Heterologous Expression of Full-Length and Truncated Human ZIP4 Zinc Transporter in Saccharomyces cerevisiae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains, Plasmids, Media, and Reagents
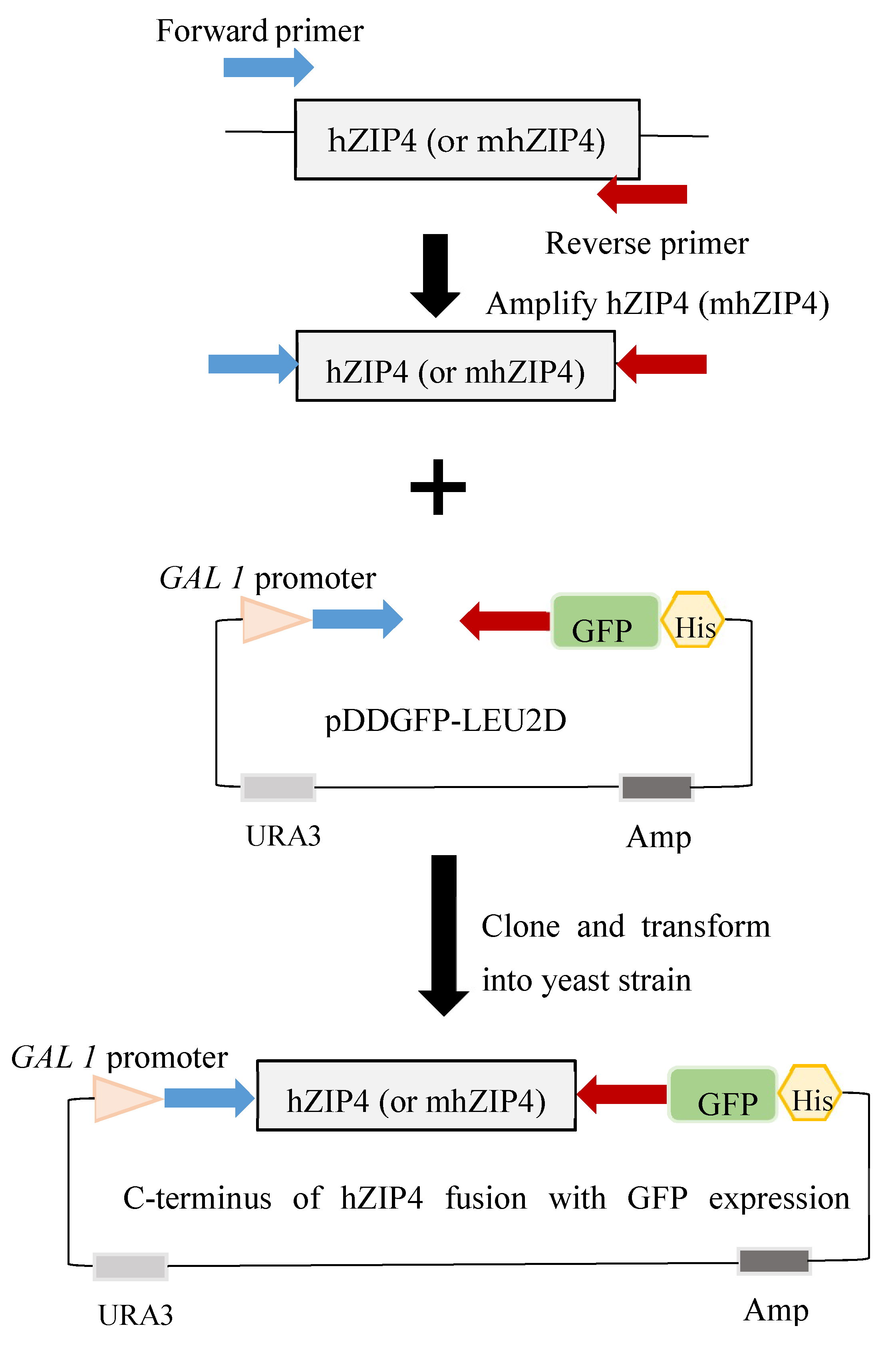
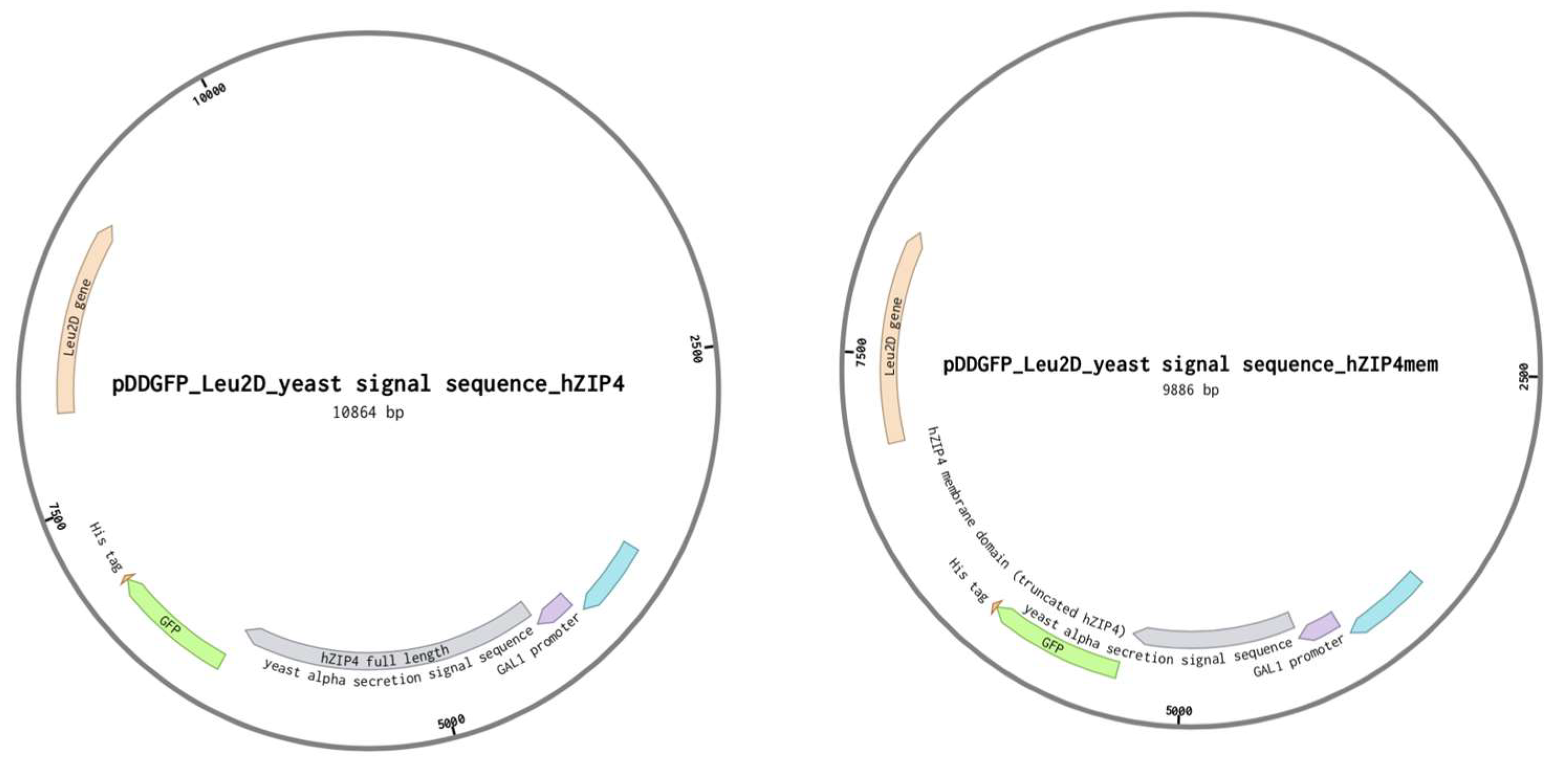
2.2. Plasmid Construction
2.3. S. cerevisiae hZIP4 Protein Expression and Localization
2.4. Measurement of Growth Curves
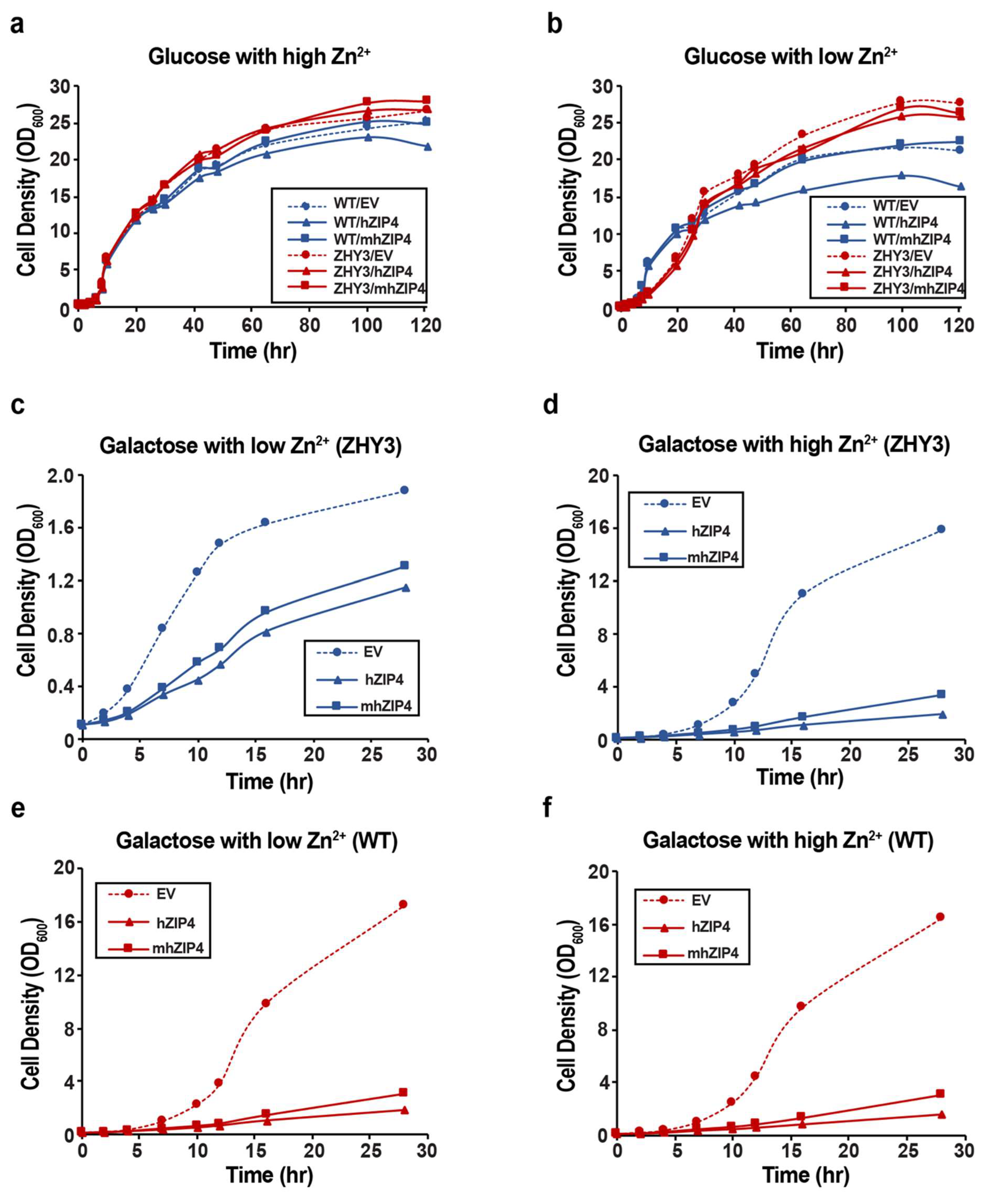
3. Results
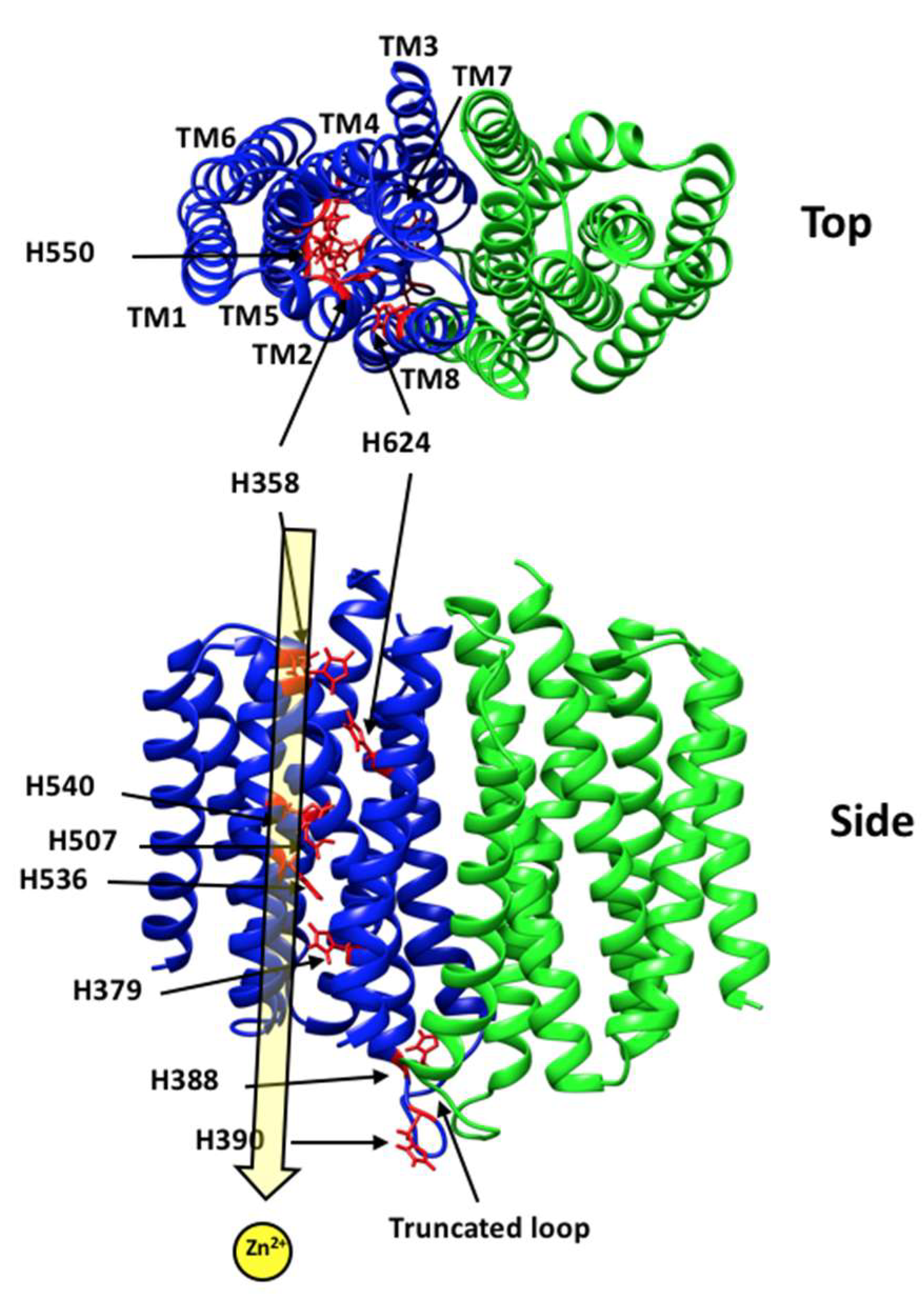
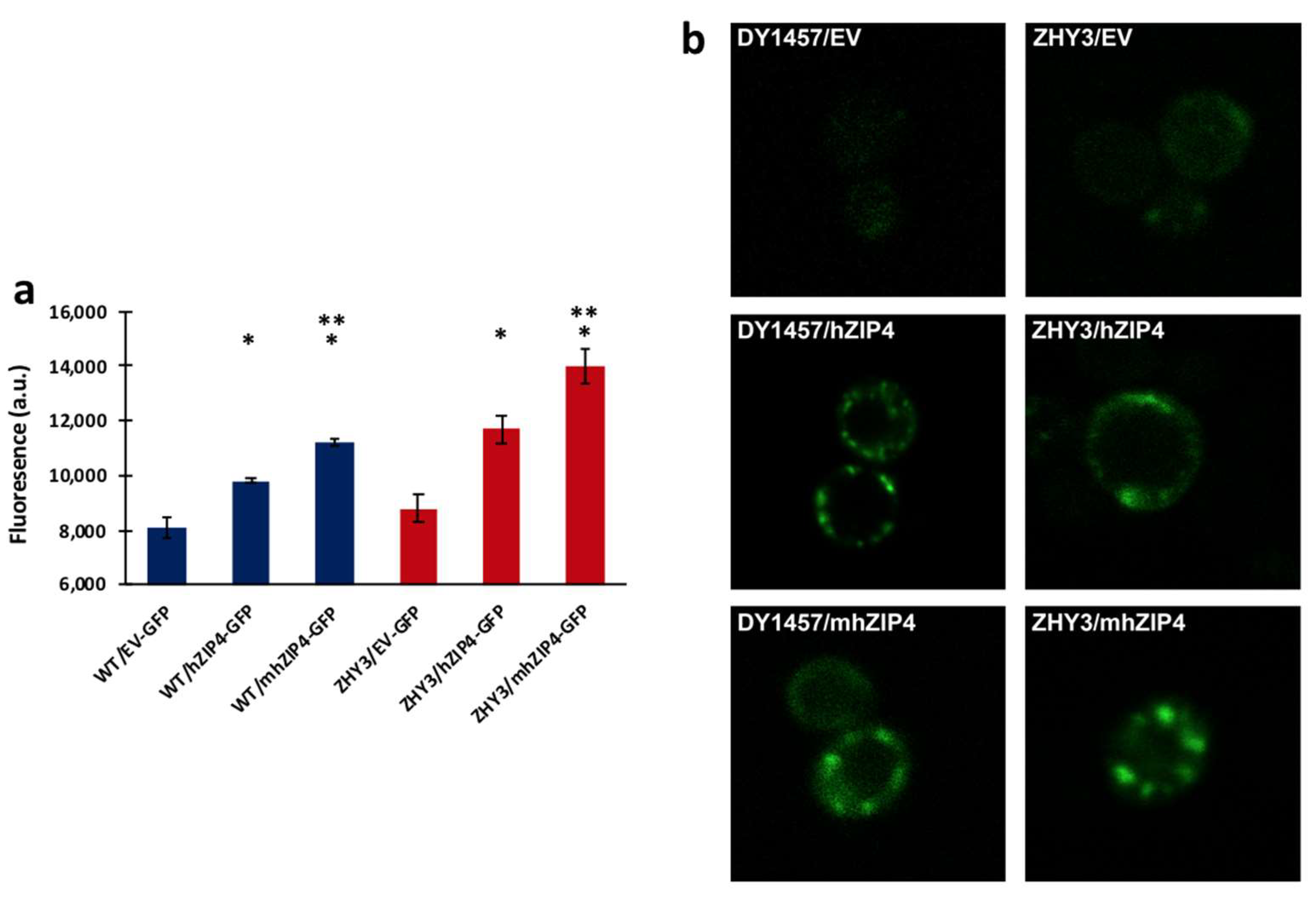
3.1. hZIP4 Was Heterologously Expressed in S. cerevisiae and Localized to the Plasma Membrane
3.2. Role of hZIP4 in Growth Rate Control
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeong, J.; Eide, D.J. The SLC39 family of zinc transporters. Mol. Asp. Med. 2013, 34, 612–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beyersmann, D.; Haase, H. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. BioMetals 2001, 14, 331–341. [Google Scholar] [CrossRef]
- Bafaro, E.; Liu, Y.; Xu, Y.; Dempski, E.R. The emerging role of zinc transporters in cellular homeostasis and cancer. Signal Transduct. Target. Ther. 2017, 2, 17029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, A.S. Discovery of Human Zinc Deficiency: Its Impact on Human Health and Disease. Adv. Nutr. Int. Rev. J. 2013, 4, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and its importance for human health: An integrative review. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2013, 18, 144–157. [Google Scholar]
- Dempski, R.E. Chapter Nine–The Cation Selectivity of the ZIP Transporters. In Current Topics in Membranes; Argüello, J.M., Lutsenko, S., Eds.; Academic Press: Cambridge, MA, USA, 2012; pp. 221–245. [Google Scholar]
- Li, M.; Zhang, Y.; Liu, Z.; Bharadwaj, U.; Wang, H.; Wang, X.; Zhang, S.; Liuzzi, J.P.; Chang, S.-M.; Cousins, R.J.; et al. Aberrant expression of zinc transporter ZIP4 (SLC39A4) significantly contributes to human pancreatic cancer pathogenesis and progression. Proc. Natl. Acad. Sci. USA 2007, 104, 18636–18641. [Google Scholar] [CrossRef] [Green Version]
- Dufner-Beattie, J.; Wang, F.; Kuo, Y.-M.; Gitschier, J.; Eide, D.; Andrews, G.K. The Acrodermatitis Enteropathica Gene ZIP4 Encodes a Tissue-specific, Zinc-regulated Zinc Transporter in Mice. J. Biol. Chem. 2003, 278, 33474–33481. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Bharadwaj, U.; Logsdon, C.D.; Chen, C.; Yao, Q.; Li, M. ZIP4 Regulates Pancreatic Cancer Cell Growth by Activating IL-6/STAT3 Pathway via Zinc Finger Transcription Factor CREB. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2010, 16, 1423–1430. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Zhou, B.; Kuo, Y.-M.; Zemansky, J.; Gitschier, J. A Novel Member of a Zinc Transporter Family Is Defective in Acrodermatitis Enteropathica. Am. J. Hum. Genet. 2002, 71, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Küry, S.; Dréno, B.; Bézieau, S.; Giraudet, S.; Kharfi, M.; Kamoun, R.; Moisan, J.-P. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat. Genet. 2002, 31, 239–240. [Google Scholar] [CrossRef]
- Weaver, B.P.; Dufner-Beattie, J.; Fau-Kambe, T.; Andrews, G.K. Novel zinc-responsive post-transcriptional mechanisms reciprocally regulate expression of the mouse Slc39a4 and Slc39a5 zinc transporters (Zip4 and Zip5). Biol. Chem. 2007, 388, 1301–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, G.K. Regulation and Function of Zip4, the Acrodermatitis Enteropathica Gene. Biochem. Soc. Trans. 2008, 36, 1242–1246. [Google Scholar] [CrossRef] [Green Version]
- Kambe, T.; Andrews, G.K. Novel Proteolytic Processing of the Ectodomain of the Zinc Transporter ZIP4 (SLC39A4) during Zinc Deficiency Is Inhibited by Acrodermatitis Enteropathica Mutations. Mol. Cell. Biol. 2009, 29, 129–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golovine, K.; Makhov, P.; Uzzo, R.G.; Shaw, T.; Kunkle, D.; Kolenko, V.M. Overexpression of the zinc uptake transporter hZIP1 inhibits nuclear factor-kappaB and reduces the malignant potential of prostate cancer cells in vitro and in vivo. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 5376–5384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaither, L.A.; Eide, D.J. Functional Expression of the Human hZIP2 Zinc Transporter. J. Biol. Chem. 2000, 275, 5560–5564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antala, S.; Ovchinnikov, S.; Kamisetty, H.; Baker, D.; Dempski, R.E. Computation and Functional Studies Provide a Model for the Structure of the Zinc Transporter hZIP4. J. Biol. Chem. 2015, 290, 17796–17805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahern, M.E.; Bafaro, E.M.; Cowan, A.; Dempski, R.E. Quantifying the Oligomeric State of hZIP4 on the Surface of Cells. Biochemistry 2019, 58, 1705–1708. [Google Scholar] [CrossRef] [PubMed]
- Hoch, E.; Levy, M.; Hershfinkel, M.; Sekler, I. Elucidating the H+ Coupled Zn2+ Transport Mechanism of ZIP4; Implications in Acrodermatitis Enteropathica. Int. J. Mol. Sci. 2020, 21, 734. [Google Scholar] [CrossRef] [Green Version]
- Bafaro, E.M.; Maciejewski, M.W.; Hoch, J.C.; Dempski, R.E. Concomitant disorder and high-affinity zinc binding in the human zinc- and iron-regulated transport protein 4 intracellular loop. Protein Sci. 2019, 28, 868–880. [Google Scholar] [CrossRef]
- Küry, S.; Kharfi, M.; Kamoun, R.; Taïeb, A.; Mallet, E.; Baudon, J.-J.; Glastre, C.; Michel, B.; Sebag, F.; Brooks, D.; et al. Mutation spectrum of human SLC39A4 in a panel of patients with acrodermatitis enteropathica. Hum. Mutat. 2003, 22, 337–338. [Google Scholar] [CrossRef]
- Mao, X.; Kim, B.-E.; Wang, F.; Eide, D.J.; Petris, M.J. A Histidine-rich Cluster Mediates the Ubiquitination and Degradation of the Human Zinc Transporter, hZIP4, and Protects against Zinc Cytotoxicity. J. Biol. Chem. 2007, 282, 6992–7000. [Google Scholar] [CrossRef] [Green Version]
- Antala, S.; Dempski, R.E. The Human ZIP4 Transporter Has Two Distinct Binding Affinities and Mediates Transport of Multiple Transition Metals. Biochemistry 2012, 51, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-E.; Wang, F.; Dufner-Beattie, J.; Andrews, G.K.; Eide, D.J.; Petris, M.J. Zn2+-stimulated Endocytosis of the mZIP4 Zinc Transporter Regulates Its Location at the Plasma Membrane. J. Biol. Chem. 2004, 279, 4523–4530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bafaro, E.M.; Antala, S.; Nguyen, T.-V.; Dzul, S.P.; Doyon, B.; Stemmler, T.L.; Dempski, R.E. The large intracellular loop of hZIP4 is an intrinsically disordered zinc binding domain. Met. Integr. Biomet. Sci. 2015, 7, 1319–1330. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Liu, J.; Fellner, M.; Zhang, C.; Sui, D.; Hu, J. Crystal structures of a ZIP zinc transporter reveal a binuclear metal center in the transport pathway. Sci. Adv. 2017, 3, e1700344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bleackley, M.R.; MacGillivray, R.T.A. Transition metal homeostasis: From yeast to human disease. BioMetals 2011, 24, 785–809. [Google Scholar] [CrossRef] [PubMed]
- Dix, D.R.; Bridgham, J.T.; A Broderius, M.; A Byersdorfer, C.; Eide, D.J. The FET4 gene encodes the low affinity Fe(II) transport protein of Saccharomyces cerevisiae. J. Biol. Chem. 1994, 269, 26092–26099. [Google Scholar] [CrossRef]
- Wu, X.; Sinani, D.; Kim, H.; Lee, J. Copper Transport Activity of Yeast Ctr1 Is Down-regulated via Its C Terminus in Response to Excess Copper. J. Biol. Chem. 2009, 284, 4112–4122. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Eide, D. The ZRT2 Gene Encodes the Low Affinity Zinc Transporter in Saccharomyces cerevisiae. J. Biol. Chem. 1996, 271, 23203–23210. [Google Scholar] [CrossRef] [Green Version]
- Drew, D.E.; Heijne, G.v.; Fau-Nordlund, P.; Nordlund, P.; Fau-de Gier, J.W.; de Gier, J.W. Green fluorescent protein as an indicator to monitor membrane protein overexpression in Escherichia coli. FEBS Lett. 2001, 507, 220–224. [Google Scholar] [CrossRef] [Green Version]
- Newstead, S.; Kim, H.; von Heijne, G.; Iwata, S.; Drew, D. High-throughput fluorescent-based optimization of eukaryotic membrane protein overexpression and purification in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2007, 104, 13936–13941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawate, T.; Gouaux, E. Fluorescence-detection size-exclusion chromatography for precrystallization screening of integral membrane proteins. Structure 2006, 14, 673–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becares, E.R.; Pedersen, P.A.; Gourdon, P.; Gotfryd, K. Overproduction of Human Zip (SLC39) Zinc Transporters in Saccharomyces cerevisiae for Biophysical Characterization. Cells 2021, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.L.; Newstead, S. Method to increase the yield of eukaryotic membrane protein expression in Saccharomyces cerevisiae for structural and functional studies. Protein Sci. 2014, 23, 1309–1314. [Google Scholar] [CrossRef]
- Drew, D.; Newstead, S.; Sonoda, Y.; Kim, H.; Von Heijne, G.; Iwata, S. GFP-based optimization scheme for the overexpression and purification of eukaryotic membrane proteins in Saccharomyces cerevisiae. Nat. Protoc. 2008, 3, 784–798. [Google Scholar] [CrossRef] [Green Version]
- Vidali, L.; Rounds, C.M.; Hepler, P.K.; Bezanilla, M. Lifeact-mEGFP reveals a dynamic apical F-actin network in tip growing plant cells. PLoS ONE 2009, 4, e5744. [Google Scholar] [CrossRef] [Green Version]
- Hermida-Matsumoto, L.; Resh, M.D. Localization of Human Immunodeficiency Virus Type 1 Gag and Env at the Plasma Membrane by Confocal Imaging. J. Virol. 2000, 74, 8670–8679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malínská, K.; Malínský, J.; Opekarová, M.; Tanner, W. Visualization of Protein Compartmentation within the Plasma Membrane of Living Yeast Cells. Mol. Biol. Cell 2003, 14, 4427–4436. [Google Scholar] [CrossRef]
- Zhao, H.; Eide, D.J. Zap1p, a metalloregulatory protein involved in zinc-responsive transcriptional regulation in Saccharomyces cerevisiae. Mol. Cell. Biol. 1997, 17, 5044–5052. [Google Scholar] [CrossRef] [Green Version]
- Johnson, P.; Federico, M.; Kirkwood, A.; Fosså, A.; Berkahn, L.; Carella, A.; d’Amore, F.; Enblad, G.; Franceschetto, A.; Fulham, M.; et al. Adapted Treatment Guided by Interim PET-CT Scan in Advanced Hodgkin’s Lymphoma. N. Engl. J. Med. 2016, 374, 2419–2429. [Google Scholar] [CrossRef]
- Lee, J.-H.; You, J.; Dobrota, E.; Skalnik, D.G. Identification and Characterization of a Novel Human PP1 Phosphatase Complex. J. Biol. Chem. 2010, 285, 24466–24476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozak, K.; Papierniak, A.; Barabasz, A.; Kendziorek, M.; Palusińska, M.; Williams, L.E.; Antosiewicz, D.M. NtZIP11, a new Zn transporter specifically upregulated in tobacco leaves by toxic Zn level. Environ. Exp. Bot. 2018, 157, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Khouja, H.R.; Abbà, S.; Lacercat-Didier, L.; Daghino, S.; Doillon, D.; Richaud, P.; Martino, E.; Vallino, M.; Perotto, S.; Chalot, M.; et al. OmZnT1 and OmFET, two metal transporters from the metal-tolerant strain Zn of the ericoid mycorrhizal fungus Oidiodendron maius, confer zinc tolerance in yeast. Fungal Genet. Biol. 2013, 52, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Sui, D.; Hu, J. Structural insights of ZIP4 extracellular domain critical for optimal zinc transport. Nat. Commun. 2016, 7, 11979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawada, H.; Inanobe, A.; Kurachi, Y. Isolation of proflavine as a blocker of G protein-gated inward rectifier potassium channels by a cell growth-based screening system. Neuropharmacology 2016, 109, 18–28. [Google Scholar] [CrossRef] [PubMed]
| Sequences | Primer Sequence (5′-3′) |
|---|---|
| hZIP4 S. cere signal sequence forward | ACCCCGGATTCTAGAACTAGTGGATCCCCCATGAGATTTCCTTCAATTTTTACTGC |
| hZIP4 full-length overlap extension forward | GAGAGGCTGAAGCTTACGTAGCGTCCCTGGTCTCGCTGGAGC |
| hZIP4 full-length overlap extension reverse | GCTCCAGCGAGACCAGGGACGCTACGTAAGCTTCAGCCTCTC |
| hZIP4 S. cere signal sequence reverse | AAATTGACCTTGAAAATATAAATTTTCCCCAGAACCACCGAAGGTGATGTCATCCTCGTAC |
| Truncated hZIP4 S. cere signal sequence forward | ACCCCGGATTCTAGAACTAGTGGATCCCCCATGAGATTTCCTTCAATTTTTACTGC |
| Truncated hZIP4 full-length overlap extension forward | GAGAGGCTGAAGCTTACGTACTGTACGGCTCCCTGGCCACGC |
| Truncated hZIP4 full-length overlap extension reverse | GCGTGGCCAGGGAGCCGTACAGTACGTAAGCTTCAGCCTCTC |
| Truncated hZIP4 S. cere signal sequence reverse | AAATTGACCTTGAAAATATAAATTTTCCCCAGAACCACCGAAGGTGATGTCATCCTCGTAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Bafaro, E.M.; Dempski, R.E. Heterologous Expression of Full-Length and Truncated Human ZIP4 Zinc Transporter in Saccharomyces cerevisiae. Biomolecules 2022, 12, 726. https://doi.org/10.3390/biom12050726
Liu Y, Bafaro EM, Dempski RE. Heterologous Expression of Full-Length and Truncated Human ZIP4 Zinc Transporter in Saccharomyces cerevisiae. Biomolecules. 2022; 12(5):726. https://doi.org/10.3390/biom12050726
Chicago/Turabian StyleLiu, Yuting, Elizabeth M. Bafaro, and Robert E. Dempski. 2022. "Heterologous Expression of Full-Length and Truncated Human ZIP4 Zinc Transporter in Saccharomyces cerevisiae" Biomolecules 12, no. 5: 726. https://doi.org/10.3390/biom12050726
APA StyleLiu, Y., Bafaro, E. M., & Dempski, R. E. (2022). Heterologous Expression of Full-Length and Truncated Human ZIP4 Zinc Transporter in Saccharomyces cerevisiae. Biomolecules, 12(5), 726. https://doi.org/10.3390/biom12050726






