Campylobacter jejuni Serine Protease HtrA Induces Paracellular Transmigration of Microbiota across Polarized Intestinal Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cultivation of Bacterial Strains
2.2. Cultivation of Caco-2 Cells Used for Infection Assays
2.3. Transwell System and Measurement of Transepithelial Electrical Resistance (TER)
2.4. Scanning Electron Microscopy
2.5. Confocal Immunofluorescence Staining
2.6. SDS-PAGE and Immunoblot Analysis
2.7. Statistics
3. Results and Discussion
3.1. C. jejuni Colonization of Apical Caco-2 Cell Surfaces
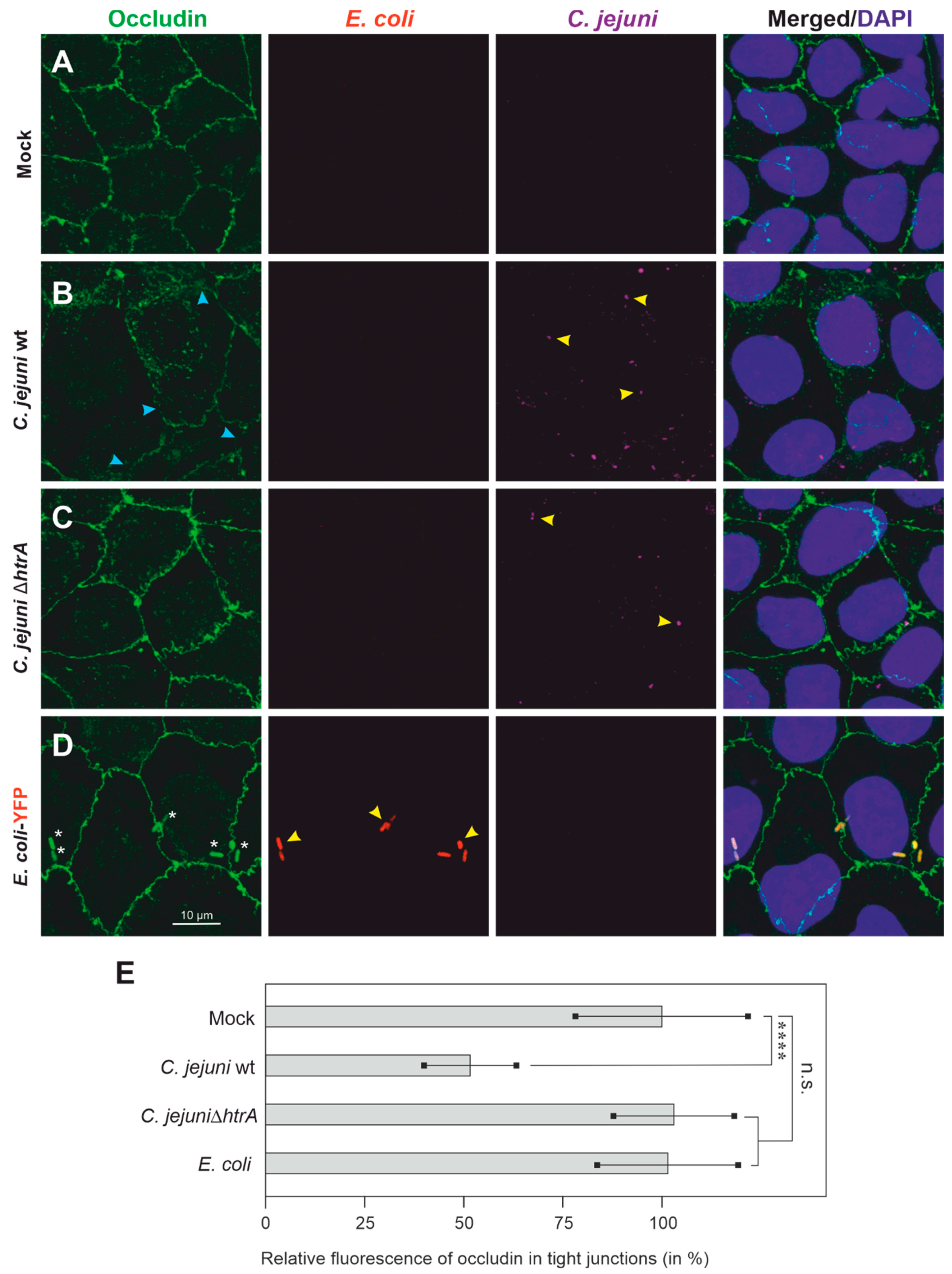
3.2. Colonization of Caco-2 Cells by C. jejuni and E. coli, and Impact on Tight Junctions
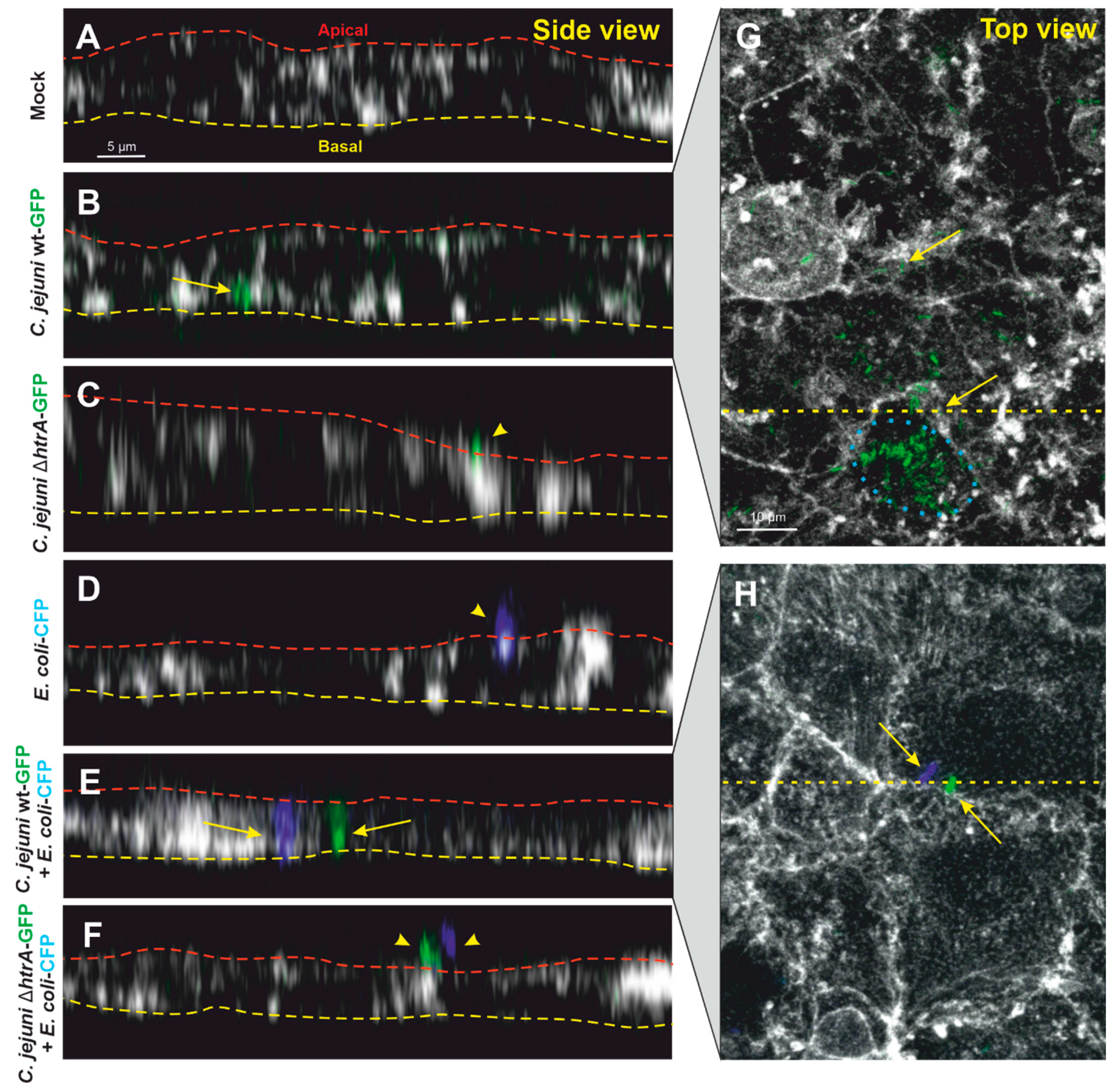
3.3. Confocal Microscopy of Co-Transmigrating C. jejuni and E. coli across Polarized Cells
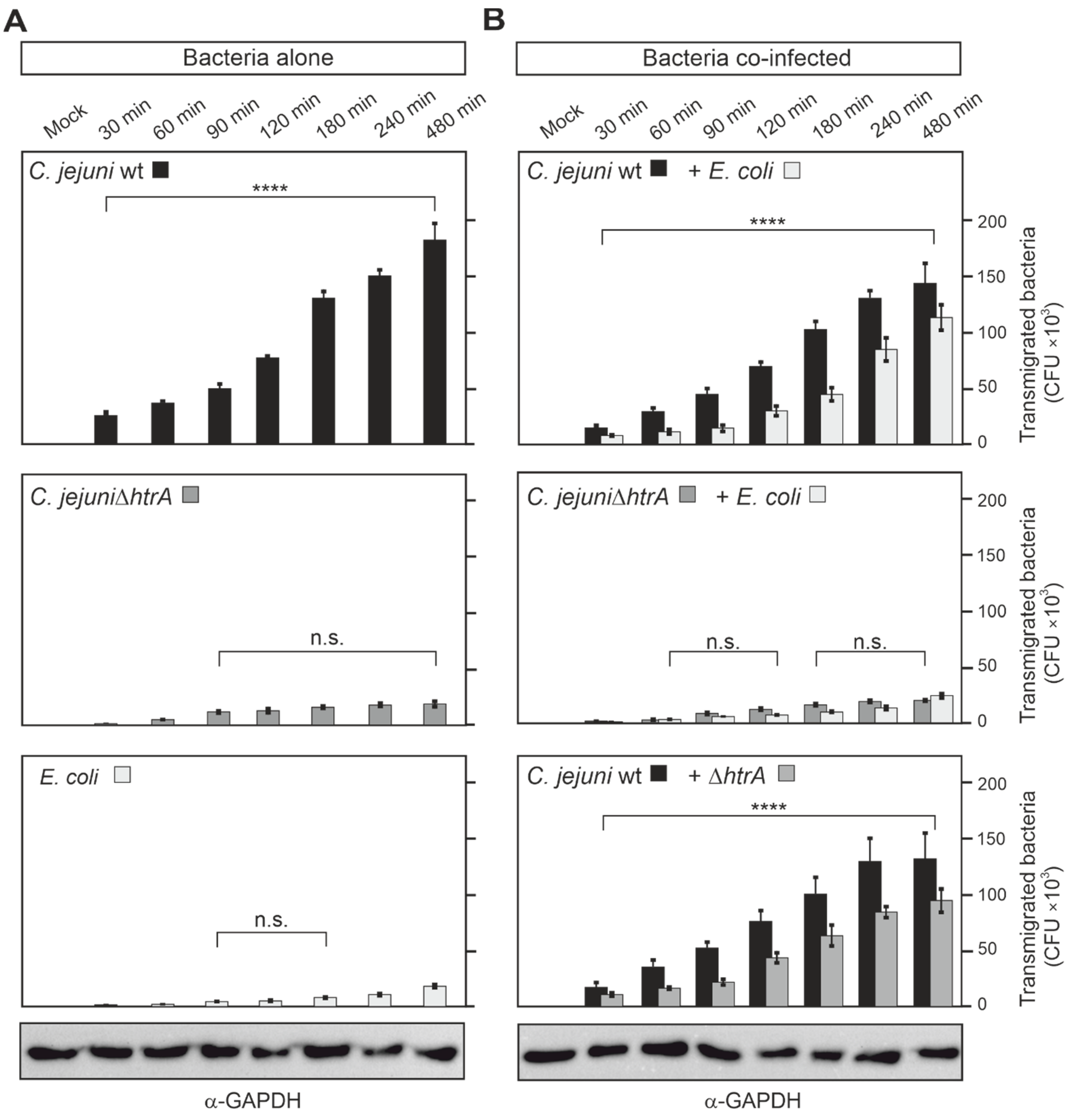
3.4. Quantification of Co-Transmigrating C. jejuni and E. coli across Polarized Caco-2 Cells
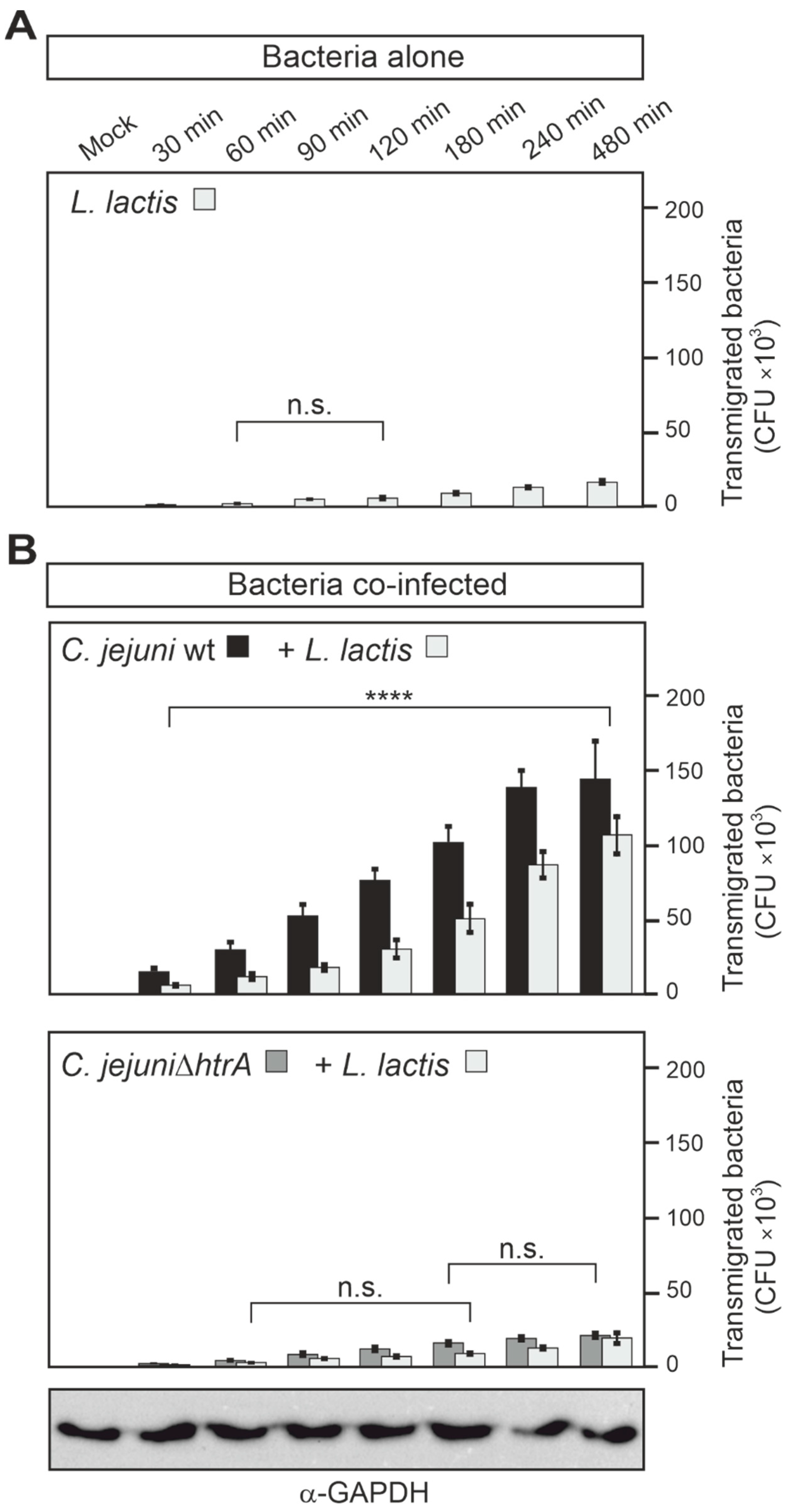
3.5. Epithelial Transmigration of Other Microbiota by HtrA-Expressing C. jejuni
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kirk, M.D.; Pires, S.M.; Black, R.E.; Caipo, M.; Crump, J.A.; Devleesschauwer, B.; Dopfer, D.; Fazil, A.; Fischer-Walker, C.L.; Hald, T.; et al. World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. PLoS Med. 2015, 12, e1001921. [Google Scholar] [CrossRef]
- Burnham, P.M.; Hendrixson, D.R. Campylobacter jejuni: Collective components promoting a successful enteric lifestyle. Nat. Rev. Microbiol. 2018, 16, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Heimesaat, M.M.; Backert, S.; Alter, T.; Bereswill, S. Human Campylobacteriosis-A Serious Infectious Threat in a One Health Perspective. Curr. Top. Microbiol. Immunol. 2021, 431, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Zbrun, M.V.; Rossler, E.; Romero-Scharpen, A.; Soto, L.P.; Berisvil, A.; Zimmermann, J.A.; Fusari, M.L.; Signorini, M.L.; Frizzo, L.S. Worldwide meta-analysis of the prevalence of Campylobacter in animal food products. Res. Vet. Sci. 2020, 132, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Dogan, O.B.; Aditya, A.; Ortuzar, J.; Clarke, J.; Wang, B. A systematic review and meta-analysis of the efficacy of processing stages and interventions for controlling Campylobacter contamination during broiler chicken processing. Compr. Rev. Food Sci. Food Saf. 2021, 21, 227–271. [Google Scholar] [CrossRef]
- Ang, C.W.; Laman, J.D.; Willison, H.J.; Wagner, E.R.; Endtz, H.P.; De Klerk, M.A.; Tio-Gillen, A.P.; Van den Braak, N.; Jacobs, B.C.; Van Doorn, P.A. Structure of Campylobacter jejuni lipopolysaccharides determines antiganglioside specificity and clinical features of Guillain-Barre, and Miller Fisher patients. Infect. Immun. 2002, 70, 1202–1208. [Google Scholar] [CrossRef][Green Version]
- Smith, J.L. Campylobacter jejuni infection during pregnancy: Long-term consequences of associated bacteremia, Guillain-Barre syndrome, and reactive arthritis. J. Food Prot. 2002, 65, 696–708. [Google Scholar] [CrossRef]
- Kalischuk, L.D.; Buret, A.G. A role for Campylobacter jejuni-induced enteritis in inflammatory bowel disease? Am. J. Physiol.-Gastrointest. Liver Physiol. 2010, 298, G1–G9. [Google Scholar] [CrossRef]
- Guan, Q.D. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef]
- O’Loughlin, J.L.; Samuelson, D.R.; Braundmeier-Fleming, A.G.; White, B.A.; Haldorson, G.J.; Stone, J.B.; Lessmann, J.J.; Eucker, T.P.; Konkel, M.E. The Intestinal Microbiota Influences Campylobacter jejuni Colonization and Extraintestinal Dissemination in Mice. Appl. Environ. Microbiol. 2015, 81, 4642–4650. [Google Scholar] [CrossRef]
- Sylte, M.J.; Shippy, D.C.; Bearson, B.L.; Bearson, S.M.D. Detection of Campylobacter jejuni liver dissemination in experimentally colonized turkey poults. Poult. Sci. 2020, 99, 4028–4033. [Google Scholar] [CrossRef] [PubMed]
- Martora, F.; Pagliuca, C.; Della Pepa, M.E.; Della Rocca, M.T.; Curto, S.; Iovene, M.R.; Vitiello, M. Campylobacter jejuni bacteremia in Italian pediatric patients with acute lymphoblastic leukemia: Report of two cases. New Microbiol. 2020, 43, 96–98. [Google Scholar] [PubMed]
- Tegtmeyer, N.; Sharafutdinov, I.; Harrer, A.; Esmaeili, D.S.; Linz, B.; Backert, S. Campylobacter Virulence Factors and Molecular Host-Pathogen Interactions. Curr. Top. Microbiol. Immunol. 2021, 431, 169–202. [Google Scholar] [CrossRef] [PubMed]
- Clausen, T.; Kaiser, M.; Huber, R.; Ehrmann, M. HTRA proteases: Regulated proteolysis in protein quality control. Nat. Rev. Mol. Cell Biol. 2011, 12, 152–162. [Google Scholar] [CrossRef]
- Backert, S.; Bernegger, S.; Skorko-Glonek, J.; Wessler, S. Extracellular HtrA serine proteases: An emerging new strategy in bacterial pathogenesis. Cell. Microbiol. 2018, 20, e12845. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, K.K. Structure and function of HtrA family proteins, the key players in protein quality control. J. Bioch. Mol. Biol. 2005, 38, 266–274. [Google Scholar] [CrossRef]
- Skorko-Glonek, J.; Zurawa-Janicka, D.; Koper, T.; Jarzab, M.; Figaj, D.; Glaza, P.; Lipinska, B. HtrA Protease Family as Therapeutic Targets. Curr. Pharm. Des. 2013, 19, 977–1009. [Google Scholar] [CrossRef]
- Neddermann, M.; Backert, S. Quantification of serine protease HtrA molecules secreted by the foodborne pathogen Campylobacter Jejuni. Gut Pathog. 2019, 11, 14. [Google Scholar] [CrossRef]
- Boehm, M.; Hoy, B.; Rohde, M.; Tegtmeyer, N.; Baek, K.T.; Oyarzabal, O.A.; Brondsted, L.; Wessler, S.; Backert, S. Rapid paracellular transmigration of Campylobacter jejuni across polarized epithelial cells without affecting TER: Role of proteolytic-active HtrA cleaving E-cadherin but not fibronectin. Gut Pathog. 2012, 4, 3. [Google Scholar] [CrossRef]
- Hoy, B.; Geppert, T.; Boehm, M.; Reisen, F.; Plattner, P.; Gadermaier, G.; Sewald, N.; Ferreira, F.; Briza, P.; Schneider, G.; et al. Distinct roles of secreted HtrA proteases from gram-negative pathogens in cleaving the junctional protein and tumor suppressor E-cadherin. J. Biol. Chem. 2012, 287, 10115–10120. [Google Scholar] [CrossRef]
- Sharafutdinov, I.; Esmaeili, D.S.; Harrer, A.; Tegtmeyer, N.; Sticht, H.; Backert, S. Campylobacter jejuni Serine Protease HtrA Cleaves the Tight Junction Component Claudin-8. Front. Cell. Infect. Microbiol. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Harrer, A.; Bucker, R.; Boehm, M.; Zarzecka, U.; Tegtmeyer, N.; Sticht, H.; Schulzke, J.D.; Backert, S. Campylobacter jejuni enters gut epithelial cells and impairs intestinal barrier function through cleavage of occludin by serine protease HtrA. Gut Pathog. 2019, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Heimesaat, M.M.; Fischer, A.; Alutis, M.; Grundmann, U.; Boehm, M.; Tegtmeyer, N.; Gobel, U.B.; Kuhl, A.A.; Bereswill, S.; Backert, S. The impact of serine protease HtrA in apoptosis, intestinal immune responses and extra-intestinal histopathology during Campylobacter jejuni infection of infant mice. Gut Pathog. 2014, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Heimesaat, M.M.; Alutis, M.; Grundmann, U.; Fischer, A.; Tegtmeyer, N.; Bohm, M.; Kuhl, A.A.; Gobel, U.B.; Backert, S.; Bereswill, S. The role of serine protease HtrA in acute ulcerative enterocolitis and extra-intestinal immune responses during Campylobacter jejuni infection of gnotobiotic IL-10 deficient mice. Front. Cell. Infect. Microbiol. 2014, 4, 77. [Google Scholar] [CrossRef]
- Boehm, M.; Simson, D.; Escher, U.; Schmidt, A.-M.; Bereswill, S.; Tegtmeyer, N.; Backert, S.; Heimesaat, M.M. Function of Serine Protease HtrA in the Lifecycle of the Foodborne Pathogen Campylobacter jejuni. Eur. J. Microbiol. Immunol. 2018, 8, 70–77. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Escher, U.; Mousavi, S.; Boehm, M.; Backert, S.; Bereswill, S.; Heimesaat, M.M. Protease Activity of Campylobacter jejuni HtrA Modulates Distinct Intestinal and Systemic Immune Responses in Infected Secondary Abiotic IL-10 Deficient Mice. Front. Cell. Infect. Microbiol. 2019, 9, 79. [Google Scholar] [CrossRef]
- Haag, L.M.; Fischer, A.; Otto, B.; Plickert, R.; Kuhl, A.A.; Gobel, U.B.; Bereswill, S.; Heimesaat, M.M. Intestinal Microbiota Shifts towards Elevated Commensal Escherichia coli Loads Abrogate Colonization Resistance against Campylobacter jejuni in Mice. PLoS ONE 2012, 7, e35988. [Google Scholar] [CrossRef]
- Aldars-Garcia, L.; Marin, A.C.; Chaparro, M.; Gisbert, J.P. The Interplay between Immune System and Microbiota in Inflammatory Bowel Disease: A Narrative Review. Int. J. Mol. Sci. 2021, 22, 3076. [Google Scholar] [CrossRef]
- Ahlawat, S.; Kumar, P.; Mohan, H.; Goyal, S.; Sharma, K.K. Inflammatory bowel disease: Tri-directional relationship between microbiota, immune system and intestinal epithelium. Crit. Rev. Microbiol. 2021, 47, 254–273. [Google Scholar] [CrossRef]
- Mukhopadhya, I.; Hansen, R.; El-Omar, E.M.; Hold, G.L. IBD-what role do Proteobacteria play? Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 219–230. [Google Scholar] [CrossRef]
- Nielsen, H.L.; Dalager-Pedersen, M.; Nielsen, H. Risk of inflammatory bowel disease after Campylobacter jejuni and Campylobacter concisus infection: A population-based cohort study. Scand. J. Gastroenterol. 2019, 54, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Mirsepasi-Lauridsen, H.C.; Vallance, B.A.; Krogfelt, K.A.; Petersen, A.M. Escherichia coli Pathobionts Associated with Inflammatory Bowel Disease. Clin. Microbiol. Rev. 2019, 32, e00060-18. [Google Scholar] [CrossRef] [PubMed]
- Kalischuk, L.D.; Inglis, G.D.; Buret, A.G. Campylobacter jejuni induces transcellular translocation of commensal bacteria via lipid rafts. Gut Pathog. 2009, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Lamb-Rosteski, J.M.; Kalischuk, L.D.; Inglis, G.D.; Buret, A.G. Epidermal growth factor inhibits Campylobacter jejuni-induced claudin-4 disruption, loss of epithelial barrier function, and Escherichia coli translocation. Infect. Immun. 2008, 76, 3390–3398. [Google Scholar] [CrossRef]
- Boehm, M.; Lind, J.; Backert, S.; Tegtmeyer, N. Campylobacter jejuni serine protease HtrA plays an important role in heat tolerance, oxygen resistance, host cell adhesion, invasion, and transmigration. Eur. J. Microbiol. Immunol. 2015, 5, 68–80. [Google Scholar] [CrossRef]
- Oyarzabal, O.A.; Backert, S.; Nagaraj, M.; Miller, R.S.; Hussain, S.K.; Oyarzabal, E.A. Efficacy of supplemented buffered peptone water for the isolation of Campylobacter jejuni and C. coli from broiler retail products. J. Microbiol. Methods 2007, 69, 129–136. [Google Scholar] [CrossRef]
- Backert, S.; Hofreuter, D. Molecular methods to investigate adhesion, transmigration, invasion and intracellular survival of the foodborne pathogen Campylobacter Jejuni. J. Microbiol. Methods 2013, 95, 8–23. [Google Scholar] [CrossRef]
- Krause-Gruszczynska, M.; Boehm, M.; Rohde, M.; Tegtmeyer, N.; Takahashi, S.; Buday, L.; Oyarzabal, O.A.; Backert, S. The signaling pathway of Campylobacter jejuni-induced Cdc42 activation: Role of fibronectin, integrin beta1, tyrosine kinases and guanine exchange factor Vav2. Cell Commun. Signal. 2011, 9, 1–18. [Google Scholar] [CrossRef]
- Krause-Gruszczynska, M.; Rohde, M.; Hartig, R.; Genth, H.; Schmidt, G.; Keo, T.; König, W.; Miller, W.G.; Konkel, M.E.; Backert, S. Role of the small Rho GTPases Rac1 and Cdc42 in host cell invasion of Campylobacter jejuni. Cell. Microbiol. 2007, 9, 2431–2444. [Google Scholar] [CrossRef]
- Miller, W.G.; Bates, A.H.; Horn, S.T.; Brandl, M.T.; Wachtel, M.R.; Mandrell, R.E. Detection on surfaces and in Caco-2 cells of Campylobacter jejuni cells transformed with new gfp, yfp, and cfp marker plasmids. Appl. Environ. Microbiol. 2000, 66, 5426–5436. [Google Scholar] [CrossRef]
- Hartung, M.L.; Gruber, D.C.; Koch, K.N.; Gruter, L.; Rehrauer, H.; Tegtmeyer, N.; Backert, S.; Muller, A.H. pylori-Induced DNA Strand Breaks Are Introduced by Nucleotide Excision Repair Endonucleases and Promote NF-kappaB Target Gene Expression. Cell Rep. 2015, 13, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Krause-Gruszczynska, M.; van Alphen, L.B.; Oyarzabal, O.A.; Alter, T.; Hanel, I.; Schliephake, A.; Konig, W.; van Putten, J.P.; Konkel, M.E.; Backert, S. Expression patterns and role of the CadF protein in Campylobacter jejuni and Campylobacter coli. FEMS Microbiol. Lett. 2007, 274, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Moese, S.; Selbach, M.; Zimny-Arndt, U.; Jungblut, P.R.; Meyer, T.F.; Backert, S. Identification of a tyrosine-phosphorylated 35 kDa carboxy-terminal fragment (p35CagA) of the Helicobacter pylori CagA protein in phagocytic cells: Processing or breakage? Proteomics 2001, 1, 618–629. [Google Scholar] [CrossRef]
- Vogelmann, R.; Amieva, M.R.; Falkow, S.; Nelson, W.J. Breaking into the epithelial apical-junctional complex--news from pathogen hackers. Curr. Opin. Cell. Biol. 2004, 16, 86–93. [Google Scholar] [CrossRef]
- Backert, S.; Boehm, M.; Wessler, S.; Tegtmeyer, N. Transmigration route of Campylobacter jejuni across polarized intestinal epithelial cells: Paracellular, transcellular or both? Cell. Commun. Signal. 2013, 11, 72. [Google Scholar] [CrossRef]
- Cróinín, T.Ó.; Backert, S. Host epithelial cell invasion by Campylobacter jejuni: Trigger or zipper mechanism? Front. Cell Infect. Microbiol. 2012, 2, 25. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharafutdinov, I.; Tegtmeyer, N.; Müsken, M.; Backert, S. Campylobacter jejuni Serine Protease HtrA Induces Paracellular Transmigration of Microbiota across Polarized Intestinal Epithelial Cells. Biomolecules 2022, 12, 521. https://doi.org/10.3390/biom12040521
Sharafutdinov I, Tegtmeyer N, Müsken M, Backert S. Campylobacter jejuni Serine Protease HtrA Induces Paracellular Transmigration of Microbiota across Polarized Intestinal Epithelial Cells. Biomolecules. 2022; 12(4):521. https://doi.org/10.3390/biom12040521
Chicago/Turabian StyleSharafutdinov, Irshad, Nicole Tegtmeyer, Mathias Müsken, and Steffen Backert. 2022. "Campylobacter jejuni Serine Protease HtrA Induces Paracellular Transmigration of Microbiota across Polarized Intestinal Epithelial Cells" Biomolecules 12, no. 4: 521. https://doi.org/10.3390/biom12040521
APA StyleSharafutdinov, I., Tegtmeyer, N., Müsken, M., & Backert, S. (2022). Campylobacter jejuni Serine Protease HtrA Induces Paracellular Transmigration of Microbiota across Polarized Intestinal Epithelial Cells. Biomolecules, 12(4), 521. https://doi.org/10.3390/biom12040521







