Regulation of Neural Circuitry under General Anesthesia: New Methods and Findings
Abstract
:1. Introduction
2. In Vivo Calcium Imaging
3. Chemogenetics
4. Optogenetics
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Antkowiak, B. How do general anaesthetics work? Naturwissenschaften 2001, 88, 201–213. [Google Scholar] [CrossRef]
- Franks, N.; Lieb, W.R. Do general anaesthetics act by competitive binding to specific receptors? Nature 1984, 310, 599–601. [Google Scholar] [CrossRef]
- Solt, K.; Forman, S.A. Correlating the clinical actions and molecular mechanisms of general anesthetics. Curr. Opin. Anaesthesiol. 2007, 20, 300–306. [Google Scholar] [CrossRef]
- Vazey, E.M.; Aston-Jones, G. Designer receptor manipulations reveal a role of the locus coeruleus noradrenergic system in isoflurane general anesthesia. Proc. Natl. Acad. Sci. USA 2014, 111, 3859–3864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, N.E.; Van Dort, C.J.; Kenny, J.D.; Pei, J.; Guidera, J.A.; Vlasov, K.Y.; Lee, J.T.; Boyden, E.S.; Brown, E.N.; Solt, K. Optogenetic activation of dopamine neurons in the ventral tegmental area induces reanimation from general anesthesia. Proc. Natl. Acad. Sci. USA 2016, 113, 12826–12831. [Google Scholar] [CrossRef] [Green Version]
- Pal, D.; Dean, J.G.; Liu, T.; Li, D.; Watson, C.J.; Hudetz, A.G.; Mashour, G.A. Differential Role of Prefrontal and Parietal Cor-tices in Controlling Level of Consciousness. Curr. Biol. 2018, 28, 2145–2152.e5. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.X.; Xiong, B.; Xu, W.; Wei, H.H.; Qu, W.M.; Hong, Z.Y.; Huang, Z.L. Activation of Parabrachial Nucleus Glutamatergic Neurons Accelerates Reanimation from Sevoflurane Anesthesia in Mice. Anesthesiology 2019, 130, 106–118. [Google Scholar] [CrossRef]
- Kelz, M.B.; Sun, Y.; Chen, J.; Cheng Meng, Q.; Moore, J.T.; Veasey, S.C.; Dixon, S.; Thornton, M.; Funato, H.; Yanagisawa, M. An essential role for orexins in emergence from general anesthesia. Proc. Natl. Acad. Sci. USA 2008, 105, 1309–1314. [Google Scholar] [CrossRef] [Green Version]
- Jiang-Xie, L.F.; Yin, L.; Zhao, S.; Prevosto, V.; Han, B.X.; Dzirasa, K.; Wang, F. A Common Neuroendocrine Substrate for Diverse General Anesthetics and Slee. Neuron 2019, 102, 1053–1065.e4. [Google Scholar] [CrossRef]
- Uschakov, A.; Gong, H.; McGinty, D.; Szymusiak, R. Efferent projections from the median preoptic nucleus to sleep- and arousal-regulatory nuclei in the rat brain. Neuroscience 2007, 150, 104–120. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.; Weber, F.; Zhong, P.; Tan, C.L.; Nguyen, T.N.; Beier, K.T.; Hörmann, N.; Chang, W.C.; Zhang, Z.; Do, J.P.; et al. Identification of preoptic sleep neurons using retrograde labelling and gene profiling. Nature 2017, 545, 477–481. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yu, T.; Yuan, J.; Yu, B.W. The ventrolateral preoptic nucleus is required for propofol-induced inhibition of locus coeruleus neuronal activity. Neurol. Sci. 2015, 36, 2177–2184. [Google Scholar] [CrossRef] [PubMed]
- Vanini, G.; Bassana, M.; Mast, M.; Mondino, A.; Cerda, I.; Phyle, M.; Chen, V.; Colmenero, A.V.; Hambrecht-Wiedbusch, V.S.; Mashour, G.A. Activation of Preoptic GABAergic or Glutamatergic Neurons Modulates Sleep-Wake Architecture, but Not Anesthetic State Transitions. Curr. Biol. 2020, 30, 779–787.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Li, Y.; Feng, Q.; Guo, Q.; Zhou, J.; Luo, M. Learning shapes the aversion and reward responses of lateral habenula neurons. Elife 2017, 6, e23045. [Google Scholar] [CrossRef]
- Vlasov, K.; Van Dort, C.J.; Solt, K. Optogenetics and Chemogenetics. Methods Enzymol. 2018, 603, 181–196. [Google Scholar]
- Oh, J.; Lee, C.; Kaang, B.K. Imaging and analysis of genetically encoded calcium indicators linking neural circuits and behaviors. Korean J. Physiol. Pharmacol. 2019, 23, 237–249. [Google Scholar] [CrossRef] [Green Version]
- Gründemann, J.; Bitterman, Y.; Lu, T.; Krabbe, S.; Grewe, B.F.; Schnitzer, M.J.; Lüthi, A. Amygdala ensembles encode behavioral states. Science 2019, 364, eaav8736. [Google Scholar] [CrossRef]
- Paredes, R.M.; Etzler, J.C.; Watts, L.T.; Zheng, W.; Lechleiter, J.D. Chemical calcium indicators. Methods 2008, 46, 143–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, T.; Goltstein, P.M.; Portugues, R.; Griesbeck, O. Putting a finishing touch on GECIs. Front. Mol. Neurosci. 2014, 7, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Liu, N.; He, Y.; Liu, Y.; Ge, L.; Zou, L.; Song, S.; Xiong, W.; Liu, X. Improved calcium sensor GCaMP-X overcomes the calcium channel perturbations induced by the calmodulin in GCaM. Nat. Commun. 2018, 9, 1504. [Google Scholar] [CrossRef]
- Grienberger, C.; Konnerth, A. Imaging calcium in neurons. Neuron 2012, 73, 862–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, M.; Takeuchi, A.; Horigane, S.I.; Ohkura, M.; Gengyo-Ando, K.; Fujii, H.; Kamijo, S.; Takemoto-Kimura, S.; Kano, M.; Nakai, J.; et al. Rational design of a high-affinity, fast, red calcium indicator R-CaMP2. Nat. Methods 2015, 12, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Ledochowitsch, P.; Knoblich, U.; Lecoq, J.; Murphy, G.J.; Reid, R.C.; de Vries, S.E.; Koch, C.; Zeng, H.; Buice, M.A.; et al. Relationship between simultaneously recorded spiking activity and fluorescence signal in GCaMP6 transgenic mice. Elife 2021, 10, e51675. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; O’Hashi, K.; Kaneko, K.; Kobayashi, S.; Ogisawa, S.; Tonogi, M.; Fujita, S.; Kobayashi, M. A new phenotype identification method with the fluorescent expression in cross-sectioned tails in Thy1-GCaMP6s transgenic mice. J. Oral. Sci. 2022, 64, 156–160. [Google Scholar] [CrossRef]
- Markowitz, J.E.; Gillis, W.F.; Beron, C.C.; Neufeld, S.Q.; Robertson, K.; Bhagat, N.D.; Peterson, R.E.; Peterson, E.; Hyun, M.; Linderman, S.W.; et al. The Striatum Organizes 3D Behavior via Moment-to-Moment Action Selection. Cell 2018, 174, 44–58.e17. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.W.; Wardill, T.J.; Sun, Y.; Pulver, S.R.; Renninger, S.L.; Baohan, A.; Schreiter, E.R.; Kerr, R.A.; Orger, M.B.; Jayaraman, V.; et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 2013, 499, 295–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zeng, J.; Zhang, J.; Yue, C.; Zhong, W.; Liu, Z.; Feng, Q.; Luo, M. Hypothalamic Circuits for Predation and Evasion. Neuron 2018, 97, 911–924.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, L.; Callaway, E.M.; Svoboda, K. Genetic Dissection of Neural Circuits: A Decade of Progress. Neuron 2018, 98, 256–281. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; Zhou, J.; Feng, Q.; Lin, R.; Gong, H.; Luo, Q.; Zeng, S.; Luo, M.; Fu, L. Multi-channel fiber photometry for population neuronal activity recording. Biomed. Opt. Express 2015, 6, 3919–3931. [Google Scholar] [CrossRef]
- Roukos, V.; Misteli, T. Deep Imaging: The next frontier in microscopy. Histochem Cell Biol. 2014, 142, 125–131. [Google Scholar] [CrossRef]
- Shuman, T.; Aharoni, D.; Cai, D.J.; Lee, C.R.; Chavlis, S.; Page-Harley, L.; Vetere, L.M.; Feng, Y.; Yang, C.Y.; Mollinedo-Gajate, I.; et al. Breakdown of spatial coding and interneuron synchronization in epileptic mice. Nat. Neurosci. 2020, 23, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liang, B.; Barbera, G.; Hawes, S.; Zhang, Y.; Stump, K.; Baum, I.; Yang, Y.; Li, Y.; Lin, D.T. Miniscope GRIN Lens System for Calcium Imaging of Neuronal Activity from Deep Brain Structures in Behaving Animals. Curr. Protoc. Neurosci. 2019, 86, e56. [Google Scholar] [CrossRef] [PubMed]
- Kislin, M.; Sword, J.; Fomitcheva, I.V.; Croom, D.; Pryazhnikov, E.; Lihavainen, E.; Toptunov, D.; Rauvala, H.; Ribeiro, A.S.; Khiroug, L.; et al. Reversible Disruption of Neuronal Mitochondria by Ischemic and Traumatic Injury Revealed by Quantitative Two-Photon Imaging in the Neocortex of Anesthetized Mice. J. Neurosci. 2017, 37, 333–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Yuste, R. In vivo imaging of neural activity. Nat. Methods 2017, 14, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Duffet, L.; Kosar, S.; Panniello, M.; Viberti, B.; Bracey, E.; Zych, A.D.; Radoux-Mergault, A.; Zhou, X.; Dernic, J.; Ravotto, L.; et al. A genetically encoded sensor for in vivo imaging of orexin neuropeptides. Nat. Methods 2022, 19, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.; Obenhaus, H.A.; Skytøen, E.R.; Eneqvist, H.; de Jong, N.L.; Vale, R.; Jorge, M.R.; Moser, M.B.; Moser, E.I. Large-scale two-photon calcium imaging in freely moving mice. Cell 2022, 185, 1240–1256.e30. [Google Scholar] [CrossRef]
- Brown, R.; Lau, H.; LeDoux, J.E. Understanding the Higher-Order Approach to Consciousness. Trends Cogn. Sci. 2019, 23, 754–768. [Google Scholar] [CrossRef]
- Mashour, G.A.; Roelfsema, P.; Changeux, J.P.; Dehaene, S. Conscious Processing and the Global Neuronal Workspace Hypothesis. Neuron 2020, 105, 776–798. [Google Scholar] [CrossRef]
- Bharioke, A.; Munz, M.; Brignall, A.; Kosche, G.; Eizinger, M.F.; Ledergerber, N.; Hillier, D.; Gross-Scherf, B.; Conzelmann, K.K.; Macé, E.; et al. General anesthesia globally synchronizes activity selectively in layer 5 cortical pyramidal neurons. Neuron 2022, 110, 2024–2040.e10. [Google Scholar] [CrossRef]
- Scammell, T.E.; Arrigoni, E.; Lipton, J.O. Neural circuitry of wakefulness and sleep. Neuron 2017, 93, 747–765. [Google Scholar] [CrossRef] [Green Version]
- Gui, H.; Liu, C.; He, H.; Zhang, J.; Chen, H.; Zhang, Y. Dopaminergic Projections from the Ventral Tegmental Area to the Nucleus Accumbens Modulate Sevoflurane Anesthesia in Mice. Front. Cell Neurosci. 2021, 15, 671473. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.; Peselow, E. The neurobiology of addictive disorders. Clin. Neuropharmacol. 2009, 32, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gui, H.; Duan, Z.; Yu, T.; Zhang, J.; Liang, X.; Liu, C. Dopamine D1 Receptor in the Nucleus Accumbens Modulates the Emergence from Propofol Anesthesia in Rat. Neurochem Res. 2021, 46, 1435–1446. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhou, X.; Zhu, Q.; Fu, B.; Cao, S.; Zhang, Y.; Zhang, L.; Zhang, Y.; Yu, T. Dopamine neurons in the ventral periaqueductal gray modulate isoflurane anesthesia in rats. CNS Neurosci. Ther. 2020, 26, 1121–1133. [Google Scholar] [CrossRef]
- Monti, J.M. Serotonin control of sleep-wake behavior. Sleep Med. Rev. 2011, 15, 269–281. [Google Scholar] [CrossRef]
- Li, A.; Li, R.; Ouyang, P.; Li, H.; Wang, S.; Zhang, X.; Wang, D.; Ran, M.; Zhao, G.; Yang, Q.; et al. Dorsal raphe serotonergic neurons promote arousal from isoflurane anesthesia. CNS Neurosci. Ther. 2021, 27, 941–950. [Google Scholar] [CrossRef]
- Du, W.J.; Zhang, R.W.; Li, J.; Zhang, B.B.; Peng, X.L.; Cao, S.; Yuan, J.; Yuan, C.D.; Yu, T.; Du, J.L. The Locus Coeruleus Modulates Intravenous General Anesthesia of Zebrafish via a Cooperative Mechanism. Cell Rep. 2018, 24, 3146–3155.e3. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Chung, S.; Zhang, S.; Zhong, P.; Ma, C.; Chang, W.C.; Weissbourd, B.; Sakai, N.; Luo, L.; Nishino, S.; et al. Basal forebrain circuit for sleep-wake control. Nat. Neurosci. 2015, 18, 1641–1647. [Google Scholar] [CrossRef]
- Kim, T.; Thankachan, S.; McKenna, J.T.; McNally, J.M.; Yang, C.; Choi, J.H.; Chen, L.; Kocsis, B.; Deisseroth, K.; Strecker, R.E.; et al. Cortically projecting basal forebrain parvalbumin neurons regulate cortical gamma band oscillations. Proc. Natl. Acad. Sci. USA 2015, 112, 3535–3540. [Google Scholar] [CrossRef] [Green Version]
- Luo, T.Y.; Cai, S.; Qin, Z.X.; Yang, S.C.; Shu, Y.; Liu, C.X.; Zhang, Y.; Zhang, L.; Zhou, L.; Yu, T.; et al. Basal Forebrain Cholinergic Activity Modulates Isoflurane and Propofol Anesthesia. Front. Neurosci. 2020, 14, 559077. [Google Scholar] [CrossRef]
- Fuller, P.; Sherman, D.; Pedersen, N.P.; Saper, C.B.; Lu, J. Reassessment of the structural basis of the ascending arousal system. J. Comp. Neurol. 2011, 519, 933–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, T.; Yu, S.; Cai, S.; Zhang, Y.; Jiao, Y.; Yu, T.; Yu, W. Parabrachial Neurons Promote Behavior and Electroencephalographic Arousal from General Anesthesia. Front. Mol. Neurosci. 2018, 11, 420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Gao, Y.; Li, Y.; Yang, J.; Zhao, H. Sleep Deprivation Influences Circadian Gene Expression in the Lateral Habenula. Behav. Neurol. 2016, 2016, 7919534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Liu, J.; Zhou, L.; He, H.; Zhang, Y.; Cai, S.; Yuan, C.; Luo, T.; Zheng, J.; Yu, T.; et al. Lateral Habenula Glutamatergic Neurons Modulate Isoflurane Anesthesia in Mice. Front. Mol. Neurosci. 2021, 14, 628996. [Google Scholar] [CrossRef]
- Wang, D.; Guo, Q.; Zhou, Y.; Xu, Z.; Hu, S.W.; Kong, X.X.; Yu, Y.M.; Yang, J.X.; Zhang, H.; Ding, H.L.; et al. GABAergic Neurons in the Dorsal-Intermediate Lateral Septum Regulate Sleep-Wakefulness and Anesthesia in Mice. Anesthesiology 2021, 135, 463–481. [Google Scholar] [CrossRef]
- Qiu, G.; Wu, Y.; Yang, Z.; Li, L.; Zhu, X.; Wang, Y.; Sun, W.; Dong, H.; Li, Y.; Hu, J. Dexmedetomidine Activation of Dopamine Neurons in the Ventral Tegmental Area Attenuates the Depth of Sedation in Mice. Anesthesiology 2020, 133, 377–392. [Google Scholar] [CrossRef]
- Hua, T.; Chen, B.; Lu, D.; Sakurai, K.; Zhao, S.; Han, B.X.; Kim, J.; Yin, L.; Chen, Y.; Lu, J.; et al. General anesthetics activate a potent central pain-suppression circuit in the amygdala. Nat. Neurosci. 2020, 23, 854–868. [Google Scholar] [CrossRef]
- Zhang, K.; Lian, N.; Ding, R.; Guo, C.; Dong, X.; Li, Y.; Wei, S.; Jiao, Q.; Yu, Y.; Shen, H. Sleep Deprivation Aggravates Cognitive Impairment by the Alteration of Hippocampal Neuronal Activity and the Density of Dendritic Spine in Isoflurane-Exposed Mice. Front. Behav. Neurosci. 2020, 14, 589176. [Google Scholar] [CrossRef]
- Chen, K.; Hu, Q.; Gupta, R.; Stephens, J.; Xie, Z.; Yang, G. Inhibition of unfolded protein response prevents post-anesthesia neuronal hyperactivity and synapse loss in aged mice. Aging Cell 2022, 21, e13592. [Google Scholar] [CrossRef]
- Marvin, J.S.; Scholl, B.; Wilson, D.E.; Podgorski, K.; Kazemipour, A.; Müller, J.A.; Schoch, S.; Quiroz, F.J.U.; Rebola, N.; Bao, H.; et al. Stability, affinity, and chromatic variants of the glutamate sensor iGluSnFR. Nat. Methods 2018, 15, 936–939. [Google Scholar] [CrossRef]
- Peng, W.; Wu, Z.; Song, K.; Zhang, S.; Li, Y.; Xu, M. Regulation of sleep homeostasis mediator adenosine by basal forebrain glutamatergic neurons. Science 2020, 369, eabb0556. [Google Scholar] [CrossRef] [PubMed]
- Strader, C.D.; Gaffney, T.; Sugg, E.E.; Candelore, M.R.; Keys, R.; Patchett, A.A.; Dixon, R.A. Allele-specific activation of genetically engineered receptors. J. Biol. Chem. 1991, 266, 5–8. [Google Scholar] [CrossRef]
- Coward, P.; Wada, H.G.; Falk, M.S.; Chan, S.D.; Meng, F.; Akil, H.; Conklin, B.R. Controlling signaling with a specifically designed Gi-coupled receptor. Proc. Natl. Acad. Sci. USA 1998, 95, 352–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armbruster, B.N.; Li, X.; Pausch, M.H.; Herlitze, S.; Roth, B.L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl. Acad. Sci. USA 2007, 104, 5163–5168. [Google Scholar] [CrossRef] [Green Version]
- Roth, B.L. DREADDs for Neuroscientists. Neuron 2016, 89, 683–694. [Google Scholar] [CrossRef] [Green Version]
- Vardy, E.; Robinson, J.E.; Li, C.; Olsen, R.H.; DiBerto, J.F.; Giguere, P.M.; Sassano, F.M.; Huang, X.P.; Zhu, H.; Urban, D.J.; et al. A New DREADD Facilitates the Multiplexed Chemogenetic Interrogation of Behavior. Neuron 2015, 86, 936–946. [Google Scholar] [CrossRef] [Green Version]
- Saika, F.; Matsuzaki, S.; Kishioka, S.; Kiguchi, N. Chemogenetic Activation of CX3CR1-Expressing Spinal Microglia Using Gq-DREADD Elicits Mechanical Allodynia in Male Mice. Cells 2021, 10, 874. [Google Scholar] [CrossRef]
- Alexander, G.M.; Rogan, S.C.; Abbas, A.I.; Armbruster, B.N.; Pei, Y.; Allen, J.A.; Nonneman, R.J.; Hartmann, J.; Moy, S.S.; Nicolelis, M.A.; et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 2009, 63, 27–39. [Google Scholar] [CrossRef] [Green Version]
- Eldridge, M.A.; Lerchner, W.; Saunders, R.C.; Kaneko, H.; Krausz, K.W.; Gonzalez, F.J.; Ji, B.; Higuchi, M.; Minamimoto, T.; Richmond, B.J. Chemogenetic disconnection of monkey orbitofrontal and rhinal cortex reversibly disrupts reward value. Nat. Neurosci. 2016, 19, 37–39. [Google Scholar] [CrossRef] [Green Version]
- Taylor, N.E.; Long, H.; Pei, J.; Kukutla, P.; Phero, A.; Hadaegh, F.; Abdelnabi, A.; Solt, K.; Brenner, G.J. The rostromedial tegmental nucleus: A key modulator of pain and opioid analgesia. Pain 2019, 160, 2524–2534. [Google Scholar] [CrossRef]
- Gomez, J.L.; Bonaventura, J.; Lesniak, W.; Mathews, W.B.; Sysa-Shah, P.; Rodriguez, L.A.; Ellis, R.J.; Richie, C.T.; Harvey, B.K.; Dannals, R.F.; et al. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 2017, 357, 503–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Chu, R.; Cao, F.; Wang, Y.; Liu, Y.; Cao, J.; Guo, Y.; Mi, W.; Tong, L. Dopaminergic Neurons in the Ventral Tegmental-Prelimbic Pathway Promote the Emergence of Rats from Sevoflurane Anesthesia. Neurosci. Bull. 2022, 38, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Berridge, C.W.; Waterhouse, B.D. The locus coeruleus-noradrenergic system: Modulation of behavioral state and state-dependent cognitive processes. Brain Res. Brain Res. Rev. 2003, 42, 33–84. [Google Scholar] [CrossRef]
- Ao, Y.; Yang, B.; Zhang, C.; Wu, B.; Zhang, X.; Xing, D.; Xu, H. Locus Coeruleus to Paraventricular Thalamus Projections Facilitate Emergence from Isoflurane Anesthesia in Mice. Front. Pharmacol. 2021, 12, 643172. [Google Scholar] [CrossRef]
- Beas, B.S.; Wright, B.J.; Skirzewski, M.; Leng, Y.; Hyun, J.H.; Koita, O.; Ringelberg, N.; Kwon, H.B.; Buonanno, A.; Penzo, M.A. The locus coeruleus drives disinhibition in the midline thalamus via a dopaminergic mechanism. Nat. Neurosci. 2018, 21, 963–973. [Google Scholar] [CrossRef]
- Peyron, C.; Tighe, D.K.; Van Den Pol, A.N.; De Lecea, L.; Heller, H.C.; Sutcliffe, J.G.; Kilduff, T.S. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 1998, 18, 9996–10015. [Google Scholar] [CrossRef] [Green Version]
- Adamantidis, A.R.; Zhang, F.; Aravanis, A.M.; Deisseroth, K.; De Lecea, L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature 2007, 450, 420–424. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, S.; Li, H.; Guo, J.; Li, J.; Wang, D.; Zhang, X.; Yin, L.; Li, R.; Li, A.; et al. Activation of Orexinergic Neurons Inhibits the Anesthetic Effect of Desflurane on Consciousness State via Paraventricular Thalamic Nucleus in Rats. Anesth. Analg. 2021, 133, 781–793. [Google Scholar] [CrossRef]
- Yin, L.; Li, L.; Deng, J.; Wang, D.; Guo, Y.; Zhang, X.; Li, H.; Zhao, S.; Zhong, H.; Dong, H. Optogenetic/Chemogenetic Activation of GABAergic Neurons in the Ventral Tegmental Area Facilitates General Anesthesia via Projections to the Lateral Hypothalamus in Mice. Front. Neural. Circuits 2019, 13, 73. [Google Scholar] [CrossRef]
- Vlasov, K.; Pei, J.; Nehs, C.J.; Guidera, J.A.; Zhang, E.R.; Kenny, J.D.; Houle, T.T.; Brenner, G.J.; Taylor, N.E.; Solt, K. Activation of GABAergic Neurons in the Rostromedial Tegmental Nucleus and Other Brainstem Regions Promotes Sedation and Facilitates Sevoflurane Anesthesia in Mice. Anesth. Analg. 2021, 132, e50–e55. [Google Scholar] [CrossRef]
- Duebel, J.; Marazova, K.; Sahel, J.A. Optogenetics. Curr. Opin. Ophthalmol. 2015, 26, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, L.A.; Yizhar, O.; Berndt, A.; Sohal, V.S.; Deisseroth, K.; Hegemann, P. Ultrafast optogenetic control. Nat. Neurosci. 2010, 13, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Chuong, A.S.; Miri, M.L.; Busskamp, V.; Matthews, G.A.; Acker, L.C.; Sørensen, A.T.; Young, A.; Klapoetke, N.C.; Henninger, M.A.; Kodandaramaiah, S.B.; et al. Noninvasive optical inhibition with a red-shifted microbial rhodopsin. Nat. Neurosci. 2014, 17, 1123–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.; Xiong, W.; Zhang, P.; Chen, L.; Fang, J.; Shields, C.; Xu, X.M.; Jin, X. Increased threshold of short-latency motor evoked potentials in transgenic mice expressing Channelrhodopsin-2. PLoS ONE 2017, 12, e0178803. [Google Scholar] [CrossRef] [Green Version]
- Boyden, E.S.; Zhang, F.; Bamberg, E.; Nagel, G.; Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005, 8, 1263–1268. [Google Scholar] [CrossRef]
- Chen, S.; Weitemier, A.Z.; Zeng, X.; He, L.; Wang, X.; Tao, Y.; Huang, A.J.; Hashimotodani, Y.; Kano, M.; Iwasaki, H.; et al. Near-infrared deep brain stimulation via upconversion nanoparticle-mediated optogenetics. Science 2018, 359, 679–684. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.; Mendoza-Halliday, D.; Ting, J.T.; Kaiser, T.; Sun, X.; Bastos, A.M.; Wimmer, R.D.; Guo, B.; Chen, Q.; Zhou, Y.; et al. An Ultra-Sensitive Step-Function Opsin for Minimally Invasive Optogenetic Stimulation in Mice and Macaques. Neuron 2020, 107, 38–51.e8. [Google Scholar] [CrossRef]
- Owen, S.F.; Liu, M.H.; Kreitzer, A.C. Thermal constraints on in vivo optogenetic manipulations. Nat. Neurosci. 2019, 22, 1061–1065. [Google Scholar] [CrossRef]
- Carter, M.E.; Yizhar, O.; Chikahisa, S.; Nguyen, H.; Adamantidis, A.; Nishino, S.; Deisseroth, K.; De Lecea, L. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat. Neurosci. 2010, 13, 1526–1533. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Wang, D.; Guo, Y.; Zhang, X.; Ran, M.; Yang, C.; Yang, Q.; Dong, H. Orexin activated emergence from isoflurane anaesthesia involves excitation of ventral tegmental area dopaminergic neurones in rats. Br. J. Anaesth. 2019, 123, 497–505. [Google Scholar] [CrossRef]
- Wang, D.; Guo, Y.; Li, H.; Li, J.; Ran, M.; Guo, J.; Yin, L.; Zhao, S.; Yang, Q.; Dong, H. Selective optogenetic activation of orexinergic terminals in the basal forebrain and locus coeruleus promotes emergence from isoflurane anaesthesia in rats. Br. J. Anaesth. 2021, 126, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Krashes, M.J. Untangling Appetite Circuits with Optogenetics and Chemogenetics, in Appetite and Food Intake: Central Control; Harris, R.B.S., Ed.; CRC Press; Taylor & Francis Group, LLC.: Boca Raton, FL, USA, 2017; pp. 91–116. [Google Scholar]
- Zhao, S.; Li, R.; Li, H.; Wang, S.; Zhang, X.; Wang, D.; Guo, J.; Li, H.; Li, A.; Tong, T.; et al. Lateral Hypothalamic Area Glutamatergic Neurons and Their Projections to the Lateral Habenula Modulate the Anesthetic Potency of Isoflurane in Mice. Neurosci. Bull. 2021, 37, 934–946. [Google Scholar] [CrossRef] [PubMed]
- Hemmings, H.C., Jr.; Riegelhaupt, P.M.; Kelz, M.B.; Solt, K.; Eckenhoff, R.G.; Orser, B.A.; Goldstein, P.A. Towards a Comprehensive Understanding of Anesthetic Mechanisms of Action: A Decade of Discovery. Trends Pharmacol. Sci. 2019, 40, 464–481. [Google Scholar] [CrossRef]
- Alam, M.A.; Kumar, S.; McGinty, D.; Alam, M.N.; Szymusiak, R. Neuronal activity in the preoptic hypothalamus during sleep deprivation and recovery slee. J. Neurophysiol. 2014, 111, 287–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassani, O.K.; Lee, M.G.; Henny, P.; Jones, B.E. Discharge profiles of identified GABAergic in comparison to cholinergic and putative glutamatergic basal forebrain neurons across the sleep-wake cycle. J. Neurosci. 2009, 29, 11828–11840. [Google Scholar] [CrossRef] [Green Version]
- Anaclet, C.; Ferrari, L.; Arrigoni, E.; Bass, C.E.; Saper, C.B.; Lu, J.; Fuller, P.M. The GABAergic parafacial zone is a medullary slow wave sleep-promoting center. Nat. Neurosci. 2014, 17, 1217–1224. [Google Scholar] [CrossRef] [Green Version]
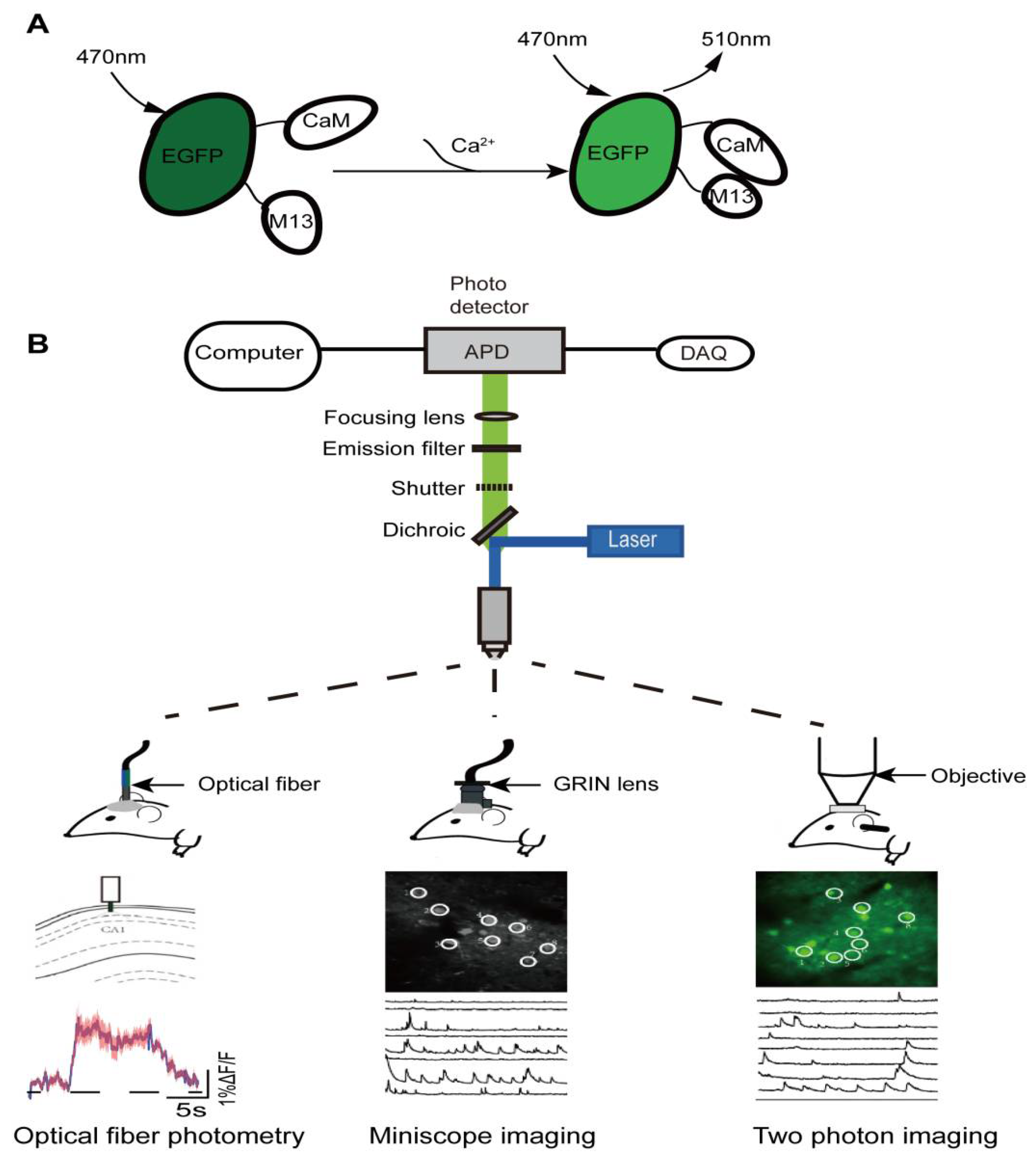
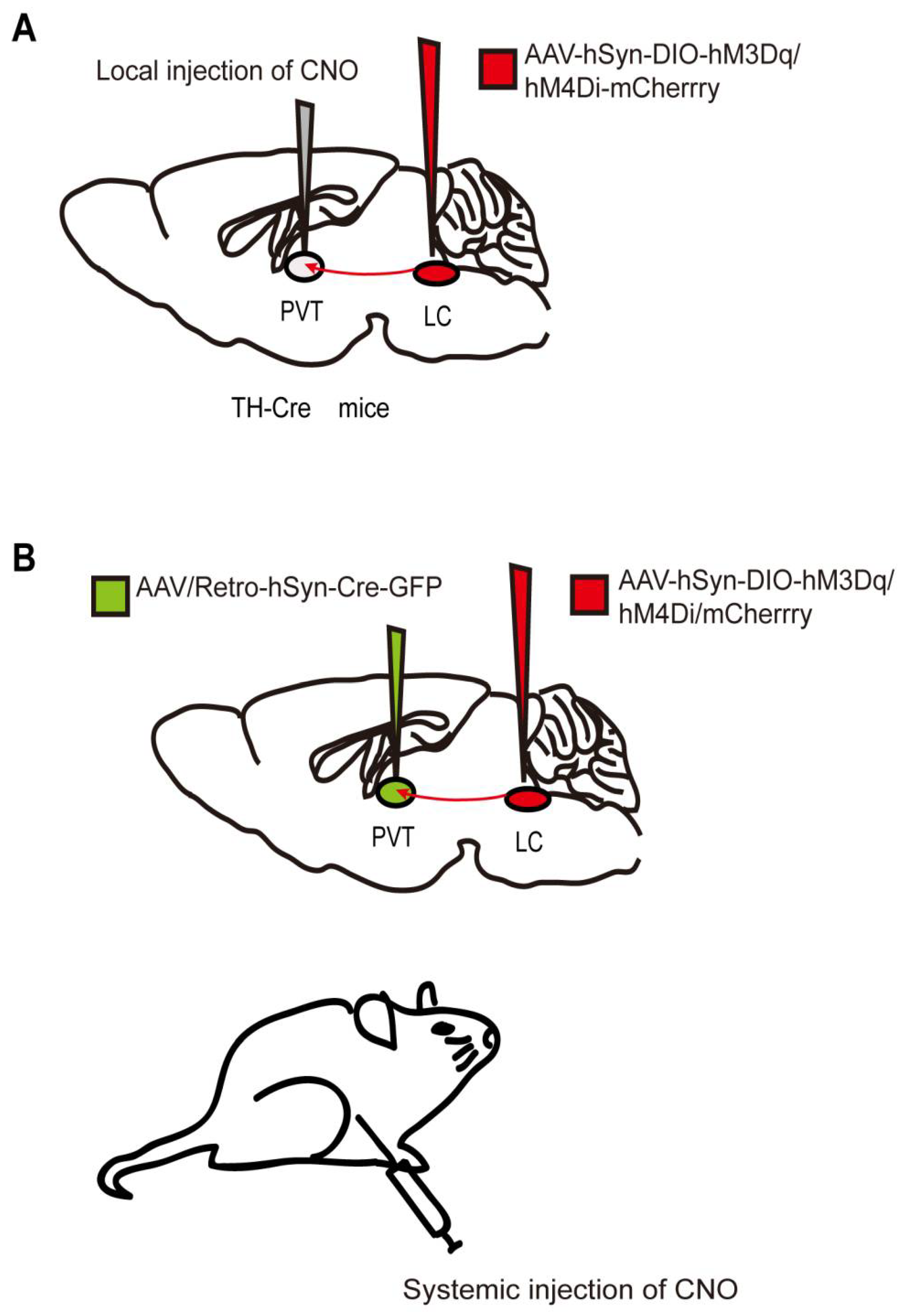
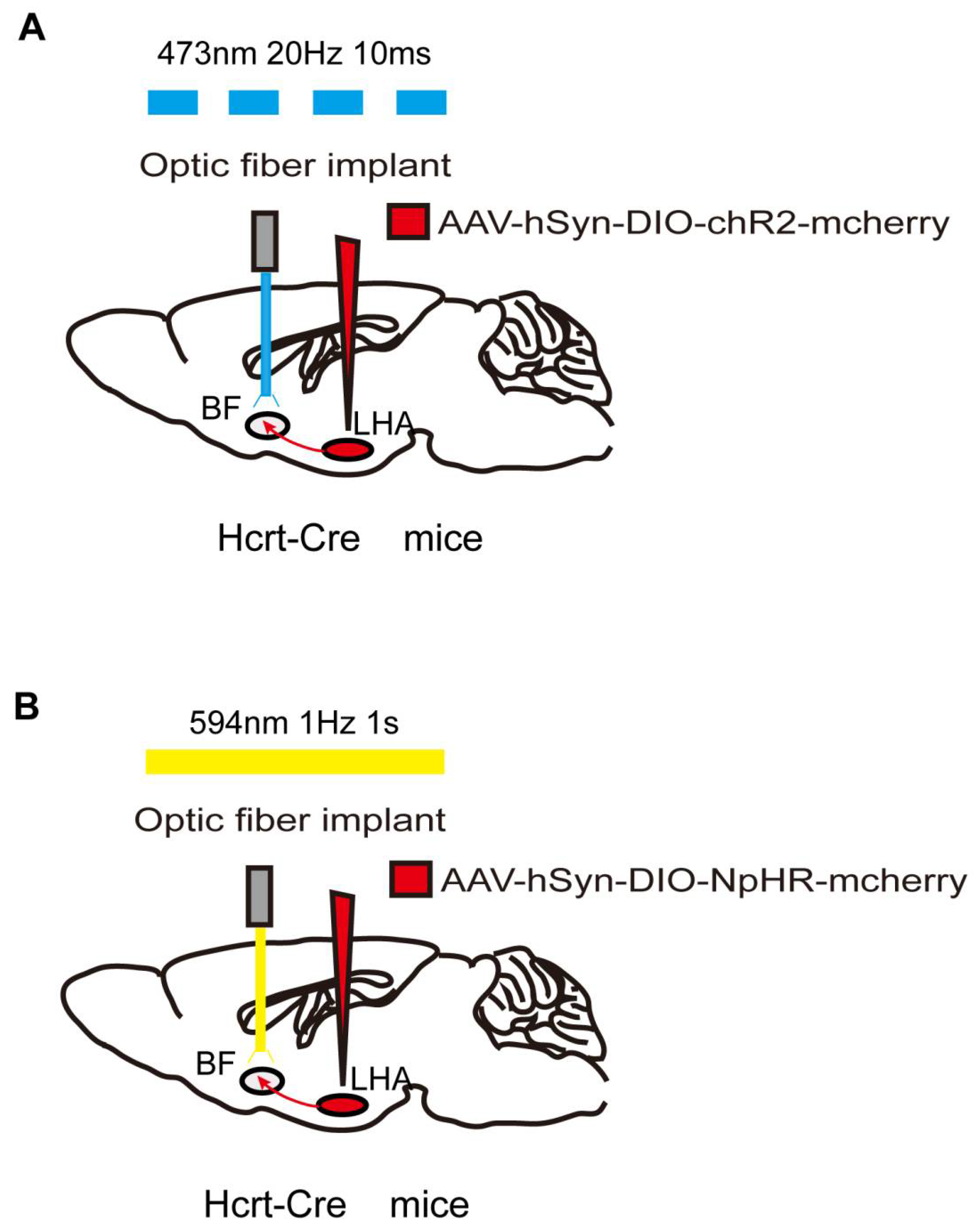

| Neuron Type of Brain Region and Its Projections | Technology | Anesthetic Method | Experimental Animals (Numbers) | The Role of Induction to or Emergence from General Anesthesia |
|---|---|---|---|---|
| Layer 5 cortical pyramidal neurons | In vivo two-photon calcium imaging | Isoflurane, Fentanyl-Medetomidine-Midazolam, and Ketamine-Xylazine | Rbp4-cre mice (22) | Both |
| VTA dopaminergic neurons and VTA-NAc and VTA-PrL dopaminergic projection | Optical-fiber photometry, optogenetics and chemogenetics | Sevoflurane | DAT-cre mice (64); Rats (67) | Both |
| NAc neurons and NAc GABAergic neurons | Optical-fiber photometry | Sevoflurane and propofol | Mice (18); Rats (12) | Both |
| vPAG dopaminergic neurons | Optical-fiber photometry | Isoflurane | Rats (12) | Both |
| DRN 5-HT neurons | Optical-fiber photometry | Isoflurane | Sert-cre mice (6) | Both |
| Chemogenetics | Sert-cre mice (24) | Emergence | ||
| LC TH neurons and LC-PVT | Chemogeneticsand optogenetics | Isoflurane | Rats (32); TH-cre mice (54) | Emergence |
| BF cholinergic neurons | Optical-fiber photometry and chemogenetics | Isoflurane and propofol | ChAT-cre mice (40) | Both |
| PBN glutamatergic neurons | Optical-fiber photometry, Chemogeneticsoptogenetics | Isoflurane and propofol | Rats (42) | Emergence |
| Sevoflurane | Vglut2-cre mice (32) | Both | ||
| LHb glutamatergic neurons | Optical-fiber photometry, chemogenetics, optogenetics | Isoflurane | Vglut2-cre mice (68) | Emergence |
| LHA glutamatergic neurons and LHA-LHb glutamatergic projection | Optogenetics andchemogenetics, | Isoflurane | Vglut2-cre mice (48) | Emergence |
| LHA orexinergic neurons and LHA-PVT orexinergic projection | Chemogenetics and optogenetics | Isoflurane | Hcrt-cre mice (83) | Emergence |
| Desflurane | Hcrt-cre mice (83) | Both | ||
| LHA orexinergic neurons, LHA-BF, LHA-LC, and LHA-VTA orexinergic projections | Optogenetics | Isoflurane | Hcrt-cre mice (69) | Emergence |
| Dorsal–intermediate lateral septum GABAergic neurons, and dorsal–intermediate lateral septum-VTA GABAergic projection | Optical-fiber photometry, chemogenetics and optogenetics | Isoflurane | Vgat-cre mice (56) | Both |
| Hypothalamus preoptic area’s GABAergic neurons | Chemogeneticsand optogenetics | Isoflurane, propofol, and ketamine | Mice (39) | Emergence |
| VTA GABAergic neurons, and VTA–LHA GABAergic projections | Chemogenetics | Isoflurane | Vgat-cre mice (30) | Both |
| RMTg GABAergic neurons | Chemogenetics | Sevoflurane | Vgat-cre mice (18) | Both |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Pan, J.; Yu, Y. Regulation of Neural Circuitry under General Anesthesia: New Methods and Findings. Biomolecules 2022, 12, 898. https://doi.org/10.3390/biom12070898
Zhang K, Pan J, Yu Y. Regulation of Neural Circuitry under General Anesthesia: New Methods and Findings. Biomolecules. 2022; 12(7):898. https://doi.org/10.3390/biom12070898
Chicago/Turabian StyleZhang, Kai, Jiacheng Pan, and Yonghao Yu. 2022. "Regulation of Neural Circuitry under General Anesthesia: New Methods and Findings" Biomolecules 12, no. 7: 898. https://doi.org/10.3390/biom12070898
APA StyleZhang, K., Pan, J., & Yu, Y. (2022). Regulation of Neural Circuitry under General Anesthesia: New Methods and Findings. Biomolecules, 12(7), 898. https://doi.org/10.3390/biom12070898





