The Novel Analogue of Modafinil CE-158 Protects Social Memory against Interference and Triggers the Release of Dopamine in the Nucleus Accumbens of Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs and Treatment
2.3. Behavioral Testing in the Social Discrimination Task
2.4. In Vivo Microdialysis in Freely Moving Mice
2.5. Quantification of Dopamine in Microdialysates
2.6. Statistics
3. Results
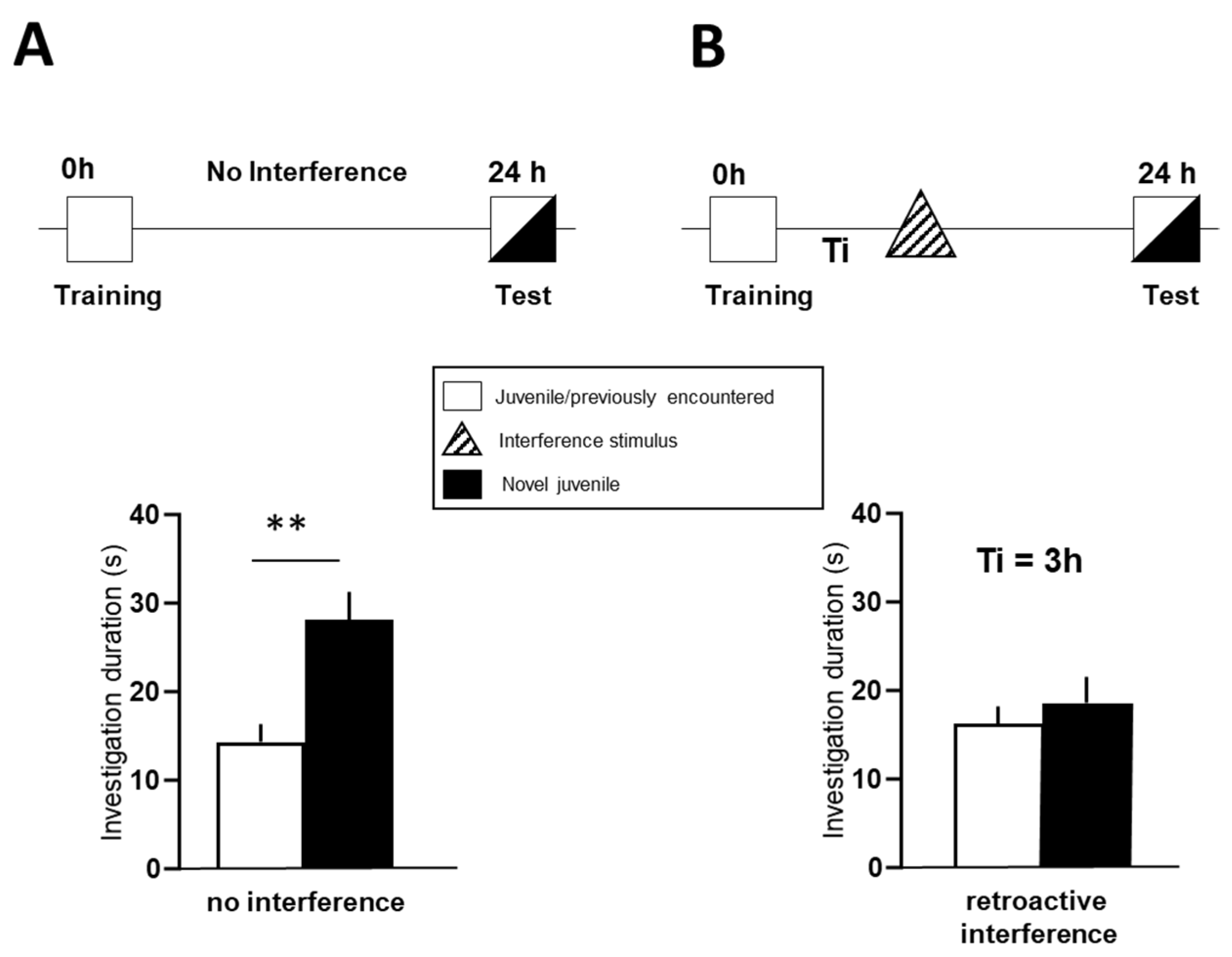
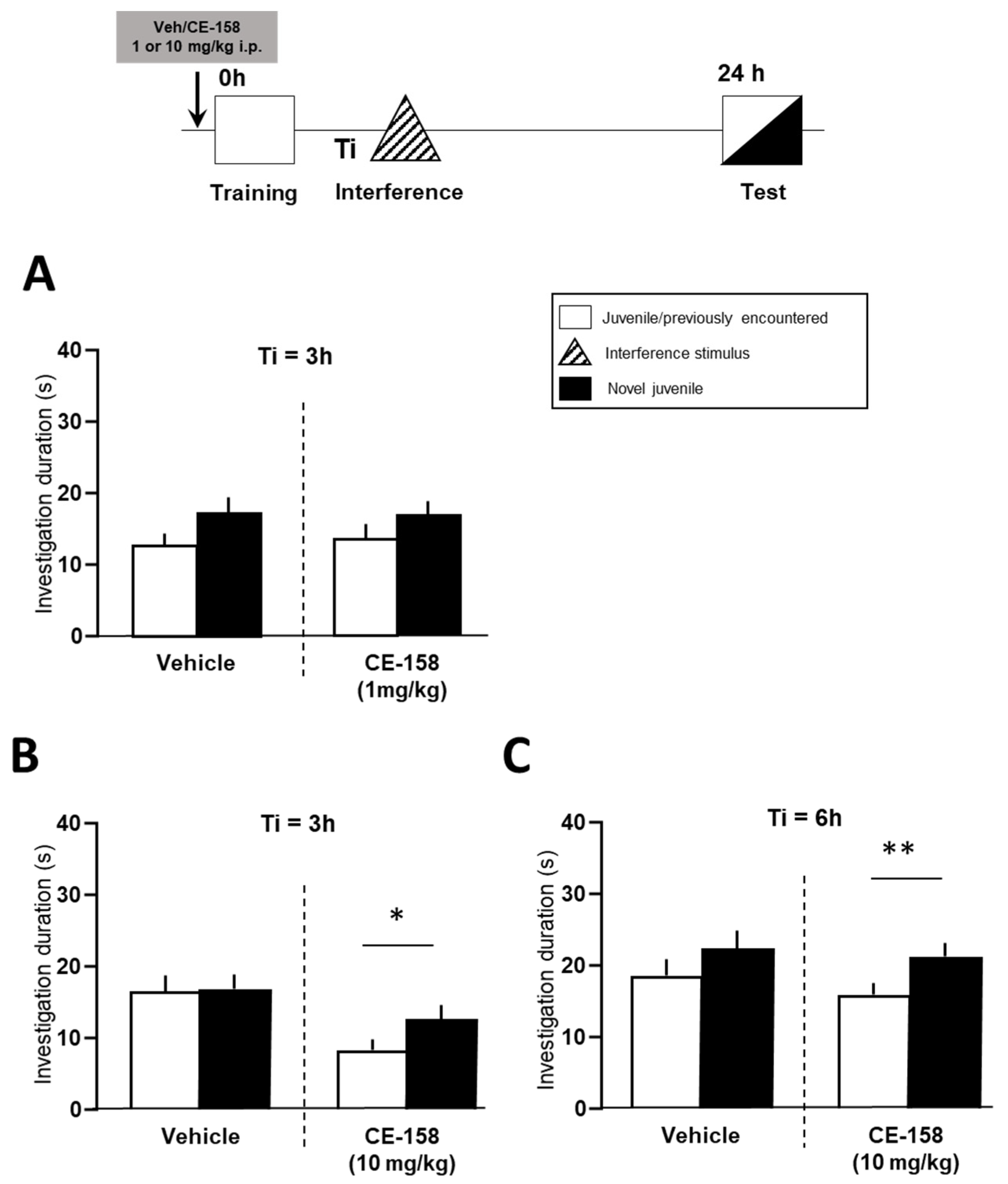
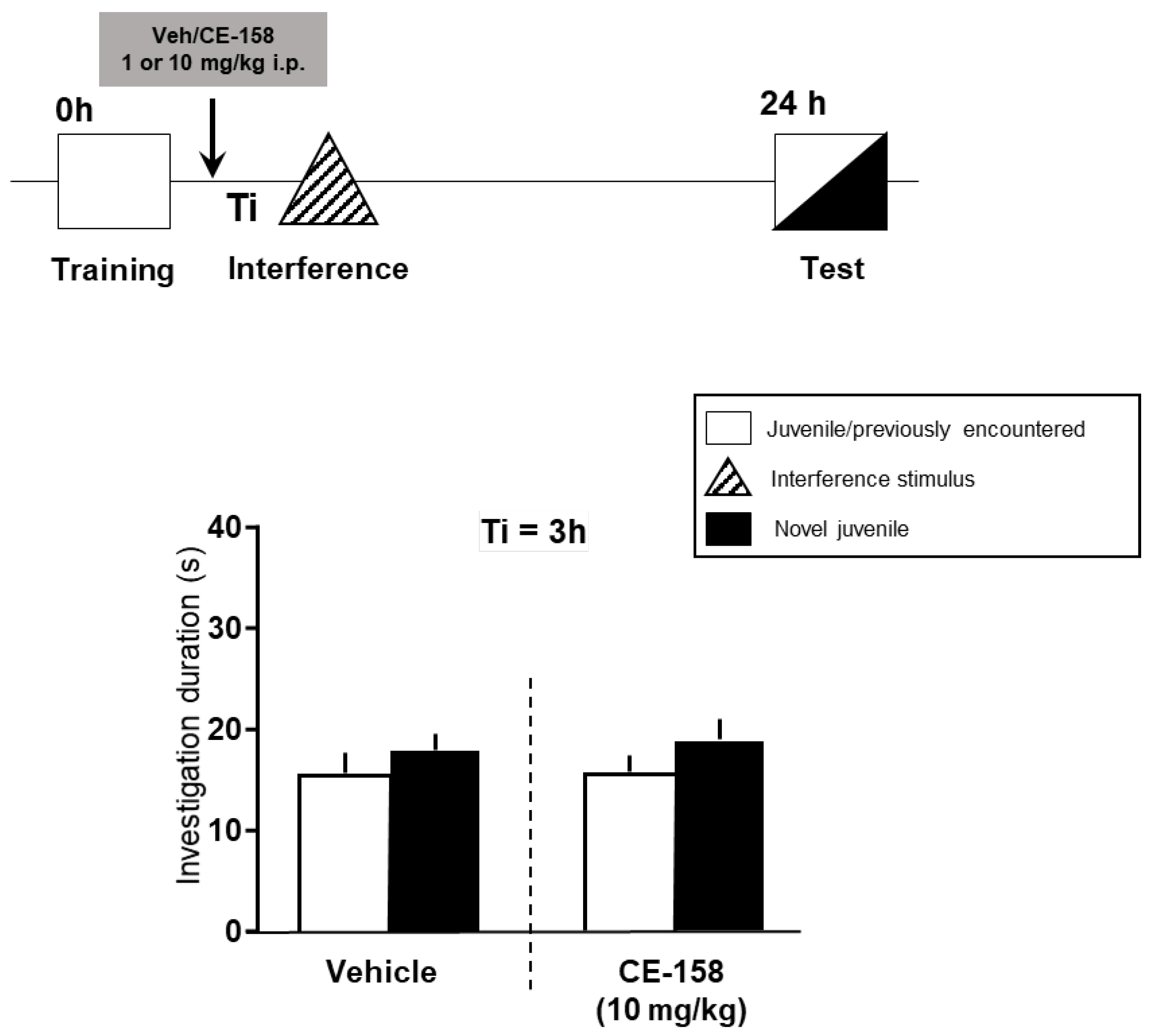
3.1. Effect of CE-158 on Retroactive Social Memory Interference
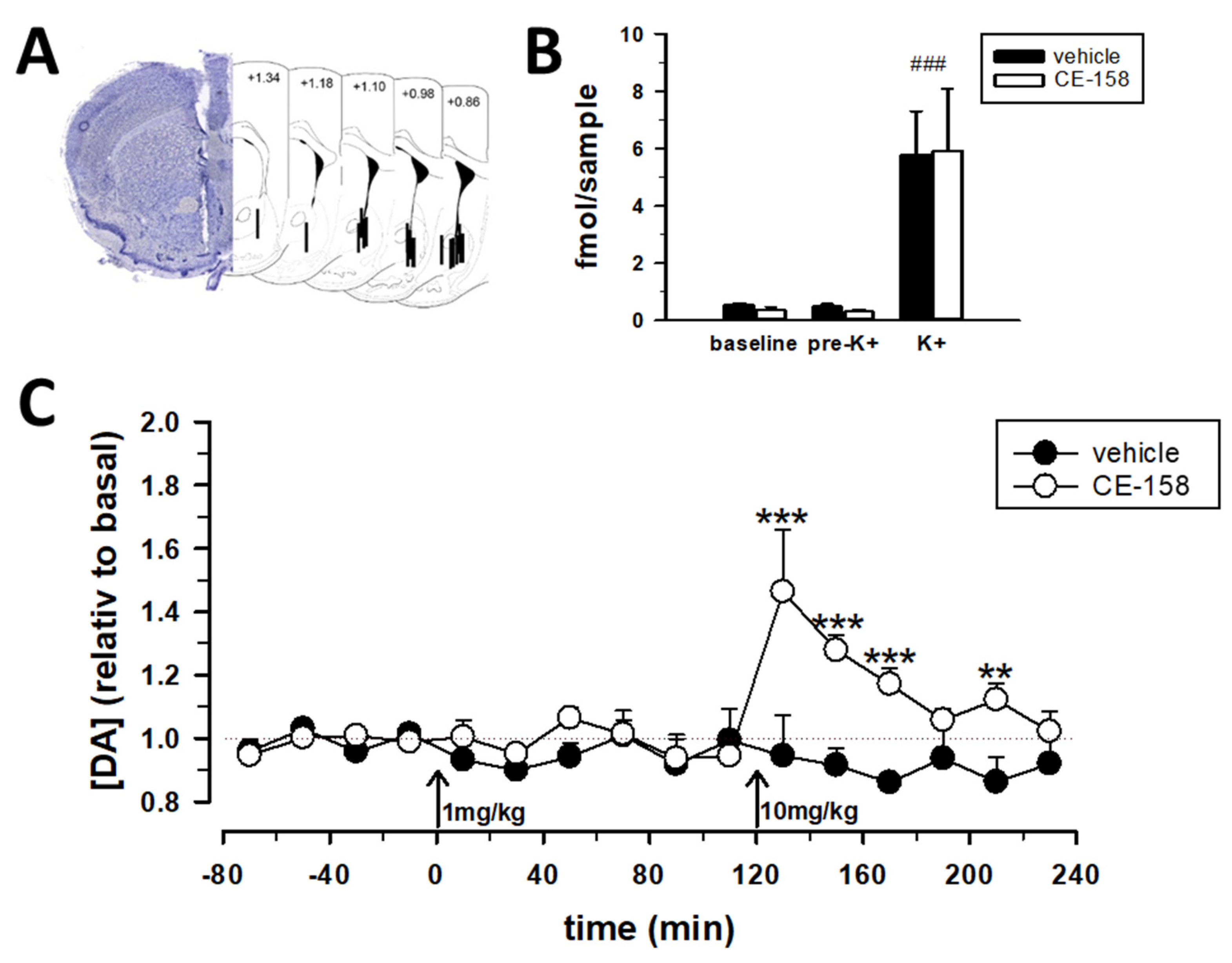
3.2. CE-158 Enhances Extracellular Dopamine Levels in the Nucleus Accumbens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, M.W.; Aggleton, J.P. Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nat. Rev. Neurosci. 2001, 2, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Steckler, T.; Drinkenburg, W.H.; Sahgal, A.; Aggleton, J.P. Recognition memory in rats—I. Concepts and classification. Prog. Neurobiol. 1998, 54, 289–311. [Google Scholar] [CrossRef]
- Camats-Perna, J.; Engelmann, M. Recognizing Others: Rodent’s Social Memories. Curr. Top. Behav. Neurosci. 2017, 30, 25–45. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, M.; Wotjak, C.T.; Landgraf, R. Social discrimination procedure: An alternative method to investigate juvenile recognition abilities in rats. Physiol. Behav. 1995, 58, 315–321. [Google Scholar] [CrossRef]
- Gabor, C.S.; Phan, A.; Clipperton-Allen, A.E.; Kavaliers, M.; Choleris, E. Interplay of oxytocin, vasopressin, and sex hormones in the regulation of social recognition. Behav. Neurosci. 2012, 126, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.A.; Huang, F.; Tsien, J.Z.; Wei, W. Social Recognition Memory Test in Rodents. Bio-Protocol 2016, 6, e1804. [Google Scholar] [CrossRef]
- Thor, D.H.; Holloway, W.R. Social Memory of the Male Laboratory Rat. J. Comp. Physiol. Psychol. 1982, 96, 1000–1006. [Google Scholar] [CrossRef]
- Richter, K.; Wolf, G.; Engelmann, M. Social recognition memory requires two stages of protein synthesis in mice. Learn. Mem. 2005, 12, 407–413. [Google Scholar] [CrossRef]
- Wanisch, K.; Wotjak, C.T.; Engelmann, M. Long-lasting second stage of recognition memory consolidation in mice. Behav. Brain Res. 2008, 186, 191–196. [Google Scholar] [CrossRef]
- Davis, R.L.; Zhong, Y. The Biology of Forgetting-A Perspective. Neuron 2017, 95, 490–503. [Google Scholar] [CrossRef]
- Hardt, O.; Nader, K.; Nadel, L. Decay happens: The role of active forgetting in memory. Trends Cogn. Sci. 2013, 17, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Sadeh, T.; Ozubko, J.D.; Winocur, G.; Moscovitch, M. How we forget may depend on how we remember. Trends Cogn. Sci. 2014, 18, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Crawford, L.; Loprinzi, P.D. Alzheimer’s Disease: Memory Interference and the Role of Exercise. In Alzheimer’s Disease; Wisniewski, T., Ed.; Codon Publications: Brisbane, Australia, 2019. [Google Scholar] [CrossRef]
- Crocco, E.; Curiel, R.E.; Acevedo, A.; Czaja, S.J.; Loewenstein, D.A. An evaluation of deficits in semantic cueing and proactive and retroactive interference as early features of Alzheimer’s disease. Am. J. Geriatr Psychiatry 2014, 22, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Dewar, M.; Pesallaccia, M.; Cowan, N.; Provinciali, L.; Della Sala, S. Insights into spared memory capacity in amnestic MCI and Alzheimer’s Disease via minimal interference. Brain Cogn. 2012, 78, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.V.; Bueno, O.F. Retroactive Interference: Forgetting as an Interruption of Memory Consolidation. Trends Psychol. 2017, 25, 1055–1067. [Google Scholar] [CrossRef]
- Müller, G.E.; Pilzecker, A. Experimentelle Beiträge zur Lehre vom Gedächtnis; Zeitschrift für Psychologie und Physiologie der Sinnesorgane EB; J.A. Barth: Leipzig, Germany, 1900; p. 300. [Google Scholar]
- Wixted, J.T. The psychology and neuroscience of forgetting. Annu. Rev. Psychol. 2004, 55, 235–269. [Google Scholar] [CrossRef]
- Camats-Perna, J.C.; Wotjak, C.T.; Stork, O.; Engelmann, M. Timing of presentation and nature of stimuli determine retroactive interference with social recognition memory in mice. Physiol. Behav. 2015, 143, 10–14. [Google Scholar] [CrossRef]
- Engelmann, M. Competition between two memory traces for long-term recognition memory. Neurobiol. Learn. Mem. 2009, 91, 58–65. [Google Scholar] [CrossRef]
- Deng, X.; Gu, L.; Sui, N.; Guo, J.; Liang, J. Parvalbumin interneuron in the ventral hippocampus functions as a discriminator in social memory. Proc. Natl. Acad. Sci. USA 2019, 116, 16583–16592. [Google Scholar] [CrossRef]
- Ploeger, G.E.; Willemen, A.P.; Cools, A.R. Role of the nucleus accumbens in social memory in rats. Brain Res. Bull. 1991, 26, 23–27. [Google Scholar] [CrossRef]
- Tzakis, N.; Holahan, M.R. Social Memory and the Role of the Hippocampal CA2 Region. Front. Behav. Neurosci. 2019, 13, 233. [Google Scholar] [CrossRef] [PubMed]
- Kerfoot, E.C.; Williams, C.L. Contributions of the Nucleus Accumbens Shell in Mediating the Enhancement in Memory Following Noradrenergic Activation of Either the Amygdala or Hippocampus. Front. Pharmacol. 2018, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Brog, J.S.; Salyapongse, A.; Deutch, A.Y.; Zahm, D.S. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: Immunohistochemical detection of retrogradely transported fluoro-gold. J. Comp. Neurol. 1993, 338, 255–278. [Google Scholar] [CrossRef] [PubMed]
- Voorn, P.; Jorritsma-Byham, B.; Van Dijk, C.; Buijs, R.M. The dopaminergic innervation of the ventral striatum in the rat: A light- and electron-microscopical study with antibodies against dopamine. J. Comp. Neurol 1986, 251, 84–99. [Google Scholar] [CrossRef] [PubMed]
- Saddoris, M.P.; Siletti, K.A.; Stansfield, K.J.; Bercum, M.F. Heterogeneous dopamine signals support distinct features of motivated actions: Implications for learning and addiction. Learn. Mem. 2018, 25, 416–424. [Google Scholar] [CrossRef]
- Schultz, W. Getting formal with dopamine and reward. Neuron 2002, 36, 241–263. [Google Scholar] [CrossRef]
- Wise, R.A. Brain reward circuitry: Insights from unsensed incentives. Neuron 2002, 36, 229–240. [Google Scholar] [CrossRef]
- Tsai, H.C.; Zhang, F.; Adamantidis, A.; Stuber, G.D.; Bonci, A.; de Lecea, L.; Deisseroth, K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 2009, 324, 1080–1084. [Google Scholar] [CrossRef]
- Camats-Perna, J.; Kalaba, P.; Ebner, K.; Sartori, S.B.; Vuyyuru, H.; Aher, N.Y.; Dragacevic, V.; Singewald, N.; Engelmann, M.; Lubec, G. Differential Effects of Novel Dopamine Reuptake Inhibitors on Interference With Long-Term Social Memory in Mice. Front. Behav. Neurosci. 2019, 13, 63. [Google Scholar] [CrossRef]
- Garrido Zinn, C.; Clairis, N.; Silva Cavalcante, L.E.; Furini, C.R.; de Carvalho Myskiw, J.; Izquierdo, I. Major neurotransmitter systems in dorsal hippocampus and basolateral amygdala control social recognition memory. Proc. Natl. Acad. Sci. USA 2016, 113, E4914–E4919. [Google Scholar] [CrossRef]
- Gonzalez, M.C.; Rossato, J.I.; Radiske, A.; Bevilaqua, L.R.M.; Cammarota, M. Dopamine controls whether new declarative information updates reactivated memories through reconsolidation. Proc. Natl. Acad. Sci. USA 2021, 118, e2025275118. [Google Scholar] [CrossRef] [PubMed]
- Hotte, M.; Naudon, L.; Jay, T.M. Modulation of recognition and temporal order memory retrieval by dopamine D1 receptor in rats. Neurobiol. Learn. Mem. 2005, 84, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.L.; Zitzman, D.L.; Smith, K.J.; Spear, L.P. Fast dopamine release events in the nucleus accumbens of early adolescent rats. Neuroscience 2011, 176, 296–307. [Google Scholar] [CrossRef]
- Kopec, A.M.; Smith, C.J.; Ayre, N.R.; Sweat, S.C.; Bilbo, S.D. Microglial dopamine receptor elimination defines sex-specific nucleus accumbens development and social behavior in adolescent rats. Nat. Commun. 2018, 9, 3769. [Google Scholar] [CrossRef]
- Kalaba, P.; Aher, N.Y.; Ilic, M.; Dragacevic, V.; Wieder, M.; Miklosi, A.G.; Zehl, M.; Wackerlig, J.; Roller, A.; Beryozkina, T.; et al. Heterocyclic Analogues of Modafinil as Novel, Atypical Dopamine Transporter Inhibitors. J. Med. Chem. 2017, 60, 9330–9348. [Google Scholar] [CrossRef]
- Kalaba, P.; Ilic, M.; Aher, N.Y.; Dragacevic, V.; Wieder, M.; Zehl, M.; Wackerlig, J.; Beyl, S.; Sartori, S.B.; Ebner, K.; et al. Structure-Activity Relationships of Novel Thiazole-Based Modafinil Analogues Acting at Monoamine Transporters. J. Med. Chem. 2020, 63, 391–417. [Google Scholar] [CrossRef]
- Aher, Y.D.; Subramaniyan, S.; Shanmugasundaram, B.; Sase, A.; Saroja, S.R.; Holy, M.; Hoger, H.; Beryozkina, T.; Sitte, H.H.; Leban, J.J.; et al. A Novel Heterocyclic Compound CE-104 Enhances Spatial Working Memory in the Radial Arm Maze in Rats and Modulates the Dopaminergic System. Front. Behav. Neurosci. 2016, 10, 20. [Google Scholar] [CrossRef][Green Version]
- Hussein, A.M.; Aher, Y.D.; Kalaba, P.; Aher, N.Y.; Dragacevic, V.; Radoman, B.; Ilic, M.; Leban, J.; Beryozkina, T.; Ahmed, A.; et al. A novel heterocyclic compound improves working memory in the radial arm maze and modulates the dopamine receptor D1R in frontal cortex of the Sprague-Dawley rat. Behav. Brain Res. 2017, 332, 308–315. [Google Scholar] [CrossRef]
- Kristofova, M.; Aher, Y.D.; Ilic, M.; Radoman, B.; Kalaba, P.; Dragacevic, V.; Aher, N.Y.; Leban, J.; Korz, V.; Zanon, L.; et al. A daily single dose of a novel modafinil analogue CE-123 improves memory acquisition and memory retrieval. Behav. Brain Res. 2018, 343, 83–94. [Google Scholar] [CrossRef]
- Saroja, S.R.; Aher, Y.D.; Kalaba, P.; Aher, N.Y.; Zehl, M.; Korz, V.; Subramaniyan, S.; Miklosi, A.G.; Zanon, L.; Neuhaus, W.; et al. A novel heterocyclic compound targeting the dopamine transporter improves performance in the radial arm maze and modulates dopamine receptors D1-D3. Behav. Brain Res. 2016, 312, 127–137. [Google Scholar] [CrossRef]
- Sase, A.; Aher, Y.D.; Saroja, S.R.; Ganesan, M.K.; Sase, S.; Holy, M.; Hoger, H.; Bakulev, V.; Ecker, G.F.; Langer, T.; et al. A heterocyclic compound CE-103 inhibits dopamine reuptake and modulates dopamine transporter and dopamine D1-D3 containing receptor complexes. Neuropharmacology 2016, 102, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Lubec, J.; Kalaba, P.; Hussein, A.M.; Feyissa, D.D.; Kotob, M.H.; Mahmmoud, R.R.; Wieder, O.; Garon, A.; Sagheddu, C.; Ilic, M.; et al. Reinstatement of synaptic plasticity in the aging brain through specific dopamine transporter inhibition. Mol. Psychiatry 2021, 26, 7076–7090. [Google Scholar] [CrossRef] [PubMed]
- Rotolo, R.A.; Kalaba, P.; Dragacevic, V.; Presby, R.E.; Neri, J.; Robertson, E.; Yang, J.H.; Correa, M.; Bakulev, V.; Volkova, N.N.; et al. Behavioral and dopamine transporter binding properties of the modafinil analog (S, S)-CE-158: Reversal of the motivational effects of tetrabenazine and enhancement of progressive ratio responding. Psychopharmacology 2020, 237, 3459–3470. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, L.A.; Grosenick, L.; Finkelstein, J.C.; Kauvar, I.V.; Fenno, L.E.; Adhikari, A.; Lammel, S.; Mirzabekov, J.J.; Airan, R.D.; Zalocusky, K.A.; et al. Natural neural projection dynamics underlying social behavior. Cell 2014, 157, 1535–1551. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, H.; Ramirez, S. Linking Social Cognition to Learning and Memory. J. Neurosci. 2020, 40, 8782–8798. [Google Scholar] [CrossRef]
- Tanimizu, T.; Kenney, J.W.; Okano, E.; Kadoma, K.; Frankland, P.W.; Kida, S. Functional Connectivity of Multiple Brain Regions Required for the Consolidation of Social Recognition Memory. J. Neurosci. 2017, 37, 4103–4116. [Google Scholar] [CrossRef]
- Engelmann, M.; Hadicke, J.; Noack, J. Testing declarative memory in laboratory rats and mice using the nonconditioned social discrimination procedure. Nat. Protoc. 2011, 6, 1152–1162. [Google Scholar] [CrossRef]
- Nikiforuk, A.; Kalaba, P.; Ilic, M.; Korz, V.; Dragacevic, V.; Wackerlig, J.; Langer, T.; Hoger, H.; Golebiowska, J.; Popik, P.; et al. A Novel Dopamine Transporter Inhibitor CE-123 Improves Cognitive Flexibility and Maintains Impulsivity in Healthy Male Rats. Front. Behav. Neurosci. 2017, 11, 222. [Google Scholar] [CrossRef]
- Rotolo, R.A.; Dragacevic, V.; Kalaba, P.; Urban, E.; Zehl, M.; Roller, A.; Wackerlig, J.; Langer, T.; Pistis, M.; De Luca, M.A.; et al. The Novel Atypical Dopamine Uptake Inhibitor (S)-CE-123 Partially Reverses the Effort-Related Effects of the Dopamine Depleting Agent Tetrabenazine and Increases Progressive Ratio Responding. Front. Pharmacol. 2019, 10, 682. [Google Scholar] [CrossRef]
- Sagheddu, C.; Pintori, N.; Kalaba, P.; Dragacevic, V.; Piras, G.; Lubec, J.; Simola, N.; De Luca, M.A.; Lubec, G.; Pistis, M. Neurophysiological and Neurochemical Effects of the Putative Cognitive Enhancer (S)-CE-123 on Mesocorticolimbic Dopamine System. Biomolecules 2020, 10, 779. [Google Scholar] [CrossRef]
- McGaugh, J.L.; Roozendaal, B. Drug enhancement of memory consolidation: Historical perspective and neurobiological implications. Psychopharmacology 2009, 202, 3–14. [Google Scholar] [CrossRef] [PubMed]
- van der Kooij, M.A.; Sandi, C. Social memories in rodents: Methods, mechanisms and modulation by stress. Neurosci. Biobehav. Rev. 2012, 36, 1763–1772. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, T.; Kitamura, T.; Roy, D.S.; Itohara, S.; Tonegawa, S. Ventral CA1 neurons store social memory. Science 2016, 353, 1536–1541. [Google Scholar] [CrossRef] [PubMed]
- Xing, B.; Mack, N.R.; Guo, K.M.; Zhang, Y.X.; Ramirez, B.; Yang, S.S.; Lin, L.; Wang, D.V.; Li, Y.C.; Gao, W.J. A Subpopulation of Prefrontal Cortical Neurons Is Required for Social Memory. Biol. Psychiatry 2021, 89, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Ryu, C.; Kim, S.; Jeong, S.J.; Koo, J.W.; Lee, Y.S.; Kim, S.J. Social isolation impairs the prefrontal-nucleus accumbens circuit subserving social recognition in mice. Cell Rep. 2021, 35, 109104. [Google Scholar] [CrossRef] [PubMed]
- Floresco, S.B. The nucleus accumbens: An interface between cognition, emotion, and action. Annu. Rev. Psychol. 2015, 66, 25–52. [Google Scholar] [CrossRef]
- Pennartz, C.M.; Berke, J.D.; Graybiel, A.M.; Ito, R.; Lansink, C.S.; van der Meer, M.; Redish, A.D.; Smith, K.S.; Voorn, P. Corticostriatal Interactions during Learning, Memory Processing, and Decision Making. J. Neurosci. 2009, 29, 12831–12838. [Google Scholar] [CrossRef]
- Setlow, B. The nucleus accumbens and learning and memory. J. Neurosci. Res. 1997, 49, 515–521. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebner, K.; Sartori, S.B.; Murau, R.; Kopel, F.; Kalaba, P.; Dragačević, V.; Leban, J.J.; Singewald, N.; Engelmann, M.; Lubec, G. The Novel Analogue of Modafinil CE-158 Protects Social Memory against Interference and Triggers the Release of Dopamine in the Nucleus Accumbens of Mice. Biomolecules 2022, 12, 506. https://doi.org/10.3390/biom12040506
Ebner K, Sartori SB, Murau R, Kopel F, Kalaba P, Dragačević V, Leban JJ, Singewald N, Engelmann M, Lubec G. The Novel Analogue of Modafinil CE-158 Protects Social Memory against Interference and Triggers the Release of Dopamine in the Nucleus Accumbens of Mice. Biomolecules. 2022; 12(4):506. https://doi.org/10.3390/biom12040506
Chicago/Turabian StyleEbner, Karl, Simone B. Sartori, Rita Murau, Fabian Kopel, Predrag Kalaba, Vladimir Dragačević, Johann J. Leban, Nicolas Singewald, Mario Engelmann, and Gert Lubec. 2022. "The Novel Analogue of Modafinil CE-158 Protects Social Memory against Interference and Triggers the Release of Dopamine in the Nucleus Accumbens of Mice" Biomolecules 12, no. 4: 506. https://doi.org/10.3390/biom12040506
APA StyleEbner, K., Sartori, S. B., Murau, R., Kopel, F., Kalaba, P., Dragačević, V., Leban, J. J., Singewald, N., Engelmann, M., & Lubec, G. (2022). The Novel Analogue of Modafinil CE-158 Protects Social Memory against Interference and Triggers the Release of Dopamine in the Nucleus Accumbens of Mice. Biomolecules, 12(4), 506. https://doi.org/10.3390/biom12040506






