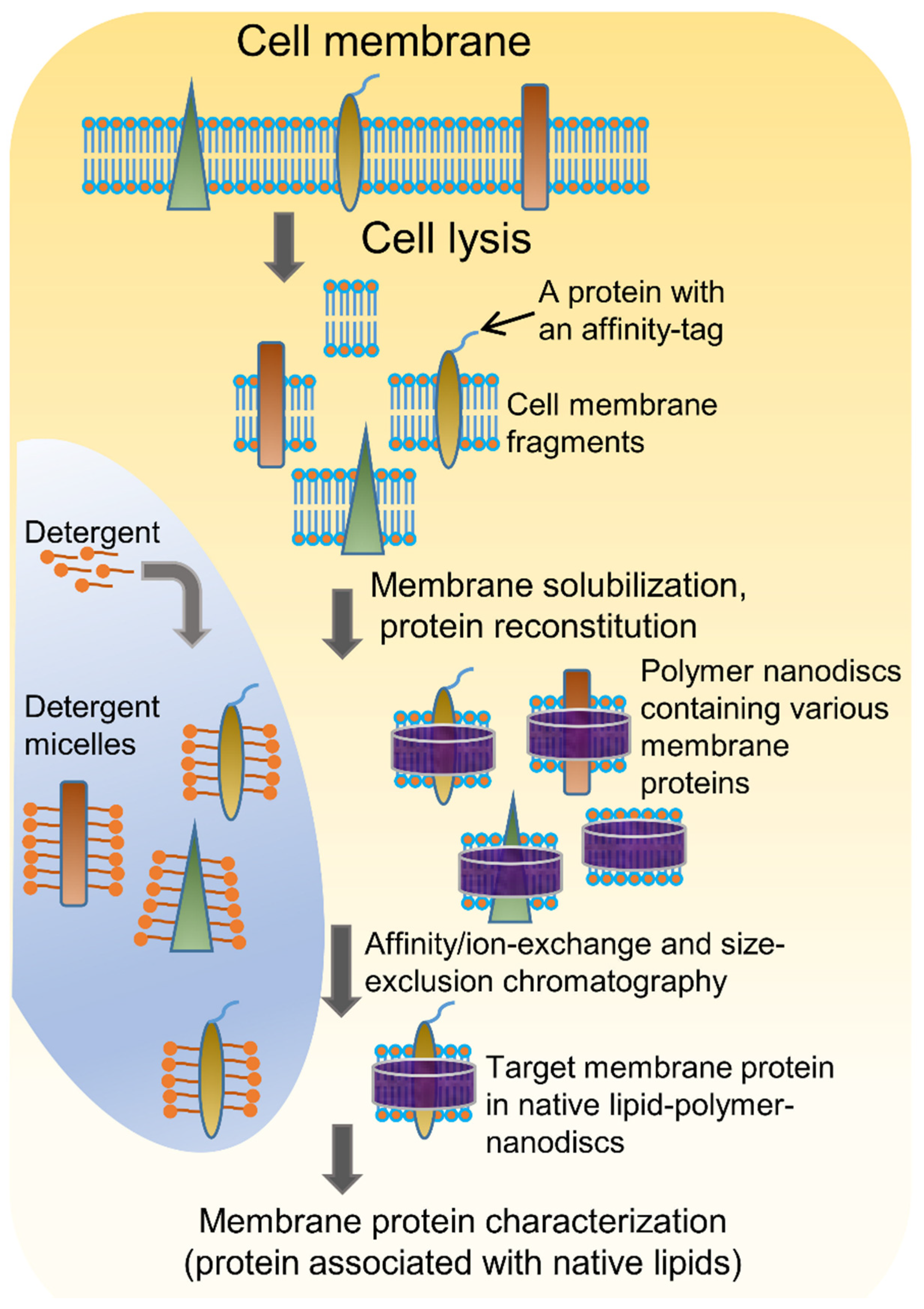
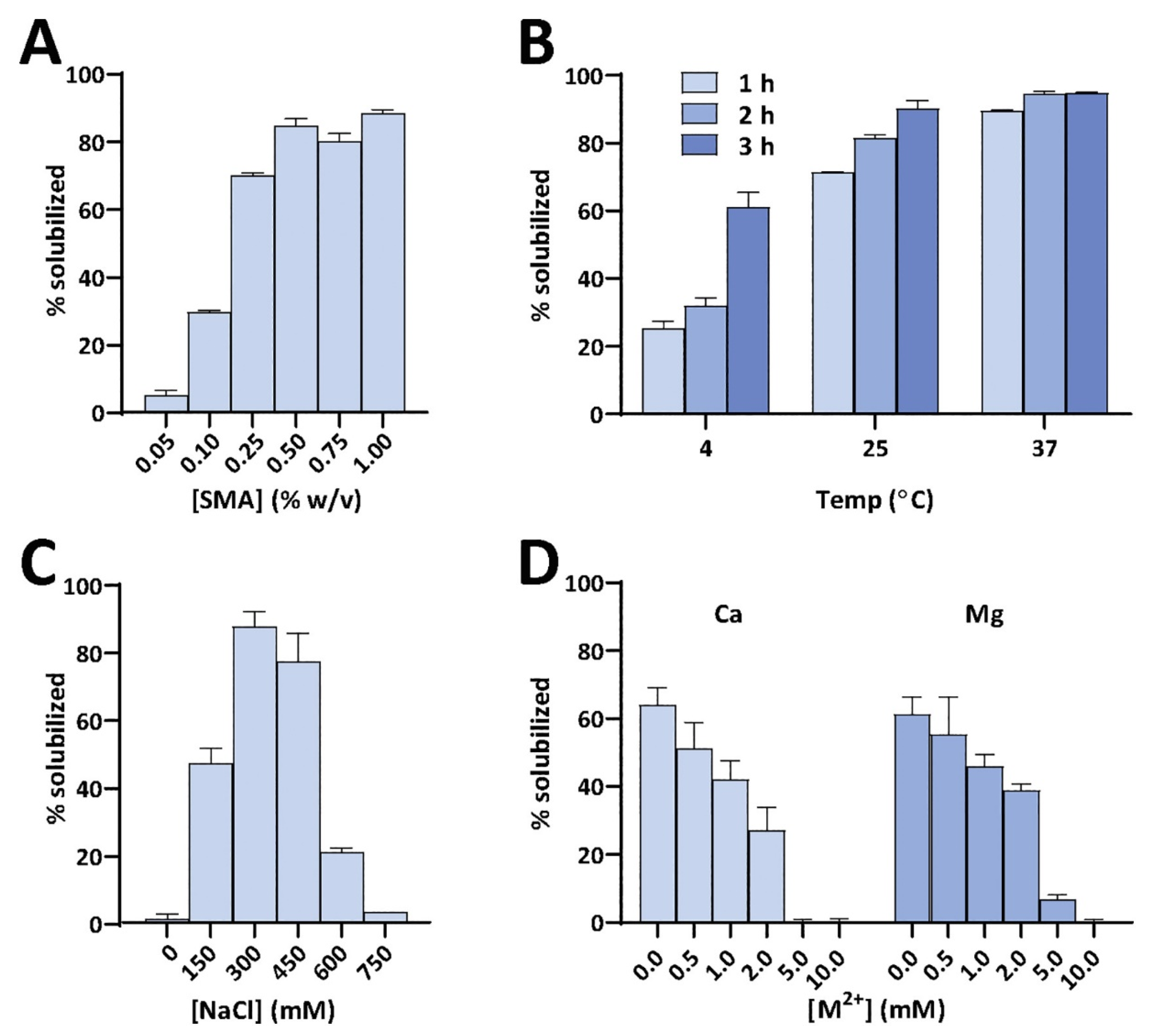
4.1. Solubilization of Cell Membranes and Isolation of Membrane Proteins into Local Lipid Polymer-Nanodiscs
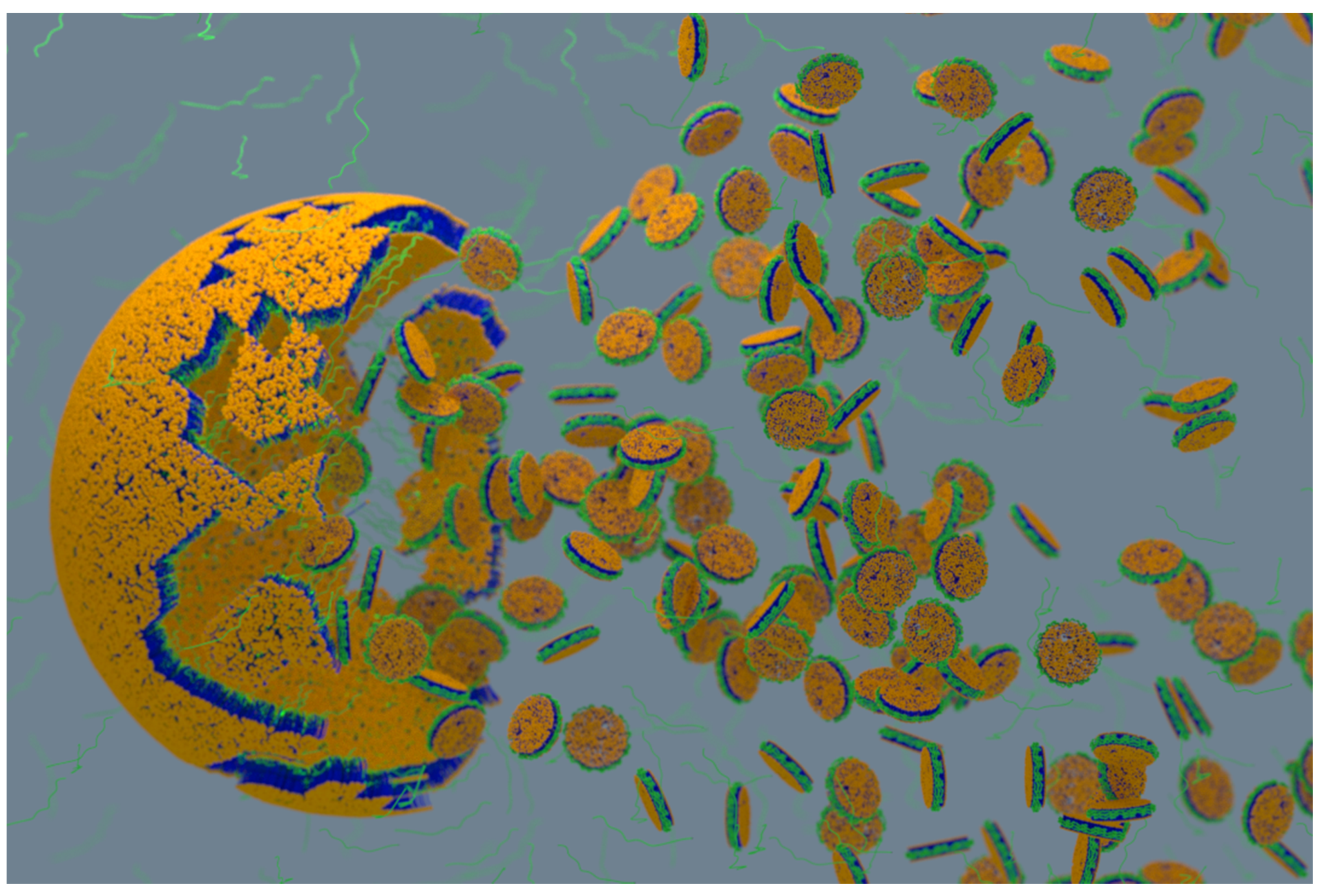
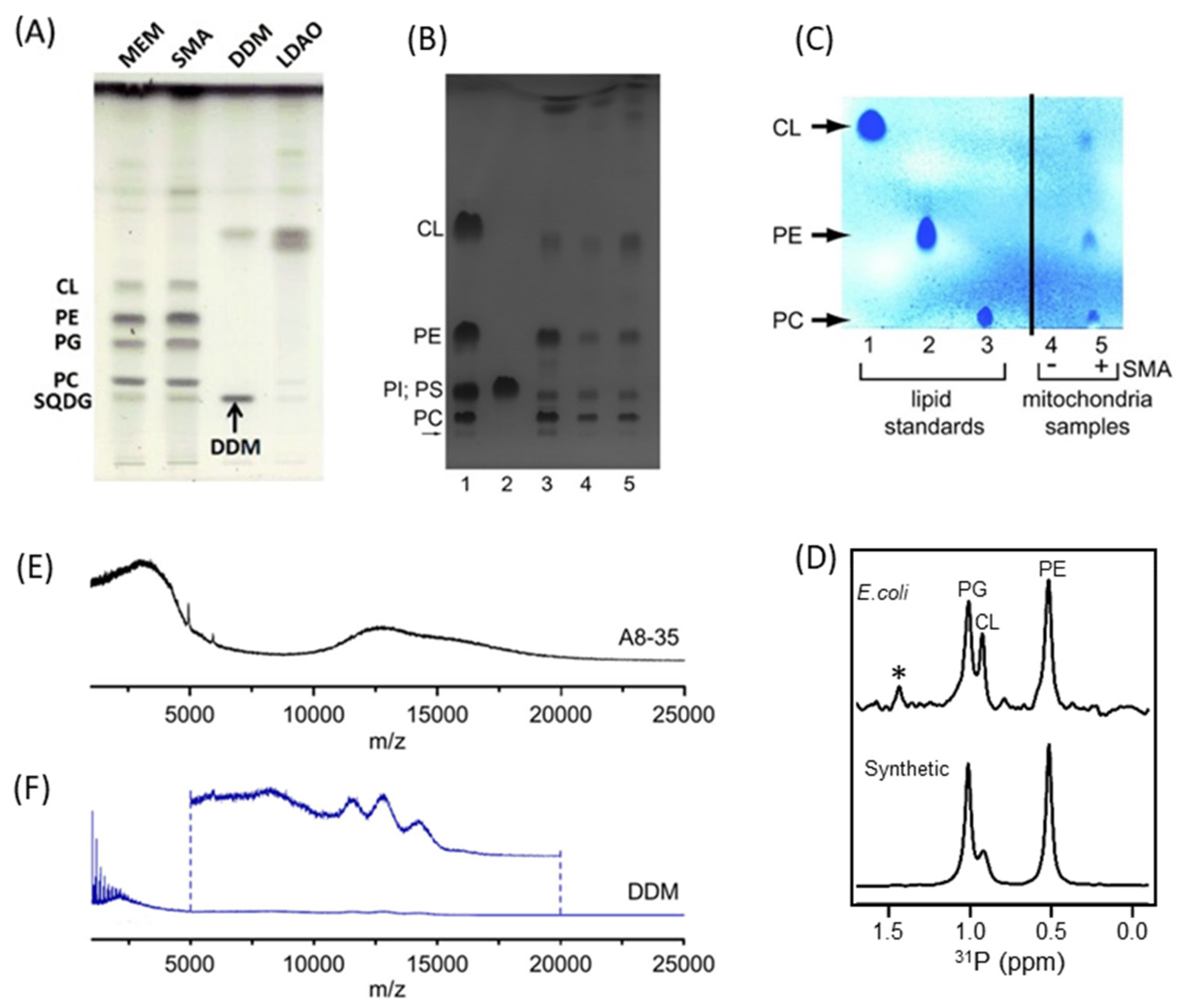
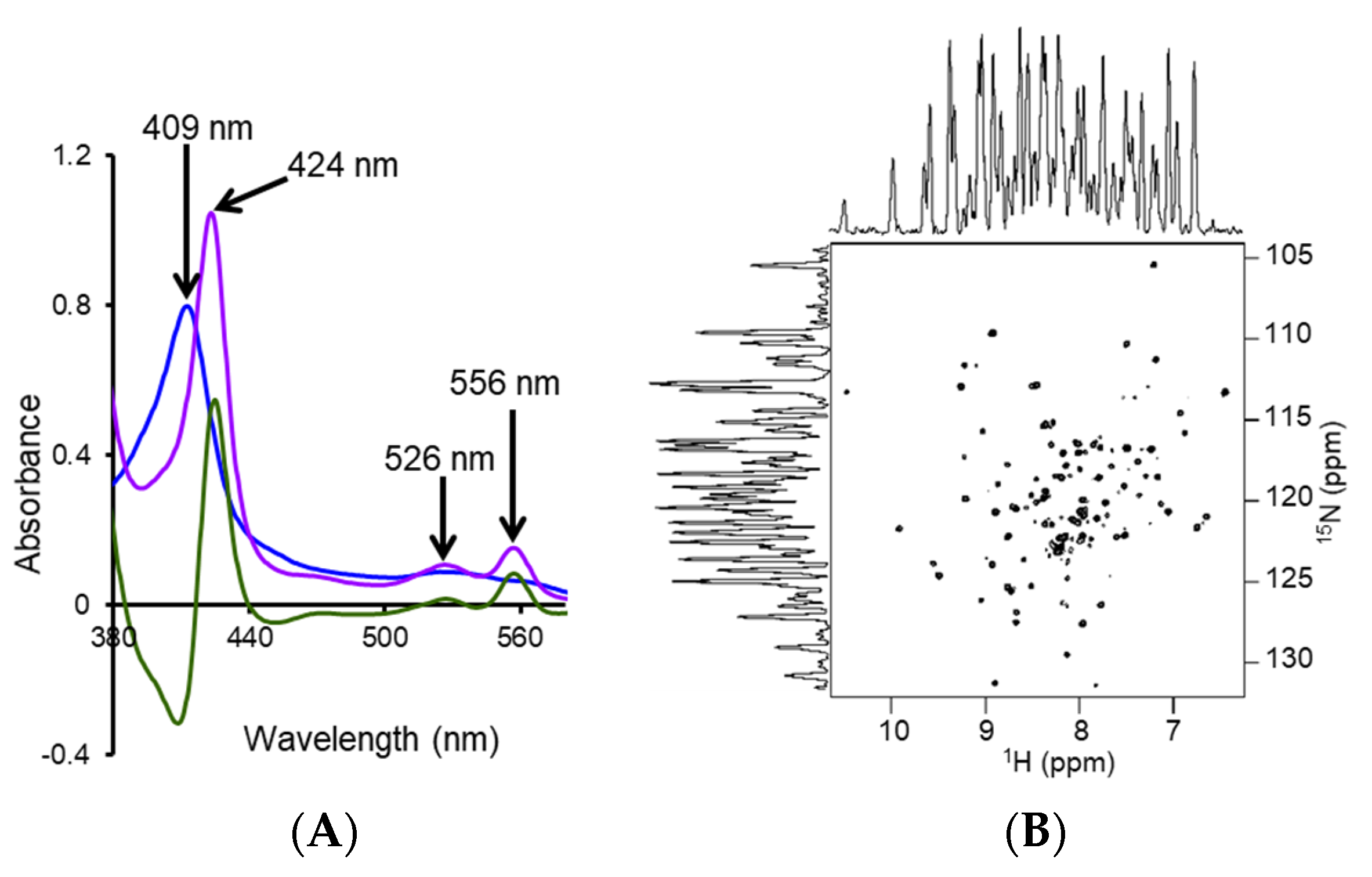
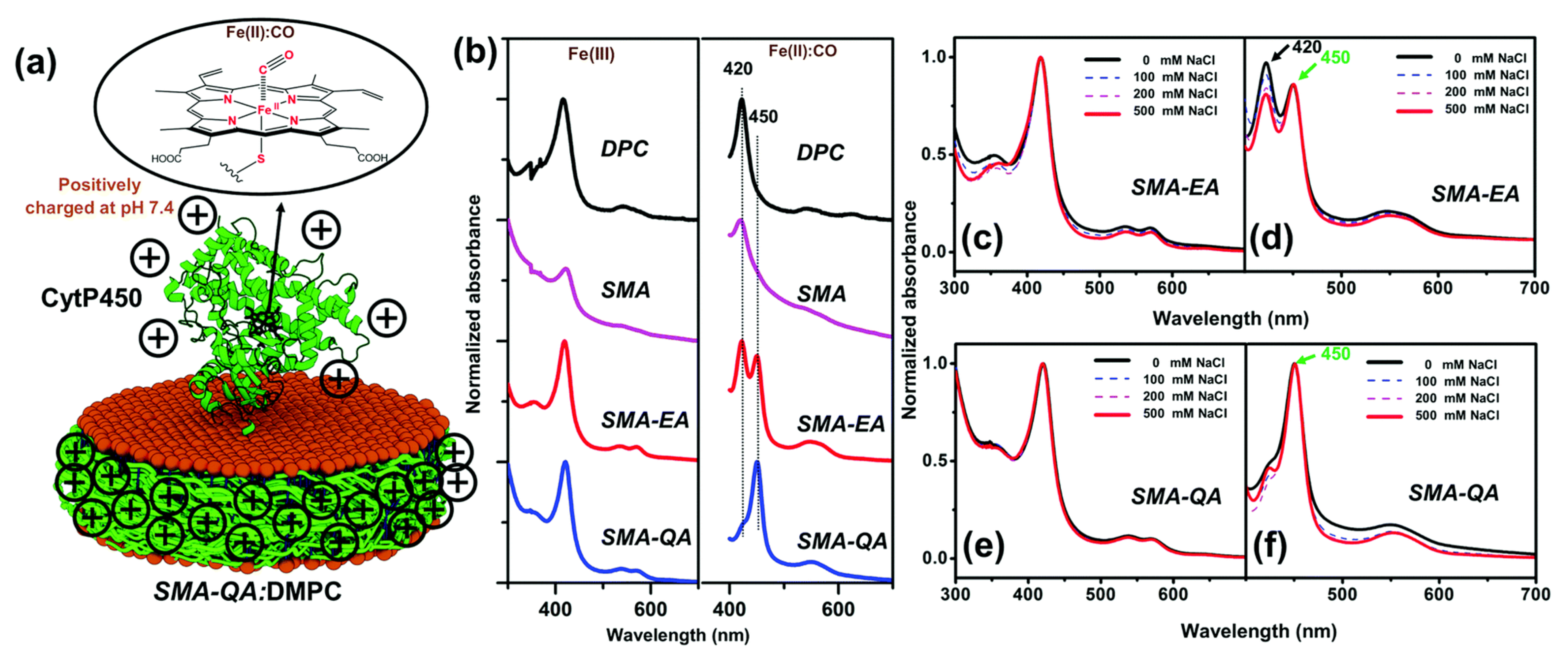
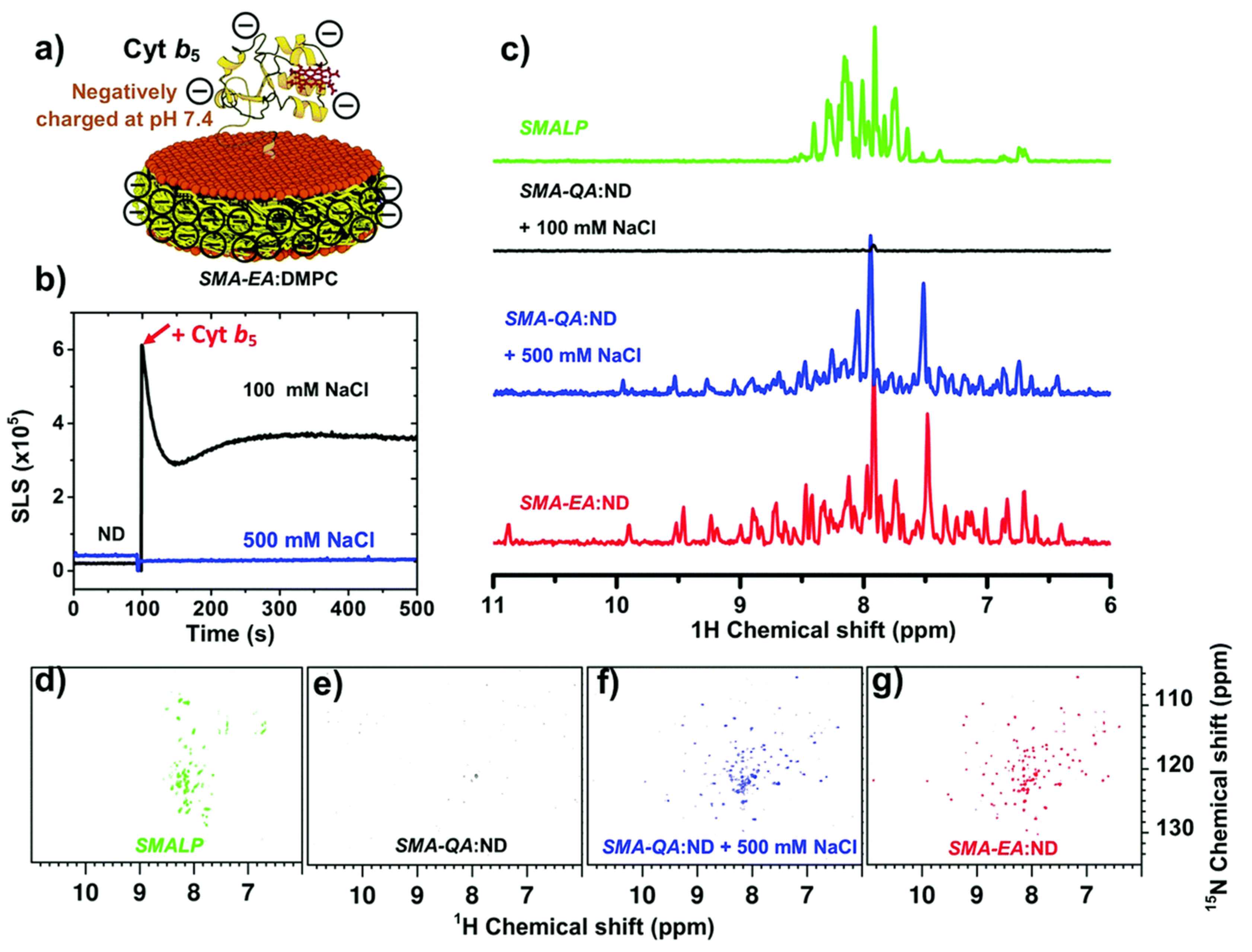
4.2. Characterization of Local Lipid Polymer-Nanodiscs
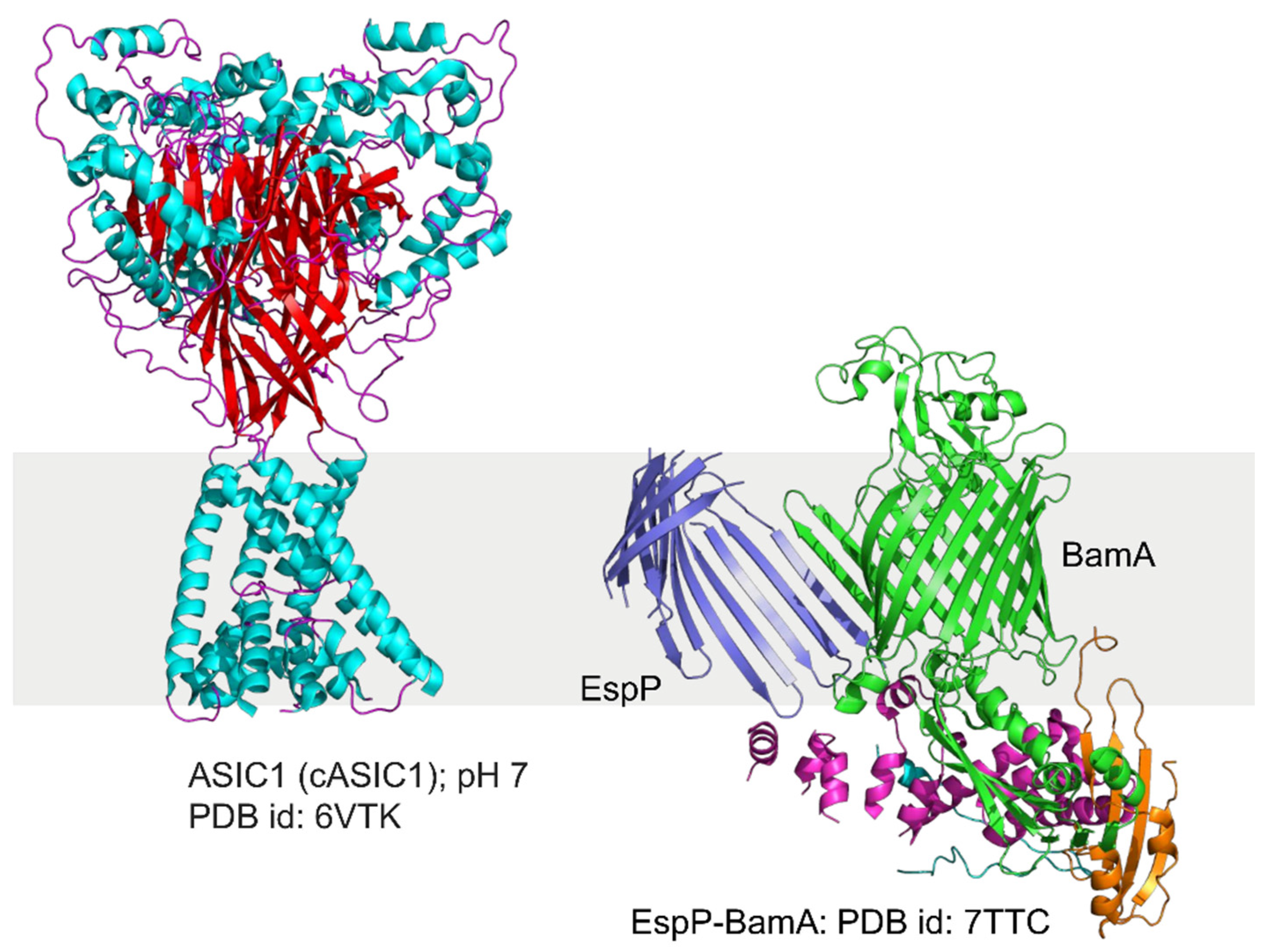
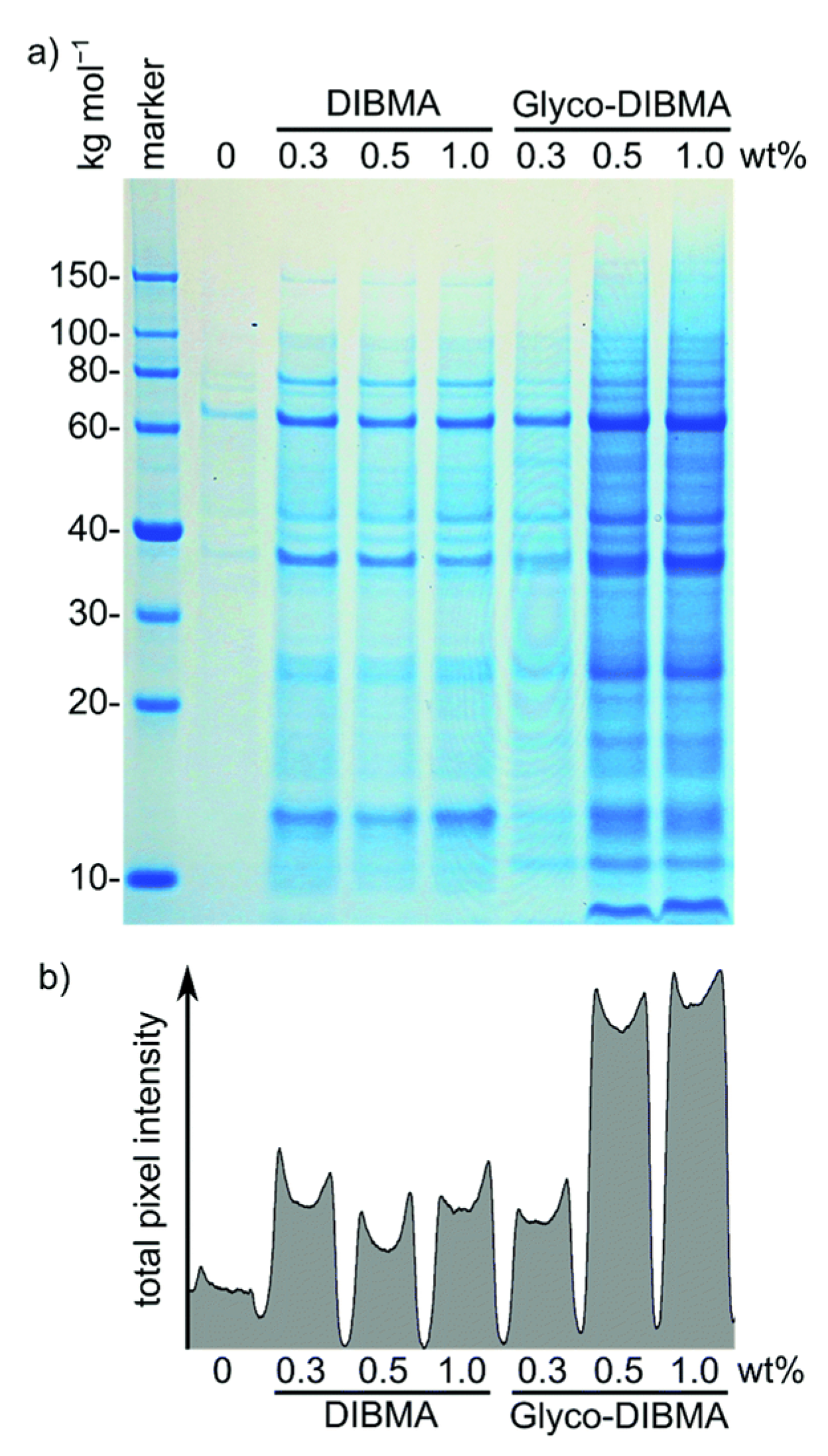
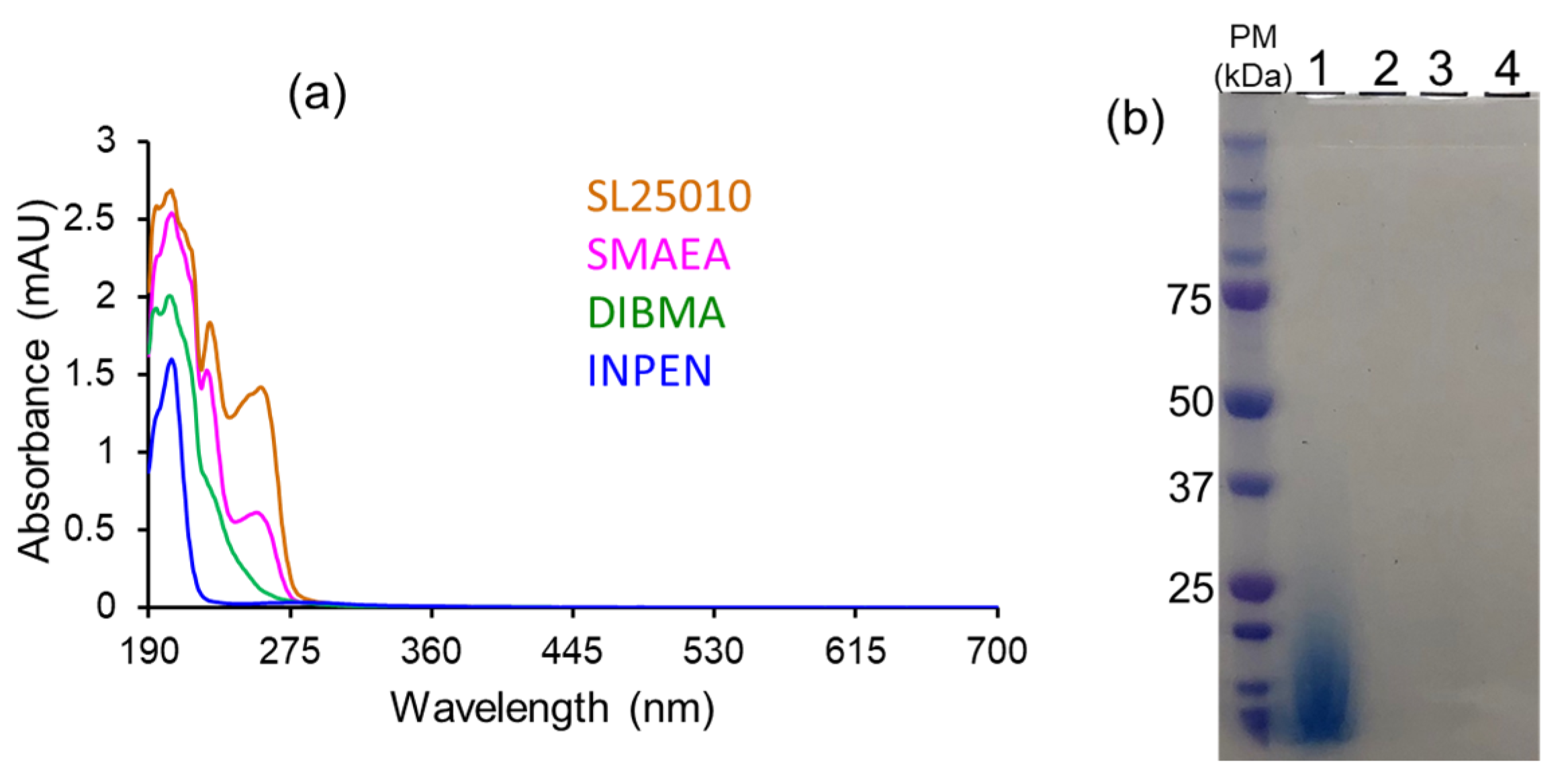
4.3. Different Types of Membrane Proteins Isolated from Various Cell Membranes Using Amphipathic Polymers
| Prokaryotic Protein | Eukaryotic Protein |
|---|---|
| SMA (Anionic) | |
| Listeria NDH-2a enzyme from the pathogenic bacteria Listeria monocytogenes, expressed in E. coli [60] | GPCR [human adenosine A2A receptor (A2AR)] expressed in P. pastoris and human embryonic kidney (HEK)293T cells [69] |
| E. coli rhomboid protease GlpG (with a six α-helix transmembrane domain) expressed in E. coli [97] | hENT1 expressed in Sf9 insect cells [98] |
| Bacterial divisome, single transmembrane protein ZipA [110] | Protein complex (CytcO along with Rcf subunits from S. cerevisiae subcellular membranes such as mitochondria) [113] |
| K+ importer A (KimA) from Bacillus subtilis and sodium–solute symporter protein (SSS) expressed in E.coli [137] | Wild-type human GPCR; dopamine receptor 1 (D1) expressed in HEK293f cells [115] |
| KcsA; it is a tetrameric potassium channel from Streptomyces lividans expressed in E. coli [117] | Human tetraspanins (four helical transmembrane domains) [116] |
| E. coli trimeric multidrug efflux transporter AcrB expressed in E. coli [156] | Protein complex from Saccharomyces cerevisiae subcellular membranes such as mitochondria [130] |
| Potassium importer KimA from Bacillus subtilis expressed in E. coli [157] | Human Pgp (P-glycoprotein; ABCB1) expressed in High Five (Trichoplusia ni) insect cells [140] |
| α-helical seven-transmembrane proton pump bacteriorhodopsin from Haloquadratum walsbyi (HwBR) expressed in E. coli [119] | Plant plasma membrane Na+/H+ antiporter SOS1 (Salt Overlay Sensitive 1) of Arabidopsis thaliana expressed in P. pastoris [158] |
| Holo-translocon (HTL)—a supercomplex of SecYEG–SecDF–YajC–YidC proteins [159] | RhD antigen from RBCs [160] |
| 2×34 kDa cation diffusion facilitator protein from Cupriavidus metallidurans CH34 for proton-detected solid-state NMR [161] | A2αR and CGRP (GPCRs) receptors expressed in P. pastoris and Cos7 cells, respectively [162] |
| PglC and PglA of Campylobacter jejuni expressed in E. coli [163] | P-glycoprotein (ABC transporter family) isolated from MCF-7/ADR cells for small molecule screening by SPR [148] |
| The membrane tether protein ZipA and the ATP binding cassette (ABC) transporter BmrA are expressed in E. coli [162] | Human multidrug resistance protein 4/ABCC4 (MRP4/ABCC4) (expressed in Sf9 cell-lines) [141] |
| Penicillin-binding protein complex PBP2/PBP2a from Staphylococcus aureus [164] | Human adenosine 2α receptor (A2αR; GPCR) expressed in Pichia pastoris [149] |
| (a) BmrA from Bacillus subtilis (a homodimer; each monomer provides six transmembrane α-helices and a cytosolic nucleotide-binding domain, (b) LeuT from Aquifex aeolicus (an amino acid:sodium symporter; comprising 12 transmembrane helices; and (c) ZipA from E. coli (a single transmembrane helix with a large cytosolic domain) [165] | (a) Full-length pore-forming α-subunits hKCNH5 and hKCNQ1 of human neuronal and cardiac voltage-gated potassium (KV) channels, (b) the fusion protein comprising of an α-subunit hKCNQ1 and its regulatory transmembrane KCNE1 β-subunit (hKCNE1-hKCNQ1), expressed in mammalian COS-1 cells [150] |
| E. coli tyrosine kinase with two transmembrane helices for 19F-NMR [166] | Dynamic dhurrin metabolon from the microsomes of Sorghum bicolor [167], photosystem I light-harvesting chlorophyll II supercomplex from spinach [168] |
| SecYEG in complex with SecA from E. coli [169] | Human ATP binding cassette ABCG2 is expressed in HEK293T cells [145] |
| AcrB from Salmonella typhimurium [170] | Melatonin MT1R (GPCR) [144] expressed in P. pastoris |
| E. coli cytochrome bo3; expressed in E. coli [171] | Human tetraspanins CD81; expressed in P. pastoris [155] |
| Bacterial pLGIC; expressed in E. coli [172] | Slow anion channel 1 (SLAC1) from Brachypodium distachyon, its structure is determined using cryo-EM at 2.97 Å [173], expressed in S. pombe |
| Thermally stable rhodopsin from Rubrobacter xylanophilus rhodopsin (RxR) and an unstable one from Halobacterium salinarum sensory rhodopsin I [174]; expressed in E. coli cells BL21 (DE3) | Rhodopsin, ABCA4, and PRPH2/ROM1 from mice [135] |
| SARS-CoV-2 S glycoprotein expressed in 293T cells | Renal outer medullary potassium channels (ROMK) are expressed in E. coli [128] |
| Mycobacterial membrane protein large 3 (Mmpl3) from Mycobacterium tuberculosis [175] | Human β2-adrenergic receptor expressed in HEK293T cell line [112] |
| BAM-MBP-76EspP co-complex; expressed in E. coli BL21 (DE3) [136] | Trimeric photosystem I from the cyanobacterium Thermosynechococcus elongates [176]. |
| Sav1866 (ABC transporter) from Staphylococcus aureus, expressed in E. coli BL21 [177] | Spinach and pea thylakoid membrane protein complexes [151] |
| KcsA expressed in E. coli BL21(λDE3), isolated suing SMA analogues [101] | Yeast transmembrane sensor Wsc1 [152] |
| Succinate dehydrogenase (Sdh4) [153] | |
| Arabidopsis thaliana cytochrome-b5 expressed in E. coli [178] | |
| BAK protein from mitochondria of BAK KO U2OS cells [179] | |
| Smoothened (SMO) GPCR protein expressed Sf9 insect cells [146] | |
| DIBMA (anionic) | |
| The membrane tether protein ZipA and the ATP Binding Cassette (ABC) transporter BmrA [162]; expressed in E. coli | GPCRs: A2αR and CGRP receptor; expressed in P. pastoris and Cos7 cells, respectively [162] |
| Bacterial OmpLA [132]; expressed in E. coli BL21 (DE3) | Human serotonin transporter (hSERT); expressed in Pichia pastoris [142] |
| E. coli rhomboid protease GlpG and Vibrio cholerae rhomboid protease [127] | |
| E. coli ZipA [180] | |
| Glyco-DIBMA (anionic) | |
| Voltage-gated K+ channel KvAP; expressed in E. coli [107] | |
| SMA-QA (cationic) and SMA-EA (anionic) | |
| Rabbit cytochrome b5; expressed in E. coli [55] | |
| Polymethacrylate (PMA) | |
| Neurotensin type 1 receptor; expressed in Sf9 cells [181] | |
| Pentyl-inulin (Non-ionic) | |
| FBD domain of CYP450-reductase; expressed in E.coli [58] | |
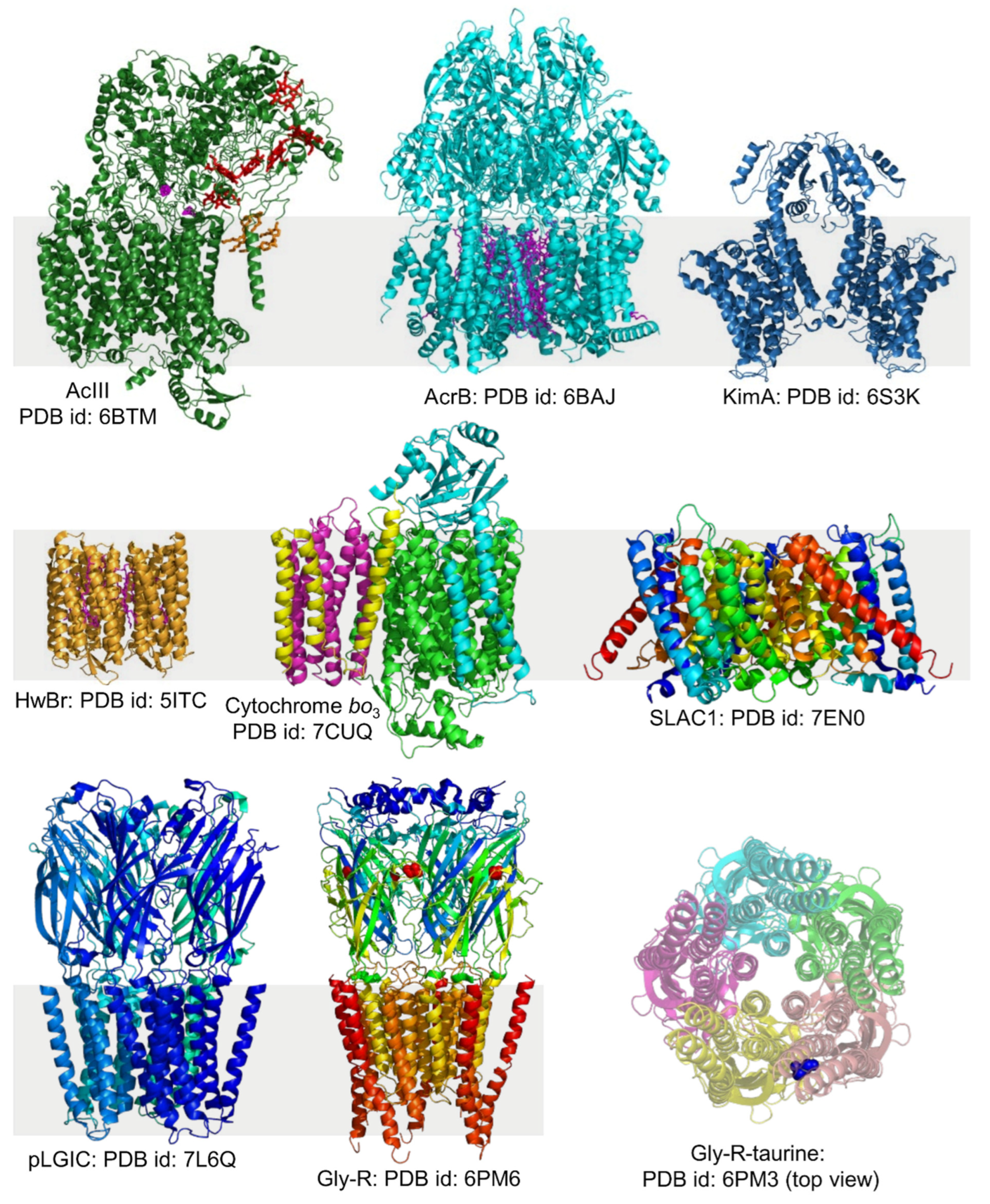
4.4. High-Resolution Structure Determination of Membrane Proteins in Local Lipid Polymer-Nanodiscs Using Cryo-EM
4.5. Other Applications of Detergent-Free Solubilization of Cell Membranes
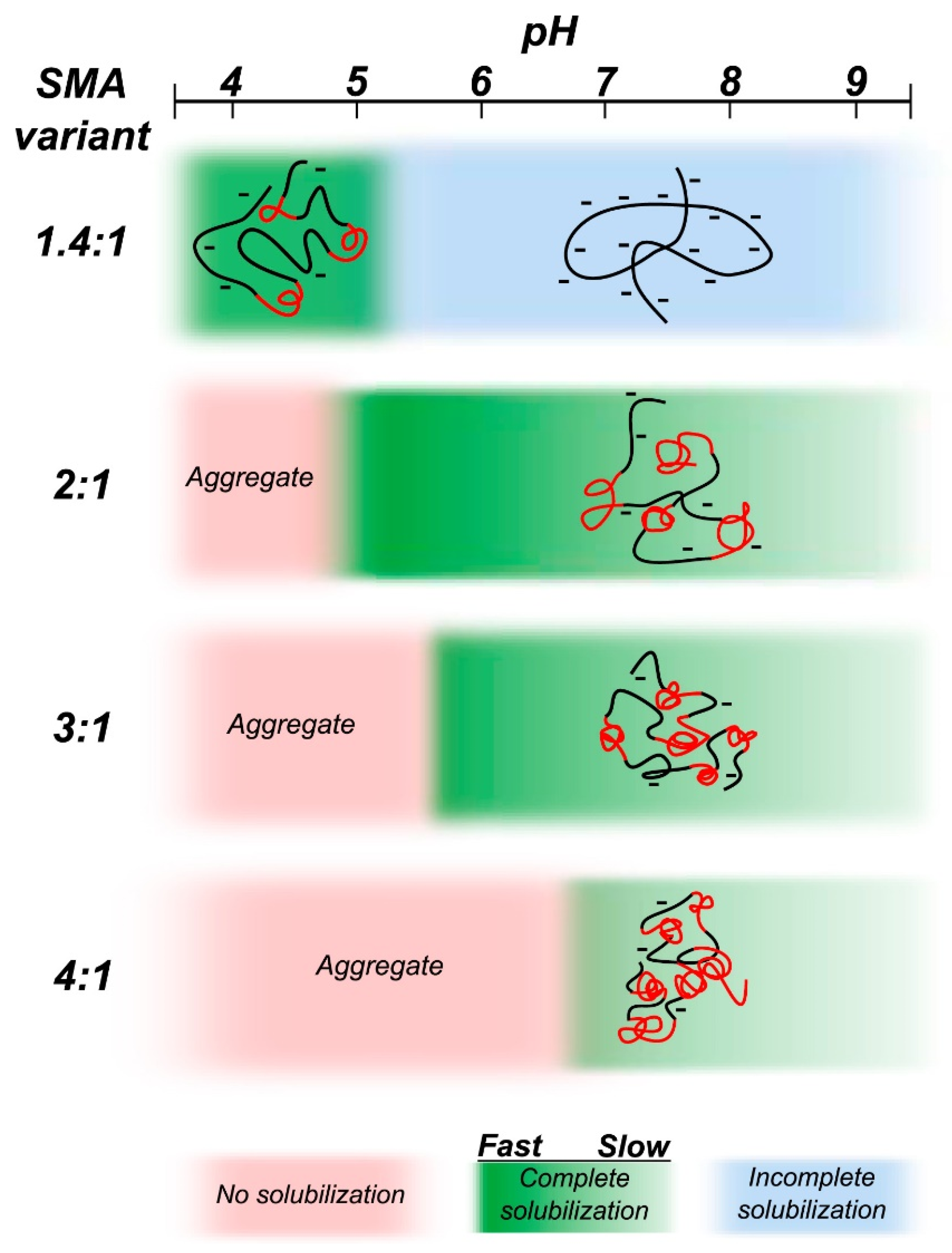
4.6. Amphipathic Polymers Resistant to Metal Ions and Different pH Conditions
5. Limitations of Ionic Polymers
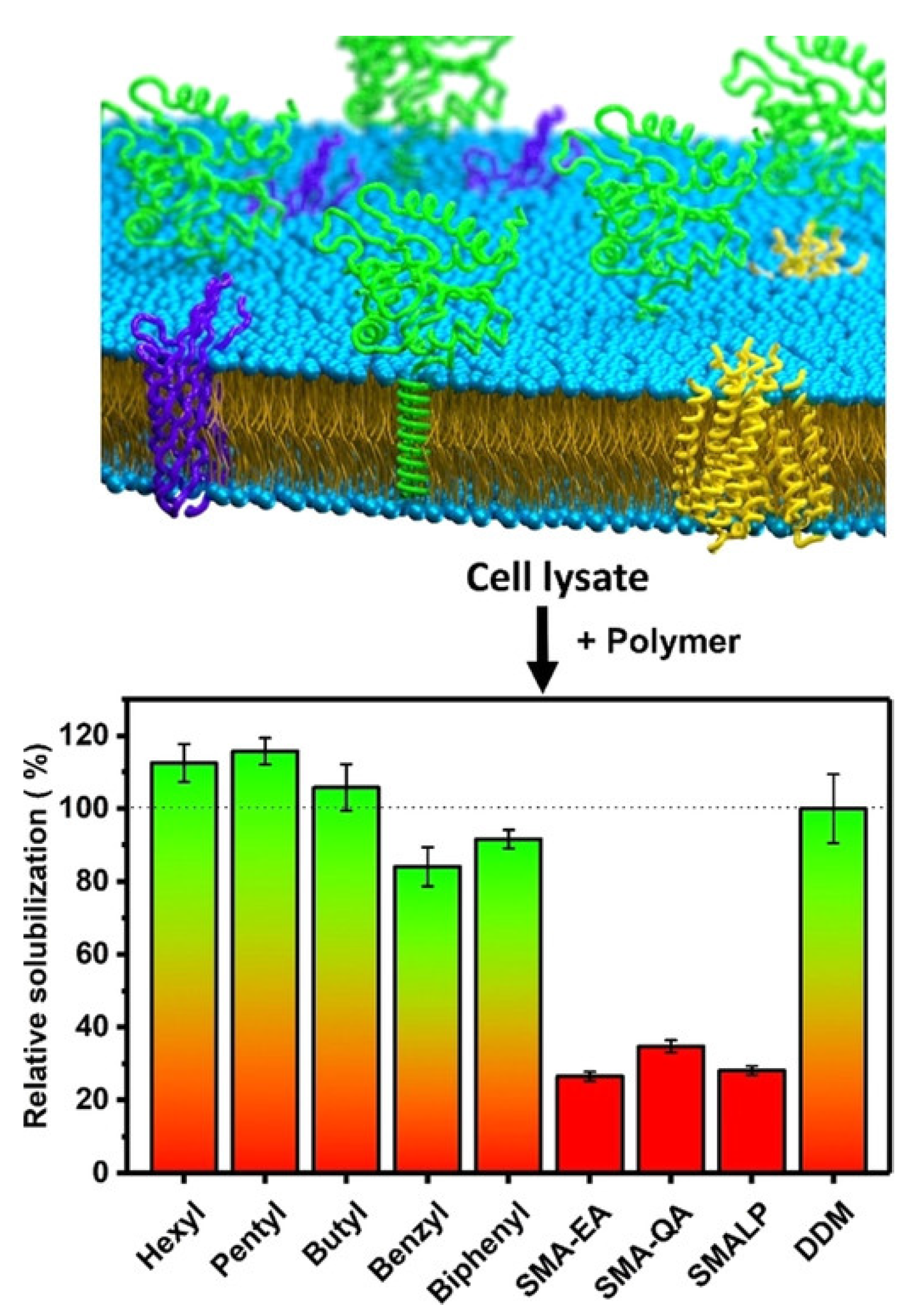
6. Non-Ionic Polymers
7. Conclusions and Future Scope
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Patents
References
- Fagerberg, L.; Jonasson, K.; von Heijne, G.; Uhlén, M.; Berglund, L. Prediction of the human membrane proteome. Proteomics 2010, 10, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Overington, J.P.; Al-Lazikani, B.; Hopkins, A.L. How many drug targets are there? Nat. Rev. Drug Discov. 2006, 5, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Dilworth, M.V.; Piel, M.S.; Bettaney, K.E.; Ma, P.; Luo, J.; Sharples, D.; Poyner, D.R.; Gross, S.R.; Moncoq, K.; Henderson, P.J.F.; et al. Microbial expression systems for membrane proteins. Methods 2018, 147, 3–39. [Google Scholar] [CrossRef] [PubMed]
- Bernaudat, F.; Frelet-Barrand, A.; Pochon, N.; Dementin, S.; Hivin, P.; Boutigny, S.; Rioux, J.-B.; Salvi, D.; Seigneurin-Berny, D.; Richaud, P.; et al. Heterologous expression of membrane proteins: Choosing the appropriate host. PLoS ONE 2011, 6, e29191. [Google Scholar] [CrossRef]
- Schlegel, S.; Hjelm, A.; Baumgarten, T.; Vikström, D.; de Gier, J.W. Bacterial-based membrane protein production. BBA-Mol. Cell Res. 2014, 1843, 1739–1749. [Google Scholar] [CrossRef]
- Chhetri, G.; Kalita, P.; Tripathi, T. An efficient protocol to enhance recombinant protein expression using ethanol in Escherichia coli. MethodsX 2015, 2, 385–391. [Google Scholar] [CrossRef]
- Miroux, B.; Walker, J.E. Over-production of proteins in Escherichia coli: Mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J. Mol. Biol. 1996, 260, 289–298. [Google Scholar] [CrossRef]
- Geertsma, E.R.; Groeneveld, M.; Slotboom, D.-J.; Poolman, B. Quality control of overexpressed membrane proteins. Proc. Natl. Acad. Sci. USA 2008, 105, 5722–5727. [Google Scholar] [CrossRef]
- Panneels, V.; Kock, I.; Krijnse-Locker, J.; Rezgaoui, M.; Sinning, I. Drosophila photoreceptor cells exploited for the production of eukaryotic membrane proteins: Receptors, transporters and channels. PLoS ONE 2011, 6, e18478. [Google Scholar] [CrossRef]
- Eroglu, C.; Cronet, P.; Panneels, V.; Beaufils, P.; Sinning, I. Functional reconstitution of purified metabotropic glutamate receptor expressed in the fly eye. EMBO Rep. 2002, 3, 491–496. [Google Scholar] [CrossRef]
- Hirz, M.; Richter, G.; Leitner, E.; Wriessnegger, T.; Pichler, H. A novel cholesterol-producing Pichia pastoris strain is an ideal host for functional expression of human Na,K-ATPase α3β1 isoform. Appl. Microbiol. Biotechnol. 2013, 97, 9465–9478. [Google Scholar] [CrossRef]
- Paila, Y.D.; Tiwari, S.; Chattopadhyay, A. Are specific nonannular cholesterol binding sites present in G-protein coupled receptors? Biochim. Biophys. Acta (BBA)-Biomembr. 2009, 1788, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, A. GPCRs: Lipid-dependent membrane receptors that act as drug targets. Adv. Biol. 2014, 2014, 143023. [Google Scholar] [CrossRef]
- Pandey, A.; Shin, K.; Patterson, R.E.; Liu, X.-Q.; Rainey, J.K. Current strategies for protein production and purification enabling membrane protein structural biology. Biochem. Cell Biol. 2016, 94, 507–527. [Google Scholar] [CrossRef] [PubMed]
- Barniol-Xicota, M.; Verhelst, S.H.L. Isolation of intramembrane proteases in membrane-like environments. Biochim. Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183193. [Google Scholar] [CrossRef]
- Shapiro, A.L.; Viñuela, E.; Maizel, J.V. Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem. Biophys. Res. Commun. 1967, 28, 815–820. [Google Scholar] [CrossRef]
- Sgro, G.G.; Costa, T.R.D. Cryo-EM grid preparation of membrane protein samples for single particle analysis. Front. Mol. Biosci. 2018, 5, 74. [Google Scholar] [CrossRef]
- McCalpin, S.D.; Ravula, T.; Ramamoorthy, A. Saponins form nonionic lipid nanodiscs for protein structural studies by Nuclear Magnetic Resonance Spectroscopy. J. Phys. Chem. Lett. 2022, 13, 1705–1712. [Google Scholar] [CrossRef]
- Diller, A.; Loudet, C.; Aussenac, F.; Raffard, G.; Fournier, S.; Laguerre, M.; Grélard, A.; Opella, S.J.; Marassi, F.M.; Dufourc, E.J. Bicelles: A natural ‘molecular goniometer’ for structural, dynamical and topological studies of molecules in membranes. Biochimie 2009, 91, 744–751. [Google Scholar] [CrossRef]
- Klöpfer, K.; Hagn, F. Beyond detergent micelles: The advantages and applications of non-micellar and lipid-based membrane mimetics for solution-state NMR. Prog. Nucl. Magn. Reson. Spectrosc. 2019, 114, 271–283. [Google Scholar] [CrossRef]
- Park, S.H.; Berkamp, S.; Cook, G.A.; Chan, M.K.; Viadiu, H.; Opella, S.J. Nanodiscs versus macrodiscs for NMR of membrane proteins. Biochemistry 2011, 50, 8983–8985. [Google Scholar] [CrossRef]
- Sanders, C.R.; Hare, B.J.; Howard, K.P.; Prestegard, J.H. Magnetically-oriented phospholipid micelles as a tool for the study of membrane-associated molecules. Prog. Nucl. Magn. Reson. Spectrosc. 1994, 26, 421–444. [Google Scholar] [CrossRef]
- Dürr, U.H.N.; Gildenberg, M.; Ramamoorthy, A. The magic of bicelles lights up membrane protein structure. Chem. Rev. 2012, 112, 6054–6074. [Google Scholar] [CrossRef] [PubMed]
- Sligar, S.G.; Denisov, I.G. Nanodiscs: A toolkit for membrane protein science. Protein Sci. 2021, 30, 297–315. [Google Scholar] [CrossRef] [PubMed]
- Warschawski, D.E.; Arnold, A.A.; Beaugrand, M.; Gravel, A.; Chartrand, É.; Marcotte, I. Choosing membrane mimetics for NMR structural studies of transmembrane proteins. Biochim. Biophys. Acta (BBA)-Biomembr. 2011, 1808, 1957–1974. [Google Scholar] [CrossRef]
- Günsel, U.; Hagn, F. Lipid nanodiscs for high-resolution NMR studies of membrane proteins. Chem. Rev. 2022, 122, 9395–9421. [Google Scholar] [CrossRef]
- Opella, S.J.; Marassi, F.M. Applications of NMR to membrane proteins. Arch. Biochem. Biophys. 2017, 628, 92–101. [Google Scholar] [CrossRef]
- Kijac, A.Z.; Li, Y.; Sligar, S.G.; Rienstra, C.M. Magic-angle spinning solid-state NMR spectroscopy of nanodisc-embedded human CYP3A4. Biochemistry 2007, 46, 13696–13703. [Google Scholar] [CrossRef]
- Raschle, T.; Hiller, S.; Yu, T.-Y.; Rice, A.J.; Walz, T.; Wagner, G. Structural and functional characterization of the integral membrane protein VDAC-1 in lipid bilayer nanodiscs. J. Am. Chem. Soc. 2009, 131, 17777–17779. [Google Scholar] [CrossRef]
- Hagn, F.; Nasr, M.L.; Wagner, G. Assembly of phospholipid nanodiscs of controlled size for structural studies of membrane proteins by NMR. Nat. Protoc. 2018, 13, 79–98. [Google Scholar] [CrossRef]
- Bibow, S.; Böhm, R.; Modaresi, S.M.; Hiller, S. Detergent titration as an efficient method for NMR resonance assignments of membrane proteins in lipid–bilayer nanodiscs. Anal. Chem. 2020, 92, 7786–7793. [Google Scholar] [CrossRef] [PubMed]
- Miehling, J.; Goricanec, D.; Hagn, F. A split-intein-based method for the efficient production of circularized nanodiscs for structural studies of membrane proteins. ChemBioChem 2018, 19, 1927–1933. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.Y.; Raschle, T.; Hiller, S.; Wagner, G. Solution NMR spectroscopic characterization of human VDAC-2 in detergent micelles and lipid bilayer nanodiscs. Biochim. Biophys. Acta 2012, 1818, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Reif, M.M.; Fischer, M.; Fredriksson, K.; Hagn, F.; Zacharias, M. The N-terminal segment of the voltage-dependent anion channel: A possible membrane-bound intermediate in pore unbinding. J. Mol. Biol. 2019, 431, 223–243. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mu, Z.; Li, Y.; Bi, Y.; Wang, Y. Smaller nanodiscs are suitable for studying protein lipid interactions by solution NMR. Protein J. 2015, 34, 205–211. [Google Scholar] [CrossRef]
- Hartmann, J.-B.; Zahn, M.; Burmann, I.M.; Bibow, S.; Hiller, S. Sequence-specific solution NMR assignments of the β-barrel insertase BamA to monitor its conformational ensemble at the atomic level. J. Am. Chem. Soc. 2018, 140, 11252–11260. [Google Scholar] [CrossRef]
- Morgado, L.; Zeth, K.; Burmann, B.M.; Maier, T.; Hiller, S. Characterization of the insertase BamA in three different membrane mimetics by solution NMR spectroscopy. J. Biomol. NMR 2015, 61, 333–345. [Google Scholar] [CrossRef]
- Hagn, F.; Etzkorn, M.; Raschle, T.; Wagner, G. Optimized phospholipid bilayer nanodiscs facilitate high-resolution structure determination of membrane proteins. J. Am. Chem. Soc. 2013, 135, 1919–1925. [Google Scholar] [CrossRef]
- Etzkorn, M.; Raschle, T.; Hagn, F.; Gelev, V.; Rice, A.J.; Walz, T.; Wagner, G. Cell-free expressed bacteriorhodopsin in different soluble membrane mimetics: Biophysical properties and NMR accessibility. Structure 2013, 21, 394–401. [Google Scholar] [CrossRef]
- Etzkorn, M.; Zoonens, M.; Catoire, L.J.; Popot, J.-L.; Hiller, S. How amphipols embed membrane proteins: Global solvent accessibility and interaction with a flexible protein terminus. J. Membr. Biol. 2014, 247, 965–970. [Google Scholar] [CrossRef]
- Pozza, A.; Giraud, F.; Cece, Q.; Casiraghi, M.; Point, E.; Damian, M.; Le Bon, C.; Moncoq, K.; Banères, J.L.; Lescop, E.; et al. Exploration of the dynamic interplay between lipids and membrane proteins by hydrostatic pressure. Nat. Commun. 2022, 13, 1780. [Google Scholar] [CrossRef] [PubMed]
- Hagn, F.; Wagner, G. Structure refinement and membrane positioning of selectively labeled OmpX in phospholipid nanodiscs. J. Biomol. NMR 2015, 61, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gui, M.; Wang, Z.-F.; Gorgulla, C.; Yu, J.J.; Wu, H.; Sun, Z.-y.J.; Klenk, C.; Merklinger, L.; Morstein, L.; et al. Cryo-EM structure of an activated GPCR–G protein complex in lipid nanodiscs. Nat. Struct. Mol. Biol. 2021, 28, 258–267. [Google Scholar] [CrossRef]
- Rodriguez Camargo, D.C.; Korshavn, K.J.; Jussupow, A.; Raltchev, K.; Goricanec, D.; Fleisch, M.; Sarkar, R.; Xue, K.; Aichler, M.; Mettenleiter, G.; et al. Stabilization and structural analysis of a membrane-associated hIAPP aggregation intermediate. Elife 2017, 6, e31226. [Google Scholar] [CrossRef] [PubMed]
- Raltchev, K.; Pipercevic, J.; Hagn, F. Production and structural analysis of membrane-anchored proteins in phospholipid nanodiscs. Chem. Eur. J. 2018, 24, 5493–5499. [Google Scholar] [CrossRef] [PubMed]
- Sperl, L.E.; Hagn, F. NMR Structural and biophysical analysis of the disease-linked inner mitochondrial membrane protein MPV17. J. Mol. Biol. 2021, 433, 167098. [Google Scholar] [CrossRef]
- Sperl, L.E.; Rührnößl, F.; Schiller, A.; Haslbeck, M.; Hagn, F. High-resolution analysis of the conformational transition of pro-apoptotic Bak at the lipid membrane. EMBO J. 2021, 40, e107159. [Google Scholar] [CrossRef]
- Yao, Y.; Dutta, S.K.; Park, S.H.; Rai, R.; Fujimoto, L.M.; Bobkov, A.A.; Opella, S.J.; Marassi, F.M. High resolution solid-state NMR spectroscopy of the Yersinia pestis outer membrane protein Ail in lipid membranes. J. Biomol. NMR 2017, 67, 179–190. [Google Scholar] [CrossRef]
- Berkamp, S.; Park, S.H.; De Angelis, A.A.; Marassi, F.M.; Opella, S.J. Structure of monomeric Interleukin-8 and its interactions with the N-terminal Binding Site-I of CXCR1 by solution NMR spectroscopy. J. Biomol. NMR 2017, 69, 111–121. [Google Scholar] [CrossRef]
- Gentry, K.A.; Prade, E.; Barnaba, C.; Zhang, M.; Mahajan, M.; Im, S.-C.; Anantharamaiah, G.M.; Nagao, S.; Waskell, L.; Ramamoorthy, A. Kinetic and structural characterization of the effects of membrane on the complex of cytochrome b5 and cytochrome c. Sci. Rep. 2017, 7, 7793. [Google Scholar] [CrossRef]
- Krishnarjuna, B.; Yamazaki, T.; Anantharamaiah, G.M.; Ramamoorthy, A. Nanodisc reconstitution of flavin mononucleotide binding domain of cytochrome-P450-reductase enables high-resolution NMR probing. Chem. Commun. 2021, 57, 4819–4822. [Google Scholar] [CrossRef] [PubMed]
- Prade, E.; Mahajan, M.; Im, S.-C.; Zhang, M.; Gentry, K.A.; Anantharamaiah, G.M.; Waskell, L.; Ramamoorthy, A. A Minimal functional complex of cytochrome P450 and FBD of cytochrome P450 reductase in nanodiscs. Angew. Chem. Int. Ed. 2018, 57, 8458–8462. [Google Scholar] [CrossRef] [PubMed]
- Gentry, K.A.; Anantharamaiah, G.M.; Ramamoorthy, A. Probing protein–protein and protein–substrate interactions in the dynamic membrane-associated ternary complex of cytochromes P450, b5, and reductase. Chem. Commun. 2019, 55, 13422–13425. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, R.; Ackermann, R.; Im, S.-C.; Waskell, L.; Schwendeman, A.; Ramamoorthy, A. Reconstitution of the Cytb5–CytP450 complex in nanodiscs for structural studies using NMR Spectroscopy. Angew. Chem. Int. Ed. 2016, 55, 4497–4499. [Google Scholar] [CrossRef] [PubMed]
- Krishnarjuna, B.; Ravula, T.; Ramamoorthy, A. Detergent-free extraction, reconstitution and characterization of membrane-anchored cytochrome-b5 in native lipids. Chem. Commun. 2020, 56, 6511–6514. [Google Scholar] [CrossRef]
- Park, S.H.; Wu, J.; Yao, Y.; Singh, C.; Tian, Y.; Marassi, F.M.; Opella, S.J. Membrane proteins in magnetically aligned phospholipid polymer discs for solid-state NMR spectroscopy. Biochim. Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183333. [Google Scholar] [CrossRef]
- Radoicic, J.; Park, S.H.; Opella, S.J. Macrodiscs comprising SMALPs for oriented sample solid-state NMR spectroscopy of membrane proteins. Biophys. J. 2018, 115, 22–25. [Google Scholar] [CrossRef]
- Krishnarjuna, B.; Ravula, T.; Ramamoorthy, A. Detergent-free isolation of CYP450-reductase’s FMN-binding domain in E. coli lipid-nanodiscs using a charge-free polymer. Chem. Commun. 2022, 58, 4913–4916. [Google Scholar] [CrossRef]
- Janaratne, T.K.; Okach, L.; Brock, A.; Lesley, S.A. Solubilization of native integral membrane proteins in aqueous buffer by noncovalent chelation with monomethoxy poly(ethylene glycol) (mPEG) polymers. Bioconjug. Chem. 2011, 22, 1513–1518. [Google Scholar] [CrossRef][Green Version]
- Nakatani, Y.; Shimaki, Y.; Dutta, D.; Muench, S.P.; Ireton, K.; Cook, G.M.; Jeuken, L.J.C. Unprecedented properties of phenothiazines unraveled by a NDH-2 bioelectrochemical assay platform. J. Am. Chem. Soc. 2020, 142, 1311–1320. [Google Scholar] [CrossRef]
- Chipot, C.; Dehez, F.; Schnell, J.R.; Zitzmann, N.; Pebay-Peyroula, E.; Catoire, L.J.; Miroux, B.; Kunji, E.R.S.; Veglia, G.; Cross, T.A.; et al. Perturbations of native membrane protein structure in alkyl phosphocholine detergents: A critical assessment of NMR and biophysical studies. Chem. Rev. 2018, 118, 3559–3607. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-X.; Cross, T.A. Influences of membrane mimetic environments on membrane protein structures. Annu. Rev. Biophys. 2013, 42, 361–392. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Yan, H.; Wu, Z.; Yan, N.; Wang, Z.; Jeffrey, P.D.; Shi, Y.J.S. Structure of a site-2 protease family intramembrane metalloprotease. Science 2007, 318, 1608–1612. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Bai, X.-c.; Ma, D.; Xie, T.; Yan, C.; Sun, L.; Yang, G.; Zhao, Y.; Zhou, R.; Scheres, S.H.W.; et al. Three-dimensional structure of human γ-secretase. Nature 2014, 512, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Hosea, N.A.; Guengerich, F.P. Oxidation of nonionic detergents by cytochrome P450 enzymes. Arch. Biochem. Biophys. 1998, 353, 365–373. [Google Scholar] [CrossRef]
- Breyton, C.; Tribet, C.; Olive, J.; Dubacq, J.-P.; Popot, J.-L. Dimer to monomer conversion of the cytochrome b6f complex: Causes and consequences. J. Biol. Chem. 1997, 272, 21892–21900. [Google Scholar] [CrossRef]
- Mandala, V.S.; McKay, M.J.; Shcherbakov, A.A.; Dregni, A.J.; Kolocouris, A.; Hong, M. Structure and drug binding of the SARS-CoV-2 envelope protein transmembrane domain in lipid bilayers. Nat. Struct. Mol. Biol. 2020, 27, 1202–1208. [Google Scholar] [CrossRef]
- Dawaliby, R.; Trubbia, C.; Delporte, C.; Masureel, M.; Van Antwerpen, P.; Kobilka, B.K.; Govaerts, C. Allosteric regulation of G protein–coupled receptor activity by phospholipids. Nat. Chem. Biol. 2016, 12, 35. [Google Scholar] [CrossRef]
- Jamshad, M.; Charlton, J.; Lin, Y.-P.; Routledge, S.J.; Bawa, Z.; Knowles, T.J.; Overduin, M.; Dekker, N.; Dafforn, T.R.; Bill, R.M.; et al. G-protein coupled receptor solubilization and purification for biophysical analysis and functional studies, in the total absence of detergent. Biosci. Rep. 2015, 35, e00188. [Google Scholar] [CrossRef]
- Muth, S.; Fries, A.; Gimpl, G. Cholesterol-induced conformational changes in the oxytocin receptor. Biochem. J. 2011, 437, 541–553. [Google Scholar] [CrossRef]
- Yen, H.-Y.; Hoi, K.K.; Liko, I.; Hedger, G.; Horrell, M.R.; Song, W.; Wu, D.; Heine, P.; Warne, T.; Lee, Y.; et al. PtdIns(4,5)P2 stabilizes active states of GPCRs and enhances selectivity of G-protein coupling. Nature 2018, 559, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Zoonens, M.; Comer, J.; Masscheleyn, S.; Pebay-Peyroula, E.; Chipot, C.; Miroux, B.; Dehez, F. Dangerous liaisons between detergents and membrane proteins. The case of mitochondrial uncoupling protein 2. J. Am. Chem. Soc. 2013, 135, 15174–15182. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.A.; Cherezov, V.; Griffith, M.T.; Roth, C.B.; Jaakola, V.P.; Chien, E.Y.; Velasquez, J.; Kuhn, P.; Stevens, R.C. A specific cholesterol binding site is established by the 2.8 A structure of the human beta2-adrenergic receptor. Structure 2008, 16, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Kellosalo, J.; Kajander, T.; Honkanen, R.; Goldman, A. Crystallization and preliminary X-ray analysis of membrane-bound pyrophosphatases. Mol. Membr. Biol. 2013, 30, 64–74. [Google Scholar] [CrossRef]
- Park, J.B.; Kim, H.J.; Ryu, P.D.; Moczydlowski, E. Effect of phosphatidylserine on unitary conductance and Ba2+ block of the BK Ca2+–activated K+ channel: Re-examination of the surface charge hypothesis. J. Gen. Physiol. 2003, 121, 375–398. [Google Scholar] [CrossRef]
- Gutmann, D.A.P.; Mizohata, E.; Newstead, S.; Ferrandon, S.; Henderson, P.J.F.; van Veen, H.W.; Byrne, B. A high-throughput method for membrane protein solubility screening: The ultracentrifugation dispersity sedimentation assay. Protein Sci. 2007, 16, 1422–1428. [Google Scholar] [CrossRef]
- Kotov, V.; Bartels, K.; Veith, K.; Josts, I.; Subhramanyam, U.K.T.; Günther, C.; Labahn, J.; Marlovits, T.C.; Moraes, I.; Tidow, H.; et al. High-throughput stability screening for detergent-solubilized membrane proteins. Sci. Rep. 2019, 9, 10379. [Google Scholar] [CrossRef]
- Matar-Merheb, R.; Rhimi, M.; Leydier, A.; Huché, F.; Galián, C.; Desuzinges-Mandon, E.; Ficheux, D.; Flot, D.; Aghajari, N.; Kahn, R.; et al. Structuring detergents for extracting and stabilizing functional membrane proteins. PLoS ONE 2011, 6, e18036. [Google Scholar] [CrossRef]
- Chae, P.S.; Rasmussen, S.G.F.; Rana, R.R.; Gotfryd, K.; Chandra, R.; Goren, M.A.; Kruse, A.C.; Nurva, S.; Loland, C.J.; Pierre, Y.; et al. Maltose-neopentyl glycol (MNG) amphiphiles for solubilization, stabilization and crystallization of membrane proteins. Nat. Methods 2010, 7, 1003–1008. [Google Scholar] [CrossRef]
- Bayburt, T.H.; Grinkova, Y.V.; Sligar, S.G. Self-assembly of discoidal phospholipid bilayer nanoparticles with membrane scaffold proteins. Nano Lett. 2002, 2, 853–856. [Google Scholar] [CrossRef]
- Marty, M.T.; Wilcox, K.C.; Klein, W.L.; Sligar, S.G. Nanodisc-solubilized membrane protein library reflects the membrane proteome. Anal. Bioanal. Chem. 2013, 405, 4009–4016. [Google Scholar] [CrossRef] [PubMed]
- Denisov, I.G.; Grinkova, Y.V.; McLean, M.A.; Camp, T.; Sligar, S.G. Midazolam as a probe for heterotropic drug-drug interactions mediated by CYP3A4. Biomolecules 2022, 12, 853. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.K.; Anantharamaiah, G.; Segrest, J.P.; Palgunachari, M.N.; Chaddha, M.; Sham, S.S.; Krishna, N.R. Association of a model class A (apolipoprotein) amphipathic α helical peptide with lipid: High resolution NMR studies of peptide·lipid discoidal complexes. J. Biol. Chem. 2006, 281, 6511–6519. [Google Scholar] [CrossRef] [PubMed]
- Abosharaf, H.A.; Sakamoto, Y.; Radwan, A.M.; Yuzu, K.; Fujimura, M.; Diab, T.; Mohamed, T.M.; Chatani, E.; Kimura, T.; Tsubaki, M. Functional assembly of Caenorhabditis elegans cytochrome b-2 (Cecytb-2) into phospholipid bilayer nanodisc with enhanced iron reductase activity. Biomolecules 2021, 11, 96. [Google Scholar] [CrossRef]
- Patel, A.M.; Koebke, K.J.; Grunkemeyer, T.J.; Riordan, C.M.; Kim, Y.; Bailey, R.C.; Marsh, E.N.G. Purification of the full-length, membrane-associated form of the antiviral enzyme viperin utilizing nanodiscs. Sci. Rep. 2022, 12, 11909. [Google Scholar] [CrossRef]
- Gao, Y.; Cao, E.; Julius, D.; Cheng, Y. TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action. Nature 2016, 534, 347–351. [Google Scholar] [CrossRef]
- Brignac-Huber, L.M.; Park, J.W.; Reed, J.R.; Backes, W.L. Cytochrome P450 organization and function are modulated by endoplasmic reticulum phospholipid heterogeneity. Drug Metab. Dispos. 2016, 44, 1859–1866. [Google Scholar] [CrossRef]
- Viegas, A.; Viennet, T.; Etzkorn, M. The power, pitfalls and potential of the nanodisc system for NMR-based studies. Biol. Chem. 2016, 397, 1335–1354. [Google Scholar] [CrossRef]
- Karsten, V.; Hegde, R.S.; Sinai, A.P.; Yang, M.; Joiner, K.A. Transmembrane Domain Modulates Sorting of Membrane Proteins in Toxoplasma gondii. J. Biol. Chem. 2004, 279, 26052–26057. [Google Scholar] [CrossRef]
- Barnaba, C.; Sahoo, B.R.; Ravula, T.; Medina-Meza, I.G.; Im, S.-C.; Anantharamaiah, G.M.; Waskell, L.; Ramamoorthy, A. Cytochrome-P450-induced ordering of microsomal membranes modulates affinity for drugs. Angew. Chem. Int. Ed. 2018, 57, 3391–3395. [Google Scholar] [CrossRef]
- Treuheit, N.A.; Redhair, M.; Kwon, H.; McClary, W.D.; Guttman, M.; Sumida, J.P.; Atkins, W.M. Membrane interactions, ligand-dependent dynamics, and stability of cytochrome P4503A4 in lipid nanodiscs. Biochemistry 2016, 55, 1058–1069. [Google Scholar] [CrossRef] [PubMed]
- Luthra, A.; Gregory, M.; Grinkova, Y.V.; Denisov, I.G.; Sligar, S.G. Nanodiscs in the studies of membrane-bound cytochrome P450 enzymes. Methods Mol. Biol. 2013, 987, 115–127. [Google Scholar] [CrossRef]
- Denisov, I.G.; Sligar, S.G. Nanodiscs for structural and functional studies of membrane proteins. Nat. Struct. Mol. Biol. 2016, 23, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Valiyaveetil, F.I.; Zhou, Y.; MacKinnon, R.J.B. Lipids in the structure, folding, and function of the KcsA K+ channel. Biochemistry 2002, 41, 10771–10777. [Google Scholar] [CrossRef] [PubMed]
- Marius, P.; de Planque, M.R.R.; Williamson, P.T.F. Probing the interaction of lipids with the non-annular binding sites of the potassium channel KcsA by magic-angle spinning NMR. Biochim. Biophys. Acta (BBA)-Biomembr. 2012, 1818, 90–96. [Google Scholar] [CrossRef]
- Palsdottir, H.; Hunte, C. Lipids in membrane protein structures. Biochim. Biophys. Acta (BBA)-Biomembr. 2004, 1666, 2–18. [Google Scholar] [CrossRef]
- Reading, E.; Hall, Z.; Martens, C.; Haghighi, T.; Findlay, H.; Ahdash, Z.; Politis, A.; Booth, P.J. Interrogating membrane protein conformational dynamics within native lipid compositions. Angew. Chem. Int. Ed. 2017, 56, 15654–15657. [Google Scholar] [CrossRef]
- Rehan, S.; Paavilainen, V.O.; Jaakola, V.-P. Functional reconstitution of human equilibrative nucleoside transporter-1 into styrene maleic acid co-polymer lipid particles. Biochim. Biophys. Acta (BBA)-Biomembr. 2017, 1859, 1059–1065. [Google Scholar] [CrossRef]
- Palsdottir, H.; Lojero, C.G.; Trumpower, B.L.; Hunte, C. Structure of the yeast cytochrome bc1 complex with a hydroxyquinone anion Qo site inhibitor bound. J. Biol. Chem. 2003, 278, 31303–31311. [Google Scholar] [CrossRef]
- Burridge, K.M.; Harding, B.D.; Sahu, I.D.; Kearns, M.M.; Stowe, R.B.; Dolan, M.T.; Edelmann, R.E.; Dabney-Smith, C.; Page, R.C.; Konkolewicz, D.; et al. Simple derivatization of RAFT-synthesized styrene-maleic anhydride copolymers for lipid disk formulations. Biomacromolecules 2020, 21, 1274–1284. [Google Scholar] [CrossRef]
- Kopf, A.H.; Lijding, O.; Elenbaas, B.O.W.; Koorengevel, M.C.; Dobruchowska, J.M.; van Walree, C.A.; Killian, J.A. Synthesis and evaluation of a library of alternating amphipathic copolymers to solubilize and study membrane proteins. Biomacromolecules 2022, 23, 743–759. [Google Scholar] [CrossRef] [PubMed]
- Harding, B.D.; Dixit, G.; Burridge, K.M.; Sahu, I.D.; Dabney-Smith, C.; Edelmann, R.E.; Konkolewicz, D.; Lorigan, G.A. Characterizing the structure of styrene-maleic acid copolymer-lipid nanoparticles (SMALPs) using RAFT polymerization for membrane protein spectroscopic studies. Chem. Phys. Lipids 2019, 218, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.F.; Clark, E.E.; Sahu, I.D.; Zhang, R.; Frantz, N.D.; Al-Abdul-Wahid, M.S.; Dabney-Smith, C.; Konkolewicz, D.; Lorigan, G.A. Tuning the size of styrene-maleic acid copolymer-lipid nanoparticles (SMALPs) using RAFT polymerization for biophysical studies. Biochim. Biophys. Acta 2016, 1858, 2931–2939. [Google Scholar] [CrossRef] [PubMed]
- Trinh, T.K.H.; Catalano, C.; Guo, Y. Membrane-active polymers: NCMNP13-x, NCMNP21-x and NCMNP21b-x for membrane protein structural biology. bioRxiv 2022. [Google Scholar] [CrossRef]
- Higgins, A.J.; Flynn, A.J.; Marconnet, A.; Musgrove, L.J.; Postis, V.L.G.; Lippiat, J.D.; Chung, C.-w.; Ceska, T.; Zoonens, M.; Sobott, F.; et al. Cycloalkane-modified amphiphilic polymers provide direct extraction of membrane proteins for CryoEM analysis. Commun. Biol. 2021, 4, 1337. [Google Scholar] [CrossRef]
- Fiori, M.C.; Jiang, Y.; Altenberg, G.A.; Liang, H. Polymer-encased nanodiscs with improved buffer compatibility. Sci. Rep. 2017, 7, 7432. [Google Scholar] [CrossRef]
- Danielczak, B.; Rasche, M.; Lenz, J.; Pérez Patallo, E.; Weyrauch, S.; Mahler, F.; Agbadaola, M.T.; Meister, A.; Babalola, J.O.; Vargas, C.; et al. A bioinspired glycopolymer for capturing membrane proteins in native-like lipid-bilayer nanodiscs. Nanoscale 2022, 14, 1855–1867. [Google Scholar] [CrossRef]
- Broadbent, L.; Depping, P.; Lodé, A.; Vaitsopoulou, A.; Hardy, D.; Ayub, H.; Mitchell-White, J.; Kerr, I.D.; Goddard, A.D.; Bill, R.M.; et al. Detergent-free membrane protein purification using SMA polymer. In Heterologous Expression of Membrane Proteins. Methods in Molecular Biology; Mus-Veteau, I., Ed.; Springer: New York, NY, USA, 2022; Volume 2507, pp. 389–404. [Google Scholar]
- Dimitrova, V.S.; Song, S.; Karagiaridi, A.; Marand, A.; Pinkett, H.W. Detergent alternatives: Membrane protein purification using synthetic nanodisc polymers. In Heterologous Expression of Membrane Proteins. Methods in Molecular Biology; Mus-Veteau, I., Ed.; Springer: New York, NY, USA, 2022; Volume 2507, pp. 375–387. [Google Scholar]
- Lee, S.C.; Knowles, T.J.; Postis, V.L.; Jamshad, M.; Parslow, R.A.; Lin, Y.P.; Goldman, A.; Sridhar, P.; Overduin, M.; Muench, S.P.; et al. A method for detergent-free isolation of membrane proteins in their local lipid environment. Nat. Protoc. 2016, 11, 1149–1162. [Google Scholar] [CrossRef]
- Kopf, A.H.; Dörr, J.M.; Koorengevel, M.C.; Antoniciello, F.; Jahn, H.; Killian, J.A. Factors influencing the solubilization of membrane proteins from Escherichia coli membranes by styrene-maleic acid copolymers. Biochim. Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183125. [Google Scholar] [CrossRef]
- Tedesco, D.; Maj, M.; Malarczyk, P.; Cingolani, A.; Zaffagnini, M.; Wnorowski, A.; Czapiński, J.; Benelli, T.; Mazzoni, R.; Bartolini, M.; et al. Application of the SMALP technology to the isolation of GPCRs from low-yielding cell lines. Biochim. Biophys. Acta (BBA)-Biomembr. 2021, 1863, 183641. [Google Scholar] [CrossRef]
- Smirnova, I.A.; Sjöstrand, D.; Li, F.; Björck, M.; Schäfer, J.; Östbye, H.; Högbom, M.; von Ballmoos, C.; Lander, G.C.; Ädelroth, P.; et al. Isolation of yeast complex IV in native lipid nanodiscs. Biochim. Biophys. Acta (BBA)-Biomembr. 2016, 1858, 2984–2992. [Google Scholar] [CrossRef] [PubMed]
- Swainsbury, D.J.K.; Proctor, M.S.; Hitchcock, A.; Cartron, M.L.; Qian, P.; Martin, E.C.; Jackson, P.J.; Madsen, J.; Armes, S.P.; Hunter, C.N. Probing the local lipid environment of the Rhodobacter sphaeroides cytochrome bc1 and Synechocystis sp. PCC 6803 cytochrome b6f complexes with styrene maleic acid. Biochim. Biophys. Acta (BBA)-Bioenergetics 2018, 1859, 215–225. [Google Scholar] [CrossRef]
- Bada Juarez, J.F.; Muñoz-García, J.C.; Inácio dos Reis, R.; Henry, A.; McMillan, D.; Kriek, M.; Wood, M.; Vandenplas, C.; Sands, Z.; Castro, L.; et al. Detergent-free extraction of a functional low-expressing GPCR from a human cell line. Biochim. Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183152. [Google Scholar] [CrossRef] [PubMed]
- Skaar, K.; Korza, H.J.; Tarry, M.; Sekyrova, P.; Högbom, M. Expression and subcellular distribution of GFP-tagged human tetraspanin proteins in Saccharomyces cerevisiae. PLoS ONE 2015, 10, e0134041. [Google Scholar] [CrossRef] [PubMed]
- Dörr, J.M.; Koorengevel, M.C.; Schäfer, M.; Prokofyev, A.V.; Scheidelaar, S.; van der Cruijsen, E.A.W.; Dafforn, T.R.; Baldus, M.; Killian, J.A. Detergent-free isolation, characterization, and functional reconstitution of a tetrameric K+ channel: The power of native nanodiscs. Proc. Natl. Acad. Sci. USA 2014, 111, 18607–18612. [Google Scholar] [CrossRef]
- Swainsbury, D.J.K.; Scheidelaar, S.; Foster, N.; van Grondelle, R.; Killian, J.A.; Jones, M.R. The effectiveness of styrene-maleic acid (SMA) copolymers for solubilisation of integral membrane proteins from SMA-accessible and SMA-resistant membranes. Biochim. Biophys. Acta (BBA)-Biomembr. 2017, 1859, 2133–2143. [Google Scholar] [CrossRef]
- Broecker, J.; Eger, B.T.; Ernst, O.P. Crystallogenesis of membrane proteins mediated by polymer-bounded lipid nanodiscs. Structure 2017, 25, 384–392. [Google Scholar] [CrossRef]
- Scheidelaar, S.; Koorengevel, M.C.; van Walree, C.A.; Dominguez, J.J.; Dörr, J.M.; Killian, J.A. Effect of polymer composition and pH on membrane solubilization by styrene-maleic acid copolymers. Biophys. J. 2016, 111, 1974–1986. [Google Scholar] [CrossRef]
- Orwick, M.C.; Judge, P.J.; Procek, J.; Lindholm, L.; Graziadei, A.; Engel, A.; Gröbner, G.; Watts, A. Detergent-free formation and physicochemical characterization of nanosized lipid–polymer complexes: Lipodisq. Angew. Chem. Int. Ed. 2012, 51, 4653–4657. [Google Scholar] [CrossRef]
- Sahoo, B.R.; Genjo, T.; Moharana, K.C.; Ramamoorthy, A. Self-assembly of polymer-encased lipid nanodiscs and membrane protein reconstitution. J. Phys. Chem. B 2019, 123, 4562–4570. [Google Scholar] [CrossRef]
- Bali, A.P.; Sahu, I.D.; Craig, A.F.; Clark, E.E.; Burridge, K.M.; Dolan, M.T.; Dabney-Smith, C.; Konkolewicz, D.; Lorigan, G.A. Structural characterization of styrene-maleic acid copolymer-lipid nanoparticles (SMALPs) using EPR spectroscopy. Chem. Phys. Lipids 2019, 220, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Sahu, I.D.; Liu, L.; Osatuke, A.; Comer, R.G.; Dabney-Smith, C.; Lorigan, G.A. Characterizing the structure of lipodisq nanoparticles for membrane protein spectroscopic studies. Biochim. Biophys. Acta (BBA)-Biomembr. 2015, 1848, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.C.L.; Tognoloni, C.; Campbell, R.A.; Richens, J.; O’Shea, P.; Terry, A.E.; Price, G.J.; Dafforn, T.R.; Edler, K.J.; Arnold, T. The interaction of styrene maleic acid copolymers with phospholipids in Langmuir monolayers, vesicles and nanodiscs; a structural study. J. Colloid Interface Sci. 2022, 625, 220–236. [Google Scholar] [CrossRef] [PubMed]
- Scheidelaar, S.; Koorengevel, M.C.; Pardo, J.D.; Meeldijk, J.D.; Breukink, E.; Killian, J.A. Molecular model for the solubilization of membranes into nanodisks by styrene maleic acid copolymers. Biophys. J. 2015, 108, 279–290. [Google Scholar] [CrossRef]
- Barniol-Xicota, M.; Verhelst, S.H.L. Stable and functional rhomboid proteases in lipid nanodiscs by using diisobutylene/maleic acid copolymers. J. Am. Chem. Soc. 2018, 140, 14557–14561. [Google Scholar] [CrossRef]
- Krajewska, M.; Koprowski, P. Solubilization, purification, and functional reconstitution of human ROMK potassium channel in copolymer styrene-maleic acid (SMA) nanodiscs. Biochim. Biophys. Acta (BBA)-Biomembr. 2021, 1863, 183555. [Google Scholar] [CrossRef]
- Ravula, T.; Ramadugu, S.K.; Di Mauro, G.; Ramamoorthy, A. Bioinspired, size-tunable self-assembly of polymer-lipid bilayer nanodiscs. Angew. Chem. Int. Ed. 2017, 56, 11466–11470. [Google Scholar] [CrossRef]
- Long, A.R.; O’Brien, C.C.; Malhotra, K.; Schwall, C.T.; Albert, A.D.; Watts, A.; Alder, N.N. A detergent-free strategy for the reconstitution of active enzyme complexes from native biological membranes into nanoscale discs. BMC Biotechnol. 2013, 13, 41. [Google Scholar] [CrossRef]
- Knowles, T.J.; Finka, R.; Smith, C.; Lin, Y.-P.; Dafforn, T.; Overduin, M. Membrane proteins solubilized intact in lipid containing nanoparticles bounded by styrene maleic acid copolymer. J. Am. Chem. Soc. 2009, 131, 7484–7485. [Google Scholar] [CrossRef]
- Oluwole, A.O.; Danielczak, B.; Meister, A.; Babalola, J.O.; Vargas, C.; Keller, S. Solubilization of membrane proteins into functional lipid-bilayer nanodiscs using a diisobutylene/maleic acid copolymer. Angew. Chem. Int. Ed. 2017, 56, 1919–1924. [Google Scholar] [CrossRef]
- Swainsbury, D.J.K.; Scheidelaar, S.; van Grondelle, R.; Killian, J.A.; Jones, M.R. Bacterial reaction centers purified with styrene maleic acid copolymer retain native membrane functional properties and display enhanced stability. Angew. Chem. Int. Ed. 2014, 53, 11803–11807. [Google Scholar] [CrossRef] [PubMed]
- Hesketh, S.J.; Klebl, D.P.; Higgins, A.J.; Thomsen, M.; Pickles, I.B.; Sobott, F.; Sivaprasadarao, A.; Postis, V.L.G.; Muench, S.P. Styrene maleic-acid lipid particles (SMALPs) into detergent or amphipols: An exchange protocol for membrane protein characterisation. Biochim. Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183192. [Google Scholar] [CrossRef] [PubMed]
- Sander, C.L.; Sears, A.E.; Pinto, A.F.M.; Choi, E.H.; Kahremany, S.; Gao, F.; Salom, D.; Jin, H.; Pardon, E.; Suh, S.; et al. Nano-scale resolution of native retinal rod disk membranes reveals differences in lipid composition. J. Cell Biol. 2021, 220, e202101063. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.T.; Jimah, J.R.; Dowdy, T.; Ohlemacher, S.I.; Larion, M.; Hinshaw, J.E.; Bernstein, H.D. Cryo-EM structures reveal multiple stages of bacterial outer membrane protein folding. Cell 2022, 185, 1143–1156.e1113. [Google Scholar] [CrossRef]
- Hellwig, N.; Peetz, O.; Ahdash, Z.; Tascón, I.; Booth, P.J.; Mikusevic, V.; Diskowski, M.; Politis, A.; Hellmich, Y.; Hänelt, I.; et al. Native mass spectrometry goes more native: Investigation of membrane protein complexes directly from SMALPs. Chem. Commun. 2018, 54, 13702–13705. [Google Scholar] [CrossRef]
- Esmaili, M.; Tancowny, B.P.; Wang, X.; Moses, A.; Cortez, L.; Sim, V.; Wille, H.; Overduin, M. Native nanodiscs formed by styrene-maleic acid copolymer derivatives help recover infectious prion multimers bound to brain-derived lipids. J. Biol. Chem. 2020, 295, 8460–8469. [Google Scholar] [CrossRef]
- Routledge, S.J.; Mikaliunaite, L.; Patel, A.; Clare, M.; Cartwright, S.P.; Bawa, Z.; Wilks, M.D.B.; Low, F.; Hardy, D.; Rothnie, A.J.; et al. The synthesis of recombinant membrane proteins in yeast for structural studies. Methods 2016, 95, 26–37. [Google Scholar] [CrossRef]
- Gulati, S.; Jamshad, M.; Knowles, T.J.; Morrison, K.A.; Downing, R.; Cant, N.; Collins, R.; Koenderink, J.B.; Ford, R.C.; Overduin, M.; et al. Detergent-free purification of ABC (ATP-binding-cassette) transporters. Biochem. J. 2014, 461, 269–278. [Google Scholar] [CrossRef]
- Hardy, D.; Bill, R.M.; Rothnie, A.J.; Jawhari, A. Stabilization of human multidrug resistance protein 4 (MRP4/ABCC4) using novel solubilization agents. SLAS Discov. 2019, 24, 1009–1017. [Google Scholar] [CrossRef]
- Dilworth, M.V.; Findlay, H.E.; Booth, P.J. Detergent-free purification and reconstitution of functional human serotonin transporter (SERT) using diisobutylene maleic acid (DIBMA) copolymer. Biochim. Biophys. Acta (BBA)-Biomembr. 2021, 1863, 183602. [Google Scholar] [CrossRef]
- Postis, V.; Rawson, S.; Mitchell, J.K.; Lee, S.C.; Parslow, R.A.; Dafforn, T.R.; Baldwin, S.A.; Muench, S.P. The use of SMALPs as a novel membrane protein scaffold for structure study by negative stain electron microscopy. Biochim. Biophys. Acta (BBA)-Biomembr. 2015, 1848, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Logez, C.; Damian, M.; Legros, C.; Dupré, C.; Guéry, M.; Mary, S.; Wagner, R.; M’Kadmi, C.; Nosjean, O.; Fould, B.; et al. Detergent-free isolation of functional G protein-coupled receptors into nanometric lipid particles. Biochemistry 2016, 55, 38–48. [Google Scholar] [CrossRef]
- Horsey, A.J.; Briggs, D.A.; Holliday, N.D.; Briddon, S.J.; Kerr, I.D. Application of fluorescence correlation spectroscopy to study substrate binding in styrene maleic acid lipid copolymer encapsulated ABCG2. Biochim. Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183218. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhao, H.; Wang, Y.; Yu, C.; Liu, J.; Li, L.; Li, Z.; Zhang, J.; Dai, H.; Wang, J.; et al. Solubilization, purification, and ligand binding characterization of G protein-coupled receptor SMO in native membrane bilayer using styrene maleic acid copolymer. PeerJ 2022, 10, e13381. [Google Scholar] [CrossRef] [PubMed]
- Korona, D.; Dirnberger, B.; Giachello, C.N.G.; Queiroz, R.M.L.; Popovic, R.; Müller, K.H.; Minde, D.-P.; Deery, M.J.; Johnson, G.; Firth, L.C.; et al. Drosophila nicotinic acetylcholine receptor subunits and their native interactions with insecticidal peptide toxins. Elife 2022, 11, e74322. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Fang, J.; Shi, Y.; Wang, H.; Chen, X.; Liu, Y.; Zhu, Z.; Cao, Y.; Hong, Z.; Chai, Y. Screening potential P-glycoprotein inhibitors by combination of a detergent-free membrane protein extraction with surface plasmon resonance biosensor. Acta Pharm. Sin. B 2022, 12, 3113–3123. [Google Scholar] [CrossRef]
- Routledge, S.J.; Jamshad, M.; Little, H.A.; Lin, Y.-P.; Simms, J.; Thakker, A.; Spickett, C.M.; Bill, R.M.; Dafforn, T.R.; Poyner, D.R.; et al. Ligand-induced conformational changes in a SMALP-encapsulated GPCR. Biochim. Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183235. [Google Scholar] [CrossRef]
- Karlova, M.G.; Voskoboynikova, N.; Gluhov, G.S.; Abramochkin, D.; Malak, O.A.; Mulkidzhanyan, A.; Loussouarn, G.; Steinhoff, H.J.; Shaitan, K.V.; Sokolova, O.S. Detergent-free solubilization of human Kv channels expressed in mammalian cells. Chem. Phys. Lipids 2019, 219, 50–57. [Google Scholar] [CrossRef]
- Korotych, O.I.; Nguyen, T.T.; Reagan, B.C.; Burch-Smith, T.M.; Bruce, B.D. Poly(styrene-co-maleic acid)-mediated isolation of supramolecular membrane protein complexes from plant thylakoids. Biochim. Biophys. Acta (BBA)-Bioenergetics 2021, 1862, 148347. [Google Scholar] [CrossRef]
- Voskoboynikova, N.; Karlova, M.; Kurre, R.; Mulkidjanian, A.Y.; Shaitan, K.V.; Sokolova, O.S.; Steinhoff, H.-J.; Heinisch, J.J. A three-dimensional model of the yeast transmembrane sensor Wsc1 obtained by SMA-based detergent-free purification and transmission electron microscopy. J. Fungi 2021, 7, 118. [Google Scholar] [CrossRef]
- Kemmerer, Z.A.; Robinson, K.P.; Schmitz, J.M.; Manicki, M.; Paulson, B.R.; Jochem, A.; Hutchins, P.D.; Coon, J.J.; Pagliarini, D.J. UbiB proteins regulate cellular CoQ distribution in Saccharomyces cerevisiae. Nat. Commun. 2021, 12, 4769. [Google Scholar] [CrossRef] [PubMed]
- Dörr, J.M.; van Coevorden-Hameete, M.H.; Hoogenraad, C.C.; Killian, J.A. Solubilization of human cells by the styrene–maleic acid copolymer: Insights from fluorescence microscopy. Biochim. Biophys. Acta (BBA)-Biomembr. 2017, 1859, 2155–2160. [Google Scholar] [CrossRef] [PubMed]
- Ayub, H.; Clare, M.; Milic, I.; Chmel, N.P.; Böning, H.; Devitt, A.; Krey, T.; Bill, R.M.; Rothnie, A.J. CD81 extracted in SMALP nanodiscs comprises two distinct protein populations within a lipid environment enriched with negatively charged headgroups. Biochim. Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183419. [Google Scholar] [CrossRef] [PubMed]
- Parmar, M.; Rawson, S.; Scarff, C.A.; Goldman, A.; Dafforn, T.R.; Muench, S.P.; Postis, V.L.G. Using a SMALP platform to determine a sub-nm single particle cryo-EM membrane protein structure. Biochim. Biophys. Acta (BBA)-Biomembr. 2018, 1860, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Tascón, I.; Sousa, J.S.; Corey, R.A.; Mills, D.J.; Griwatz, D.; Aumüller, N.; Mikusevic, V.; Stansfeld, P.J.; Vonck, J.; Hänelt, I. Structural basis of proton-coupled potassium transport in the KUP family. Nat. Commun. 2020, 11, 626. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Esmaili, M.; Overduin, M.; Fliegel, L. Expression and detergent free purification and reconstitution of the plant plasma membrane Na+/H+ antiporter SOS1 overexpressed in Pichia pastoris. Biochim. Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183111. [Google Scholar] [CrossRef] [PubMed]
- Komar, J.; Alvira, S.; Schulze, R.J.; Martin, R.; Lycklama a Nijeholt, J.A.; Lee, S.C.; Dafforn, T.R.; Deckers-Hebestreit, G.; Berger, I.; Schaffitzel, C.; et al. Membrane protein insertion and assembly by the bacterial holo-translocon SecYEG–SecDF–YajC–YidC. Biochem. J. 2016, 473, 3341–3354. [Google Scholar] [CrossRef]
- Desrames, A.; Genetet, S.; Delcourt, M.P.; Goossens, D.; Mouro-Chanteloup, I. Detergent-free isolation of native red blood cell membrane complexes. Biochim. Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183126. [Google Scholar] [CrossRef]
- Bersch, B.; Dörr, J.M.; Hessel, A.; Killian, J.A.; Schanda, P. Proton-detected solid-state NMR spectroscopy of a zinc diffusion facilitator protein in native nanodiscs. Angew. Chem. Int. Ed. 2017, 56, 2508–2512. [Google Scholar] [CrossRef]
- Gulamhussein, A.A.; Uddin, R.; Tighe, B.J.; Poyner, D.R.; Rothnie, A.J. A comparison of SMA (styrene maleic acid) and DIBMA (di-isobutylene maleic acid) for membrane protein purification. Biochim. Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183281. [Google Scholar] [CrossRef]
- Swiecicki, J.-M.; Santana, J.T.; Imperiali, B. A strategic approach for fluorescence imaging of membrane proteins in a native-like environment. Cell Chem. Biol. 2020, 27, 245–251.e243. [Google Scholar] [CrossRef] [PubMed]
- Paulin, S.; Jamshad, M.; Dafforn, T.R.; Garcia-Lara, J.; Foster, S.J.; Galley, N.F.; Roper, D.I.; Rosado, H.; Taylor, P.W. Surfactant-free purification of membrane protein complexes from bacteria: Application to the staphylococcal penicillin-binding protein complex PBP2/PBP2a. Nanotechnology 2014, 25, 285101. [Google Scholar] [CrossRef]
- Morrison, K.A.; Akram, A.; Mathews, A.; Khan, Z.A.; Patel, J.H.; Zhou, C.; Hardy, D.J.; Moore-Kelly, C.; Patel, R.; Odiba, V.; et al. Membrane protein extraction and purification using styrene–maleic acid (SMA) copolymer: Effect of variations in polymer structure. Biochem. J. 2016, 473, 4349–4360. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, J.; Zhuang, Y.; Zhang, L.; Xiong, Y.; Shi, P.; Tian, C. Nano-size uni-lamellar lipodisq improved in situ auto-phosphorylation analysis of E. coli tyrosine kinase using 19F nuclear magnetic resonance. Protein Cell 2015, 6, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Laursen, T.; Borch, J.; Knudsen, C.; Bavishi, K.; Torta, F.; Martens, H.J.; Silvestro, D.; Hatzakis, N.S.; Wenk, M.R.; Dafforn, T.R.; et al. Characterization of a dynamic metabolon producing the defense compound dhurrin in sorghum. Science 2016, 354, 890–893. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.J.; Frankel, L.K.; Bricker, T.M. High yield non-detergent isolation of photosystem I-light-harvesting chlorophyll II membranes from spinach thylakoids: Implications for the organization of the PS I antennae in higher plants. J. Biol. Chem. 2015, 290, 18429–18437. [Google Scholar] [CrossRef]
- Prabudiansyah, I.; Kusters, I.; Caforio, A.; Driessen, A.J.M. Characterization of the annular lipid shell of the Sec translocon. Biochim. Biophys. Acta (BBA)-Biomembr. 2015, 1848, 2050–2056. [Google Scholar] [CrossRef]
- Johnson, R.M.; Fais, C.; Parmar, M.; Cheruvara, H.; Marshall, R.L.; Hesketh, S.J.; Feasey, M.C.; Ruggerone, P.; Vargiu, A.V.; Postis, V.L.G.; et al. Cryo-EM structure and molecular dynamics analysis of the fluoroquinolone resistant mutant of the AcrB transporter from Salmonella. Microorganisms 2020, 8, 943. [Google Scholar] [CrossRef]
- Li, J.; Han, L.; Vallese, F.; Ding, Z.; Choi, S.K.; Hong, S.; Luo, Y.; Liu, B.; Chan, C.K.; Tajkhorshid, E.; et al. Cryo-EM structures of Escherichia coli cytochrome bo3 reveal bound phospholipids and ubiquinone-8 in a dynamic substrate binding site. Proc. Natl. Acad. Sci. USA 2021, 118, e2106750118. [Google Scholar] [CrossRef]
- Kumar, P.; Cymes, G.D.; Grosman, C. Structure and function at the lipid–protein interface of a pentameric ligand-gated ion channel. Proc. Natl. Acad. Sci. USA 2021, 118, e2100164118. [Google Scholar] [CrossRef]
- Deng, Y.-n.; Kashtoh, H.; Wang, Q.; Zhen, G.-x.; Li, Q.-y.; Tang, L.-h.; Gao, H.-l.; Zhang, C.-r.; Qin, L.; Su, M.; et al. Structure and activity of SLAC1 channels for stomatal signaling in leaves. Proc. Natl. Acad. Sci. USA 2021, 118, e2015151118. [Google Scholar] [CrossRef] [PubMed]
- Ueta, T.; Kojima, K.; Hino, T.; Shibata, M.; Nagano, S.; Sudo, Y. Applicability of styrene-maleic acid copolymer for two microbial rhodopsins, RxR and HsSRI. Biophys. J. 2020, 119, 1760–1770. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Guo, Y. Analysis of the oligomeric state of mycobacterial membrane protein large 3 and its interaction with SQ109 with native cell membrane nanoparticles system. Biochim. Biophys. Acta (BBA)-Biomembr. 2022, 1864, 183793. [Google Scholar] [CrossRef]
- Brady, N.G.; Workman, C.E.; Cawthon, B.; Bruce, B.D.; Long, B.K. Protein extraction efficiency and selectivity of esterified styrene–maleic acid copolymers in thylakoid membranes. Biomacromolecules 2021, 22, 2544–2553. [Google Scholar] [CrossRef] [PubMed]
- Pollock, N.L.; Lloyd, J.; Montinaro, C.; Rai, M.; Dafforn, T.R. Conformational trapping of an ABC transporter in polymer lipid nanoparticles. Biochem. J. 2022, 479, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, T.; Das, D. Biochemical investigation of membrane-bound cytochrome b5 and the catalytic domain of cytochrome b5 reductase from Arabidopsis thaliana. Biochemistry 2022, 61, 909–921. [Google Scholar] [CrossRef]
- Cosentino, K.; Hertlein, V.; Jenner, A.; Dellmann, T.; Gojkovic, M.; Peña-Blanco, A.; Dadsena, S.; Wajngarten, N.; Danial, J.S.H.; Thevathasan, J.V.; et al. The interplay between BAX and BAK tunes apoptotic pore growth to control mitochondrial-DNA-mediated inflammation. Mol. Cell 2022, 82, 933–949.e939. [Google Scholar] [CrossRef]
- Ball, L.E.; Riley, L.J.; Hadasha, W.; Pfukwa, R.; Smith, C.J.I.; Dafforn, T.R.; Klumperman, B. Influence of DIBMA polymer length on lipid nanodisc formation and membrane protein extraction. Biomacromolecules 2021, 22, 763–772. [Google Scholar] [CrossRef]
- Lavington, S.; Watts, A. Detergent-free solubilisation & purification of a G protein coupled receptor using a polymethacrylate polymer. Biochim. Biophys. Acta (BBA)-Biomembr. 2021, 1863, 183441. [Google Scholar] [CrossRef]
- Qiu, W.; Fu, Z.; Xu, G.G.; Grassucci, R.A.; Zhang, Y.; Frank, J.; Hendrickson, W.A.; Guo, Y. Structure and activity of lipid bilayer within a membrane-protein transporter. Proc. Natl. Acad. Sci. USA 2018, 115, 12985–12990. [Google Scholar] [CrossRef]
- Guo, Y. Be cautious with crystal structures of membrane proteins or complexes prepared in detergents. Crystals 2020, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Benlekbir, S.; Venkatakrishnan, P.; Wang, Y.; Hong, S.; Hosler, J.; Tajkhorshid, E.; Rubinstein, J.L.; Gennis, R.B. Structure of the alternative complex III in a supercomplex with cytochrome oxidase. Nature 2018, 557, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhu, H.; Lape, R.; Greiner, T.; Du, J.; Lü, W.; Sivilotti, L.; Gouaux, E. Mechanism of gating and partial agonist action in the glycine receptor. Cell 2021, 184, 957–968.e921. [Google Scholar] [CrossRef]
- Yoder, N.; Gouaux, E. The His-Gly motif of acid-sensing ion channels resides in a reentrant ‘loop’ implicated in gating and ion selectivity. Elife 2020, 9, e56527. [Google Scholar] [CrossRef]
- Gonen, T.; Cheng, Y.; Sliz, P.; Hiroaki, Y.; Fujiyoshi, Y.; Harrison, S.C.; Walz, T. Lipid-protein interactions in double-layered two-dimensional AQP0 crystals. Nature 2005, 438, 633–638. [Google Scholar] [CrossRef]
- Schmidt, V.; Sturgis, J.N. Modifying styrene-maleic acid co-polymer for studying lipid nanodiscs by direct fluorescent labeling. Bio-Protocol 2018, 8, e2969. [Google Scholar] [CrossRef]
- Ravula, T.; Hardin, N.Z.; Ramadugu, S.K.; Cox, S.J.; Ramamoorthy, A. Formation of pH-resistant monodispersed polymer-lipid nanodiscs. Angew. Chem. Int. Ed. 2018, 57, 1342–1345. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, D.O.; Cabiaux, V.; Choe, S.; Eisenberg, D.; Collier, R.J. pH-dependent insertion of proteins into membranes: B-chain mutation of diphtheria toxin that inhibits membrane translocation, Glu-349 → Lys. Proc. Natl. Acad. Sci. USA 1992, 89, 6202–6206. [Google Scholar] [CrossRef]
- Andreev, O.A.; Karabadzhak, A.G.; Weerakkody, D.; Andreev, G.O.; Engelman, D.M.; Reshetnyak, Y.K. pH (low) insertion peptide (pHLIP) inserts across a lipid bilayer as a helix and exits by a different path. Proc. Natl. Acad. Sci. USA 2010, 107, 4081–4086. [Google Scholar] [CrossRef]
- Hall, S.C.L.; Tognoloni, C.; Charlton, J.; Bragginton, E.C.; Rothnie, A.J.; Sridhar, P.; Wheatley, M.; Knowles, T.J.; Arnold, T.; Edler, K.J.; et al. An acid-compatible co-polymer for the solubilization of membranes and proteins into lipid bilayer-containing nanoparticles. Nanoscale 2018, 10, 10609–10619. [Google Scholar] [CrossRef]
- Danielczak, B.; Meister, A.; Keller, S. Influence of Mg2+ and Ca2+ on nanodisc formation by diisobutylene/maleic acid (DIBMA) copolymer. Chem. Phys. Lipids 2019, 221, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Grime, R.L.; Logan, R.T.; Nestorow, S.A.; Sridhar, P.; Edwards, P.C.; Tate, C.G.; Klumperman, B.; Dafforn, T.R.; Poyner, D.R.; Reeves, P.J.; et al. Differences in SMA-like polymer architecture dictate the conformational changes exhibited by the membrane protein rhodopsin encapsulated in lipid nano-particles. Nanoscale 2021, 13, 13519–13528. [Google Scholar] [CrossRef] [PubMed]
- Szundi, I.; Pitch, S.G.; Chen, E.; Farrens, D.L.; Kliger, D.S. Styrene-maleic acid copolymer effects on the function of the GPCR rhodopsin in lipid nanoparticles. Biophys. J. 2021, 120, 4337–4348. [Google Scholar] [CrossRef]
- Hawkins, O.P.; Jahromi, C.P.T.; Gulamhussein, A.A.; Nestorow, S.; Bahra, T.; Shelton, C.; Owusu-Mensah, Q.K.; Mohiddin, N.; O’Rourke, H.; Ajmal, M.; et al. Membrane protein extraction and purification using partially-esterified SMA polymers. Biochim. Biophys. Acta (BBA)-Biomembr. 2021, 1863, 183758. [Google Scholar] [CrossRef] [PubMed]
- Esmaili, M.; Brown, C.J.; Shaykhutdinov, R.; Acevedo-Morantes, C.; Wang, Y.L.; Wille, H.; Gandour, R.D.; Turner, S.R.; Overduin, M. Homogeneous nanodiscs of native membranes formed by stilbene–maleic-acid copolymers. Nanoscale 2020, 12, 16705–16709. [Google Scholar] [CrossRef]
- Fiori, M.C.; Zheng, W.; Kamilar, E.; Simiyu, G.; Altenberg, G.A.; Liang, H. Extraction and reconstitution of membrane proteins into lipid nanodiscs encased by zwitterionic styrene-maleic amide copolymers. Sci. Rep. 2020, 10, 9940. [Google Scholar] [CrossRef]
- Ravula, T.; Hardin, N.Z.; Bai, J.; Im, S.C.; Waskell, L.; Ramamoorthy, A. Effect of polymer charge on functional reconstitution of membrane proteins in polymer nanodiscs. Chem. Commun. 2018, 54, 9615–9618. [Google Scholar] [CrossRef]
- Barniol-Xicota, M.; Verhelst, S.H.L. Lipidomic and in-gel analysis of maleic acid co-polymer nanodiscs reveals differences in composition of solubilized membranes. Commun. Biol. 2021, 4, 218. [Google Scholar] [CrossRef]
- Harayama, T.; Riezman, H. Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol. 2018, 19, 281–296. [Google Scholar] [CrossRef]
- Ravula, T.; Ramamoorthy, A. Synthesis, characterization, and nanodisc formation of non-ionic polymers. Angew. Chem. Int. Ed. 2021, 60, 16885–16888. [Google Scholar] [CrossRef]
- Krishnarjuna, B.; Im, S.-C.; Ravula, T.; Marte, J.; Auchus, R.; Ramamoorthy, A. Non-ionic inulin-based polymer-nanodiscs enable functional reconstitution of a redox complex composed of oppositely charged CYP450 and CPR in a lipid bilayer membrane. 2022; submitted. [Google Scholar]
- Lloris-Garcerá, P.; Klinter, S.; Chen, L.; Skynner, M.J.; Löving, R.; Frauenfeld, J. DirectMX—One-step reconstitution of membrane proteins from crude cell membranes into Salipro nanoparticles. Front. Bioeng. Biotechnol. 2020, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Lee, A. Lipid-protein interactions in biological membranes: A structural perspective. Biochim. Biophys. Acta (BBA)-Biomembr. 2003, 1612, 1–40. [Google Scholar] [CrossRef]
- Ong, S.T.; Bajaj, S.; Tanner, M.R.; Chang, S.C.; Krishnarjuna, B.; Ng, X.R.; Morales, R.A.V.; Chen, M.W.; Luo, D.; Patel, D.J.; et al. Modulation of lymphocyte potassium channel KV1.3 by membrane-penetrating, joint-targeting immunomodulatory plant defensin. ACS Pharmacol. Transl. Sci. 2020, 3, 720–736. [Google Scholar] [CrossRef] [PubMed]
- Sunanda, P.; Krishnarjuna, B.; Peigneur, S.; Mitchell, M.L.; Estrada, R.; Villegas-Moreno, J.; Pennington, M.W.; Tytgat, J.; Norton, R.S. Identification, chemical synthesis, structure, and function of a new KV1 channel blocking peptide from Oulactis sp. Pept. Sci. 2018, 110, e24073. [Google Scholar] [CrossRef]
- Jaipuria, G.; Krishnarjuna, B.; Mondal, S.; Dubey, A.; Atreya, H. Amino Acid Selective Labeling and Unlabeling for Protein Resonance Assignments. In Isotope Labeling in Biomolecular NMR; Advances in Experimental Medicine and, Biology; Atreya, H.S., Ed.; Springer: Dordrecht, The Netherlands, 2012; Volume 992, pp. 95–118. [Google Scholar]
- Krishnarjuna, B.; Sunanda, P.; Villegas-Moreno, J.; Csoti, A.; Morales, R.A.V.; Wai, D.C.C.; Panyi, G.; Prentis, P.; Norton, R.S. A disulfide-stabilised helical hairpin fold in acrorhagin I: An emerging structural motif in peptide toxins. J. Struct. Biol. 2021, 213, 107692. [Google Scholar] [CrossRef]
- Elnahriry, K.A.; Wai, D.C.C.; Krishnarjuna, B.; Badawy, N.N.; Chittoor, B.; MacRaild, C.A.; Williams-Noonan, B.J.; Surm, J.M.; Chalmers, D.K.; Zhang, A.H.; et al. Structural and functional characterisation of a novel peptide from the Australian sea anemone Actinia tenebrosa. Toxicon 2019, 168, 104–112. [Google Scholar] [CrossRef]
- Krishnarjuna, B.; MacRaild, C.A.; Sunanda, P.; Morales, R.A.V.; Peigneur, S.; Macrander, J.; Yu, H.H.; Daly, M.; Raghothama, S.; Dhawan, V.; et al. Structure, folding and stability of a minimal homologue from Anemonia sulcata of the sea anemone potassium channel blocker ShK. Peptides 2018, 99, 169–178. [Google Scholar] [CrossRef]
- Krishnarjuna, B.; Villegas-Moreno, J.; Mitchell, M.L.; Csoti, A.; Peigneur, S.; Amero, C.; Pennington, M.W.; Tytgat, J.; Panyi, G.; Norton, R.S. Synthesis, folding, structure and activity of a predicted peptide from the sea anemone Oulactis sp. with an ShKT fold. Toxicon 2018, 150, 50–59. [Google Scholar] [CrossRef]
- Eagles, D.A.; Saez, N.J.; Krishnarjuna, B.; Bradford, J.J.; Chin, Y.K.-Y.; Starobova, H.; Mueller, A.; Reichelt, M.E.; Undheim, E.A.B.; Norton, R.S.; et al. A peptide toxin in ant venom mimics vertebrate EGF-like hormones to cause long-lasting hypersensitivity in mammals. Proc. Natl. Acad. Sci. USA 2022, 119, e2112630119. [Google Scholar] [CrossRef]
- Genton, B.; Betuela, I.; Felger, I.; Al-Yaman, F.; Anders, R.F.; Saul, A.; Rare, L.; Baisor, M.; Lorry, K.; Brown, G.V.; et al. A recombinant blood-stage malaria vaccine reduces Plasmodium falciparum density and exerts selective pressure on parasite populations in a phase 1-2b trial in Papua New Guinea. J. Infect. Dis. 2002, 185, 820–827. [Google Scholar] [CrossRef]
- Krishnarjuna, B.; Andrew, D.; MacRaild, C.A.; Morales, R.A.V.; Beeson, J.G.; Anders, R.F.; Richards, J.S.; Norton, R.S. Strain-transcending immune response generated by chimeras of the malaria vaccine candidate merozoite surface protein 2. Sci. Rep. 2016, 6, 20613. [Google Scholar] [CrossRef] [PubMed]
- MacRaild, C.A.; Zachrdla, M.; Andrew, D.; Krishnarjuna, B.; Nováček, J.; Žídek, L.; Sklenář, V.; Richards, J.S.; Beeson, J.G.; Anders, R.F.; et al. Conformational dynamics and antigenicity in the disordered malaria antigen merozoite surface protein 2. PLoS ONE 2015, 10, e0119899. [Google Scholar] [CrossRef] [PubMed]
- Das, S.C.; Morales, R.A.; Seow, J.; Krishnarjuna, B.; Dissanayake, R.; Anders, R.F.; MacRaild, C.A.; Norton, R.S. Lipid interactions modulate the structural and antigenic properties of the C-terminal domain of the malaria antigen merozoite surface protein 2. FEBS J. 2017, 284, 2649–2662. [Google Scholar] [CrossRef] [PubMed]
- Bozelli, J.C.; Aulakh, S.S.; Epand, R.M. Membrane shape as determinant of protein properties. Biophys. Chem. 2021, 273, 106587. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Kazama, A.; Fukuhara, K.; Sato, H.; Tamai, N.; Ito, H.-O.; Matsuki, H. Membrane fusion of phospholipid bilayers under high pressure: Spherical and irreversible growth of giant vesicles. Biophys. Chem. 2021, 277, 106639. [Google Scholar] [CrossRef]
- Miguel, V.; Sánchez-Borzone, M.E.; Mariani, M.E.; García, D.A. Modulation of membrane physical properties by natural insecticidal ketones. Biophys. Chem. 2021, 269, 106526. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bhattacharyya, D.; Bhunia, A. Host-membrane interacting interface of the SARS coronavirus envelope protein: Immense functional potential of C-terminal domain. Biophys. Chem. 2020, 266, 106452. [Google Scholar] [CrossRef]
- Hussey, G.A.; Thomas, N.E.; Henzler-Wildman, K.A. Highly coupled transport can be achieved in free-exchange transport models. J. Gen. Physiol. 2020, 152, e201912437. [Google Scholar] [CrossRef]
- Morrison, E.A.; Robinson, A.E.; Liu, Y.; Henzler-Wildman, K.A. Asymmetric protonation of EmrE. J. Gen. Physiol. 2015, 146, 445–461. [Google Scholar] [CrossRef]
- Doltchinkova, V.; Stoylov, S.; Angelova, P.R. Viper toxins affect membrane characteristics of human erythrocytes. Biophys. Chem. 2021, 270, 106532. [Google Scholar] [CrossRef]
- Tsubone, T.M.; Baptista, M.S.; Itri, R. Understanding membrane remodelling initiated by photosensitized lipid oxidation. Biophys. Chem. 2019, 254, 106263. [Google Scholar] [CrossRef]
- Perissinotto, F.; Rondelli, V.; Parisse, P.; Tormena, N.; Zunino, A.; Almásy, L.; Merkel, D.G.; Bottyán, L.; Sajti, S.; Casalis, L. GM1 Ganglioside role in the interaction of Alpha-synuclein with lipid membranes: Morphology and structure. Biophys. Chem. 2019, 255, 106272. [Google Scholar] [CrossRef] [PubMed]
- de Vlugt, J.E.; Xiao, P.; Munro, R.; Charchoglyan, A.; Brewer, D.; Al-Abdul-Wahid, M.S.; Brown, L.S.; Ladizhansky, V. Identifying lipids tightly bound to an integral membrane protein. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183345. [Google Scholar] [CrossRef]
- Shukla, R.; Medeiros-Silva, J.; Parmar, A.; Vermeulen, B.J.A.; Das, S.; Paioni, A.L.; Jekhmane, S.; Lorent, J.; Bonvin, A.M.J.J.; Baldus, M.; et al. Mode of action of teixobactins in cellular membranes. Nat. Commun. 2020, 11, 2848. [Google Scholar] [CrossRef] [PubMed]
- Rivière, G.; Jaipuria, G.; Andreas, L.B.; Leonov, A.; Giller, K.; Becker, S.; Zweckstetter, M. Membrane-embedded TSPO: An NMR view. Eur. Biophys. 2021, 50, 173–180. [Google Scholar] [CrossRef]
- Sun, Z.; Xu, Y.; Zhang, D.; McDermott, A.E. Probing allosteric coupling in a constitutively open mutant of the ion channel KcsA using solid-state NMR. Proc. Natl. Acad. Sci. USA 2020, 117, 7171–7175. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kishore, A.; Maity, D.; Sunanda, P.; Krishnarjuna, B.; Vappala, S.; Raghothama, S.; Kenyon, L.C.; Pal, D.; Das Sarma, J. A proline insertion-deletion in the spike glycoprotein fusion peptide of mouse hepatitis virus strongly alters neuropathology. J. Biol. Chem. 2019, 294, 8064–8087. [Google Scholar] [CrossRef]


| (1) Ionic Detergents |
| Sodium dodecyl sulfate (SDS; anionic) |
| Deoxycholate (anionic; bile acid salt) |
| Sodium cholate (anionic; bile acid salt) |
| Calixarene (anionic) |
| N-lauryl sarcosine or sarkosyl (anionic) |
| Cetyltrimethylammonium bromide (CTAB; cationic) |
| Hexadecyltrimethylammonium bromide (cationic) |
| (2) Zwitterionic detergents |
| 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) |
| 3-[(3-cholamidopropyl)dimethylammonio]-2-hydroxy-1-propanesulfonate (CHAPSO) |
| n-dodecyl-N, N-dimethylamine-N-oxide (LDAO) |
| n-dodecyl phosphocholine (DPC) |
| (3) Nonionic detergents a |
| Poly-oxyethyleneglycol lauryl ether |
| n-dodecyl-β-D-maltoside (DDM) |
| n-nonyl-β-D-glucoside (NG) |
| n-octylglucoside (OG) |
| Polyethylene glycol tert-octyl phenyl ether (Triton X-100) |
| Undecyl-β-D-maltoside (UDM) |
| Digitonin |
| Maltose neopentyl glycol (MNG) |
| Hecameg [6-O-(N-heptylcarbamoyl)-methyl-α-D-glucopyranoside (HG) |
| Protein(s) Reconstituted | Nanodisc Type | Ref. |
|---|---|---|
| Human cytochrome P450 3A4 (CYP3A4) | MSP | [28] |
| Human voltage-dependent anion channel-1 (VDAC-1) | MSP | [29,30,31,32] |
| Human voltage-dependent anion channel-2 (VDAC-2) | MSP | [33] |
| VDAC N-terminal segment (NTS) | MSP | [34] |
| The transmembrane domain of stromal interaction molecule (STIM1-TM) | MSP | [35] |
| Bacterial β-barrel assembly machinery-A (BamA) | MSP | [31,36,37] |
| Bacteriorhodopsin | MSP | [38,39,40] |
| Outer membrane protein X (OmpX) | MSP | [30,31,32,38,40,41,42] |
| α–helical BLT2 G protein-coupled receptor | MSP | [41] |
| NTS8–13–NTSR1–Gαi1β1γ1 complex | MSP | [43] |
| hIAPP | MSP | [44] |
| Anti-apoptotic protein BclxL | MSP | [45] |
| Inner mitochondrial MPV17 | MSP | [46] |
| Bak transmembrane helix | MSP | [47] |
| Y. pestis Omp adhesion invasion locus (Ail) | MSP | [48] |
| Human interleukin-8 (IL-8)-CXCR1(1–38) complex | MSP | [49] |
| Rabbit cytochrome-b5 + horse cytochrome C | 4F peptide | [50] |
| Rabbit CYP450 2B4, rat CYP450 reductase FMN-binding domain, and rabbit cytochrome-b5 | 4F peptide | [51,52,53] |
| Cytochrome-b5 + CYP450 | 22A peptide | [54] |
| Pf1, p7 from human hepatitis C virus and human chemokine receptor CXCR1 (GPCR) | 18A peptide | [21] |
| Rabbit cytochrome-b5 | SMA-EA | [55] |
| Pf1 coat protein | SMA | [56,57] |
| MerFt, CXCR1 and Ail | SMA | [56] |
| Rat CYP450 reductase FMN-binding domain | Pentyl-inulin | [58] |
| (1) Ionic Polymers |
| Styrene maleic acid copolymer (SMA) (1:1, 2:1, 3:1, 2.3:1, 1.2:1) |
| SMA-QA and SMA-EA |
| poly(styrene-co-maleimide) (SMI) |
| Diisobutylene maleic acid co-polymer (DIBMA) |
| (2) Zwitterionic polymers |
| zSMA, SMA-ED and SMA-Neut |
| (3) Nonionic polymers |
| Inulin functionalized with different hydrophobic moieties |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krishnarjuna, B.; Ramamoorthy, A. Detergent-Free Isolation of Membrane Proteins and Strategies to Study Them in a Near-Native Membrane Environment. Biomolecules 2022, 12, 1076. https://doi.org/10.3390/biom12081076
Krishnarjuna B, Ramamoorthy A. Detergent-Free Isolation of Membrane Proteins and Strategies to Study Them in a Near-Native Membrane Environment. Biomolecules. 2022; 12(8):1076. https://doi.org/10.3390/biom12081076
Chicago/Turabian StyleKrishnarjuna, Bankala, and Ayyalusamy Ramamoorthy. 2022. "Detergent-Free Isolation of Membrane Proteins and Strategies to Study Them in a Near-Native Membrane Environment" Biomolecules 12, no. 8: 1076. https://doi.org/10.3390/biom12081076
APA StyleKrishnarjuna, B., & Ramamoorthy, A. (2022). Detergent-Free Isolation of Membrane Proteins and Strategies to Study Them in a Near-Native Membrane Environment. Biomolecules, 12(8), 1076. https://doi.org/10.3390/biom12081076







