Attenuation of the Counter-Regulatory Glucose Response in CVLM C1 Neurons: A Possible Explanation for Anorexia of Aging
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Animals
2.3. Animal Treatment, Sample Collection, and Preparation
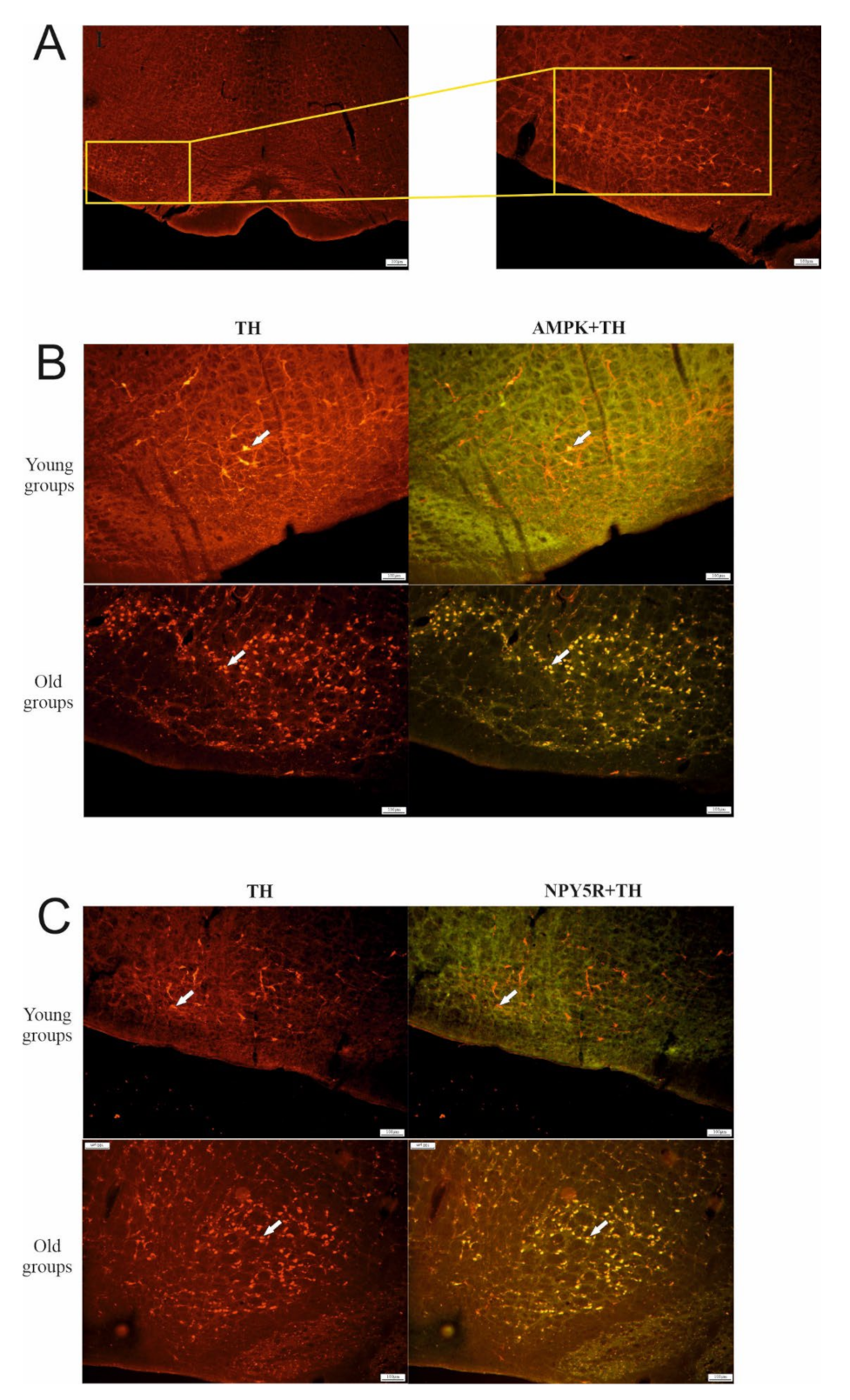
3. Immunofluorescence
4. Western Blot Analysis
5. Statistical Analysis
6. Results
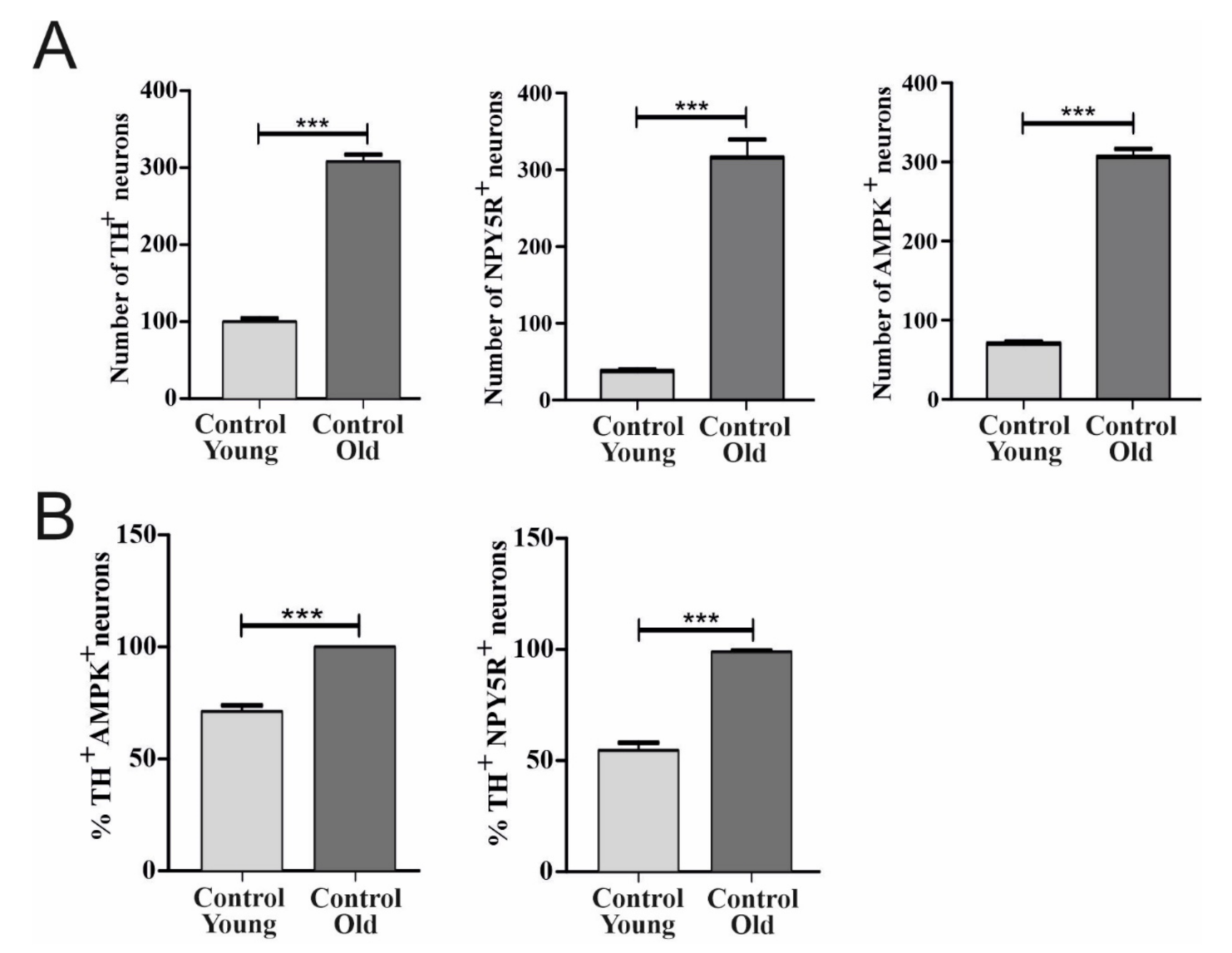
6.1. Effect of Aging on TH, AMPK, and NPY5R Immunoreactivities in the CVLM
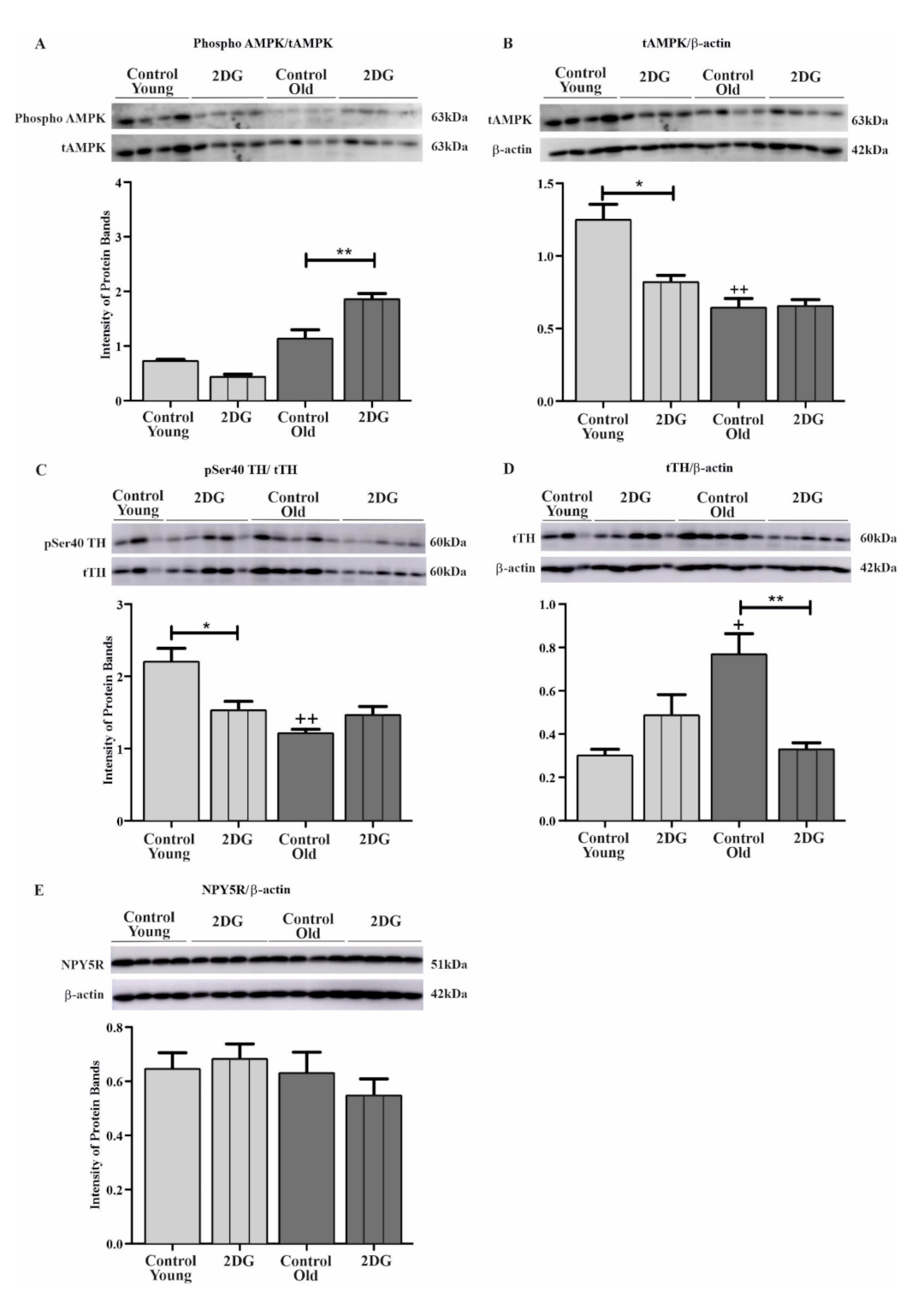
6.2. Effect of Aging and Glucoprivation on pSer40 TH, Total TH (tTH), Phosphorylated AMPK, Total AMPK (tAMPK), and NPY5R in the CVLM
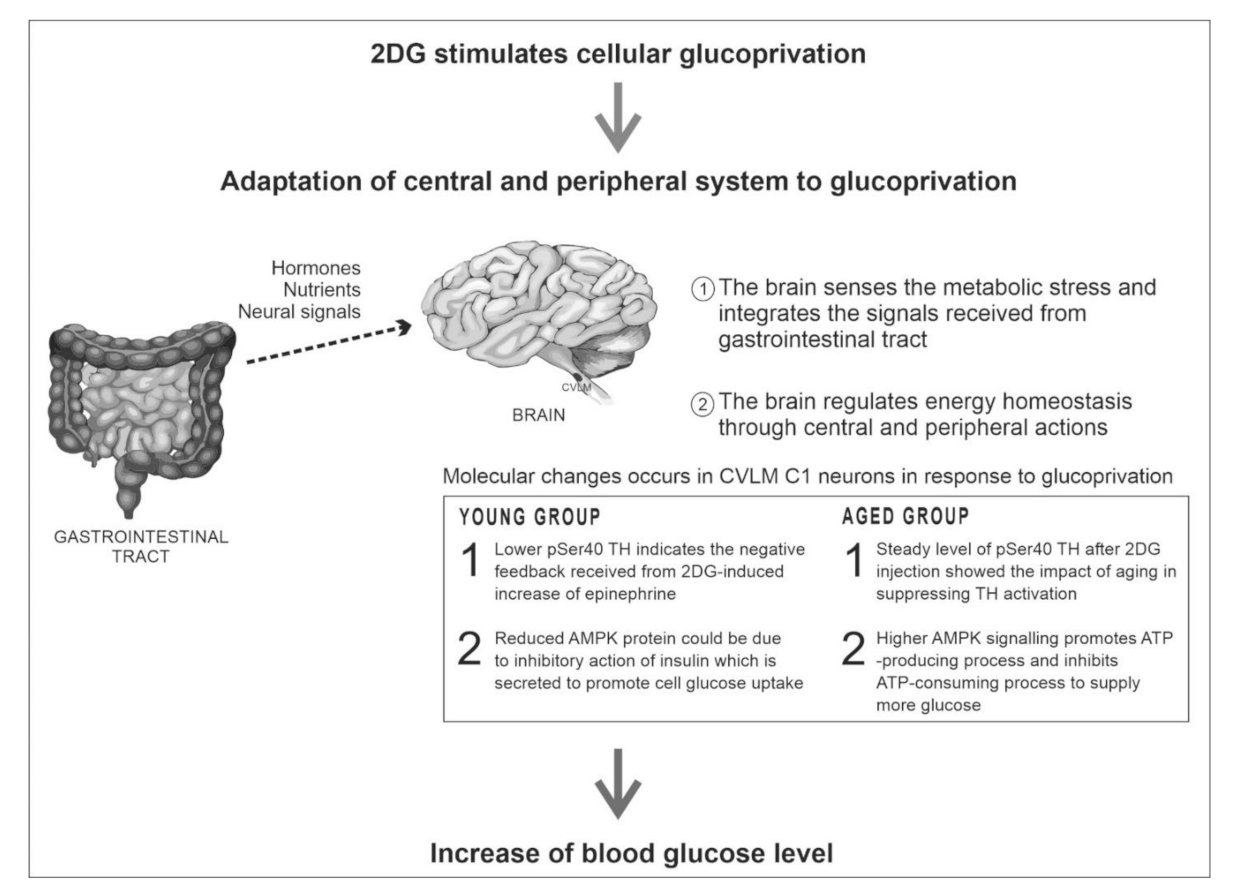
7. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morley, J.E.; Silver, A.J. Anorexia in the elderly. Neurobiol. Aging 1988, 9, 9–16. [Google Scholar] [CrossRef]
- Morley, J.E. Pathophysiology of the anorexia of aging. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Luis, D.; Huang, X.; Sjögren, P.; Risérus, U.; Ärnlöv, J.; Lindholm, B.; Cederholm, T.; Carrero, J.J. Renal function associates with energy intake in elderly community-dwelling men. Br. J. Nutr. 2014, 111, 2184–2189. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Picca, A.; Calvani, R.; Marzetti, E. Anorexia of aging: Assessment and management. Clin. Geriatr. Med. 2017, 33, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Laviano, A.; Cruz-Jentoft, A.J. The Anorexia of aging: Is it a geriatric syndrome? J. Am. Med. Dir. Assoc. 2010, 11, 153–156. [Google Scholar] [CrossRef]
- Morley, J.E. Anorexia of aging: A true geriatric syndrome. J. Nutr. Health Aging 2012, 16, 422–425. [Google Scholar] [CrossRef]
- Li, A.J.; Wang, Q.; Dinh, T.T.; Ritter, S. Simultaneous silencing of NPY and DBH expression in hindbrain A1/C1 catecholamine cells suppresses glucoprivic feeding. J. Neurosci. 2009, 29, 280–287. [Google Scholar] [CrossRef]
- Li, A.-J.; Wang, Q.; Ritter, S. Selective pharmacogenetic activation of catecholamine subgroups in the ventrolateral medulla elicits key glucoregulatory responses. Endocrinology 2017, 159, 341–355. [Google Scholar] [CrossRef]
- Sergeyev, V.; Broberger, C.; Gorbatyuk, O.; Hokfelt, T. Effect of 2-mercaptoacetate and 2-deoxy-D-glucose administration on the expression of NPY, AGRP, POMC, MCH and hypocretin/orexin in the rat hypothalamus. Neurochemistry 2000, 11, 117–121. [Google Scholar] [CrossRef]
- Mravec, B.; Vargovič, P.; Filipcik, P.; Novak, M.; Kvetnansky, R. Effect of a single and repeated stress exposure on gene expression of catecholamine biosynthetic enzymes in brainstem catecholaminergic cell groups in rats. Eur. J. Neurosci. 2015, 42, 1872–1886. [Google Scholar] [CrossRef]
- Li, A.-J.; Wang, Q.; Ritter, S. Participation of hindbrain AMP-activated protein kinase in glucoprivic feeding. Diabetes 2011, 60, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.S.; Cho, H.; Cho, J.H.; Yu, S.-W.; Kim, E.-K. Hypothalamic AMPK-induced autophagy increases food intake by regulating NPY and POMC expression. Autophagy 2016, 12, 2009–2025. [Google Scholar] [CrossRef] [PubMed]
- Damanhuri, H.A.; Burke, P.G.R.; Ong, L.K.; Bobrovskaya, L.; Dickson, P.W.; Dunkley, P.R.; Goodchild, A.K. Tyrosine hydroxylase phosphorylation in catecholaminergic brain regions: A marker of activation following acute hypotension and glucoprivation. PLoS ONE 2012, 7, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Li, A.-J.; Wang, Q.; Dinh, T.T.; Wiater, M.F.; Eskelsen, A.K.; Ritter, S. Hindbrain catecholamine neurons control rapid switching of metabolic substrate use during glucoprivation in male rats. Endocrinology 2013, 154, 4570–4579. [Google Scholar] [CrossRef][Green Version]
- Li, A.-J.; Wang, Q.; Ritter, S. Differential responsiveness of dopamine-β-hydroxylase gene expression to glucoprivation in different catecholamine cell groups. Endocrinology 2006, 147, 3428–3434. [Google Scholar] [CrossRef][Green Version]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 5th ed.; Elsevier: Amsterdam, The Netherlands, 1982. [Google Scholar]
- Olympus CellSens Standard Software, Version 1.14; Olympus Corporation: Tokyo, Japan, 2017.
- Ramlan, H.; Damanhuri, H.A. Effects of age on feeding response: Focus on the rostral C1 neuron and its glucoregulatory proteins. Exp. Gerontol. 2020, 129, 1–9. [Google Scholar] [CrossRef]
- Muda, N.A.; Ramlan, H.; Damanhuri, H.A. Effects of age on the glucoregulatory response following acute glucoprivation induced by 2-deoxyglucose (2DG) in the adrenal medulla of Sprague Dawley rats. Neuroendocrinol. Lett. 2017, 38, 101–112. [Google Scholar] [PubMed]
- Strong, R.; Moore, M.A.; Hale, C.; Wessels-Reiker, M.; Armbrecht, H.J.; Richardson, A. Modulation of tyrosine hydroxylase gene expression in the rat adrenal gland by age and reserpine. Brain Res. 1990, 525, 126–132. [Google Scholar] [CrossRef]
- Türner, N.; Broxson, C.S.; LaRochelle, J.; Scarpace, P.J. Induction of tyrosine hydroxylase and neuropeptide Y by carbachol: Modulation with age. J. Gerontol. Ser. A 1999, 54, B418–B423. [Google Scholar] [CrossRef]
- Wilkie, F.L.; Halter, J.B.; Prinz, P.N.; Benedetti, C.; Eisdorfer, C.; Atwood, B.; Yamasaki, D. Age-related changes in venous catecholamines basally and during epinephrine infusion in man. J. Gerontol. 1985, 40, 133–140. [Google Scholar] [CrossRef]
- Abd-Allah, N.M.; Hassan, F.H.; Esmat, A.Y.; Hammad, S.A. Age dependence of the levels of plasma norepinephrine, aldosterone, renin activity and urinary vanillylmandelic acid in normal and essential hypertensives. Biol. Res. 2004, 37, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Dela Cruz, C.P.; Revilla, E.; Venero, J.L.; Ayala, A.; Cano, J.; MacHado, A. Oxidative inactivation of tyrosine hydroxylase in substantia nigra of aged rat. Free Radic. Biol. Med. 1996, 20, 53–61. [Google Scholar] [CrossRef]
- Ramos, B.P.; Birnbaum, S.G.; Lindenmayer, I.; Newton, S.S.; Duman, R.S.; Arnsten, A.F. Dysregulation of protein kinase a signaling in the aged prefrontal cortex: New strategy for treating age-related cognitive decline. Neuron 2003, 40, 835–845. [Google Scholar] [CrossRef]
- Veyrat-Durebex, C.; Quirion, R.; Ferland, G.; Dumont, Y.; Gaudreau, P. Aging and long-term caloric restriction regulate neuropeptide Y receptor subtype densities in the rat brain. Neuropeptides 2013, 47, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Pavia, J.M.; Morris, M.J. Age-related changes in neuropeptide y content in brain and peripheral tissues of spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. 1994, 21, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.J.; Pavia, J.M. Lack of effect of age on the cardiovascular response to neuropeptide Y injection in the rat nucleus tractus solitarius. Clin. Exp. Pharmacol. Physiol. 1997, 24, 162–165. [Google Scholar] [CrossRef]
- Macrae, I.M.; Reid, J.L. Cardiovascular significance of neuropeptide Y in the caudal ventrolateral medulla of the rat. Brain Res. 1988, 456, 1–8. [Google Scholar] [CrossRef]
- Cheng, S.; Xanthakis, V.; Sullivan, L.; Vasan, R.S. Blood pressure tracking over the adult life course: Patterns and correlates in the framingham heart study. Hypertension 2012, 60, 1393–1399. [Google Scholar] [CrossRef]
- Mahadir, N.B.; Id, N.; Fadhli, M.; Yusoff, M.; Id, S.A.; Sahril, N.; Aris, T. Factors associated with the severity of hypertension among Malaysian adults. PLoS ONE 2019, 14, 1–16. [Google Scholar] [CrossRef]
- Anderson, G.H., Jr. Effect of age on hypertension: Analysis of over 4800 referred hypertensive patients. Saudi J. Kidney Dis. Transplant. 1999, 10, 286–297. [Google Scholar]
- Qiang, W.; Weiqiang, K.; Qing, Z.; Pengju, Z.; Yi, L. Aging impairs insulin-stimulated glucose uptake in rat skeletal muscle via suppressing AMPKα. Exp. Mol. Med. 2007, 39, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Barton, G.P.; Sepe, J.J.; McKiernan, S.H.; Aiken, J.; Diffee, G.M. Mitochondrial and metabolic gene expression in the aged rat heart. Front. Physiol. 2016, 7, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Hardman, S.E.; Hall, D.E.; Cabrera, A.J.; Hancock, C.R.; Thomson, D.M. The effects of age and muscle contraction on AMPK activity and heterotrimer composition. Exp. Gerontol. 2014, 55, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, J.D.; Gonzalez, A.A.; Kumar, R.; Davis, A.J.; Saupe, K.W. Aging elevates basal adenosine monophosphate-activated protein kinase (AMPK) activity and eliminates hypoxic activation of AMPK in mouse liver. J. Gerontol. Ser. A 2005, 60, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Benashski, S.E.; Persky, R.; Xu, Y.; Li, J.; McCullough, L.D. Age-related changes in AMP-activated protein kinase after stroke. Age 2012, 34, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Carling, D.; Carlson, M. The AMP-activated/Snf1 protein kinase subfamily: Metabolic sensors of the eukaryotic cell? Annu. Rev. Biochem. 1998, 67, 821–855. [Google Scholar] [CrossRef] [PubMed]
- Levadoux, E.; Morio, B.; Montaurier, C.; Puissant, V.; Boirie, Y.; Fellmann, N.; Picard, B.; Rousset, P.; Beaufrere, B.; Ritz, P. Reduced whole-body fat oxidation in women and in the elderly. Int. J. Obes. 2001, 25, 39–44. [Google Scholar] [CrossRef]
- Kuhla, A.; Blei, T.; Jaster, R.; Vollmar, B. Aging Is associated with a shift of fatty metabolism toward lipogenesis. J. Gerontol. Ser. A 2011, 66, 1192–1200. [Google Scholar] [CrossRef]
- Wang, B.Z.; Yang, J.J.; Zhang, H.; A Smith, C.; Jin, K. AMPK signaling regulates the age-related decline of hippocampal neurogenesis. Aging Dis. 2019, 10, 1058–1074. [Google Scholar] [CrossRef]
- Senthilkumaran, M.; Johnson, M.E.; Bobrovskaya, L. The effects of insulin-induced hypoglycaemia on tyrosine hydroxylase phosphorylation in rat brain and adrenal gland. Neurochem. Res. 2016, 41, 1612–1624. [Google Scholar] [CrossRef]
- Ritter, S.; Llewellyn-Smith, I.; Dinh, T.T. Subgroups of hindbrain catecholamine neurons are selectively activated by 2-deoxy-d-glucose induced metabolic challenge. Brain Res. 1998, 805, 41–54. [Google Scholar] [CrossRef]
- Tumer, N.; Hale, C.; Lawler, J.; Strong, R. Modulation of tyrosine hydroxylase gene expression in the rat adrenal gland by exercise: Effects of age. Mol. Brain Res. 1992, 14, 51–56. [Google Scholar] [CrossRef]
- Kawashima, J.; Alquier, T.; Tsuji, Y.; Peroni, O.D.; Kahn, B.B. Ca2+/Calmodulin-dependent protein kinase kinase is not involved in hypothalamic AMP-activated protein kinase activation by neuroglucopenia. PLoS ONE 2012, 7, 1–7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McCrimmon, R.J.; Shaw, M.; Fan, X.; Cheng, H.; Ding, Y.; Vella, M.C.; Zhou, L.; McNay, E.C.; Sherwin, R.S. Key role for AMP-activated protein kinase in the ventromedial hypothalamus in regulating counterregulatory hormone responses to acute hypoglycemia. Diabetes 2008, 57, 444–450. [Google Scholar] [CrossRef]
- Kawai, K.; Yokota, C.; Ohashi, S.; Watanabe, Y.; Yamashita, K. Evidence that glucagon stimulates insulin secretion through its own receptor in rats. Diabetologia 1995, 38, 274–276. [Google Scholar] [CrossRef]
- Song, G.; Pacini, G.; Ahren, B.; D’Argenio, D.Z. Glucagon increases insulin levels by stimulating insulin secretion without effect on insulin clearance in mice. Peptides 2017, 88, 74–79. [Google Scholar] [CrossRef]
- Uemura, E.; Greenlee, H.W. Insulin regulates neuronal glucose uptake by promoting translocation of glucose transporter GLUT3. Exp. Neurol. 2006, 198, 48–53. [Google Scholar] [CrossRef]
- Halberg, N.; Henriksen, M.; Söderhamn, N.; Stallknecht, B.; Ploug, T.; Schjerling, P.; Dela, F. Effect of intermittent fasting and refeeding on insulin action in healthy men. J. Appl. Physiol. 2005, 99, 2128–2136. [Google Scholar] [CrossRef]
- Minokoshi, Y.; Alquier, T.; Furukawa, N.; Kim, Y.-B.; Lee, A.; Xue, B.; Mu, J.; Foufelle, F.; Ferré, P.; Birnbaum, M.J.; et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 2004, 428, 569–574. [Google Scholar] [CrossRef]
- Lee, K.; Li, B.; Xi, X.; Suh, Y.; Martin, R.J. Role of neuronal energy status in the regulation of adenosine 5′-monophosphate-activated protein kinase, orexigenic neuropeptides expression, and feeding behavior. Endocrinology 2005, 146, 3–10. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Q.; Whim, M.D. Fasting induces a form of autonomic synaptic plasticity that prevents hypoglycemia. Proc. Natl. Acad. Sci. USA 2016, 113, E3029–E3038. [Google Scholar] [CrossRef] [PubMed]
- Li, A.-J.; Ritter, S. Glucoprivation increases expression of neuropeptide Y mRNA in hindbrain neurons that innervate the hypothalamus. Eur. J. Neurosci. 2004, 19, 2147–2154. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Tan, H.Y.; Fogarty, M.J.; Andrews, Z.B.; Veldhuis, J.D.; Herzog, H.; Steyn, F.J.; Chen, C. Actions of NPY, and Its Y1 and Y2 Receptors on Pulsatile Growth Hormone Secretion during the Fed and Fasted State. J. Neurosci. 2014, 34, 16309–16319. [Google Scholar] [CrossRef] [PubMed]
- Vecteezy, Human Organ Anatomy Set. 2020. Available online: https://www.vecteezy.com/free-vector/human-organs (accessed on 13 February 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramlan, H.; Damanhuri, H.A. Attenuation of the Counter-Regulatory Glucose Response in CVLM C1 Neurons: A Possible Explanation for Anorexia of Aging. Biomolecules 2022, 12, 449. https://doi.org/10.3390/biom12030449
Ramlan H, Damanhuri HA. Attenuation of the Counter-Regulatory Glucose Response in CVLM C1 Neurons: A Possible Explanation for Anorexia of Aging. Biomolecules. 2022; 12(3):449. https://doi.org/10.3390/biom12030449
Chicago/Turabian StyleRamlan, Hajira, and Hanafi Ahmad Damanhuri. 2022. "Attenuation of the Counter-Regulatory Glucose Response in CVLM C1 Neurons: A Possible Explanation for Anorexia of Aging" Biomolecules 12, no. 3: 449. https://doi.org/10.3390/biom12030449
APA StyleRamlan, H., & Damanhuri, H. A. (2022). Attenuation of the Counter-Regulatory Glucose Response in CVLM C1 Neurons: A Possible Explanation for Anorexia of Aging. Biomolecules, 12(3), 449. https://doi.org/10.3390/biom12030449





