Lack of Ecto-5′-Nucleotidase Protects Sensitized Mice against Allergen Challenge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Allergic Sensitization and Challenge
2.3. Immunoblot Analysis for CD73
2.4. Measurement of Airway Responsiveness
2.5. Measurement of AMPase Activity
2.6. Enzyme-Linked Immunosorbent Assay
2.7. Lung Histology
2.8. Flow Cytometry Analysis
2.9. Statistical Analysis
3. Results
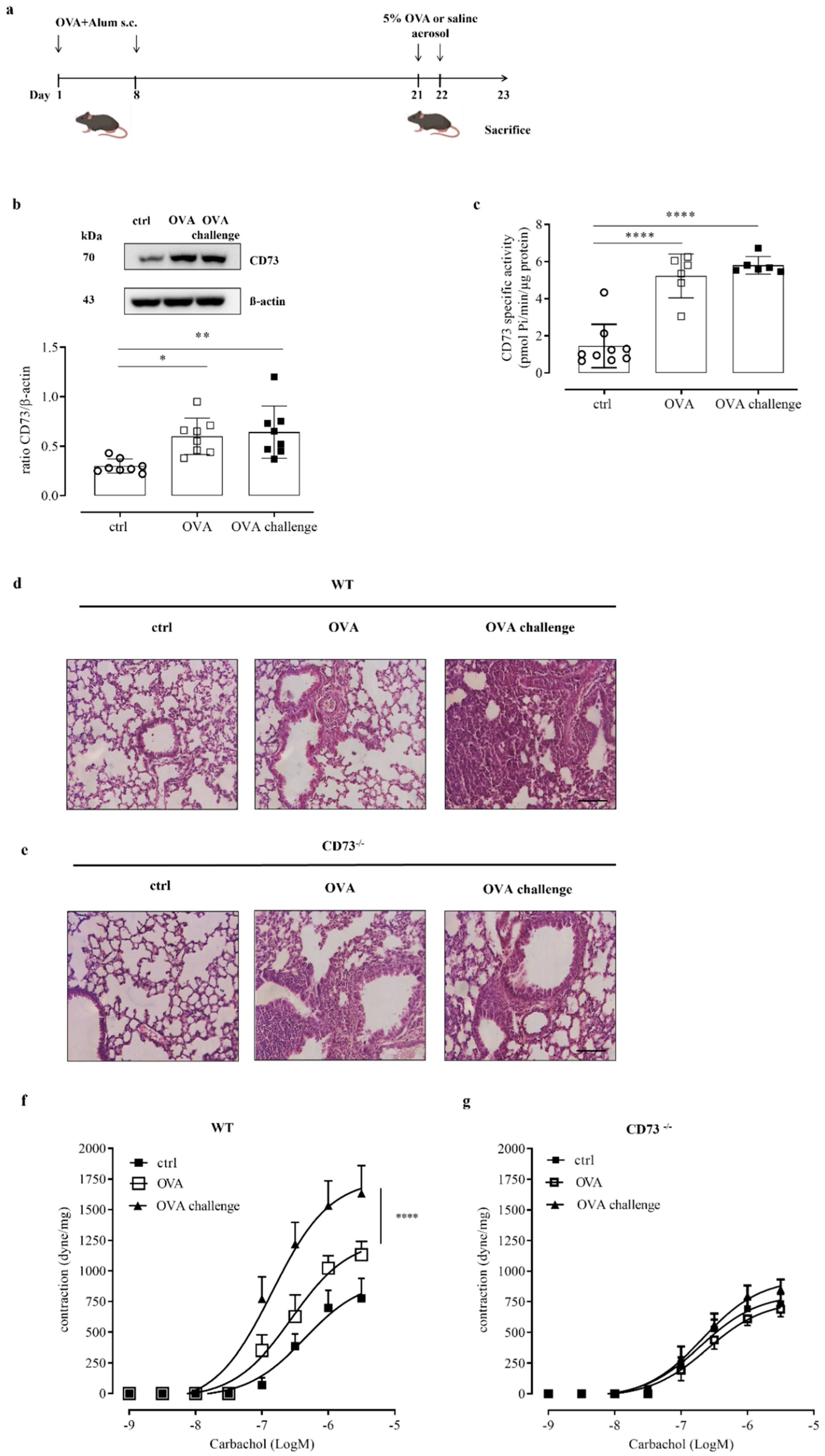
3.1. Pulmonary Tissues from Ovalbumin-Challenged Mice Show High Levels of CD73 Expression and Activity
3.2. Ovalbumin Challenge Does Not Increase Pulmonary Inflammatory Cell Infiltration in CD73−/− Mice
3.3. Ovalbumin Challenge Does Not Increase Bronchial Hyperreactivity to Carbachol in CD73−/− Mice
3.4. Ovalbumin Challenge Enhances IgE Production in All Mice Groups
3.5. OVA Challenge Increases Pulmonary Infiltration of CD23+B Cells and IL-4+ T Cells
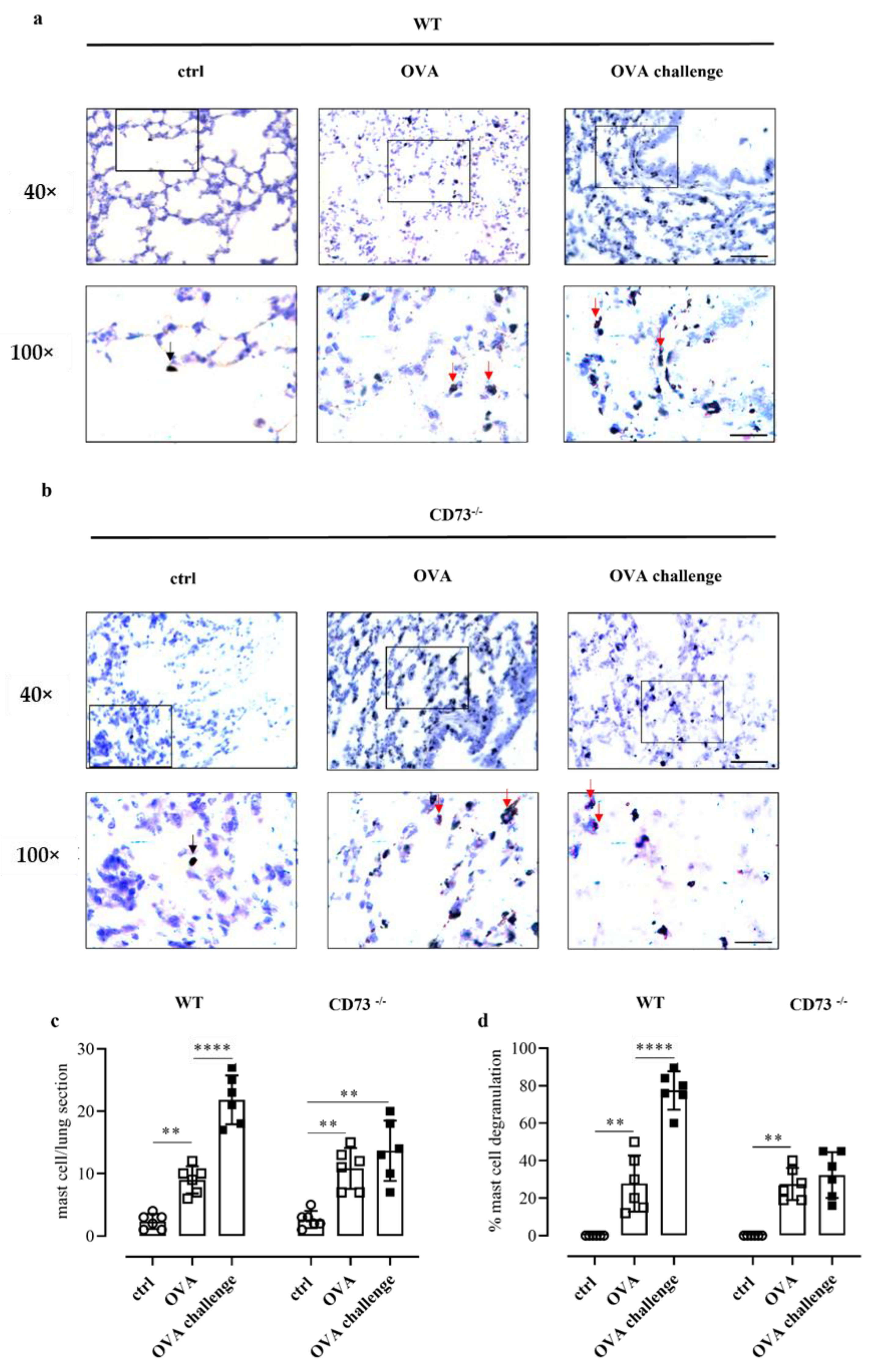
3.6. Ovalbumin Challenge Does Not Increase Mast Cell Degranulation in the Lung of CD73−/− Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holgate, S.T.; Wenzel, S.; Postma, D.S.; Weiss, S.T.; Renz, H.; Sly, P.D. Asthma. Nat. Rev. Dis. Primers 2015, 1, 15025. [Google Scholar] [CrossRef] [PubMed]
- Ree, R.; Hummelshøj, L.; Plantinga, M.; Poulsen, L.K.; Swindle, E. Allergic sensitization: Host-immune factors. Clin. Transl. Allergy 2014, 4, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, H.; Zebisch, M.; Sträter, N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012, 8, 437–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Currie, G.P.; Jackson, C.M.; Lee, D.K.C.; Lipworth, B.J. Allergen sensitization and bronchial hyper-responsiveness to adenosine monophosphate in asthmatic patients. Clin. Exp. Allergy 2003, 33, 1405–1408. [Google Scholar] [CrossRef]
- Wilson, C.N. Adenosine receptors and asthma in humans. J. Cereb. Blood Flow Metab. 2008, 155, 475–486. [Google Scholar] [CrossRef] [Green Version]
- Alfieri, A.; Parisi, A.; Maione, F.; Grassia, G.; Morello, S.; Ialenti, A.; Mascolo, N.; Cicala, C. Hyperresponsiveness to adenosine in sensitized Wistar rats over-expressing A1 receptor. Eur. J. Pharmacol. 2012, 695, 120–125. [Google Scholar] [CrossRef]
- Cicala, C.; Ialenti, A. Adenosine signaling in airways: Toward a promising antiasthmatic approach. Eur. J. Pharmacol. 2013, 714, 522–525. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Garcia, L.; Olle, L.; Martin, M.; Roca-Ferrer, J.; Muñoz-Cano, R. Adenosine Signaling in Mast Cells and Allergic Diseases. Int. J. Mol. Sci. 2021, 22, 5203. [Google Scholar] [CrossRef]
- Eckle, T.; Füllbier, L.; Wehrmann, M.; Khoury, J.; Mittelbronn, M.; Ibla, J.; Rosenberger, P.; Eltzschig, H.K. Identification of Ectonucleotidases CD39 and CD73 in Innate Protection during Acute Lung Injury. J. Immunol. 2007, 178, 8127–8137. [Google Scholar] [CrossRef]
- Wang, L.-L.; Tang, H.-P.; Shi, G.-C.; Wan, H.-Y.; Tang, W.; Hou, X.-X.; Pan, L.-N.; Shi, B.-Y.; Tao, L.-Q. CD39/CD73 and the imbalance of Th17 cells and regulatory T cells in allergic asthma. Mol. Med. Rep. 2013, 8, 1432–1438. [Google Scholar] [CrossRef] [Green Version]
- Neuberger, A.; Ring, S.; Silva-Vilches, C.; Schrader, J.; Enk, A.; Mahnke, K. Expression of CD73 slows down migration of skin dendritic cells, affecting the sensitization phase of contact hypersensitivity reactions in mice. J. Dermatol. Sci. 2017, 87, 292–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caiazzo, E.; Morello, S.; Carnuccio, R.; Ialenti, A.; Cicala, C. The Ecto-5’-Nucleotidase/CD73 Inhibitor, α,β-Methylene Adenosine 5’-Diphosphate, Exacerbates Carrageenan-Induced Pleurisy in Rat. Front. Pharmacol. 2019, 10, 775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, E.; Winzer, R.; Rissiek, A.; Ricklefs, I.; Meyer-Schwesinger, C.; Ricklefs, F.L.; Bauche, A.; Behrends, J.; Reimer, R.; Brenna, S.; et al. CD73-mediated adenosine production by CD8 T cell-derived extracellular vesicles constitutes an intrinsic mechanism of immune suppression. Nat. Commun. 2021, 12, 5911. [Google Scholar] [CrossRef] [PubMed]
- Caiazzo, E.; Cerqua, I.; Riemma, M.A.; Turiello, R.; Ialenti, A.; Schrader, J.; Fiume, G.; Caiazza, C.; Roviezzo, F.; Morello, S.; et al. Exacerbation of Allergic Airway Inflammation in Mice Lacking ECTO-5′-Nucleotidase (CD73). Front. Pharmacol. 2020, 11, 589343. [Google Scholar] [CrossRef]
- Morello, S.; Vellecco, V.; Roviezzo, F.; Maffia, P.; Cuzzocrea, S.; Cirino, G.; Cicala, C. A protective role for proteinase activated receptor 2 in airways of lipopolysaccharide-treated rats. Biochem. Pharmacol. 2005, 71, 223–230. [Google Scholar] [CrossRef]
- Roviezzo, F.; Rossi, A.; Caiazzo, E.; Orlando, P.; Riemma, M.A.; Iacono, V.M.; Guarino, A.; Ialenti, A.; Cicala, C.; Peritore, A.; et al. Palmitoylethanolamide Supplementation during Sensitization Prevents Airway Allergic Symptoms in the Mouse. Front. Pharmacol. 2017, 8, 857. [Google Scholar] [CrossRef] [Green Version]
- Engeroff, P.; Vogel, M. The role of CD23 in the regulation of allergic responses. Allergy 2021, 76, 1981–1989. [Google Scholar] [CrossRef] [PubMed]
- Schena, F.; Volpi, S.; Faliti, C.E.; Penco, F.; Santi, S.; Proietti, M.; Schenk, U.; Damonte, G.; Salis, A.; Bellotti, M.; et al. Dependence of Immunoglobulin Class Switch Recombination in B Cells on Vesicular Release of ATP and CD73 Ectonucleotidase Activity. Cell Rep. 2013, 3, 1824–1831. [Google Scholar] [CrossRef] [Green Version]
- Schreiber, R.; Castrop, H.; Kunzelmann, K. Allergen-induced airway hyperresponsiveness is absent in ecto-5′-nucleotidase (CD73)-deficient mice. Pflügers Arch. -Eur. J. Physiol. 2008, 457, 431–440. [Google Scholar] [CrossRef]
- Conrad, D.H.; Ford, J.W.; Sturgill, J.; Gibb, D.R. CD23: An overlooked regulator of allergic disease. Curr. Allergy Asthma Rep. 2007, 7, 331–337. [Google Scholar] [CrossRef]
- Cheng, L.E.; Wang, Z.-E.; Locksley, R.M. Murine B Cells Regulate Serum IgE Levels in a CD23-Dependent Manner. J. Immunol. 2010, 185, 5040–5047. [Google Scholar] [CrossRef] [PubMed]
- Selb, R.; Eckl-Dorna, J.; Neunkirchner, A.; Schmetterer, K.; Marth, K.; Gamper, J.; Jahn-Schmid, B.; Pickl, W.F.; Valenta, R.; Niederberger, V. CD23 surface density on B cells is associated with IgE levels and determines IgE-facilitated allergen uptake, as well as activation of allergen-specific T cells. J. Allergy Clin. Immunol. 2017, 139, 290–299.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poole, J.A.; Meng, J.; Reff, M.; Spellman, M.C.; Rosenwasser, L.J. Anti-CD23 monoclonal antibody, lumiliximab, inhibited allergen-induced responses in antigen-presenting cells and T cells from atopic subjects. J. Allergy Clin. Immunol. 2005, 116, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.M.; Tomayko, M.M.; Ahuja, A.; Haberman, A.M.; Shlomchik, M.J. New markers for murine memory B cells that define mutated and unmutated subsets. J. Exp. Med. 2007, 204, 2103–2114. [Google Scholar] [CrossRef] [Green Version]
- Conter, L.J.; Song, E.; Shlomchik, M.J.; Tomayko, M.M. CD73 Expression Is Dynamically Regulated in the Germinal Center and Bone Marrow Plasma Cells Are Diminished in Its Absence. PLoS ONE 2014, 9, e92009. [Google Scholar] [CrossRef] [Green Version]
- Gomez, G.; Zhao, W.; Schwartz, L.B. Disparity in FcεRI-Induced Degranulation of Primary Human Lung and Skin Mast Cells Exposed to Adenosine. J. Clin. Immunol. 2011, 31, 479–487. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caiazzo, E.; Cerqua, I.; Turiello, R.; Riemma, M.A.; De Palma, G.; Ialenti, A.; Roviezzo, F.; Morello, S.; Cicala, C. Lack of Ecto-5′-Nucleotidase Protects Sensitized Mice against Allergen Challenge. Biomolecules 2022, 12, 697. https://doi.org/10.3390/biom12050697
Caiazzo E, Cerqua I, Turiello R, Riemma MA, De Palma G, Ialenti A, Roviezzo F, Morello S, Cicala C. Lack of Ecto-5′-Nucleotidase Protects Sensitized Mice against Allergen Challenge. Biomolecules. 2022; 12(5):697. https://doi.org/10.3390/biom12050697
Chicago/Turabian StyleCaiazzo, Elisabetta, Ida Cerqua, Roberta Turiello, Maria Antonietta Riemma, Giacomo De Palma, Armando Ialenti, Fiorentina Roviezzo, Silvana Morello, and Carla Cicala. 2022. "Lack of Ecto-5′-Nucleotidase Protects Sensitized Mice against Allergen Challenge" Biomolecules 12, no. 5: 697. https://doi.org/10.3390/biom12050697
APA StyleCaiazzo, E., Cerqua, I., Turiello, R., Riemma, M. A., De Palma, G., Ialenti, A., Roviezzo, F., Morello, S., & Cicala, C. (2022). Lack of Ecto-5′-Nucleotidase Protects Sensitized Mice against Allergen Challenge. Biomolecules, 12(5), 697. https://doi.org/10.3390/biom12050697








