Cytokine Profile and Anti-Inflammatory Activity of a Standardized Conditioned Medium Obtained by Coculture of Monocytes and Mesenchymal Stromal Cells (PRS CK STORM)
Abstract
1. Introduction
2. Materials and Methods
2.1. Primary Cell Isolation, Expansion, and Coculture
2.1.1. MSCs Isolation
2.1.2. PBMC and Monocyte Isolation
2.1.3. Phenotypic Characterization of MSCs and M2 Macrophages by Flow Cytometry
2.1.4. Characterization of the Secretome (PRS CK STORM)
2.1.5. Anti-Inflammatory Potency Tests
2.1.6. MTT Assay
2.1.7. Statistics and Images
3. Results
3.1. Isolation of MSCs and Monocytes
3.2. Phenotypic Characterization of MSCs and M2 Macrophages
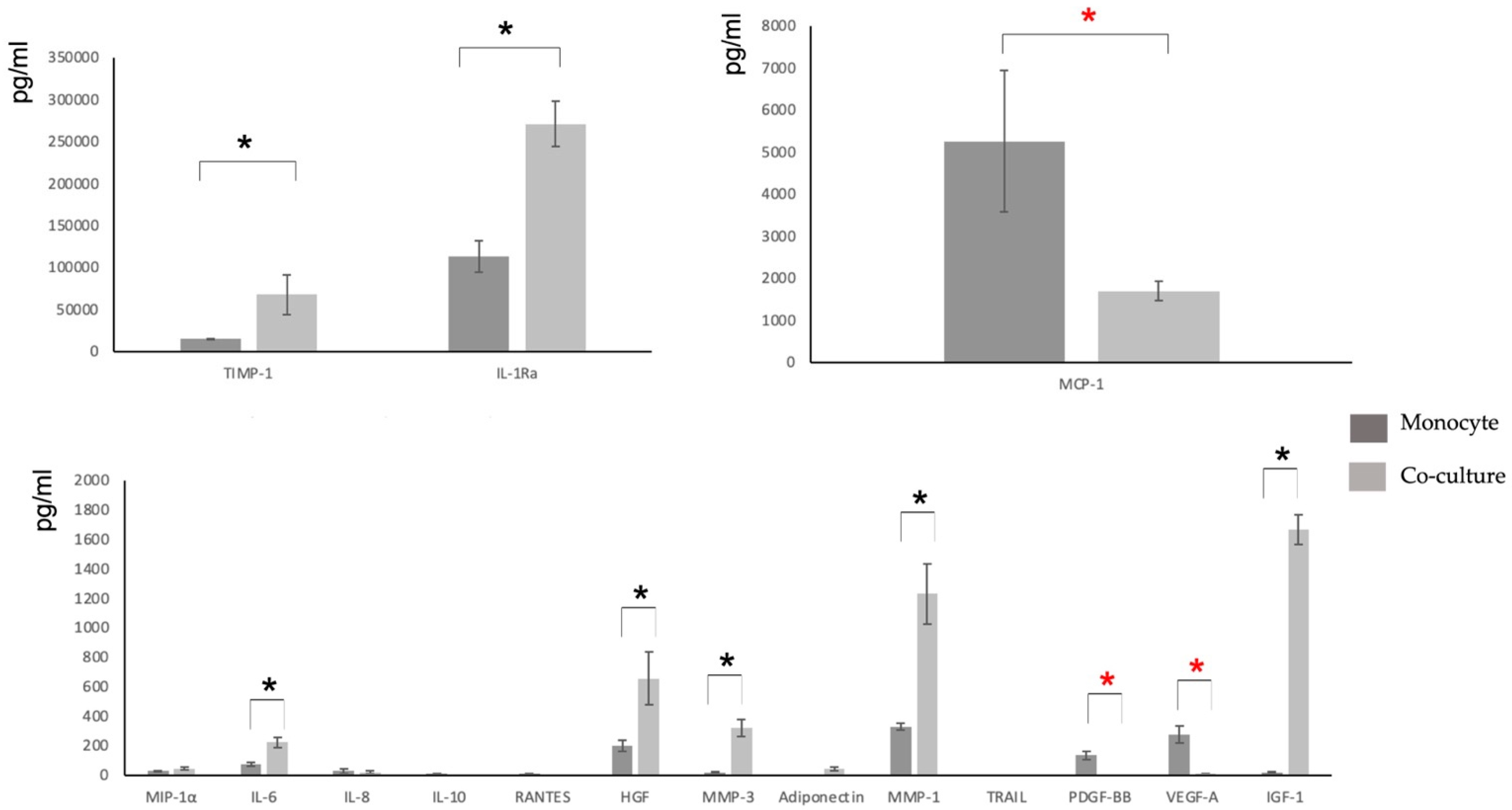
3.3. Characterization of the Secretome
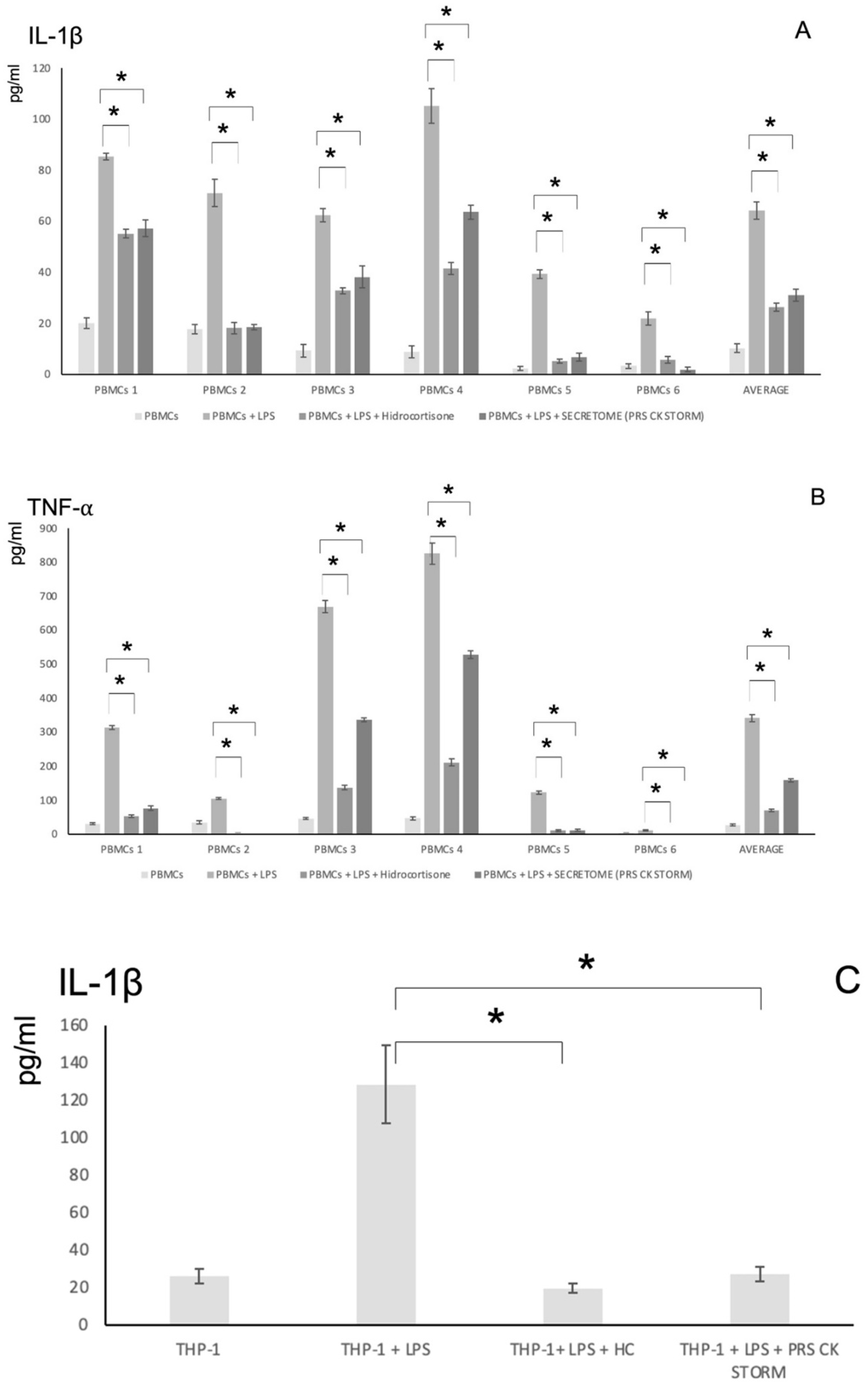
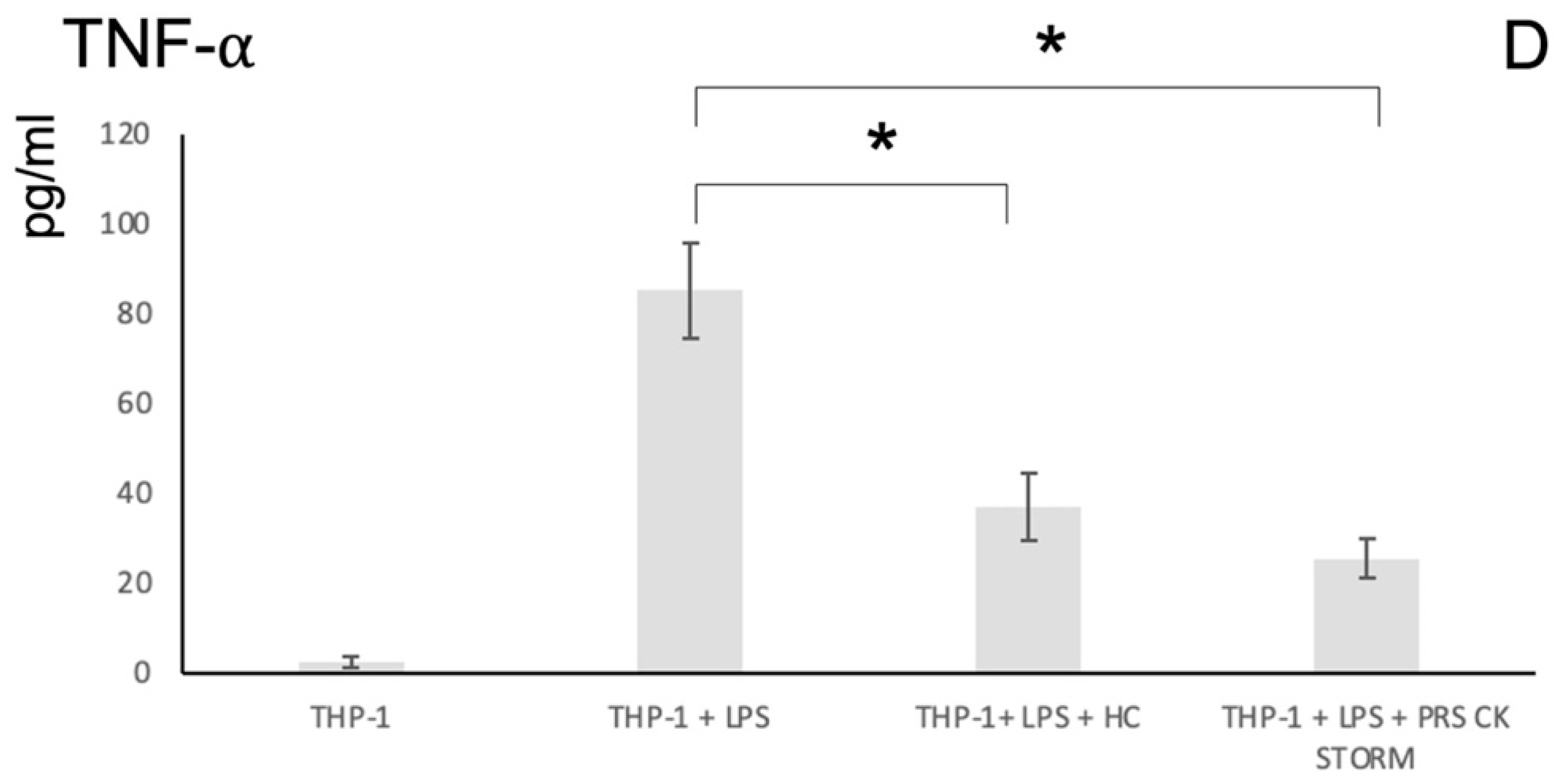
3.4. Pro-Inflammatory Cytokine Release Assays to Assess the Anti-Inflammatory Potency of the Secretome
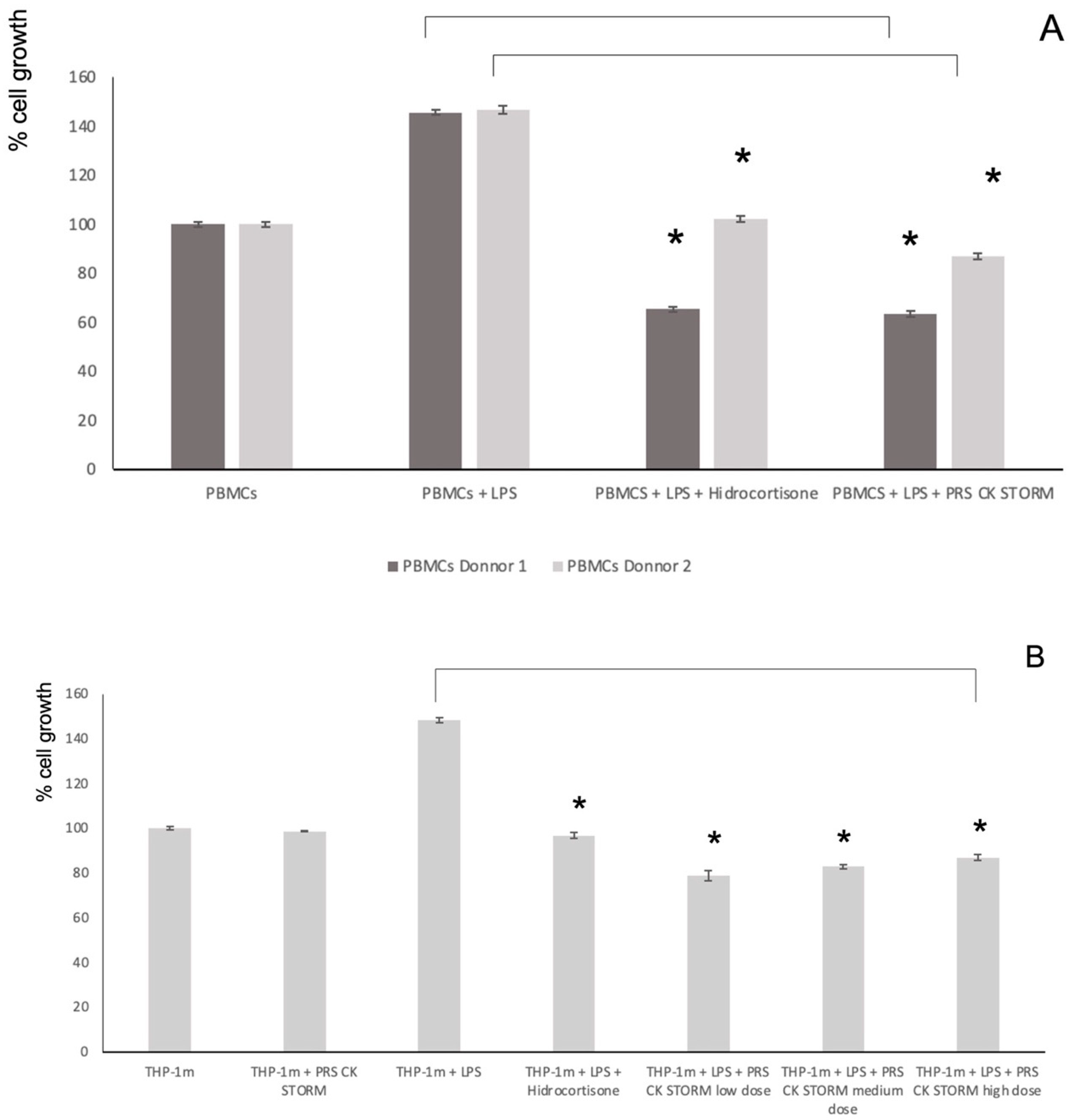
3.5. Effect of the Secretome on Cell Viability and on LPS-Induced Growth
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vallés, G.; Bensiamar, F.; Crespo, L.; Arruebo, M.; Vilaboa, N.; Saldaña, L. Topographical cues regulate the crosstalk between MSCs and macrophages. Biomaterials 2014, 37, 124–133. [Google Scholar] [CrossRef] [PubMed]
- English, K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol. Cell Biol. 2012, 91, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Melief, S.M.; Geutskens, S.B.; Fibbe, W.E.; Roelofs, H. Multipotent stromal cells skew monocytes towards an anti-inflammatory interleukin-10-producing phenotype by production of interleukin-6. Haematologica 2013, 98, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Saldaña, L.; Bensiamar, F.; Vallés, G.; Mancebo, F.J.; García-Rey, E.; Vilaboa, N. Immunoregulatory potential of mesenchymal stem cells following activation by macrophage-derived soluble factors. Stem Cell Res. Ther. 2019, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Luz-Crawford, P.; Djouad, F.; Toupet, K.; Bony, C.; Franquesa, M.; Hoogduijn, M.J.; Jorgensen, C.; Noël, D. Mesenchymal Stem Cell-Derived Interleukin 1 Receptor Antagonist Promotes Macrophage Polarization and Inhibits B Cell Differentiation. Stem Cells 2015, 34, 483–492. [Google Scholar] [CrossRef]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage activation and polarization. Front. Biosci. 2008, 13, 453–461. [Google Scholar] [CrossRef]
- Banerjee, S.; Cui, H.; Xie, N.; Tan, Z.; Yang, S.; Icyuz, M.; Thannickal, V.J.; Abraham, E.; Liu, G. miR-125a-5p regulates differential activation of macrophages and inflammation. J. Biol. Chem. 2013, 288, 35428–35436. [Google Scholar] [CrossRef]
- Ge, W.; Jiang, J.; Arp, J.; Liu, W.; Garcia, B.; Wang, H. Regulatory T-Cell Generation and Kidney Allograft Tolerance Induced by Mesenchymal Stem Cells Associated with Indoleamine 2,3-Dioxygenase Expression. Transplantation 2010, 90, 1312–1320. [Google Scholar] [CrossRef]
- Himanshu, K.; Taro, K.; Shizuo, A. Pathogen Recognition by the Innate Immune System. Int. Rev. Immunol. 2011, 30, 16–34. [Google Scholar]
- Taro, K.; Shizuo, A. Toll-like Receptors and Their Crosstalk with Other Innate Receptors in Infection and Immunity. Immunity 2011, 34, 637–650. [Google Scholar]
- Komal, D.; Manoj, B.; Gourango, B.; Atul, B.; Sangita, B. TLRs/NLRs: Shaping the landscape of host immunity. Int. Rev. Immunol. 2011, 37, 3–19. [Google Scholar]
- Root-Bernstein, R. Synergistic Activation of Toll-Like and NOD Receptors by Complementary Antigens as Facilitators of Autoimmune Disease: Review, Model and Novel Predictions. Int. J. Mol. Sci. 2020, 21, 4645. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.M.; Majidi, J.; Baradaran, B.; Yousefi, M. Toll-Like Receptors in the Pathogenesis of Autoimmune Diseases. Adv. Pharm. Bull. 2015, 5, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Dabbagh, K.; Lewis, D.B. Toll-like receptors and T-helper-1/T-helper-2 responses. Curr. Opin. Infect. Dis. 2003, 16, 199–204. [Google Scholar] [CrossRef]
- Tukhvatulin, A.I.; Gitlin, I.I.; Shcheblyakov, D.V.; Artemicheva, N.M.; Burdelya, L.G.; Shmarov, M.M.; Naroditsky, B.S.; Gudkov, A.V.; Gintsburg, A.L.; Logunov, D.Y. Combined Stimulation of Toll-Like Receptor 5 and NOD1 Strongly Potentiates Activity of NF-kβ, Resulting in Enhanced Innate Immune Reactions and Resistance to Salmonella enterica Serovar Typhimurium Infection. Infect. Immun. 2013, 81, 3855–3864. [Google Scholar] [CrossRef][Green Version]
- Moreira, L.O.; Zamboni, D.S. NOD1 and NOD2 Signaling in Infection and Inflammation. Front. Immunol. 2012, 3, 328. [Google Scholar] [CrossRef]
- Di-Benedetto, P.; Ruscitti, P.; Vadasz, Z.; Toubi, E.; Giacomelli, R. Macrophages with regulatory functions, a possible new therapeutic perspective in autoimmune diseases. Autoimmun. Rev. 2019, 18, 102369. [Google Scholar] [CrossRef]
- Locati, M.; Mantovani, A.; Sica, A. Macrophage activation and polarization as an adaptive component of innate immunity. In Advances in Immunology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 120, pp. 163–184. [Google Scholar]
- Wang, X.-F.; Wang, H.-S.; Zhang, F.; Guo, Q.; Wang, H.; Wang, K.-F.; Zhang, G.; Bu, X.-Z.; Cai, S.-H.; Du, J. Nodal promotes the generation of M2-like macrophages and downregulates the expression of IL-12. Eur. J. Immunol. 2013, 44, 173–183. [Google Scholar] [CrossRef]
- Giacomelli, R.; Ruscitti, P.; Alvaro, S.; Ciccia, F.; Liakouli, V.; Di-Benedetto, P.; Guggino, G.; Berardicurti, O.; Carubbi, F.; Triolo, G.; et al. IL-1β at the crossroad between rheumatoid arthritis and type 2 diabetes: May we kill two birds with one stone? Expert. Rev. Clin. Immunol. 2016, 12, 849–855. [Google Scholar] [CrossRef]
- Schett, G.; Elewaut, D.; McInnes, I.B.; Dayer, J.M.; Neurath, M.F. How cytokine networks fuel inflammation: Toward a cytokine-based disease taxonomy. Nat. Med. 2013, 19, 822–824. [Google Scholar] [CrossRef]
- Navegantes, K.C.; de-Souza Gomes, R.; Pereira, P.A.T.; Czaikoski, P.G.; Azevedo, C.H.M.; Monteiro, M.C. Immune modulation of some autoimmune diseases: The critical role of macrophages and neutrophils in the innate and adaptive immunity. J. Transl. Med. 2017, 15, 36. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Andreev, D.; Oeser, K.; Krljanac, B.; Hueber, A.; Kleyer, A.; Voehringer, D.; Schett, G.; Bozec, A. Th2 and eosinophil responses suppress inflammatory arthritis. Nat. Commun. 2016, 7, 11596. [Google Scholar] [CrossRef] [PubMed]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Hu, G.; Su, J.; Li, W.; Chen, Q.; Shou, P.; Xu, C.; Chen, X.; Huang, Y.; Zhu, Z.; et al. Mesenchymal stem cells: A new strategy for immunosuppression and tissue repair. Cell Res. 2010, 20, 510–518. [Google Scholar] [CrossRef]
- Lapuente, J.P.; Dos-Anjos, S.; Blázquez-Martínez, A. Intra-articular infiltration of adipose-derived stromal vascular fraction cells slows the clinical progression of moderate-severe knee osteoarthritis: Hypothesis on the regulatory role of intra-articular adipose tissue. J. Orthop. Surg. Res. 2020, 15, 137. [Google Scholar] [CrossRef]
- Jiang, X.X.; Zhang, Y.I.; Liu, B.; Zhang, S.X.; Wu, Y.; Yu, X.D.; Mao, N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 2005, 105, 4120–4126. [Google Scholar] [CrossRef]
- Ramasamy, R.; Fazekasova, H.; Lam, E.W.; Soeiro, I.; Lombardi, G.; Dazzi, F. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation 2007, 83, 71–76. [Google Scholar] [CrossRef]
- Iseri, K.; Iyoda, M.; Ohtaki, H.; Matsumoto, K.; Wada, Y.; Suzuki, T.; Yamamoto, Y.; Saito, T.; Hihara, K.; Tachibana, S.; et al. Therapeutic effects and mechanism of conditioned media from human mesenchymal stem cells on anti-GBM glomerulonephritis in WKY rats. Am. J. Physiol. Ren. Physiol. 2016, 310, F1182–F1191. [Google Scholar] [CrossRef]
- Ko, J.H.; Kim, H.J.; Jeong, H.J.; Lee, H.J.; Oh, J.Y. Mesenchymal Stem and Stromal Cells Harness Macrophage-Derived Amphiregulin to Maintain Tissue Homeostasis. Cell Rep. 2020, 30, 3806–3820.e6. [Google Scholar] [CrossRef]
- Dahbour, S.; Jamali, F.; Alhattab, D.; Al-Radaideh, A.; Ababneh, O.; Al-Ryalat, N.; Al Bdour, M.; Hourani, B.; Msallam, M.; Rasheed, M.; et al. Mesenchymal stem cells and conditioned media in the treatment of multiple sclerosis patients: Clinical, ophthalmological and radiological assessments of safety and efficacy. CNS Neurosci. Ther. 2017, 23, 866–874. [Google Scholar] [CrossRef]
- Laggner, M.; Gugerell, A.; Bachmann, C.; Hofbauer, H.; Vorstandlechner, V.; Seibold, M.; Gouya Lechner, G.; Peterbauer, A.; Madlener, S.; Demyanets, S.; et al. Reproducibility of GMP-compliant production of therapeutic stressed peripheral blood mononuclear cell-derived secretomes, a novel class of biological medicinal products. Stem Cell Res. Ther. 2020, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Risdall, R.J.; McKenna, R.W.; Nesbit, M.E.; Krivit, W.; Balfour, H.H., Jr.; Simmons, R.L.; Simmons, R.L.; Brunning, R.D. Virus-associated haemophagocytic syndrome: A benign histiocytic proliferation distinct from malignant histiocytosis. Cancer 1979, 44, 993–1002. [Google Scholar] [CrossRef]
- Guo, X.Z.J.; Thomas, P.G. New fronts emerge in the influenza cytokine storm. Semin. Immunopathol. 2017, 39, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.T.; Sheng, W.H.; Lin, B.H.; Lin, C.W.; Wang, J.T.; Chen, Y.C.; Chang, S.C. Causes, clinical symptoms, and outcomes of infectious diseases associated with hemophagocytic lymphohistiocytosis in Taiwanese adults. Journal of Microbiology. Immunol. Infect. 2011, 44, 191–197. [Google Scholar]
- Cascio, A.; Pernice, L.M.; Barberi, G.; Delfino, D.; Biondo, C.; Beninati, C.; Mancuso, G.; Rodriguez-Morales, A.J.; Iaria, C. Secondary haemophagocytic lymphohistiocytosis in zoonoses. A systematic review. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 1324–1337. [Google Scholar]
- Singh, Z.N.; Rakheja, D.; Yadav, T.P.; Shome, D.K. Infection-associated haemophagocytosis: The tropical spectrum. Int. J. Lab. Hematol. 2005, 27, 312–315. [Google Scholar] [CrossRef]
- Teijaro, J.R. Cytokine storms in infection diseases. Semin. Immunopathol. 2017, 39, 501–503. [Google Scholar] [CrossRef]
- Davila, M.L.; Riviere, I.; Wang, X.; Bartido, S.; Park, J.; Curran, K.; Chung, S.S.; Stefanski, J.; Borquez-Ojeda, O.; Olszewska, M.; et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 2014, 6, 224. [Google Scholar] [CrossRef]
- Siddiqi, H.K.; Mehra, M.R. COVID-19 illness in native and immunosuppressed states: A clinical–therapeutic staging proposal. J. Heart Lung Transplant. 2020, 39, 405–407. [Google Scholar] [CrossRef]
- Hauner, H.; Schmid, P.; Pfeiffer, E.F. Glucocorticoids and Insulin Promote the Differentiation of Human Adipocyte Precursor Cells into Fat Cells. J. Clin. Endocrinol. Metab. 1987, 64, 832–835. [Google Scholar] [CrossRef]
- Katz, A.J.; Llull, R.; Hedrick, M.H.; Futrell, J. Emerging Approaches to The Tissue Engineering of Fat. Clin. Plast. Surg. 1999, 26, 587–603. [Google Scholar] [CrossRef]
- Launay, D.; Dutoit-Lefevre, V.; Faure, E.; Robineau, O.; Hauspie, C.; Sobanski, V.; Hachulla, E.; Labalette, M.; Hatron, P.-Y.; Dubucquoi, S. Effect of In Vitro and In Vivo Anakinra on Cytokines Production in Schnitzler Syndrome. PLoS ONE 2013, 8, e59327. [Google Scholar] [CrossRef] [PubMed]
- Park, E.K.; Jung, H.S.; Yang, H.I.; Yoo, M.C.; Kim, C.; Kim, K.S. Optimized THP-1 differentiation is required for the detection of responses to weak stimuli. Inflamm. Res. 2007, 56, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sugiyama, K.; Inamura, M.; Tanaka, S.; Onda, K.; Yin, H.; Hirano, T. Effects of insulin on pharmacodynamics of immunosuppressive drugs against mitogen-activated human peripheral blood mononuclear cells. Immunopharmacol. Immunotoxicol. 2016, 38, 372–378. [Google Scholar] [CrossRef]
- Kristiansen, M.; Graversen, J.H.; Jacobsen, C.; Sonne, O.; Hoffman, H.J.; Law, S.A.; Moestrup, S.K. Identification of the haemoglobin scavenger receptor. Nature 2001, 409, 198–201. [Google Scholar] [CrossRef]
- Fujisaka, S.; Usui, I.; Bukhari, A.; Ikutani, M.; Oya, T.; Kanatani, Y.; Tsuneyama, K.; Nagai, Y.; Takatsu, K.; Urakaze, M.; et al. Regulatory Mechanisms for Adipose Tissue M1 and M2 Macrophages in Diet-Induced Obese Mice. Diabetes 2009, 58, 2574–2582. [Google Scholar] [CrossRef]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [CrossRef]
- Choudhery, M.S.; Badowski, M.; Muise, A.; Pierce, J.; Harris, D.T. Subcutaneous Adipose Tissue-Derived Stem Cell Utility Is Independent of Anatomical Harvest Site. Biores. Open Access. 2015, 4, 131–145. [Google Scholar] [CrossRef]
- West, C.C.; Hardy, W.R.; Murray, I.R.; James, A.W.; Corselli, M.; Pang, S.; Black, C.; Lobo, S.E.; Sukhija, K.; Liang, P.; et al. Prospective purification of perivascular presumptive perivascular mesenchymal stem cells from human adipose tissue: Process optimization and cell population metrics across a large cohort of diverse demographics. Stem Cell Res Ther. 2016, 7, 47. [Google Scholar] [CrossRef]
- Arnalich-Montiel, F.; Pastor, S.; Blazquez-Martinez, A.; Fernandez-Delgado, J.; Nistal, M.; Alio, J.L.; De Miguel, M.P. Adipose-Derived Stem Cells Are a Source for Cell Therapy of the Corneal Stroma. Stem Cells 2007, 26, 570–579. [Google Scholar] [CrossRef]
- Nasef, A.; Ashammakhi, N.; Fouillard, L. Immunomodulatory effect of mesenchymal stromal cells: Possible mechanisms. Regen. Med. 2008, 3, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Moll, G.; Alm, J.J.; Davies, L.C.; von Bahr, L.; Heldring, N.; Stenbeck-Funke, L.; Hamad, O.A.; Hinsch, R.; Ignatowicz, L.; Locke, M.; et al. Do cryopreserved mesenchymal stromal cells display impaired immunomodulatory and therapeutic properties? Stem Cells 2014, 32, 2430–2442. [Google Scholar] [CrossRef] [PubMed]
- Stab, B.R., 2nd; Martinez, L.; Grismaldo, A.; Lerma, A.; Gutiérrez, M.L.; Barrera, L.A.; Sutachan, J.J.; Albarracín, S.L. Mitochondrial Functional Changes Characterization in Young and Senescent Human Adipose Derived MSCs. Front. Aging Neurosci. 2016, 8, 299. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.; Wiley, C.D.; Velarde, M.C. Mitochondrial effectors of cellular senescence: Beyond the free radical theory of aging. Aging Cell 2014, 14, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sareen, N.; Sequiera, G.L.; Chaudhary, R.; Abu-El-Rub, E.; Chowdhury, S.R.; Sharma, V.; Surendran, A.; Moudgil, M.; Fernyhough, P.; Ravandi, A.; et al. Early passaging of mesenchymal stem cells does not instigate significant modifications in their immunological behavior. Stem Cell Res. Ther. 2018, 9, 121. [Google Scholar] [CrossRef]
- Van Wilgenburg, B.; Browne, C.; Vowles, J.; Cowley, S.A. Efficient, long term production of monocyte-derived macrophages from human pluripotent stem cells under partly-defined and fully-defined conditions. PLoS ONE 2013, 8, e71098. [Google Scholar] [CrossRef]
- Delben, P.B.; Zomer, H.D.; da Silva, C.A.; Gomes, R.S.; Melo, F.R.; Dillenburg-Pilla, P.; Trentin, A.G. Human adipose-derived mesenchymal stromal cells from face and abdomen undergo replicative senescence and loss of genetic integrity after long-term culture. Exp. Cell Res. 2021, 406, 112740. [Google Scholar] [CrossRef]
- Maruri, A.; Cruzans, P.R.; Lorenzo, M.S.; Tello, M.F.; Teplitz, G.M.; Carou, M.C.; Lombardo, D.M. Embryotrophic effect of a short-term embryo coculture with bovine luteal cells. Theriogenology 2018, 119, 143–149. [Google Scholar] [CrossRef]
- Barros, M.H.M.; Hauck, F.; Dreyer, J.H.; Kempkes, B.; Niedobitek, G. Macrophage Polarisation: An Immunohistochemical Approach for Identifying M1 and M2 Macrophages. PLoS ONE 2013, 8, e80908. [Google Scholar] [CrossRef]
- Meital, L.T.; Coward, A.S.; Windsor, M.T.; Bailey, T.G.; Kuballa, A.; Russell, F.D. A simple and effective method for the isolation and culture of human monocytes from small volumes of peripheral blood. J. Immunol. Methods 2019, 472, 75–78. [Google Scholar] [CrossRef]
- Kim, J.; Hematti, P. Mesenchymal stem cell-educated macrophages: A novel type of alternatively activated macrophages. Exp. Hematol. 2009, 37, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Gribben, J.G.; Devereux, S.; Thomas, N.S.; Keim, M.; Jones, H.M.; Goldstone, A.H.; Linch, D.C. Development of antibodies to unprotected glycosylation sites on recombinant human GM-CSF. Lancet 1990, 335, 8687. [Google Scholar] [CrossRef]
- Opal, S.M.; DePalo, V.A. Anti-inflammatory cytokines. Chest 2000, 117, 4. [Google Scholar] [CrossRef] [PubMed]
- Parsons, A.M.; Ciombor, D.M.; Liu, P.Y.; Darling, E.M. Regenerative Potential and Inflammation-Induced Secretion Profile of Human Adipose-Derived Stromal Vascular Cells Are Influenced by Donor Variability and Prior Breast Cancer Diagnosis. Stem Cell Rev. Rep. 2018, 14, 546–557. [Google Scholar] [CrossRef]
- Oh, S.-J.; Choi, K.-U.; Choi, S.-W.; Kim, S.-D.; Kong, S.-K.; Lee, S.; Cho, K.-S. Comparative Analysis of Adipose-Derived Stromal Cells and Their Secretome for Auricular Cartilage Regeneration. Stem Cells Int. 2020, 2020, 8595940. [Google Scholar] [CrossRef]
- Ferreira, J.R.; Teixeira, G.Q.; Santos, S.G.; Barbosa, M.A.; Almeida-Porada, G.; Gonçalves, R.M. Mesenchymal stromal cell secretome: Influencing therapeutic potential by cellular pre-conditioning. Front. Immunol. 2018, 9, 2837. [Google Scholar] [CrossRef]
- Fang, H.; Judd, R.L. Adiponectin Regulation and Function. Compr. Physiol. 2018, 8, 1031–1063. [Google Scholar]
- Bosshart, H.; Heinzelmann, M. THP-1 cells as a model for human monocytes. Ann. Transl. Med. 2016, 4, 438. [Google Scholar] [CrossRef]
- Yang, N.; Sin, D.D.; Dorscheid, D.R. Various factors affect lipopolysaccharide sensitization in cell cultures. BioTechniques 2020, 69, 126–132. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Barnes, P.J.; Karin, M. Nuclear factor-kappaB: A pivotal transcription factor in chronic inf1ammatory diseases. N. Engl. J. Med. 1997, 336, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Zhang, L.; Zhao, X.; Xu, G.; Zhang, Y.; Roberts, A.I.; Zhao, R.C.; Shi, Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008, 2, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Amrani, Y.; Panettieri, R.A.; Ramos-Ramirez, P.; Schaafsma, D.; Kaczmarek, K.; Tliba, O. Important lessons learned from studies on the pharmacology of glucocorticoids in human airway smooth muscle cells: Too much of a good thing may be a problem. Pharmacol. Ther. 2020, 213, 107589. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, C.; Hu, F.; Wang, J.; Zhao, Q.; Gale, R.P.; Liang, Y. Impact of corticosteroid therapy on outcomes of persons with SARS-CoV-2, SARS-CoV, or MERS-CoV infection: A systematic review and meta-analysis. Leukemia 2020, 34, 1503–1511. [Google Scholar] [CrossRef]

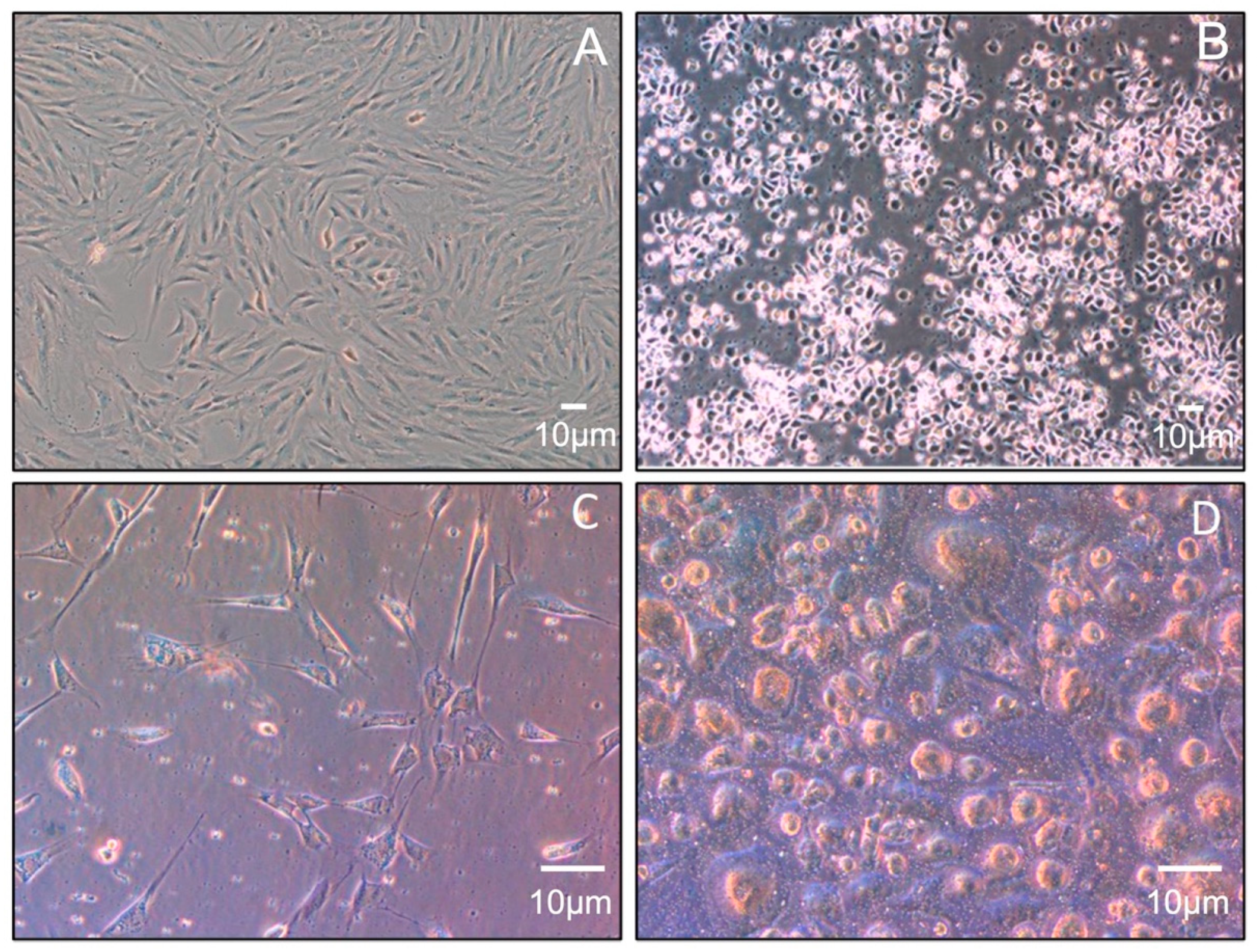
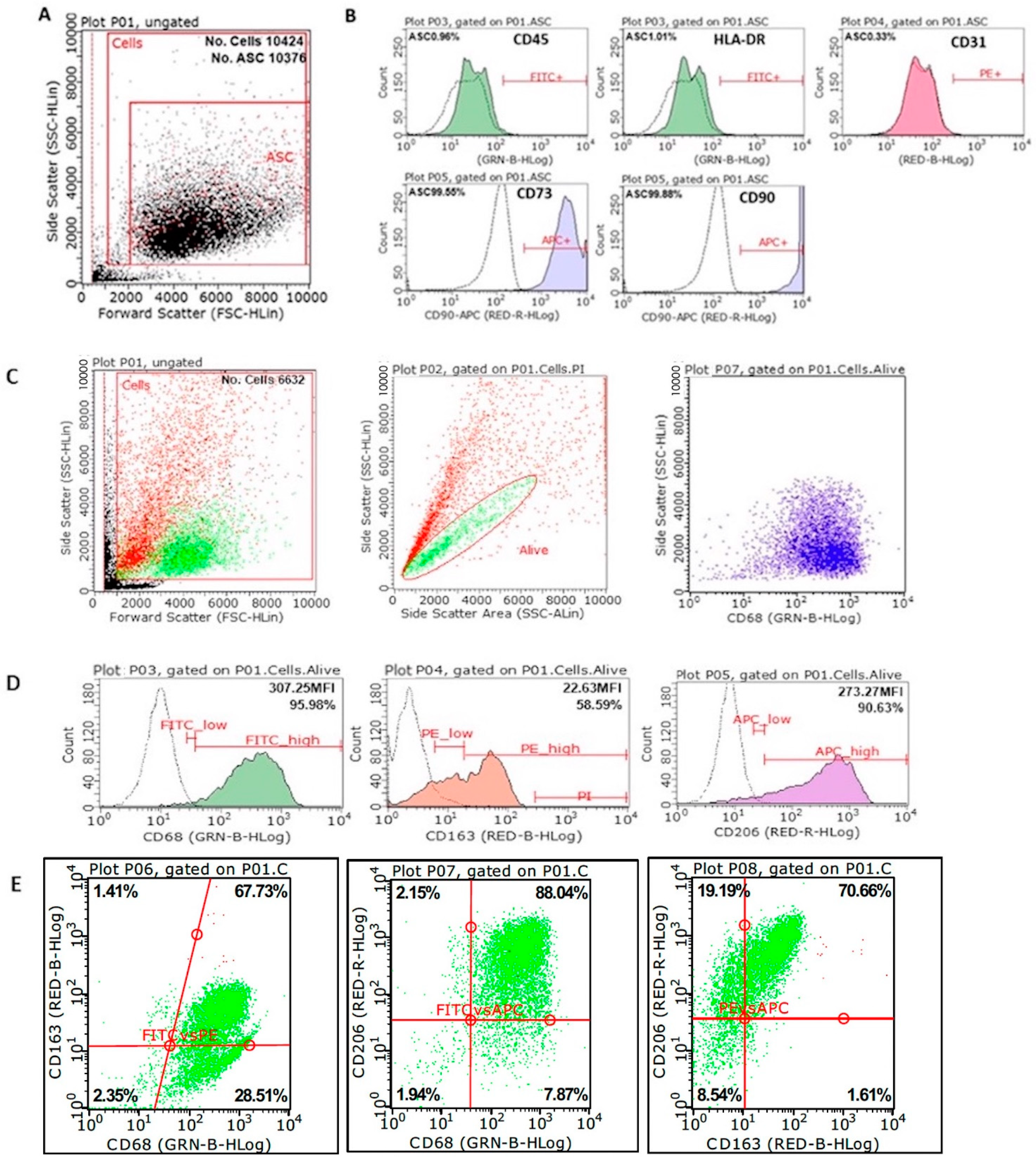
| Sample | Co-Cultivation Time | CD68 | CD163 | CD206 |
|---|---|---|---|---|
| 1 | 7 days | 94.48 | 42.04 | 73.21 |
| 14 days | 96.96 | 83.77 | 93.25 | |
| 28 days | 82.82 | 71.21 | 82.33 | |
| 2 | 7 days | 93.51 | 73.25 | 64.2 |
| 14 days | 86.8 | 65.22 | 57.63 | |
| 28 days | 75.9 | 50.17 | 76.77 | |
| 3 | 7days | 55.79 | 16.37 | 21.62 |
| 14days | 78.31 | 30.53 | 22.14 | |
| 28days | 63.69 | 46.51 | 35.06 |
| MIP-1α | IL-2 | IL-6 | TIMP-1 | IL-8 | IL-10 | |
| Monocyte control | 28.51 (SD 5.05) | <7.21 | 75.4 (SD 11.5) | 14786.45 (SD 989.56) | 28.26 (SD 15.71) | 10.03 (SD 1.67) |
| MSC Control | <2.234 | <7.21 | 24.56 (SD 9.68) | 26789.32 (SD 678.67) | 18.77 (SD 7.09) | 0.06 (SD 0.67) |
| Co-culture MSC-M2 | 46.07 (SD 9.89) | <7.21 | 222.61 (SD 35.8) | 67788.33 (SD 18776.9) | 26.71 (SD 9.91) | 1.36 (SD 1.12) |
| IL-12p70 | IL-1Ra | RANTES | GM-CSF | IL-18 | HGF | |
| Monocyte control | <4.71 | 113304 (SD 23456.1) | 10 (SD 1.46) | <13.79 | <12.21 | 199.23 (SD 36.98) |
| MSC Control | 1.08 (SD 0.78) | <35 | 0.06 (SD 0.09) | <13.79 | <3.42 | 11.03 (SD 2.81) |
| Co-culture MSC-M2 | <4.71 | 271130 (SD 27345.2) | 2.92 (SD 0.89) | <7.21 | <12.21 | 654.78 (SD 178.45) |
| MMP-3 | MCP-1 | BNGF | EGF | Adiponectin | TNF-α | |
| Monocyte control | 18.9 (SD 2.81) | 5260 (SD 1678.1) | <6.14 | <1.78 | <6.14 | <1.45 |
| MSC Control | 94.42 (SD 21.09) | 193.88 (SD 31.89) | 1.84 (SD 1.01) | <2.1 | 23.45 (SD 4.88) | 6.8 (SD 1.81) |
| Co-culture MSC-M2 | 320.01 (SD 56.67) | 1687 (SD 231.45) | <6.14 | <1.78 | 44.16 (SD 12.66) | 3.19 (SD 1.23) |
| MMP-1 | TRAIL | FGF-2 | PDGF-BB | VEGF-A | IGF-1 | |
| Monocyte control | 329.0 (SD 23.12) | 1.13 (SD 0.36) | 6.53 (SD 1.67) | 135.33 (SD 26.52) | 275.58 (SD 59.12) | 19.32 (SD 5.96) |
| MSC Control | 180.26 (SD 28.61) | 1.23 (SD 0.48) | 0.44 (SD 0.15) | 3.12 (SD 1.36) | 241.24 (SD 45.71) | 434.5 (SD 201.47) |
| Co-culture MSC-M2 | 1230.08 (SD 204.96) | 3.24 (SD 1.2) | <2.72 | 3.95 (SD 1.37) | 9.82 (SD 3.01) | 1667.4 (SD 102.78) |
| BMP-6 | IL-1β | IL-4 | TGF-β1 | TGF-β3 | VEGF-C | |
| Monocyte control | <6.14 | <2.16 | <10.49 | < 10.49 | <2.5 | <12.21 |
| MSC Control | <6.14 | <2.16 | <10.49 | 12.24 (SD 3.39) | <2.5 | <12.21 |
| Co-culture MSC-M2 | <6.14 | <2.16 | <10.49 | 450.85 (SD 105.1) | <2.5 | <12.21 |
 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapuente, J.P.; Blázquez-Martínez, A.; Marco-Brualla, J.; Gómez, G.; Desportes, P.; Sanz, J.; Fernández, P.; García-Gil, M.; Bermejo, F.; San Martín, J.V.; et al. Cytokine Profile and Anti-Inflammatory Activity of a Standardized Conditioned Medium Obtained by Coculture of Monocytes and Mesenchymal Stromal Cells (PRS CK STORM). Biomolecules 2022, 12, 534. https://doi.org/10.3390/biom12040534
Lapuente JP, Blázquez-Martínez A, Marco-Brualla J, Gómez G, Desportes P, Sanz J, Fernández P, García-Gil M, Bermejo F, San Martín JV, et al. Cytokine Profile and Anti-Inflammatory Activity of a Standardized Conditioned Medium Obtained by Coculture of Monocytes and Mesenchymal Stromal Cells (PRS CK STORM). Biomolecules. 2022; 12(4):534. https://doi.org/10.3390/biom12040534
Chicago/Turabian StyleLapuente, Juan Pedro, Alejandro Blázquez-Martínez, Joaquín Marco-Brualla, Gonzalo Gómez, Paula Desportes, Jara Sanz, Pablo Fernández, Mario García-Gil, Fernando Bermejo, Juan V. San Martín, and et al. 2022. "Cytokine Profile and Anti-Inflammatory Activity of a Standardized Conditioned Medium Obtained by Coculture of Monocytes and Mesenchymal Stromal Cells (PRS CK STORM)" Biomolecules 12, no. 4: 534. https://doi.org/10.3390/biom12040534
APA StyleLapuente, J. P., Blázquez-Martínez, A., Marco-Brualla, J., Gómez, G., Desportes, P., Sanz, J., Fernández, P., García-Gil, M., Bermejo, F., San Martín, J. V., Algaba, A., De Gregorio, J. C., Lapuente, D., De Gregorio, A., Lapuente, B., Andrés, M. d. l. V., & Anel, A. (2022). Cytokine Profile and Anti-Inflammatory Activity of a Standardized Conditioned Medium Obtained by Coculture of Monocytes and Mesenchymal Stromal Cells (PRS CK STORM). Biomolecules, 12(4), 534. https://doi.org/10.3390/biom12040534







