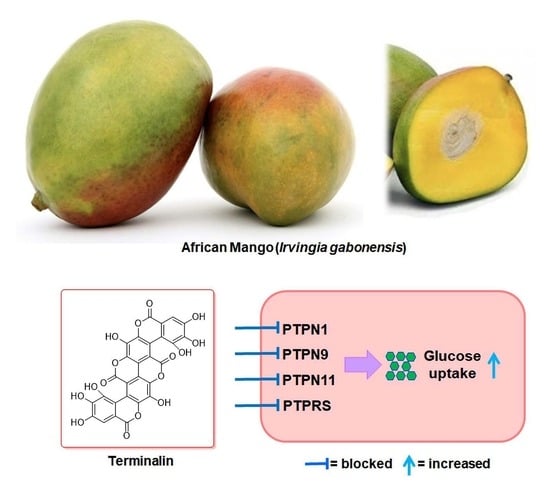
Terminalin from African Mango (Irvingia gabonensis) Stimulates Glucose Uptake through Inhibition of Protein Tyrosine Phosphatases
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Material
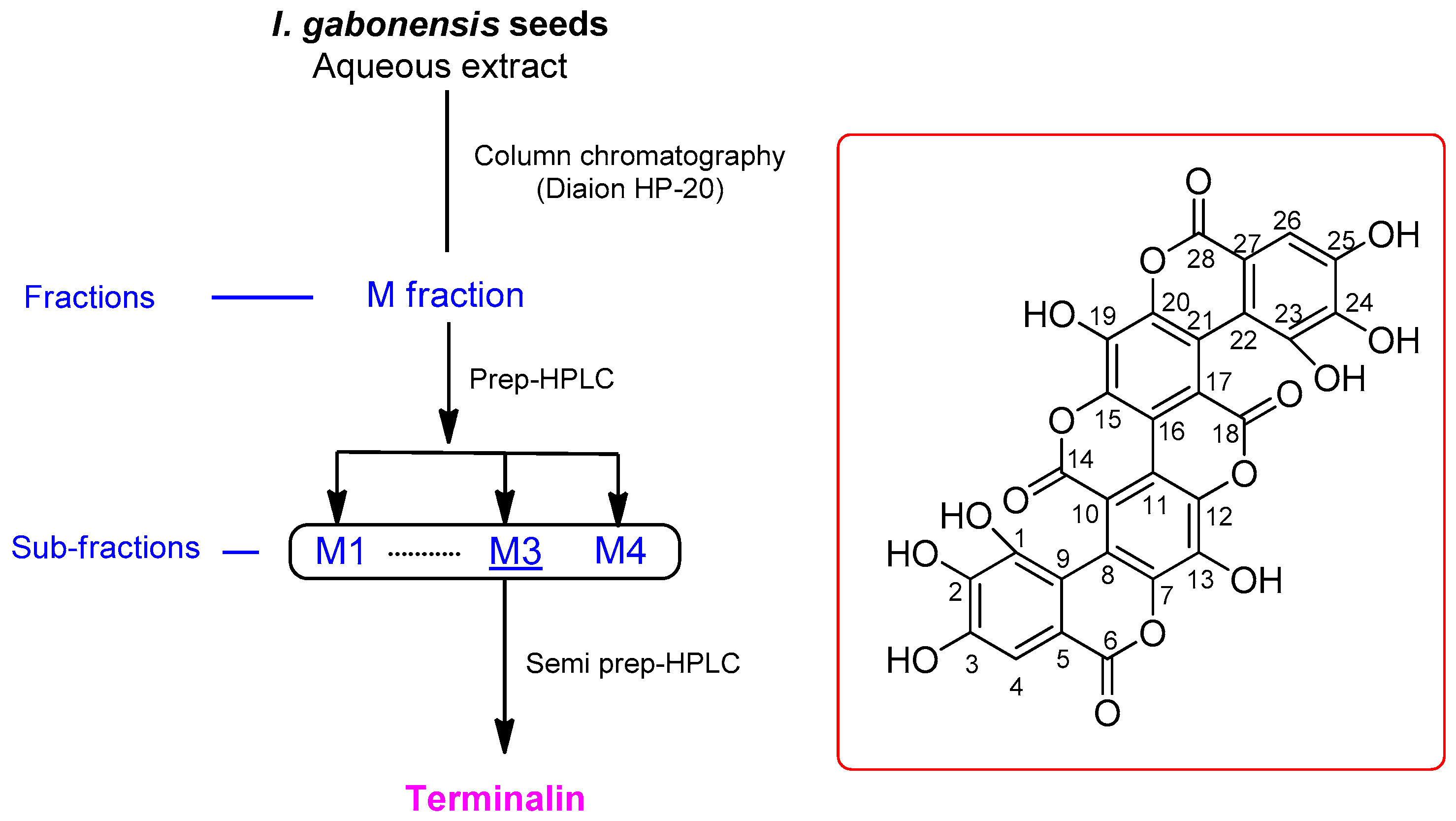
2.3. Extraction and Isolation
Terminalin
2.4. Measurement of the Effect of Terminalin on PTP Catalytic Activity
2.5. Cell Culture
2.6. Cell Differentiation
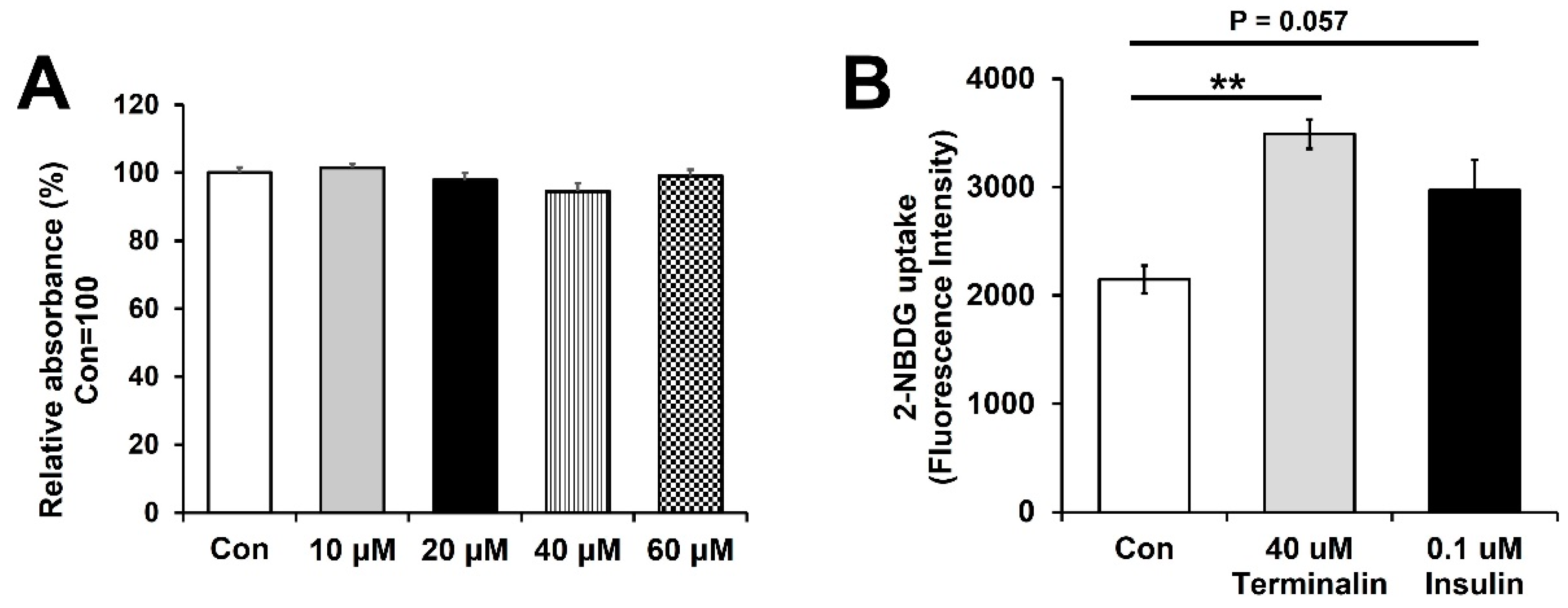
2.7. Cell Viability Assay
2.8. Glucose Uptake Assay
2.9. Statistical Analysis
3. Results and Discussion
3.1. Isolation of Terminalin
3.2. Structural Elucidation of the Isolated Compound
3.3. Terminalin Shows Inhibitory Effect on PTPs Relevant to Insulin Resistance
3.4. Terminalin Increases Glucose Uptake in C2C12 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tonks, N.K. Protein tyrosine phosphatases: From genes, to function, to disease. Nat. Rev. Mol. Cell Biol. 2006, 7, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Sevillano, J.; Sánchez-Alonso, M.G.; Pizarro-Delgado, J.; Ramos-Álvarez, M.D.P. Role of Receptor Protein Tyrosine Phos-phatases (RPTPs) in Insulin Signaling and Secretion. Int. J. Mol. Sci. 2021, 22, 5812. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wu, L.; Noh, S.J.; Guan, K.L.; Zhang, Z.Y. Altering the nucleophile specificity of a protein-tyrosine phospha-tase-catalyzed reaction. Probing the function of the invariant glutamine residues. J. Biol. Chem. 1998, 273, 5484–5492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bollu, L.; Mazumdar, A.; Savage, M.I.; Brown, P.H. Molecular Pathways: Targeting Protein Tyrosine Phosphatases in Cancer. Clin. Cancer Res. 2017, 23, 2136–2142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, R.-J.; Yu, Z.-H.; Zhang, R.-Y.; Zhang, Z.-Y. Protein tyrosine phosphatases as potential therapeutic targets. Acta Pharmacol. Sin. 2014, 35, 1227–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, C.; Kim, Y.; Ahn, D.; Chung, S.J. Protein tyrosine phosphatases (PTPs) in diabetes: Causes and therapeutic opportu-nities. Arch. Pharm. Res. 2021, 44, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.H.; Uddin, Z.; Jin, Y.M.; Li, Z.; Long, M.; Kim, K.D.; Cho, J.K.; Park, K.H. Inhibition of protein tyrosine phosphatase (PTP1B) and α-glucosidase by geranylated flavonoids from Paulownia tomentosa. J. Enzym. Inhib. Med. Chem. 2017, 32, 1195–1202. [Google Scholar] [CrossRef] [Green Version]
- Galic, S.; Klingler-Hoffmann, M.; Fodero-Tavoletti, M.T.; Puryer, M.A.; Meng, T.-C.; Tonks, N.K.; Tiganis, T. Regulation of Insulin Receptor Signaling by the Protein Tyrosine Phosphatase TCPTP. Mol. Cell. Biol. 2003, 23, 2096–2108. [Google Scholar] [CrossRef] [Green Version]
- Okoronkwo, C.; Agoha, E.; Ogodo, A.; Nwachukwu, N. Physical and chemical characteristics of the African bush mango (Ir-vingia gabonensis var garbonensis) seed oil. Int. J. Adv. Eng. Manag. 2014, 1, 28–31. [Google Scholar]
- Fungo, R.; Muyonga, J.H.; Kabahenda, M.; Okia, C.A.; Snook, L. Factors influencing consumption of nutrient rich forest foods in rural Cameroon. Appetite 2016, 97, 176–184. [Google Scholar] [CrossRef]
- Ofundem, T.; Ndip, N.R.; Abdon, A.; Patrice, L. Bush mango (Irvingia spp.): Forest and on-farm resource availability and market chains in the Southwest Region of Cameroon. For. Trees Livelihoods 2017, 26, 170–182. [Google Scholar] [CrossRef]
- Reguengo, L.M.; Barbosa-Pereira, L.; Rembangouet, W.; Bertolino, M.; Giordano, M.; Rojo-Poveda, O.; Zeppa, G. Food applications of Irvingia gabonensis (Aubry-Lecomte ex. O’Rorke) Baill., the ‘bush mango’: A review. Crit. Rev. Food Sci. Nutr. 2019, 60, 2446–2459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oben, J.E.; Ngondi, J.L.; Momo, C.N.; Agbor, G.A.; Sobgui, C.S.M. The use of a Cissus quadrangularis/Irvingia gabonensis combination in the management of weight loss: A double-blind placebo-controlled study. Lipids Health Dis. 2008, 7, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obianime, A.; Uche, F. The phytoconstituents and the comparative effects of aqueous extract of Irvingia gabonensis seeds and proviron on the biochemical parameters of male guinea pigs. Asian Pac. J. Trop. Med. 2010, 3, 101–104. [Google Scholar] [CrossRef] [Green Version]
- Ngondi, J.L.; Oben, J.E.; Minka, S.R. The effect of Irvingia gabonensis seeds on body weight and blood lipids of obese subjects in Cameroon. Lipids Health Dis. 2005, 4, 12. [Google Scholar] [CrossRef] [Green Version]
- Giami, S.Y.; Okonkwo, V.I.; Akusu, M.O. Chemical composition and functional properties of raw, heat-treated and partially proteolysed wild mango (Irvingia gabonensis) seed flour. Food Chem. 1994, 49, 237–243. [Google Scholar] [CrossRef]
- Arogba, S.; Omede, A. Comparative Antioxidant Activity of Processed Mango (Mangifera indica) and Bush Mango (Irvingia gabonensis) Kernels. Niger. Food J. 2012, 30, 17–21. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.R.; Kang, H.; Yoo, M.J.; Yu, J.S.; Lee, S.; Yi, S.A.; Beemelmanns, C.; Lee, J.; Kim, K.H. Anti-adipogenic pregnane steroid from a Hydractinia-associated fungus, Cladosporium sphaerospermum SW67. Nat. Prod. Sci. 2020, 26, 230–235. [Google Scholar]
- Lee, S.; Ryoo, R.; Choi, J.H.; Kim, J.H.; Kim, S.H.; Kim, K.H. Trichothecene and tremulane sesquiterpenes from a halluci-nogenic mushroom Gymnopilus junonius and their cytotoxicity. Arch. Pharm. Res. 2020, 43, 214–223. [Google Scholar] [CrossRef]
- Ha, J.W.; Kim, J.; Kim, H.; Jang, W.; Kim, K.H. Mushrooms: An important source of natural bioactive compounds. Nat. Prod. Sci. 2020, 26, 118–131. [Google Scholar]
- Yu, J.S.; Park, M.; Pang, C.; Rashan, L.; Jung, W.H.; Kim, K.H. Antifungal Phenols from Woodfordia uniflora Collected in Oman. J. Nat. Prod. 2020, 83, 2261–2268. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Kim, J.K.; Yu, J.S.; Jeong, S.Y.; Choi, J.H.; Kim, J.-C.; Ko, Y.-J.; Kim, S.-H.; Kim, K.H. Ginkwanghols A and B, osteogenic coumaric acid-aliphatic alcohol hybrids from the leaves of Ginkgo biloba. Arch. Pharm Res. 2021, 44, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, C.S.; Yu, J.S.; Kang, H.; Yoo, M.J.; Youn, U.J.; Ryoo, R.; Bae, H.Y.; Kim, K.H. Ergopyrone, a Styrylpyrone-Fused Steroid with a Hexacyclic 6/5/6/6/6/5 Skeleton from a Mushroom Gymnopilus orientispectabilis. Org. Lett. 2021, 23, 3315–3319. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kim, W.; Lee, Y.G.; Kang, H.J.; Lee, S.-H.; Park, S.Y.; Min, J.-K.; Chung, S.J. Identification of sennoside A as a novel inhibitor of the slingshot (SSH) family proteins related to cancer metastasis. Pharmacol. Res. 2017, 119, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.Y.; Ahn, D.; Hwang, J.Y.; Kang, M.J.; Chung, S.J. Linoleic acid exerts antidiabetic effects by inhibiting protein ty-rosine phosphatases associated with insulin resistance. J. Funct. Foods 2021, 83, 104532. [Google Scholar] [CrossRef]
- Yoon, S.-Y.; Kang, H.J.; Ahn, D.; Hwang, J.Y.; Kwon, S.J.; Chung, S.J. Identification of chebulinic acid as a dual targeting inhibitor of protein tyrosine phosphatases relevant to insulin resistance. Bioorganic Chem. 2019, 90, 103087. [Google Scholar] [CrossRef]
- Oelrichs, P.B.; Pearce, C.M.; Zhu, J.; Filippich, L.J. Isolation and structure determination of terminalin a toxic condensed tannin fromTerminalia oblongata. Nat. Toxins 1994, 2, 144–150. [Google Scholar] [CrossRef]
- Hirano, Y.; Kondo, R.; Sakai, K. 5A-Reductase inhibitory tannin-related compounds isolated from Shorea laeviforia. J. Wood Sci. 2003, 49, 339–343. [Google Scholar] [CrossRef]
- Furihata, K.; Seto, H. Decoupled HMBC (D-HMBC), an improved technique of HMBC. Tetrahedron Lett. 1995, 36, 2817–2820. [Google Scholar] [CrossRef]
- Orgil, O.; Schwartz, E.; Baruch, L.; Matityahu, I.; Mahajna, J.; Amir, R. The antioxidative and anti-proliferative potential of non-edible organs of the pomegranate fruit and tree. LWT 2014, 58, 571–577. [Google Scholar] [CrossRef]
- Reddy, M.K.; Gupta, S.K.; Jacob, M.R.; Khan, S.I.; Ferreira, D. Antioxidant, antimalarial and antimicrobial activities of tan-nin-rich fractions, ellagitannins and phenolic acids from Punica granatum L. Planta Med. 2007, 73, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.S.; Zhang, Y.; Seeram, N.P.; Heber, D.; Chen, S. Pomegranate ellagitannin-derived compounds exhibit antiprolif-erative and antiaromatase activity in breast cancer cells in vitro. Cancer Prev. Res. 2010, 3, 108–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lansky, E.P.; Newman, R.A. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J. Ethnopharmacol. 2007, 109, 177–206. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.Y.; Kim, D.H.; Min Roh, K.; Ahn, D.; Jin Kang, H.; Chung, S.J. Identification of Vaccinia-H1 Related Phosphatase as an Anticancer Target for 1,2,3,4,6-O-Pentagalloylglucose. Chem. Biodivers. 2020, 17, e1900414. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, W.J.; Pulido, R. Protein tyrosine phosphatase variants in human hereditary disorders and disease susceptibilities. Biochim. et Biophys. Acta (BBA)-Mol. Basis Dis. 2013, 1832, 1673–1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCullough, B.S.; Batsomboon, P.; Hutchinson, K.B.; Dudley, G.B.; Barrios, A.M. Synthesis and PTP Inhibitory Activity of Illudalic Acid and Its Methyl Ether, with Insights into Selectivity for LAR PTP over Other Tyrosine Phosphatases under Physio-logically Relevant Conditions. J. Nat. Prod. 2019, 82, 3386–3393. [Google Scholar] [CrossRef]
- Li, Y.; Duan, B.; Li, Y.; Yu, S.; Wang, Y. The isoflavonoid calycosin inhibits inflammation and enhances beta cell function in gestational diabetes mellitus by suppressing RNF38 expression. Immunopharmacol. Immunotoxicol. 2020, 42, 366–372. [Google Scholar] [CrossRef]
- Lambadiari, V.; Triantafyllou, K.; Dimitriadis, G.D. Insulin action in muscle and adipose tissue in type 2 diabetes: The sig-nificance of blood flow. World, J. Diabetes 2015, 6, 626–633. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Hur, H.J.; Kwon, D.Y.; Hwang, J.-T. Tangeretin stimulates glucose uptake via regulation of AMPK signaling pathways in C2C12 myotubes and improves glucose tolerance in high-fat diet-induced obese mice. Mol. Cell. Endocrinol. 2012, 358, 127–134. [Google Scholar] [CrossRef]
- Long, Y.C.; Zierath, J.R. AMP-activated protein kinase signaling in metabolic regulation. J. Clin. Invest. 2006, 116, 1776–1783. [Google Scholar] [CrossRef] [Green Version]
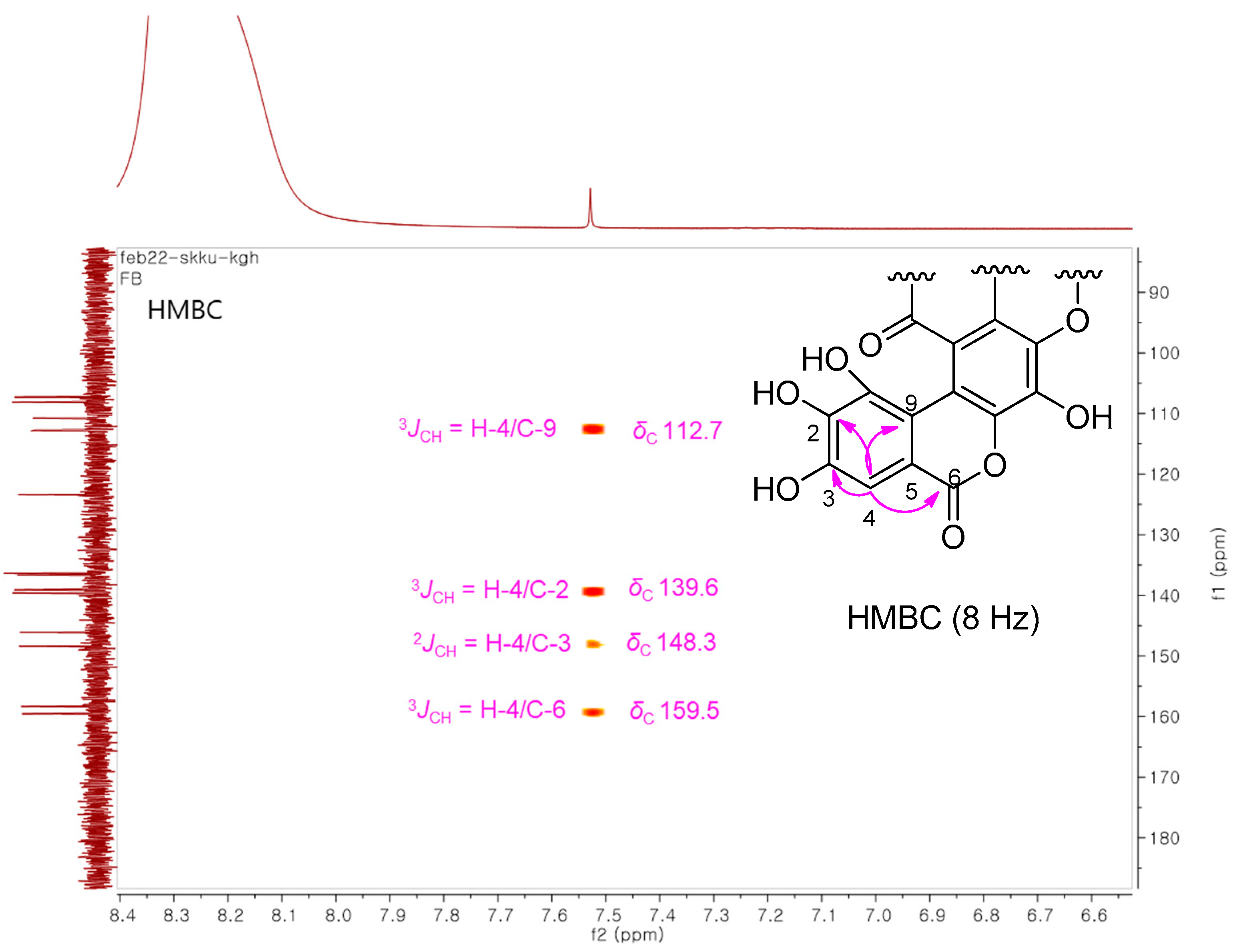
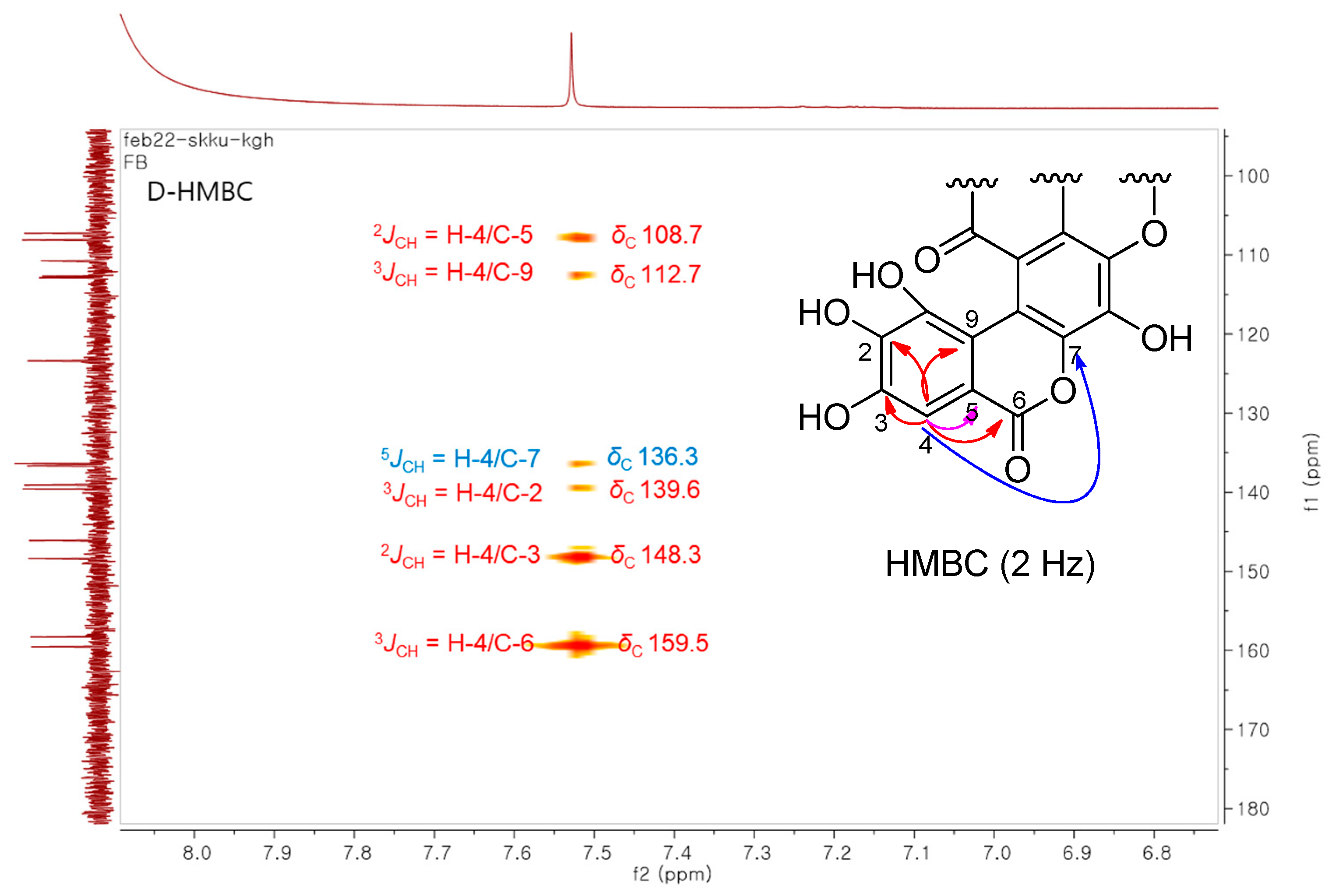

| Position | Terminalin | |
|---|---|---|
| δH (J in Hz) | δC | |
| 1, 23 | 139.0 s | |
| 2, 24 | 139.6 s | |
| 3, 25 | 148.3 s | |
| 4, 26 | 7.52 s | 110.7 d |
| 5, 27 | 108.7 s | |
| 6, 28 | 159.5 s | |
| 7, 20 | 136.3 s | |
| 8, 21 | 123.3 s | |
| 9, 22 | 112.7 s | |
| 10, 17 | 107.2 s | |
| 11, 16 | 112.8 s | |
| 12, 15 | 136.6 s | |
| 13, 19 | 146.1 s | |
| 14, 18 | 158.3 s | |
| [E] (nM) | KM (µM) | |
|---|---|---|
| PTPN1 | 0.5 | 163.1 |
| PTPN9 | 0.1 | 200 |
| PTPN11 | 1.5 | 74 |
| PTPRS | 0.45 | 56 |
| PTPRF | 0.6 | 150 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, S.-Y.; Kim, J.; Lee, B.S.; Baek, S.C.; Chung, S.J.; Kim, K.H. Terminalin from African Mango (Irvingia gabonensis) Stimulates Glucose Uptake through Inhibition of Protein Tyrosine Phosphatases. Biomolecules 2022, 12, 321. https://doi.org/10.3390/biom12020321
Yoon S-Y, Kim J, Lee BS, Baek SC, Chung SJ, Kim KH. Terminalin from African Mango (Irvingia gabonensis) Stimulates Glucose Uptake through Inhibition of Protein Tyrosine Phosphatases. Biomolecules. 2022; 12(2):321. https://doi.org/10.3390/biom12020321
Chicago/Turabian StyleYoon, Sun-Young, Jinsoo Kim, Bum Soo Lee, Su Cheol Baek, Sang J. Chung, and Ki Hyun Kim. 2022. "Terminalin from African Mango (Irvingia gabonensis) Stimulates Glucose Uptake through Inhibition of Protein Tyrosine Phosphatases" Biomolecules 12, no. 2: 321. https://doi.org/10.3390/biom12020321
APA StyleYoon, S.-Y., Kim, J., Lee, B. S., Baek, S. C., Chung, S. J., & Kim, K. H. (2022). Terminalin from African Mango (Irvingia gabonensis) Stimulates Glucose Uptake through Inhibition of Protein Tyrosine Phosphatases. Biomolecules, 12(2), 321. https://doi.org/10.3390/biom12020321