Inhibition of STAT6 Activation by AS1517499 Inhibits Expression and Activity of PPARγ in Macrophages to Resolve Acute Inflammation in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animal Protocols
2.3. Induction of Acute Sterile Inflammation and Treatment
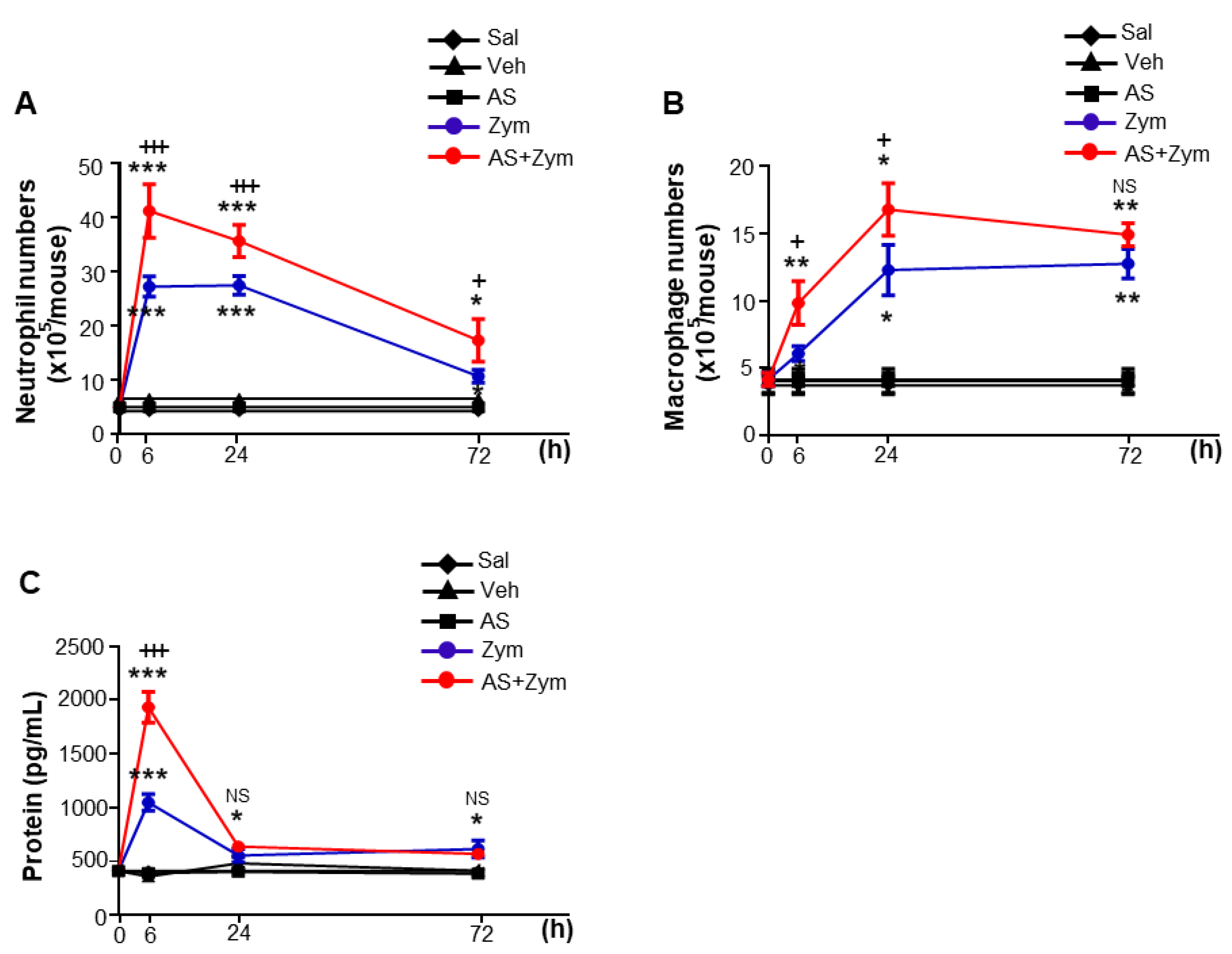
2.4. Isolation of Peritoneal Lavage Cells and Spleen
2.5. Preparation of Peritoneal Macrophages
2.6. Measurement of Total Protein in Lavage Samples
2.7. Western Blotting
2.8. Real-Time Quantitative PCR
2.9. Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. Immunocytochemistry
2.11. Induction of Apoptosis
2.12. Efferocytosis Assay
2.13. In Vitro Exposure of BMDM and Peritoneal Macrophages to Stimulants
2.14. Statistical Analysis
3. Results
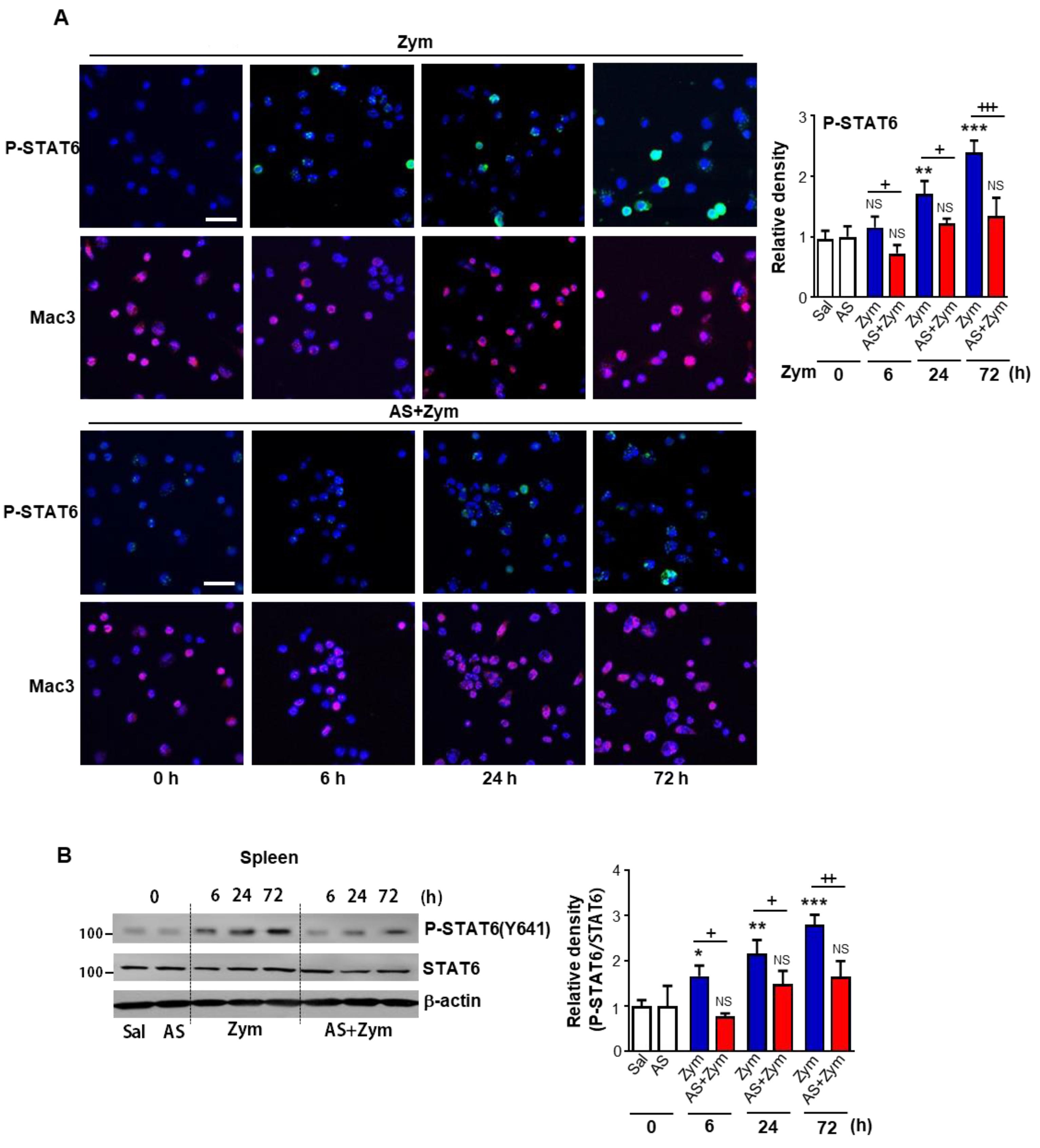
3.1. Administration of AS1517499 Inhibited STAT6 Activation in Peritoneal Macrophages and Spleen after Zymosan Injection
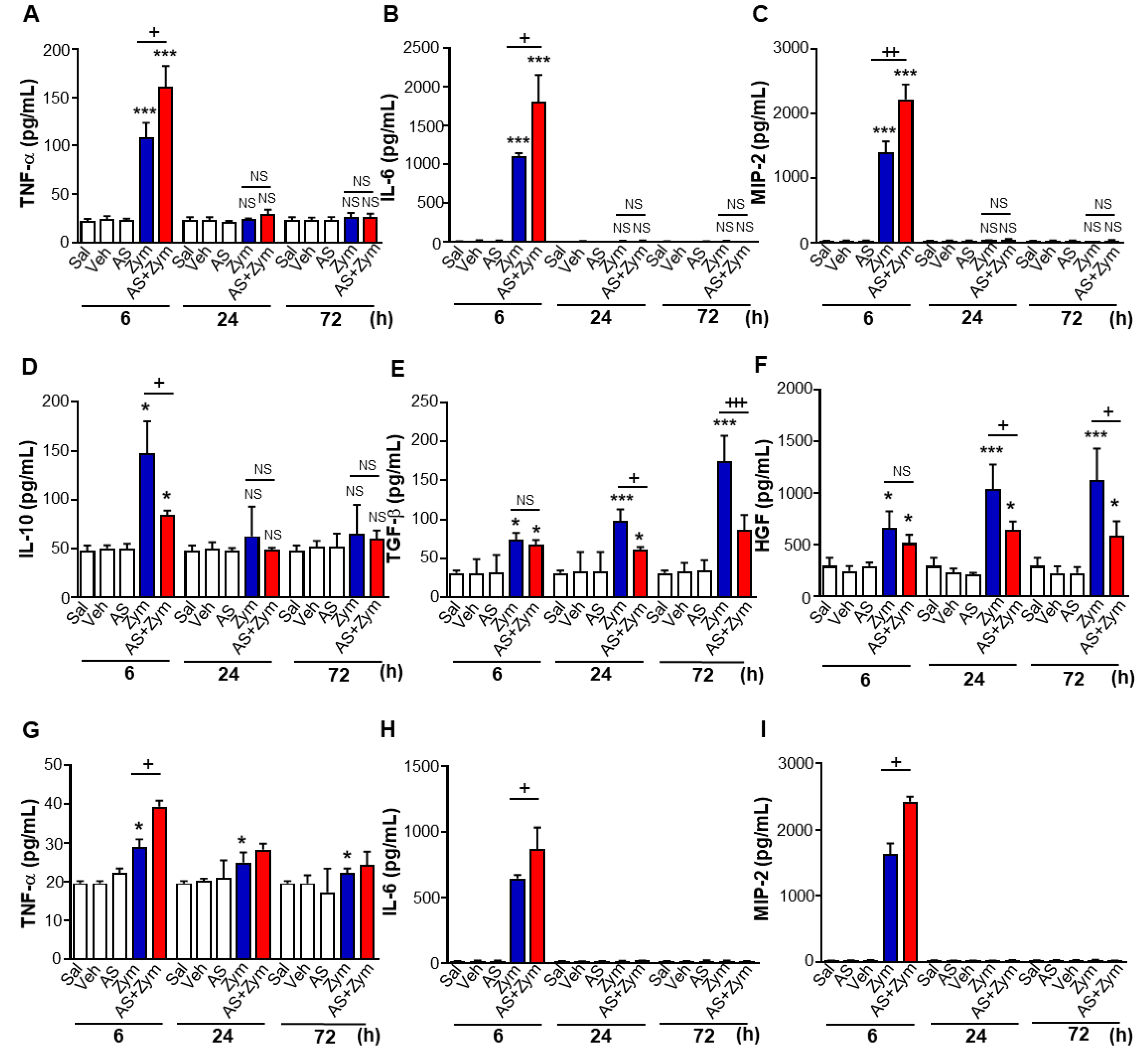
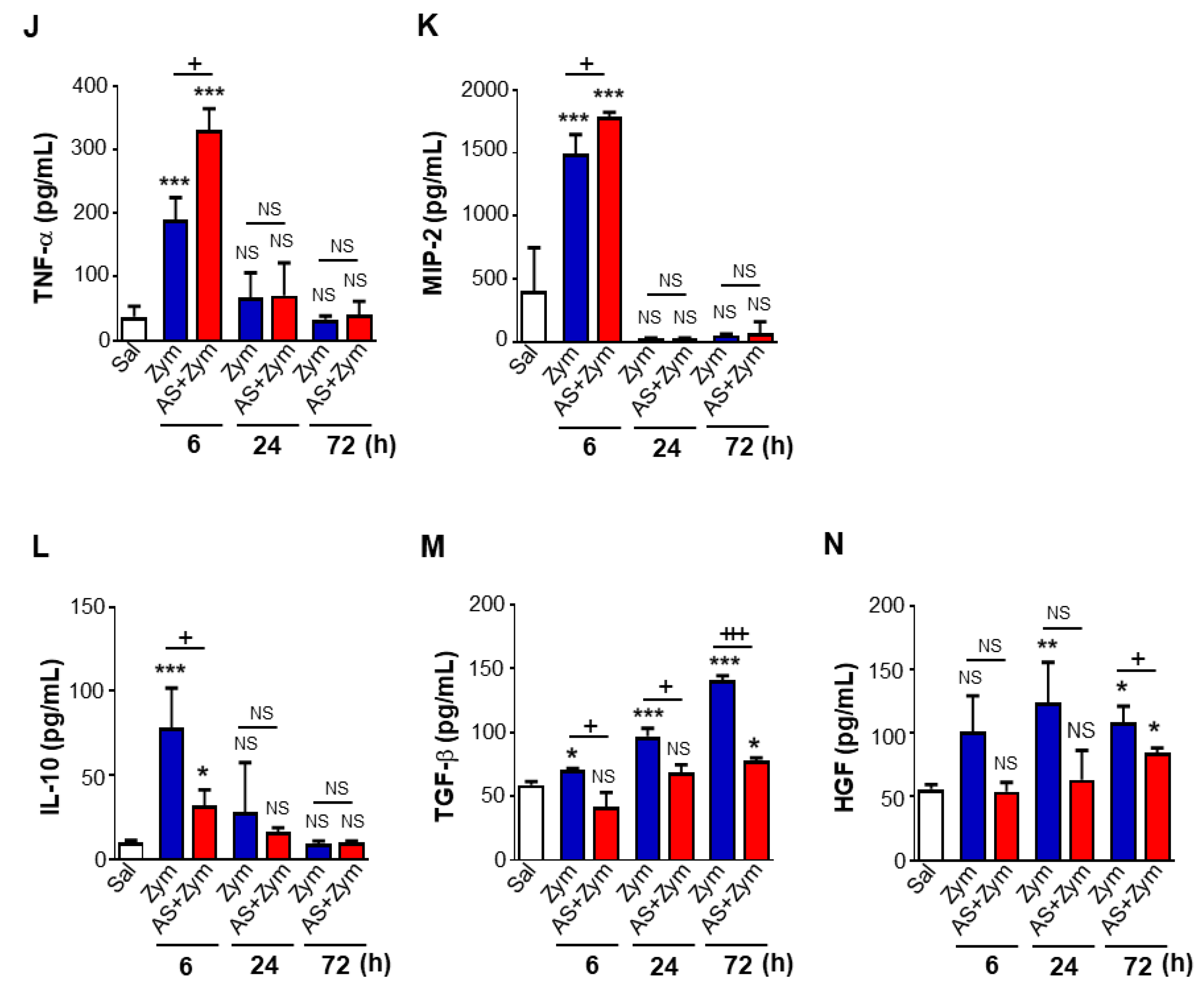
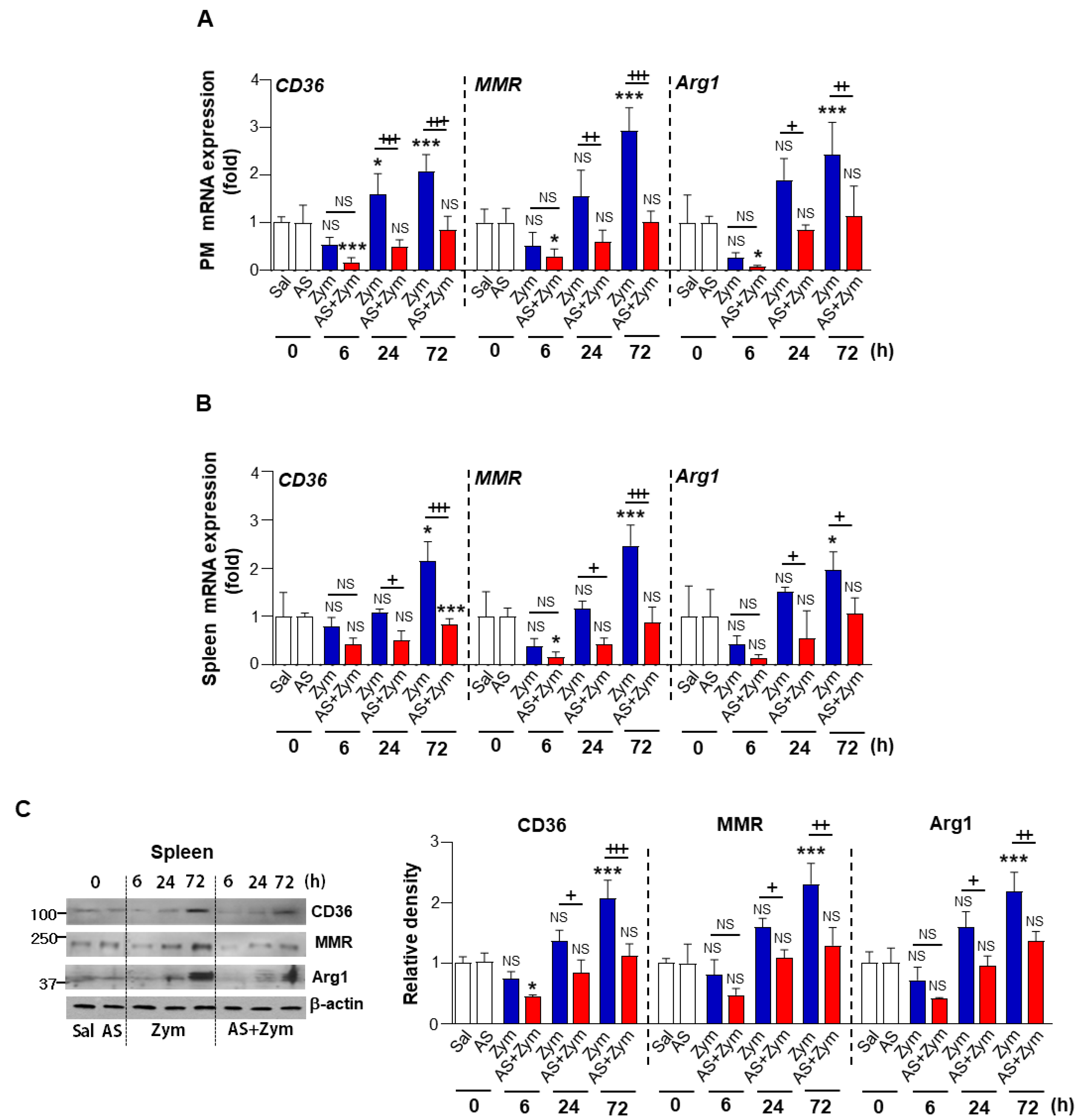
3.2. AS1517499 Enhanced Pro-Inflammatory Cytokine Production and Reduced Pro-Resolving Inflammatory Cytokines
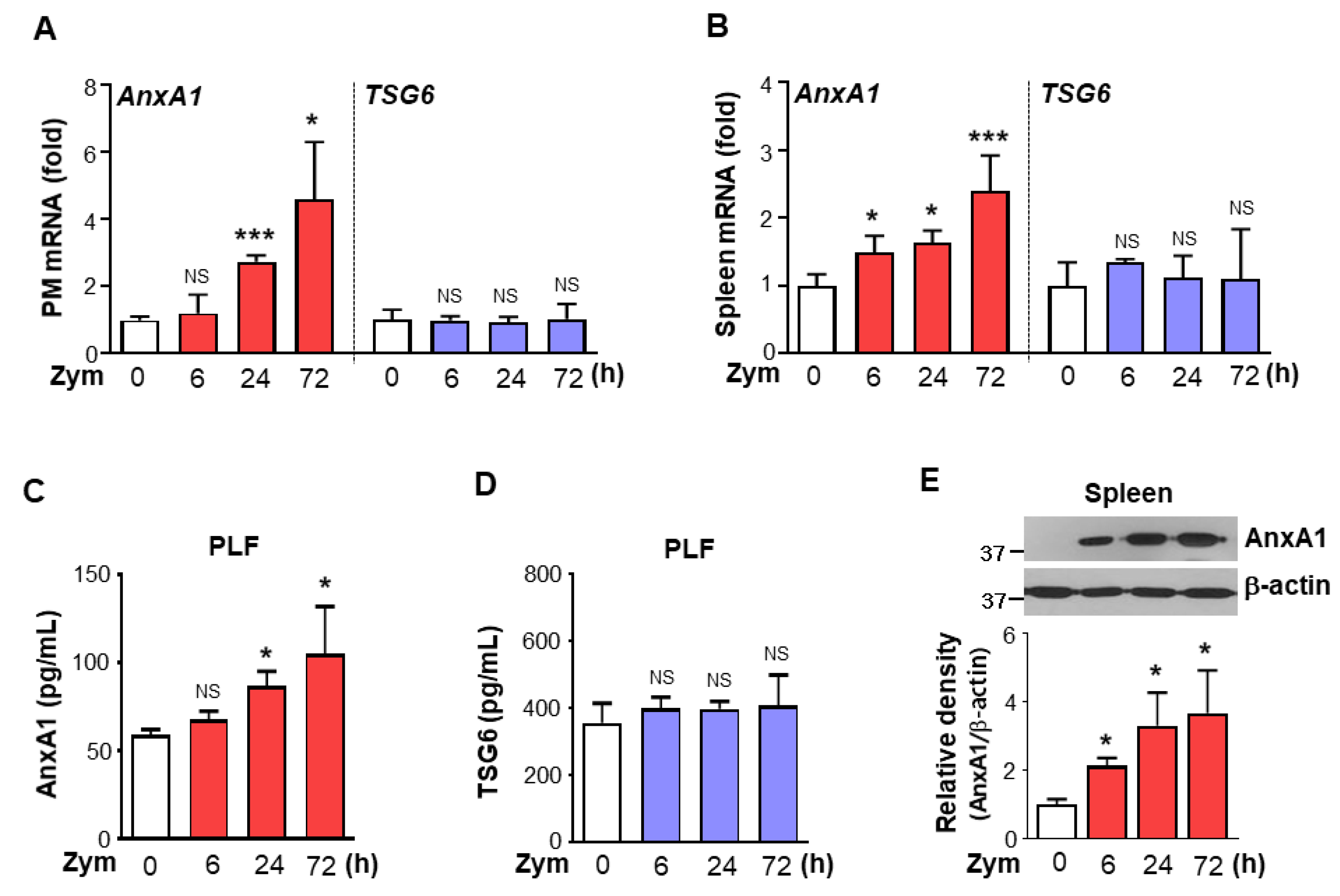
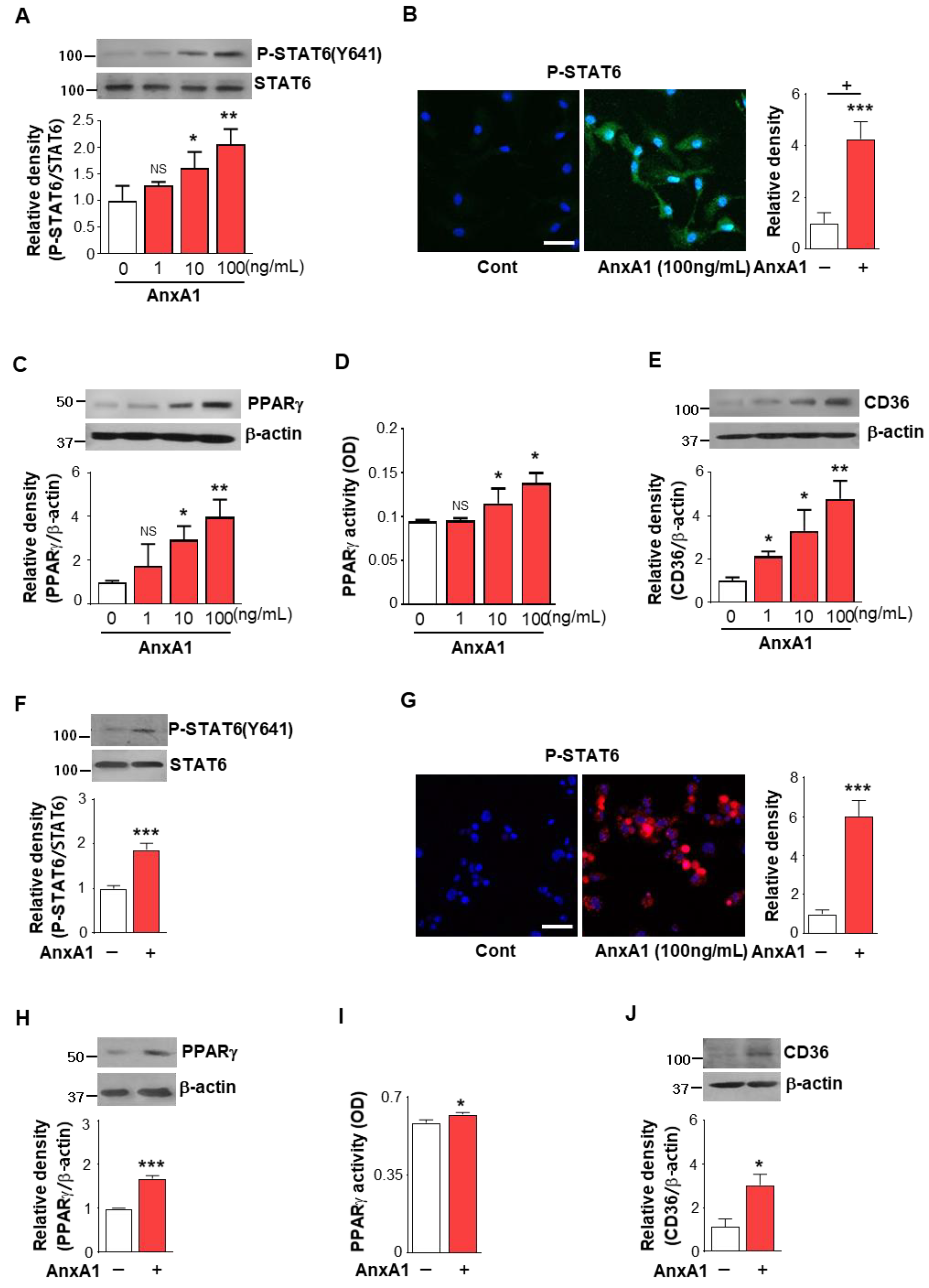
3.3. AnxA1 Was Involved in STAT6 Phosphorylation by Macrophages
3.4. AS1517499 Repressed PPARγ Expression and Activation
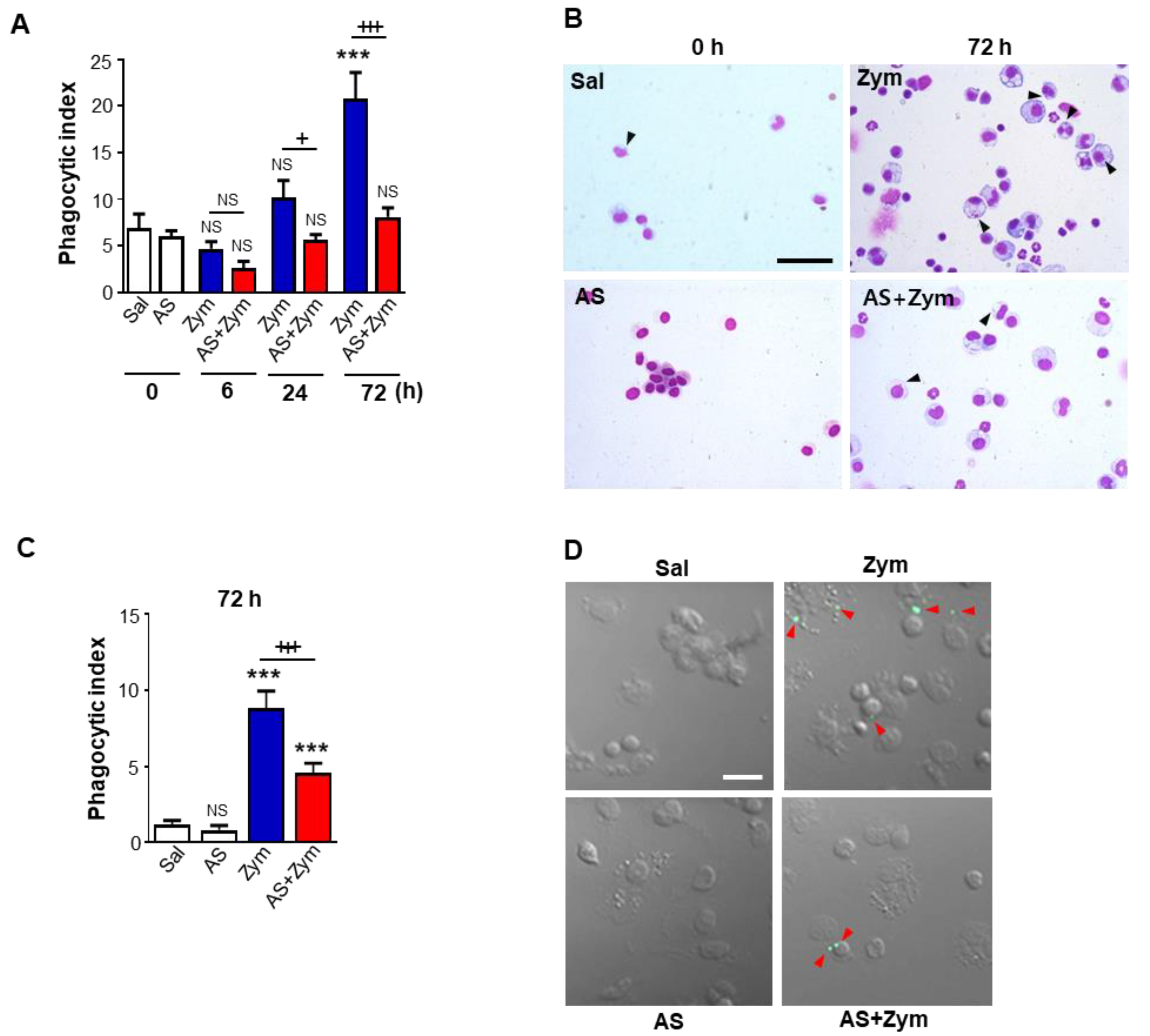
3.5. AS1517499 Inhibited Efferocytic Ability of Macrophages after Zymosan Injection In Vivo and Ex Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liao, X.; Sharma, N.; Kapadia, F.; Zhou, G.; Lu, Y.; Hong, H.; Paruchuri, K.; Mahabeleshwar, G.H.; Dalmas, E.; Venteclef, N.; et al. Krüppel-like factor 4 regulates macrophage polarization. J. Clin. Investig. 2011, 121, 2736–2749. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Shukla, M.; Yakubenko, V.P.; Mulya, A.; Kundu, S.; Cathcart, M.K. IL-4 and IL-13 employ discrete signaling pathways for target gene expression in alternatively activated monocytes/macrophages. Free Radic. Biol. Med. 2013, 54, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Van Dyken, S.J.; Locksley, R.M. Interleukin-4- and Interleukin-13-mediated alternatively activated macrophages: Role in homeostasis and disease. Annu. Rev. Immunol. 2013, 31, 317–343. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, A.K.; Walter, T.; Erkel, G. The fungal metabolite cyclonerodiol inhibits IL-4/IL-13 induced Stat6-signaling through blocking the association of Stat6 with p38, ERK1/2 and p300. Int. Immunopharmacol. 2018, 65, 392–401. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S.M.; Heller, N.M. Commentary: IL-4 and IL-13 receptors and signaling. Cytokine 2015, 75, 38–50. [Google Scholar] [CrossRef]
- Hershey, G.K. IL-13 receptors and signaling pathways: An evolving web. J. Allergy Clin. Immunol. 2003, 111, 677–690. [Google Scholar] [CrossRef]
- Zimmermann, N.; Hershey, G.K.; Foster, P.S.; Rothenberg, M.E. Chemokines in asthma: Cooperative interaction between chemokines and IL-13. J. Allergy Clin. Immunol. 2003, 111, 227–242. [Google Scholar] [PubMed]
- Wurster, A.L.; Tanaka, T.; Grusby, M.J. The biology of Stat4 and Stat6. Oncogene 2000, 19, 2577–2584. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Kaplan, M.H. Transcriptional regulation by Stat6. Immunol. Res. 2011, 50, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Nepal, S.; Tiruppathi, C.; Tsukasaki, Y.; Farahany, J.; Mittal, M.; Rehman, J.; Prockop, D.J.; Malik, A.B. STAT6 induces expression of Gas6 in macrophages to clear apoptotic neutrophils and resolve inflammation. Proc. Natl. Acad. Sci. USA 2019, 116, 16513–16518. [Google Scholar] [CrossRef]
- da Rocha, G.H.O.; Loiola, R.A.; Pantaleão, L.D.N.; Reutelingsperger, C.; Solito, E.; Farsky, S.H.P. Control of expression and activity of peroxisome proliferated--activated receptor γ by Annexin A1 on microglia during efferocytosis. Cell. Biochem. Funct. 2019, 37, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Szanto, A.; Balint, B.L.; Nagy, Z.S.; Barta, E.; Dezso, B.; Pap, A.; Szeles, L.; Poliska, S.; Oros, M.; Evans, R.M.; et al. STAT6 transcription factor is a facilitator of the nuclear receptor PPARgamma-regulated gene expression in macrophages and dendritic cells. Immunity 2010, 33, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Ferré, P. The biology of peroxisome proliferator-activated receptors: Relationship with lipid metabolism and insulin sensitivity. Diabetes 2004, 53 Suppl. 1, S43–S50. [Google Scholar] [CrossRef] [PubMed]
- Asada, K.; Sasaki, S.; Suda, T.; Chida, K.; Nakamura, H. Antiinflammatory roles of peroxisome proliferator-activated receptor gamma in human alveolar macrophages. Am. J. Respir. Crit. Care Med. 2004, 169, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Odegaard, J.I.; Ricardo-Gonzalez, R.R.; Goforth, M.H.; Morel, C.R.; Subramanian, V.; Mukundan, L.; Eagle, A.R.; Vats, D.; Brombacher, F.; Ferrante, A.W.; et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature 2007, 447, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Lentsch, A.B.; Kato, A.; Davis, B.; Wang, W.; Chao, C.; Edwards, M.J. STAT4 and STAT6 regulate systemic inflammation and protect against lethal endotoxemia. J. Clin. Investig. 2001, 108, 1475–1482. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, B.M.; Ahn, Y.H.; Choi, J.H.; Choi, Y.H.; Kang, J.L. STAT6 Signaling Mediates PPARγ Activation and Resolution of Acute Sterile Inflammation in Mice. Cells 2021, 10, 501. [Google Scholar] [CrossRef]
- Fernandez-Boyanapalli, R.; Frasch, S.C.; Riches, D.W.; Vandivier, R.W.; Henson, P.M.; Bratton, D.L. PPARgamma activation normalizes resolution of acute sterile inflammation in murine chronic granulomatous disease. Blood 2010, 116, 4512–4522. [Google Scholar] [CrossRef]
- Choi, J.Y.; Seo, J.Y.; Yoon, Y.S.; Lee, Y.J.; Kim, H.S.; Kang, J.L. Mer signaling increases the abundance of the transcription factor LXR to promote the resolution of acute sterile inflammation. Sci. Signal. 2015, 8, ra21. [Google Scholar] [CrossRef] [PubMed]
- Chiba, Y.; Todoroki, M.; Nishida, Y.; Tanabe, M.; Misawa, M. A novel STAT6 inhibitor AS1517499 ameliorates antigen-induced bronchial hypercontractility in mice. Am. J. Respir. Cell Mol. Biol. 2009, 41, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Lee, Y.J.; Yoon, Y.S.; Lim, J.H.; Park, E.M.; Chong, Y.H.; Kang, J.L. A STAT6 Inhibitor AS1517499 Reduces Preventive Effects of Apoptotic Cell Instillation on Bleomycin-Induced Lung Fibrosis by Suppressing PPARgamma. Cell. Physiol. Biochem. 2018, 45, 1863–1877. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zha, B.; Liu, X.; Liu, R.; Liu, J.; Huang, E.; Qian, T.; Liu, J.; Wang, Z.; Zhang, D.; et al. STAT6 deficiency ameliorates Graves’ disease severity by suppressing thyroid epithelial cell hyperplasia. Cell Death Dis. 2016, 7, e2506. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lane, F.C.; Mehta, J.R. In vitro human tumor sensitivity assay using cell counting and sizing. Am. Biotechnol. Lab. 1990, 8, 12–27. [Google Scholar] [PubMed]
- Castranova, V.; Jones, T.; Barger, M.W.; Afshari, A.; Frazer, D.J. Pulmonary responses of guinea pigs to consecutive exposures to cotton dust. In Proceedings of the 14th Cotton Dust Research Conference, Las Vegas, NV, USA, 12–13 January 1990; Jacobs, R.R., Wakelyn, P.J., Domelsmith, L.N., Eds.; National Cotton Council: Memphis, TN, USA, 1990; pp. 131–135. [Google Scholar]
- Porter, D.W.; Barger, M.; Robinson, V.A.; Leonard, S.S.; Landsittel, D.; Castranova, V. Comparison of low doses of aged and freshly fractured silica on pulmonary inflammation and damage in the rat. Toxicology 2002, 175, 63–71. [Google Scholar] [CrossRef]
- Kim, B.M.; Lee, Y.J.; Choi, Y.H.; Park, E.M.; Kang, J.L. Gas6 ameliorates inflammatory response and apoptosis in bleomycin-induced acute lung injury. Biomedicines 2021, 9, 1674. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Han, J.Y.; Byun, J.; Park, H.J.; Park, E.M.; Chong, Y.H.; Cho, M.S.; Kang, J.L. Inhibiting Mer receptor tyrosine kinase suppresses STAT1, SOCS1/3, and NF-κB activation and enhances inflammatory responses in lipopolysaccharide-induced acute lung injury. J Leukoc. Biol. 2012, 91, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Raja, R.M.; Kramer, M.S.; Barber, K. Solute transport and ultrafiltration during peritonitis in CAPD patients. ASAIO J. 1984, 7, 8–11. [Google Scholar]
- Krediet, R.T.; Zuyderhoudt, F.M.J.; Boeschoten, E.W.; Arisz, L. Alterations in the peritoneal transport of water and solutes during peritonitis in continuous ambulatory peritoneal dialysis patients. Eur. J. Clin. Investig. 1987, 17, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Dulaney, J.T.; Hatch, F.E., Jr. Peritoneal dialysis and loss of proteins: A review. Kidney Int. 1982, 26, 253–262. [Google Scholar] [CrossRef]
- Brown, J.R.; Goldblatt, D.; Buddle, J.; Morton, L.; Thrasher, A.J. Diminished production of anti-inflammatory mediators during neutrophil apoptosis and macrophage phagocytosis in chronic granulomatous disease (CGD). J. Leukoc. Biol. 2003, 73, 591–599. [Google Scholar] [CrossRef]
- Lee, Y.J.; Moon, C.; Lee, S.H.; Park, H.J.; Seoh, J.Y.; Cho, M.S.; Kang, J.L. Apoptotic cell instillation after bleomycin attenuates lung injury through hepatocyte growth factor induction. Eur. Respir. J. 2012, 40, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.S.; Kim, S.Y.; Kim, M.J.; Lim, J.H.; Cho, M.S.; Kang, J.L. PPARγ activation following apoptotic cell instillation promotes resolution of lung inflammation and fibrosis via regulation of efferocytosis and proresolving cytokines. Muc. Immunol. 2015, 8, 1031–1046. [Google Scholar] [CrossRef]
- Richens, T.R.; Linderman, D.J.; Horstmann, S.A.; Lambert, C.; Xiao, Y.Q.; Keith, R.L.; Boe, D.M.; Morimoto, K.; Bowler, R.P.; Day, B.J.; et al. Cigarette smoke impairs clearance of apoptotic cells through oxidant-dependent activation of RhoA. Am. J. Respir. Crit. Care Med. 2009, 179, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, M.; Thorsson, V.; Li, B.; Rust, A.G.; Korb, M.; Roach, J.C.; Kennedy, K.; Hai, T.; Bolouri, H.; Aderem, A. Systems biology approaches identify ATF3 as a negative regulator of Toll-like receptor 4. Nature 2006, 441, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Cailhier, J.F.; Partolina, M.; Vuthoori, S.; Wu, S.; Ko, K.; Watson, S.; Savill, J.; Hughes, J.; Lang, R.A. Conditional macrophage ablation demonstrates that resident macrophages initiate acute peritoneal inflammation. J. Immunol. 2005, 174, 2336–2342. [Google Scholar] [CrossRef]
- Schwab, J.M.; Chiang, N.; Arita, M.; Serhan, C.N. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 2007, 447, 869–874. [Google Scholar] [CrossRef]
- Ivanovska, N.D.; Dimitrova, P.A.; Luckett, J.C.; El-Rachkidy Lonnen, R.; Schwaeble, W.J.; Stover, C.M. Properdin deficiency in murine models of nonseptic shock. J. Immunol. 2008, 180, 6962–6969. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Ma, J.; Peng, Y.; Sun, M.; Xu, K.; Wu, R.; Lin, J. Autocrine Production of Interleukin-34 Promotes the Development of Endometriosis through CSF1R/JAK3/STAT6 signaling. Sci. Rep. 2019, 9, 16781. [Google Scholar] [CrossRef]
- Heuvel, F.O.; Holl, S.; Chandrasekar, A.; Li, Z.; Wang, Y.; Rehman, R.; Förstner, P.; Sinske, D.; Palmer, A.; Wiesner, D.; et al. STAT6 mediates the effect of ethanol on neuroinflammatory response in TBI. Brain Behav Immun. 2019, 81, 228–246. [Google Scholar] [CrossRef] [PubMed]
- Binnemars-Postma, K.; Bansal, R.; Storm, G.; Prakash, J. Targeting the Stat6 pathway in tumor-associated macrophages reduces tumor growth and metastatic niche formation in breast cancer. FASEB J. 2018, 32, 969–978. [Google Scholar] [CrossRef]
- Perretti, M.; D’Acquisto, F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat. Rev. Immunol. 2009, 9, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Gavins, F.N.E.; Hickey, M. Annexin A1 and the regulation of innate and adaptive immunity. Front. Immunol. 2012, 3, 354. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Tiruppathi, C.; Nepal, S.; Zhao, Y.Y.; Grzych, D.; Soni, D.; Prockop, D.J.; Malik, A.B. TNFα-stimulated gene-6 (TSG6) activates macrophage phenotype transition to prevent inflammatory lung injury. Proc. Natl. Acad. Sci. USA 2016, 113, E8151–E8158. [Google Scholar] [CrossRef] [PubMed]
- Daniel, B.; Nagy, G.; Horvath, A.; Czimmerer, Z.; Cuaranta-Monroy, I.; Poliska, S.; Hays, T.T.; Sauer, S.; Francois-Deleuze, J.; Nagy, L. The IL-4/STAT6/PPARγ signaling axis is driving the expansion of the RXR heterodimer cistrome, providing complex ligand responsiveness in macrophages. Nucleic Acids Res. 2018, 46, 4425–4439. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Dai, X.; Chen, J.; Zhao, J.; Xu, M.; Zhang, L.; Yang, B.; Zhang, W.; Rocha, M.; Nakao, T.; et al. STAT6/Arg1 promotes microglia/ macrophage efferocytosis and inflammation resolution in stroke mice. JCI Insight 2019, 4, e131355. [Google Scholar] [CrossRef] [PubMed]
- Croasdell, A.; Duffney, P.F.; Kim, N.; Lacy, S.H.; Sime, P.J.; Phipps, R.P. PPARγ and the Innate Immune System Mediate the Resolution of Inflammation. PPAR Res. 2015, 2015, 549691. [Google Scholar] [CrossRef] [PubMed]
- Berry, A.; Balard, P.; Coste, A.; Olagnier, D.; Lagane, C.; Authier, H.; Benoit-Vical, F.; Lepert, J.C.; Séguéla, J.P.; Magnaval, J.F.; et al. IL-13 induces expression of CD36 in human monocytes through PPARγ activation. Eur. J. Immunol. 2007, 37, 1642–1652. [Google Scholar] [CrossRef] [PubMed]
- Serghides, L.; Kain, K.C. Peroxisome Proliferator-Activated Receptor γ-Retinoid X Receptor Agonists Increase CD36-Dependent Phagocytosis of Plasmodium falciparum-Parasitized Erythrocytes and Decrease Malaria-Induced TNF-α Secretion by Monocytes/Macrophages. J. Immunol. 2001, 166, 6742–6748. [Google Scholar] [CrossRef]
- Zhao, X.R.; Gonzales, N.; Aronowsk, J. Pleiotropic Role of PPARγ in Intracerebral Hemorrhage: An Intricate System Involving Nrf2, RXR, and NF-κB. CNS Neurosc. Ther. 2015, 21, 357–366. [Google Scholar] [CrossRef]
- Galès, A.; Conduché, A.; Bernad, J.; Lefevre, L.; Olagnier, D.; Béraud, M.; Martin-Blondel, G.; Linas, M.D.; Auwerx, J.; Coste, A.; et al. PPARγ Controls Dectin-1 Expression Required for Host Antifungal Defense against Candida albicans. PLoS Pathog. 2010, 6, e1000714. [Google Scholar] [CrossRef] [PubMed]
- Kurisu, K.; Zheng, Z.; Kim, J.Y.; Shi, J.; Kanoke, A.; Liu, J.; Hsieh, C.L.; Yenari, M.A. Triggering receptor expressed on myeloid cells-2 expression in the brain is required for maximal phagocytic activity and improved neurological outcomes following experimental stroke. J. Cereb. Blood Flow Metab. 2018, 39, 1906–1918. [Google Scholar] [CrossRef] [PubMed]
- Fadok, V.A.; Bratton, D.L.; Guthrie, L.; Henson, P.M. Differential effects of apoptotic versus lysed cells on macrophage production of cytokines: Role of proteases. J. Immunol. 2001, 166, 6847–6854. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.C.; Wu, H.L.; Chen, S.H.; Wang, Y.T.; Wu, C.C. Thrombomodulin facilitates peripheral nerve regeneration through regulating M1/M2 switching. J. Neuroinflammation 2020, 17, 240. [Google Scholar] [CrossRef] [PubMed]
- Majai, G.; Sarang, Z.; Csomo’s, K.; Zahuczky, G.; Fésüs, L. PPARgamma-dependent regulation of human macrophages in phagocytosis of apoptotic cells. Eur. J. Immunol. 2007, 37, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- Ricote, M.; Glass, C.K. PPARs and molecular mechanisms of transrepression. Biochim. Biophys. Acta 2007, 1771, 926–935. [Google Scholar]
- Rizzo, G.; Fiorucci, S. PPARs and other nuclear receptors in inflammation. Curr. Opin. Pharmacol. 2006, 6, 421–427. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-J.; Kim, K.; Kim, M.; Ahn, Y.-H.; Kang, J.L. Inhibition of STAT6 Activation by AS1517499 Inhibits Expression and Activity of PPARγ in Macrophages to Resolve Acute Inflammation in Mice. Biomolecules 2022, 12, 447. https://doi.org/10.3390/biom12030447
Lee Y-J, Kim K, Kim M, Ahn Y-H, Kang JL. Inhibition of STAT6 Activation by AS1517499 Inhibits Expression and Activity of PPARγ in Macrophages to Resolve Acute Inflammation in Mice. Biomolecules. 2022; 12(3):447. https://doi.org/10.3390/biom12030447
Chicago/Turabian StyleLee, Ye-Ji, Kiyoon Kim, Minsuk Kim, Young-Ho Ahn, and Jihee Lee Kang. 2022. "Inhibition of STAT6 Activation by AS1517499 Inhibits Expression and Activity of PPARγ in Macrophages to Resolve Acute Inflammation in Mice" Biomolecules 12, no. 3: 447. https://doi.org/10.3390/biom12030447
APA StyleLee, Y.-J., Kim, K., Kim, M., Ahn, Y.-H., & Kang, J. L. (2022). Inhibition of STAT6 Activation by AS1517499 Inhibits Expression and Activity of PPARγ in Macrophages to Resolve Acute Inflammation in Mice. Biomolecules, 12(3), 447. https://doi.org/10.3390/biom12030447






