By Modulating the Hormonal Balance and Ribonuclease Activity of Tomato Plants Bacillus subtilis Induces Defense Response against Potato Virus X and Potato Virus Y
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Objects
2.2. 16S rRNA Gene Sequencing
2.3. Experimental Design
2.4. Assessment of Viral Diseases
2.4.1. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) to Investigate Virus Accumulation
2.4.2. Double-Antibody Sandwich Enzyme-Linked Immunosorbent Assay (DAS-ELISA)
2.4.3. Western Blotting Assays
2.5. Assessment of the Endophytic Properties of the Bacillus Strains
2.6. Assessment of the Growth, Fresh and Dry Weight and Yield of Tomato Plants
2.7. Assessment of Ribonuclease Activity in the Liquid Culture Medium of Bacteria and in Tomato Plants
2.8. Isolation of RNA and Performing the Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) to Investigate Defense Gene Expression of Tomato Plants
2.9. Assessment of the Phytohormone Content in the Liquid Culture Medium of Bacteria and in Tomato Plants
2.9.1. Cytokinins Assay
2.9.2. IAA and ABA Assays
2.10. Statistics
3. Results
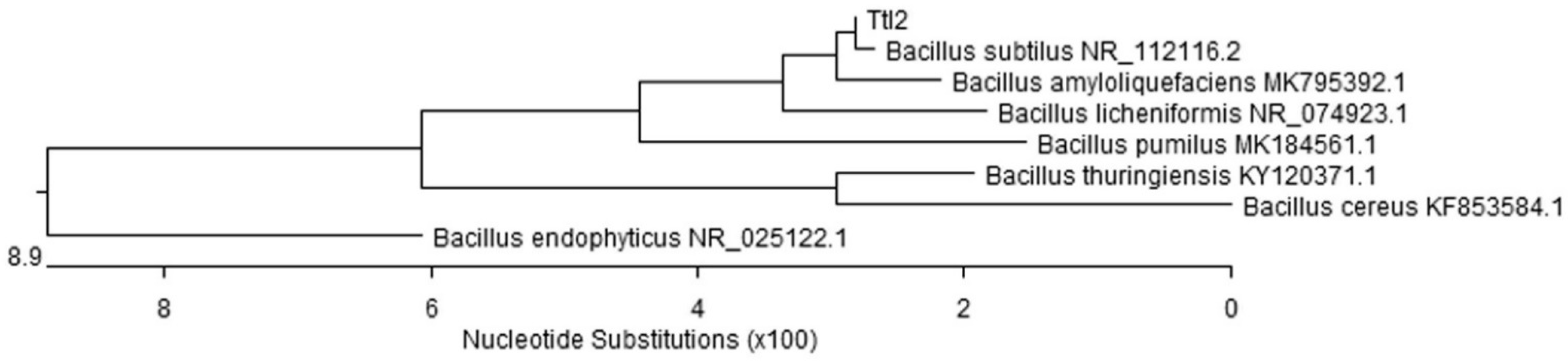
3.1. Characterization of the B. subtilis 26D and B. subtilis Ttl2 Strains
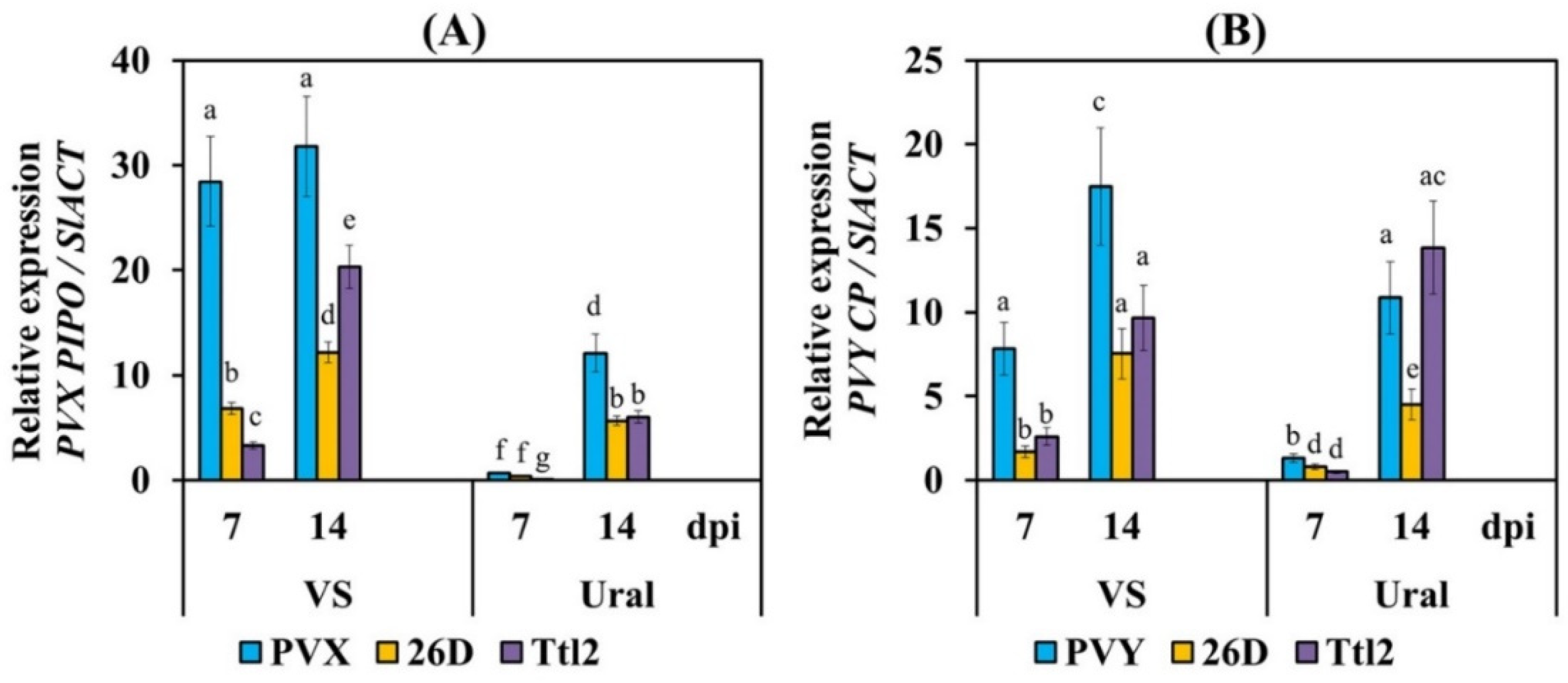
3.2. Endophytic Strains B. subtilis 26D and B. subtilis Ttl2 Suppress the Accumulation of PVX and PVY in Tomato Plants
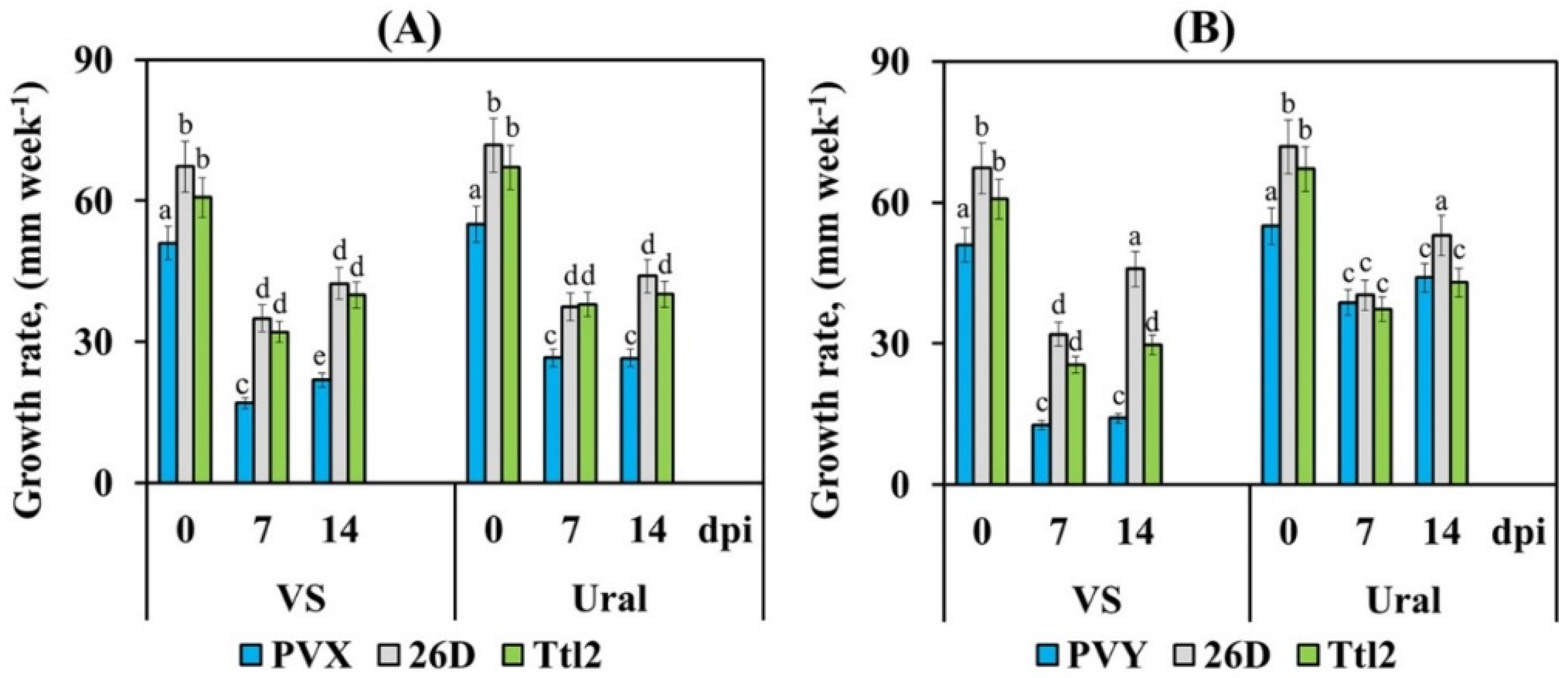
3.3. Endophytic Strains B. subtilis 26D and B. subtilis Ttl2 Promote Plant Growth, Biomass Accumulation and Fruit Yield of Tomato Plants Infected with PVX and PVY
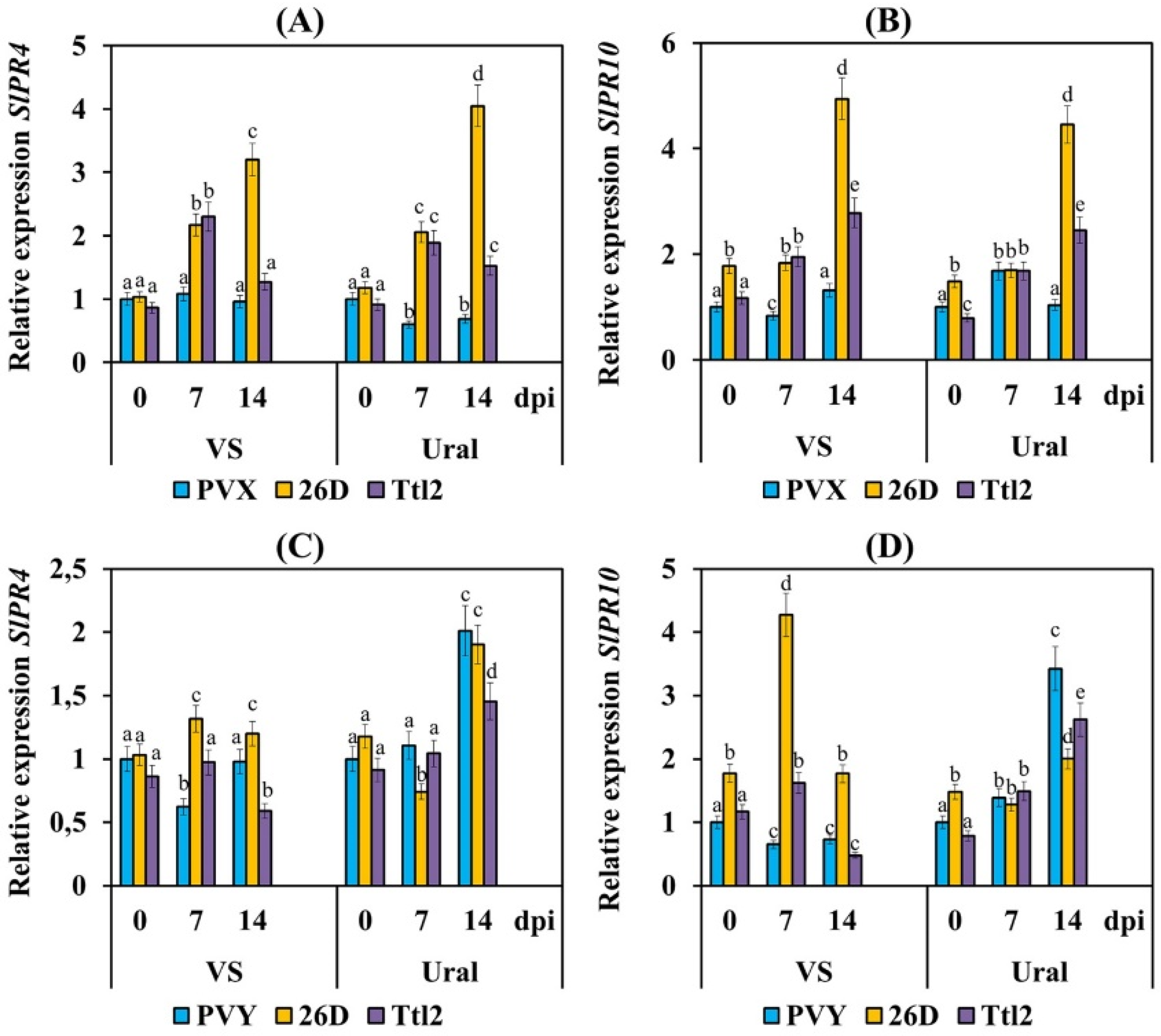
3.4. Endophytic Strains B. subtilis 26D and B. subtilis Ttl2 Increase Ribonuclease and Transcriptional Activity of the Pathogenesis-Related Genes PR4 and PR10 in Tomato Plants Infected with PVX and PVY
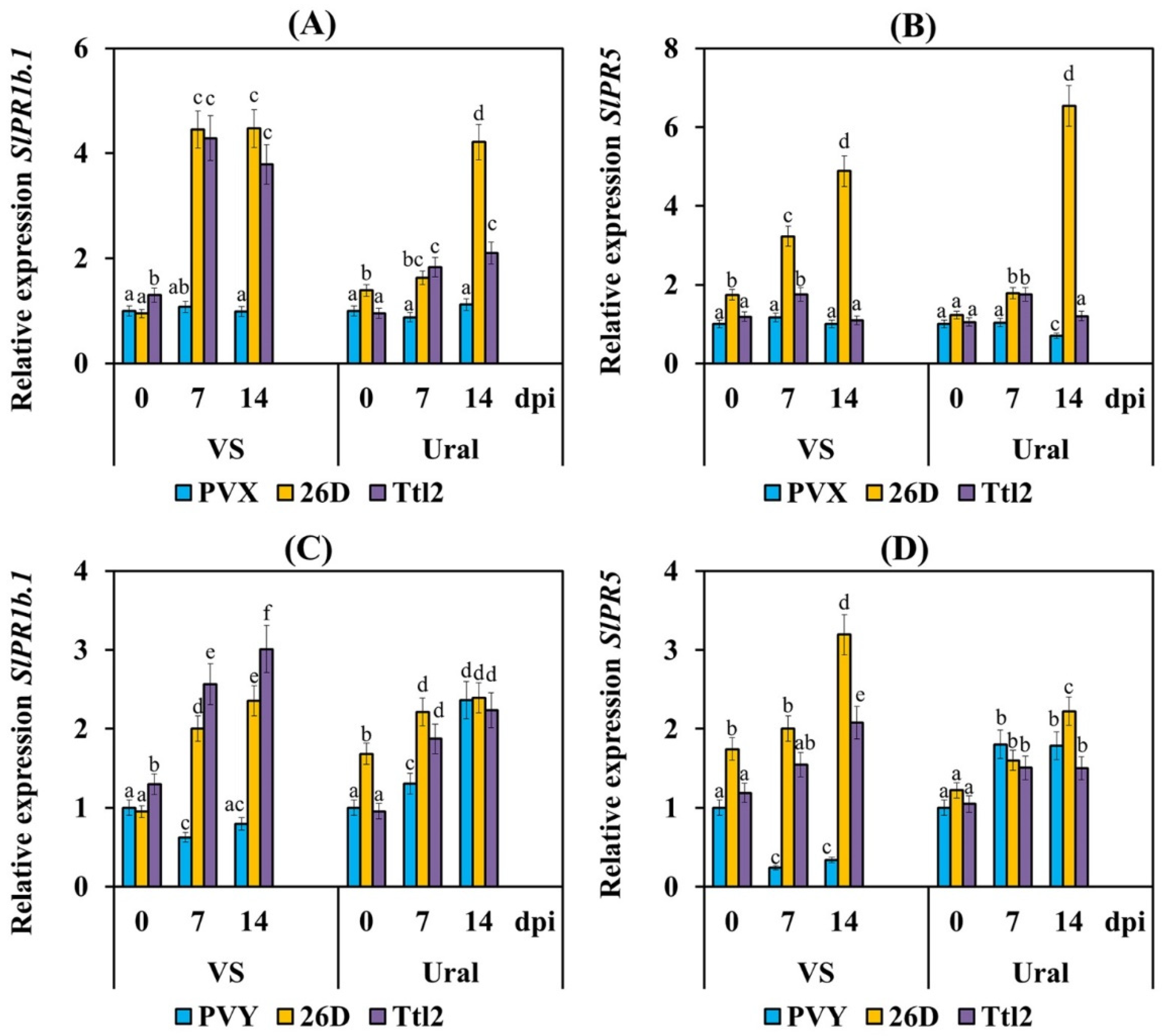
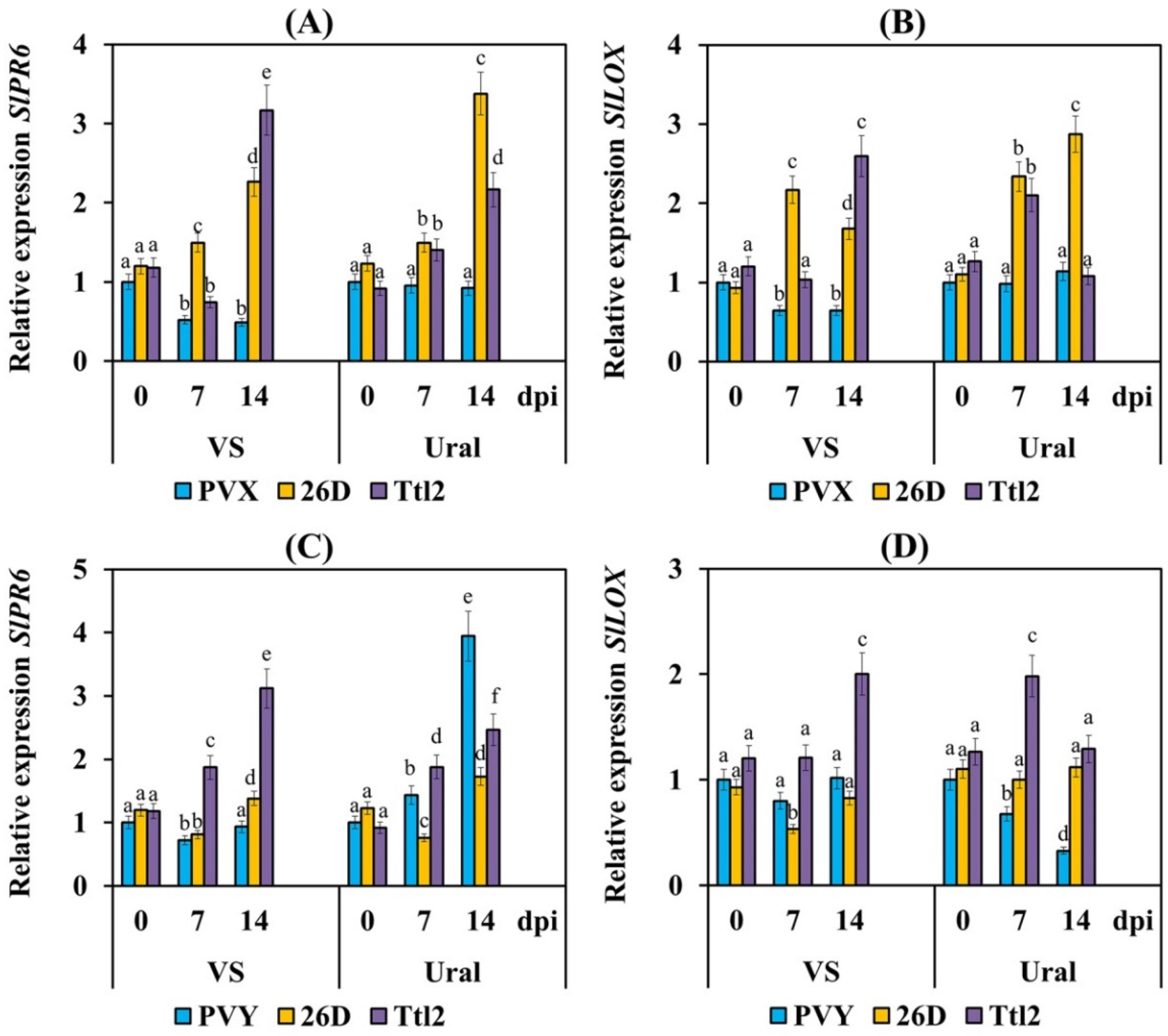
3.5. Endophytic Strains B. subtilis 26D and B. subtilis Ttl2 Induce Systemic Resistance in Tomato Plants Infected with PVX and PVY
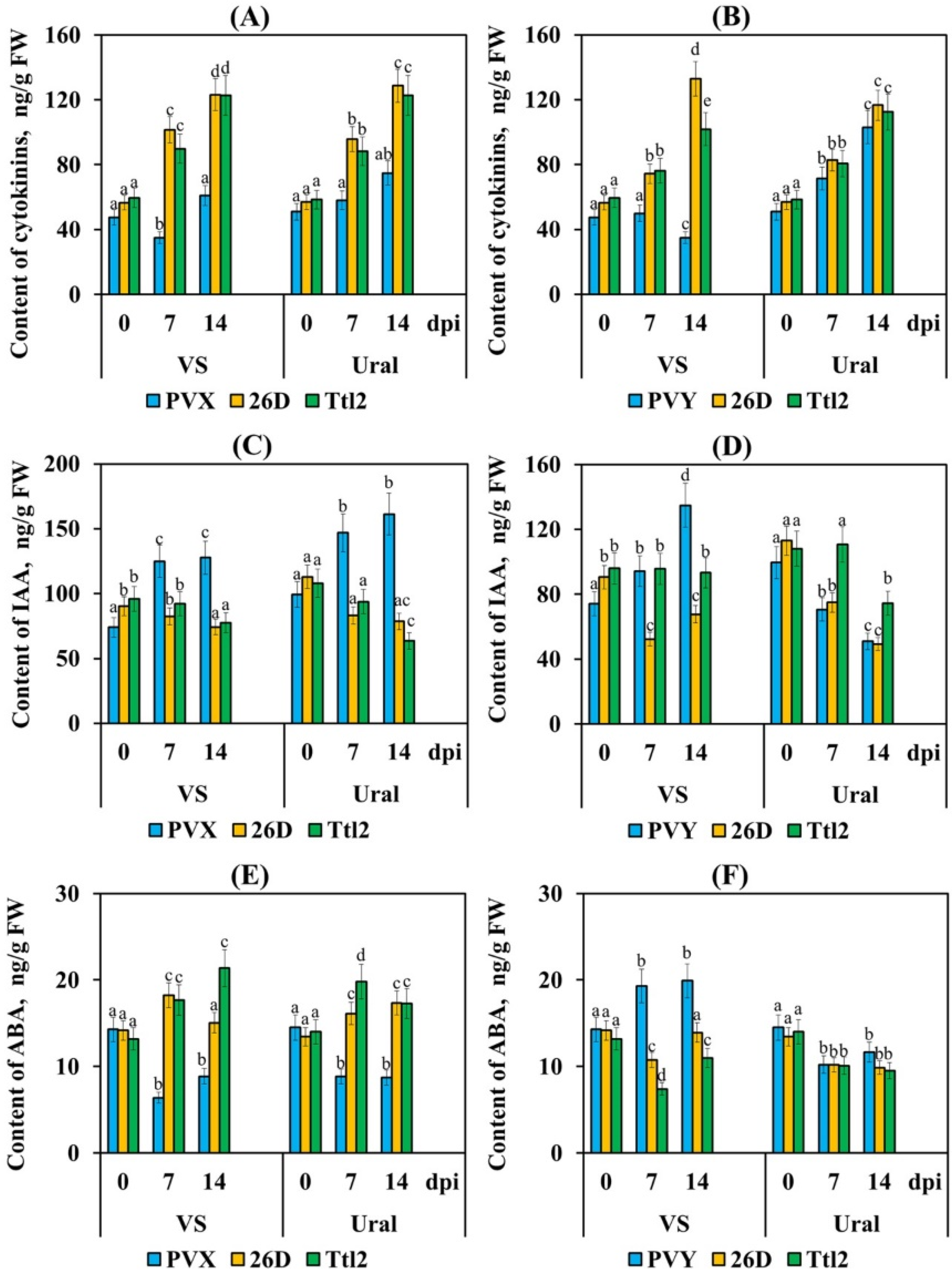
3.6. Endophytic Strains B. subtilis 26D and B. subtilis Ttl2 Regulate the Level of Phytohormones in Tomato Plants Infected with PVX and PVY
4. Discussion
4.1. Effect of Potato Virus X and Potato Virus Y on Tomato Plants
4.2. Antiviral Activity of the Endophytic Strains B. subtilis 26D and B. subtilis Ttl2
4.3. The Hormone-Producing Endophytic Strains B. subtilis 26D and B. subtilis Ttl2 Increase the Growth and Fruit Yield of Tomato Plants Infected with PVX and PVY
4.4. Effect of the Endophytic Strains B. subtilis 26D and B. subtilis Ttl2 on the Ribonucleases of Tomato Plants Infected with PVX and PVY
4.5. Effect of the Endophytic Strains B. subtilis 26D and B. subtilis Ttl2 on Triggering the Induced Systemic Resistance of Tomato Plants Infected with PVX and PVY
4.6. Effect of the Endophytic Strains B. subtilis 26D and B. subtilis Ttl2 on the Hormonal Balance of Tomato Plants Infected with PVX and PVY
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, R.A.C. Global plant virus disease pandemics and epidemics. Plants 2021, 10, 233. [Google Scholar] [CrossRef]
- Cheng, J.; Xie, J.; Luo, X. Synthesis and antiviral activity against Coxsackie virus B3 of some novel benzimidazole derivatives. Bioorg. Med. Chem. Lett. 2005, 15, 267–269. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Bakker, P.A.H.M.; Pieterse, C.M.J. Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 1998, 36, 453–483. [Google Scholar] [CrossRef] [PubMed]
- Baebler, Š.; Coll, A.; Gruden, K. Plant molecular responses to potato virus Y: A continuum of outcomes from sensitivity and tolerance to resistance. Viruses 2020, 12, 217. [Google Scholar] [CrossRef]
- Prins, M.; Laimer, M.; Noris, E.; Schubert, J.; Wassenegger, M.; Tepfer, M. Strategies for anti- viral resistance in transgenic plants. Mol. Plant Pathol. 2008, 9, 73–83. [Google Scholar] [CrossRef]
- Raupach, G.S.; Kloepper, J.W. Mixtures of plant growth-promoting rhizobacteria enhance biological control of multiple cucumber pathogens. Phytopathology 1998, 88, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Maksimov, I.V.; Sorokan, A.V.; Burkhanova, G.F.; Veselova, S.V.; Alekseev, V.Y.; Shein, M.Y.; Avalbaev, A.M.; Dhaware, P.D.; Mehetre, G.T.; Singh, B.P.; et al. Mechanisms of plant tolerance to RNA viruses induced by plant-growth-promoting microorganisms. Plants 2019, 8, 575. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Ryu, C.-M. Spraying of leaf-colonizing Bacillus amyloliquefaciens protects pepper from Cucumber mosaic virus. Plant Dis. 2016, 100, 2099–2105. [Google Scholar] [CrossRef]
- Kumar, S.; Chauhan, P.S.; Agrawal, L.; Raj, R.; Srivastava, A.; Gupta, S.; Mishra, S.K.; Yadav, S.; Singh, P.C.; Raj, S.K.; et al. Paenibacillus lentimorbus inoculation enhances tobacco growth and extenuates the virulence of Cucumber mosaic virus. PLoS ONE 2016, 11, e0149980. [Google Scholar] [CrossRef]
- Beris, D.; Theologidis, I.; Skandalis, N.; Vassilakos, N. Bacillus amyloliquefaciens strain MBI600 induces salicylic acid-dependent resistance in tomato plants against Tomato spotted wilt virus and Potato virus Y. Sci. Rep. 2018, 8, 10320. [Google Scholar] [CrossRef]
- Harish, S.; Kavino, M.; Kumar, N.; Balasubramanian, P.; Samiyappan, R. Induction of defense-related proteins by mixtures of plant growth promoting endophytic bacteria against Banana bunchy top virus. Appl. Soil Ecol. 2008, 39, 187–200. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Al-Askar, A.A.; Behiry, S.I. Bacillus licheniformis strain POT1 mediated polyphenol biosynthetic pathways genes activation and systemic resistance in potato plants against Alfalfa mosaic virus. Sci. Rep. 2020, 10, 16120. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, A.; Singh, R.; Pandey, K.D. Endophytic bacteria: A new source of bioactive compounds. 3 Biotech 2017, 7, 315. [Google Scholar] [CrossRef] [PubMed]
- Rana, K.L.; Kour, D.; Kaur, T.; Devi, R.; Yadav, A.N.; Yadav, N.; Dhaliwal, H.S.; Saxena, A.K. Endophytic microbes: Biodiversity, plant growth-promoting mechanisms and potential applications for agricultural sustainability. Antonie Leeuwenhoek 2020, 113, 1075–1107. [Google Scholar] [CrossRef]
- Le Cocq, K.; Gurr, S.J.; Hirsch, P.R.; Mauchline, T.H. Exploitation of endophytes for sustainable agricultural intensification. Mol. Plant Pathol. 2017, 18, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Zehnder, G.W.; Yao, C.; Murphy, J.F.; Sikora, E.R.; Kloepper, J.W. Induction of resistance in tomato against Cucumber mosaic cucumovirus by plant growth-promoting rhizobacteria. Biocontrol 2000, 45, 127–137. [Google Scholar] [CrossRef]
- Murphy, J.F.; Zehnder, G.W.; Schuster, D.J.; Sikora, E.J.; Polston, J.E.; Kloepper, J.W. Plant growth-promoting rhizobacterial mediated protection in tomato against Tomato mottle virus. Plant Dis. 2000, 84, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Megahed, A.A.; El-Dougdoug, K.A.; Othman, B.A.; Lashin, S.M.; Ibrahim, M.A.; Sofy, A.R. Induction of resistance in tomato plants against tomato mosaic tobamovirus using beneficial microbial isolates. Pak. J. Biol. Sci. 2013, 16, 385–390. [Google Scholar] [CrossRef]
- Ryu, C.-M.; Murphy, J.F.; Mysore, K.S.; Kloepper, J.W. Plant growth-promoting rhizobacteria systemically protect Arabidopsis thaliana against Cucumber mosaic virus by a salicylic acid and NPR1-independent and jasmonic acid-dependent signaling pathway. Plant J. 2004, 39, 381–392. [Google Scholar] [CrossRef]
- Elsharkawy, M.M.; Shimizu, M.; Takahashi, H.; Ozaki, K.; Hyakumachi, M. Induction of systemic resistance against Cucumber mosaic virus in Arabidopsis thaliana by Trichoderma asperellum SKT-1. Plant Pathol. J. 2013, 29, 193–200. [Google Scholar] [CrossRef]
- Khalimi, K.; Temaja, I.; Suprapta, D. Systemic resistance induced by Stenotrophomonas maltophilia Sg3 against Cucumber mosaic virus in tobacco plant. Intl. J. Agric. Biol. 2020, 23, 149–154. [Google Scholar] [CrossRef]
- Taha, M.; Ghaly, M.; Atwa, H.; Askoura, M. Evaluation of the effectiveness of soil Streptomyces isolates for induction of plant resistance against Tomato mosaic virus (ToMV). Curr. Microbiol. 2021, 78, 3032–3043. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-S.; Paul, D.; Ryu, K.-R.; Kim, E.-Y.; Kim, Y.-K. Bacillus vallismortis strain EXTN-1 mediated systemic resistance against Potato virus Y and X in the field. Plant Pathol. J. 2006, 22, 360. [Google Scholar] [CrossRef]
- Sorokan, A.; Cherepanova, E.; Burkhanova, G.; Veselova, S.; Rumyantsev, S.; Alekseev, V.; Mardanshin, I.; Sarvarova, E.; Khairullin, R.; Benkovskaya, G.; et al. Endophytic Bacillus spp. as a prospective biological tool for control of viral diseases and non-vector Leptinotarsa decemlineata Say. in Solanum tuberosum L. Front. Microbiol. 2020, 11, 569457. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.W.; Niu, T.G. Purification and some properties of an extracellular ribonuclease withantiviral activity against tobacco mosaic virus from Bacillus cereus. Biotechnol. Lett. 2009, 31, 101–105. [Google Scholar] [CrossRef]
- Trifonova, E.A.; Ibragimova, S.M.; Volkova, O.A.; Shumny, V.K.; Kochetov, A.V. Ribonuclease activity as a new prospective disease resistance marker in potato. Vavilov J. Genet. Breed. 2018, 22, 987–991. [Google Scholar] [CrossRef]
- Miljakovic, D.; Marinkovic, J.; Baleševic-Tubic, S. The significance of Bacillus spp. in disease suppression and growth promotion of field and vegetable crops. Microorganisms 2020, 8, 1037. [Google Scholar] [CrossRef]
- Goodfellow, S.; Zhang, D.; Wang, M.B.; Zhang, R. Bacterium-mediated RNA interference: Potential application in plant protection. Plants 2019, 8, 572. [Google Scholar] [CrossRef]
- Maksimov, I.V.; Veselova, S.V.; Nuzhnaya, T.V.; Sarvarova, E.R.; Khairullin, R.M. Plant growth promoting bacteria in regulation of plant resistance to stress factors. Russ. J. Plant Physiol. 2015, 62, 715–726. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; van Wees, S.C.; Bakker, P.A. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Y. Current understanding of the interplays between host hormones and plant viral infections. PLoS Pathol. 2021, 17, e1009242. [Google Scholar] [CrossRef] [PubMed]
- Robert-Seilaniantz, A.; Grantm, M.; Jones, J.D. Hormone crosstalk in plant disease and defense: More than just jasmonate-salicylate antagonism. Annu. Rev. Phytopathol. 2011, 49, 317–343. [Google Scholar] [CrossRef] [PubMed]
- Müllender, M.; Varrelmann, M.; Savenkov, E.I.; Liebe, S. Manipulation of auxin signalling by plant viruses. Mol. Plant Pathol. 2021, 22, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Alazem, M.; Lin, N.-S. Interplay between ABA signaling and RNA silencing in plant viral resistance. Curr. Opin. Virol. 2020, 42, 1–7. [Google Scholar] [CrossRef]
- Wu, X.; Ye, J. Manipulation of jasmonate signaling by plant viruses and their insect vectors. Viruses 2020, 12, 148. [Google Scholar] [CrossRef]
- Sorokan, A.; Veselova, S.; Benkovskaya, G.; Maksimov, I. Endophytic strain Bacillus subtilis 26D increases levels of phytohormones and repairs growth of potato plants after Colorado potato beetle damage. Plants 2021, 10, 923. [Google Scholar] [CrossRef]
- Santner, A.; Estelle, M. Recent advances and emerging trends in plant hormone signalling. Nature 2009, 459, 1071–1078. [Google Scholar] [CrossRef]
- Sharipova, M.; Rockstroh, A.; Balaban, N.; Mardanova, A.; Toymentseva, A.; Tikhonova, A. Antiviral Effect of Ribonuclease from Bacillus pumilus against phytopathogenic RNA-Viruses. Agric. Sci. 2015, 6, 1357–1366. [Google Scholar] [CrossRef][Green Version]
- Khalaf, E.M.; Raizada, M.N. Bacterial seed endophytes of domesticated cucurbits antagonize fungal and oomycete pathogens including powdery mildew. Front. Microbiol. 2018, 9, 42. [Google Scholar] [CrossRef]
- Ulyanova, V.; Mahmud, R.S.; Dudkina, E.; Vershinina, V.; Domann, E.; Ilinskaya, O. Phylogenetic distribution of extracellular guanyl-preferring ribonucleases renews taxonomic status of two Bacillus strains. J. Gen. Appl. Microbiol. 2016, 62, 181–188. [Google Scholar] [CrossRef][Green Version]
- Ilinskaya, O.; Ulyanova, V.; Lisevich, I.; Dudkina, E.; Zakharchenko, N.; Kusova, A.; Faizullin, D.; Zuev, Y. The native monomer of Bacillus pumilus ribonuclease does not exist extracellularly. BioMed Res. Int. 2018, 2018, 4837623. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, A.A.; Azzami, K.; Ryabchikova, E.I.; Spitsyna, Y.E.; Silnikov, V.N.; Ritter, W.; Gross, H.J.; Tautz, J.; Vlassov, V.V.; Beier, H.; et al. Inactivation of a non-enveloped RNA virus by artificial ribonucleases: Honey bees and acute bee paralysis virus as a new experimental model for in vivo antiviral activity assessment. Antivir. Res. 2011, 91, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S.; Rao, A.L.N. Riboproteomics: A versatile approach for the identification of host protein interaction network in plant pathogenic noncoding RNAs. PLoS ONE 2017, 12, e0186703. [Google Scholar] [CrossRef] [PubMed]
- Sokurenko, Y.; Nadyrova, A.; Ulyanova, V.; Ilinskaya, O. Extracellular ribonuclease from Bacillus licheniformis (balifase), a new member of the N1/T1 RNase superfamily. BioMed Res. Int. 2016, 2016, 4239375. [Google Scholar] [CrossRef]
- Linskaya, O.N.; Karamova, N.S.; Ivanchenco, O.B.; Kipenskaya, L.V. SOS-inducing ability of native and mutant microbial ribonucleases. Mutat. Res. 1996, 354, 203–209. [Google Scholar] [CrossRef]
- Veselov, S.Y.; Timergalina, L.N.; Akhiyarova, G.R.; Kudoyarova, G.R.; Korobova, A.V.; Ivanov, I.; Arkhipova, T.N.; Prinsen, E. Study of cytokinin transport from shoots to roots of wheat plants is informed by a novel method of differential localization of free cytokinin bases or their ribosylated forms by means of their specific fixation. Protoplasma 2018, 255, 1581–1594. [Google Scholar] [CrossRef]
- Arkhipova, T.N.; Veselov, S.U.; Melentiev, A.I.; Martynenko, E.V.; Kudoyarova, G.R. Ability of bacterium Bacillus subtilis to produce cytokinins and to influence the growth and endogenous hormone content of lettuce plants. Plant Soil 2005, 272, 201–209. [Google Scholar] [CrossRef]
- Bouizgarne, B. Bacteria for plant growth promotion and disease management. In Bacteria in Agrobiology: Disease Management; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; Chapter 2; pp. 15–46. [Google Scholar]
- Kudoyarova, G.R.; Veselov, D.S.; Sharipova, G.V.; Akhiyarova, R.G.; Dodd, I.C.; Veselov, S.Y. Water relations and growth of original barley plants and its ABA-deficient mutants at increased air temperature. Russ. J. Plant Physiol. 2014, 61, 188–193. [Google Scholar] [CrossRef]
- Kudoyarova, G.R.; Korobova, A.V.; Akhiyarova, G.R.; Arkhipova, T.N.; Zaytsev, D.Y.; Prinsen, E.; Egutkin, N.L.; Medvedev, S.S.; Veselov, S.Y. Accumulation of cytokinins in roots and their export to the shoots of durum wheat plants treated with the protonophore carbonyl cyanide m-chlorophenylhydrazone (CCCP). J. Exp. Bot. 2014, 65, 2287–2294. [Google Scholar] [CrossRef]
- Maksimov, I.V.; Blagova, D.K.; Veselova, S.V.; Sorokan, A.V.; Burkhanova, G.F.; Cherepanova, E.A.; Sarvarova, E.R.; Rumyantsev, S.D.; Alekseev, V.Y.; Khayrullin, R.M. Recombinant Bacillus subtilis 26DCryChS line with gene Btcry1Ia encoding Cry1Ia toxin from Bacillus thuringiensis promotes integrated wheat defense against pathogen Stagonospora nodorum Berk. and greenbug Schizaphis graminum Rond. Biocontrol 2020, 144, 104242. [Google Scholar] [CrossRef]
- Musidlak, O.; Nawrot, R.; Goździcka-Józefiak, A. Which plant proteins are involved in antiviral defense? Review on in vivo and in vitro activities of selected plant proteins against viruses. Int. J. Mol. Sci. 2017, 18, 2300. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.J.; Antoniw, J.F.; Bar-Joseph, M.; Brunt, A.A.; Candresse, T.; Foster, G.D.; Martelli, G.P.; Milne, R.G.; Zavriev, S.K.; Fauquet, C.M. The new plant virus family Flexiviridae and assessment of molecular criteria for species demarcation. Arch. Virol. 2004, 149, 1045–1060. [Google Scholar] [CrossRef]
- Cong, Q.Q.; Wang, Y.; Liu, J.; Guo, J.G.; Yang, X.-D.; Li, X.; Tian, Y.P. Evaluation of Potato virus X mild mutants for cross protection against severe infection in China. Virol. J. 2019, 16, 36. [Google Scholar] [CrossRef] [PubMed]
- Huisman, M.J.; Linthorst, H.J.; Bol, J.F.; Cornelissen, J.C. The complete nucleotide sequence of potato virus X and its homologies at the amino acid level with various plus-stranded RNA viruses. J. Gen. Virol. 1988, 69, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Morozov, S.Y.; Solovyev, A.G. Triple gene block: Modular design of a multifunctional machine for plant virus movement. J. Gen. Virol. 2003, 84, 1351–1366. [Google Scholar] [CrossRef]
- Bayne, E.H.; Rakitina, D.V.; Morozov, S.Y.; Baulcombe, D.C. Cell-to-cell movement of Potato Potexvirus X is dependent on suppression of RNA silencing. Plant J. 2005, 44, 471–482. [Google Scholar] [CrossRef]
- Chiu, M.H.; Chen, I.H.; Baulcombe, D.C.; Tsai, C.H. The silencing suppressor P25 of Potato virus X interacts with Argonaute1 and mediates its degradation through the proteasome pathway. Mol. Plant Pathol. 2010, 11, 641–649. [Google Scholar] [CrossRef]
- Yang, X.; Lu, Y.; Wang, F.; Chen, Y.; Tian, Y.; Jiang, L.; Peng, J.; Zheng, H.; Lin, L.; Yan, C.; et al. Involvement of the chloroplast gene ferredoxin 1 in multiple responses of Nicotiana benthamiana to Potato virus X infection. J. Exp. Bot. 2020, 71, 2142–2156. [Google Scholar] [CrossRef]
- Chung, B.Y.-W.; Miller, W.A.; Atkins, J.F.; Firth, A.E. An overlapping essential gene in the Potyviridae. Proc. Natl. Acad. Sci. USA 2008, 105, 5897–5902. [Google Scholar] [CrossRef]
- Hanssen, I.M.; Lapidot, M.; Thomma, B.P. Emerging viral diseases of tomato crops. Mol. Plant Microbe Interact. 2010, 23, 539–548. [Google Scholar] [CrossRef]
- Kwon, J.; Kasai, A.; Maoka, T.; Masuta, C.; Sano, T.; Nakahara, K.S. RNA silencing-related genes contribute to tolerance of infection with potato virus X and Y in a susceptible tomato plant. Virol. J. 2020, 17, 149. [Google Scholar] [CrossRef] [PubMed]
- De Santi Ferrara, F.I.; Oliveira, Z.M.; Gonzales, H.H.S.; Floh, E.I.S.; Barbosa, H.R. Endophytic and rhizospheric enterobacteria isolated from sugar cane have different potentials for producing plant growth-promoting substances. Plant Soil 2012, 353, 409–417. [Google Scholar] [CrossRef]
- Perez-Flores, P.; Valencia-Cantero, E.; Altamirano-Hernandez, J.; Pelagio-Flores, R.; Lopez-Bucio, J.; Garcia-Juarez, P. Bacillus methylotrophicus M4-96 isolated from maize (Zea mays) rhizoplane increases growth and auxin content in Arabidopsis thaliana via emission of volatiles. Protoplasma 2017, 254, 2201–2213. [Google Scholar] [CrossRef] [PubMed]
- Kudoyarova, G.; Arkhipova, T.; Korshunova, T.; Bakaeva, M.; Loginov, O.; Dodd, I.C. Phytohormone mediation of interactions between plants and non-symbiotic growth promoting bacteria under edaphic stresses. Front. Plant Sci. 2019, 10, 1368. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ji, B.; Su, X.; Li, H.; Dong, C.; Chen, S.; Zhu, Y.; Feng, W. Isolation of endophytic bacteria from Rehmannia glutinosa Libosch and their potential to promote plant growth. J. Gen. Appl. Microbiol. 2020, 66, 279–288. [Google Scholar] [CrossRef]
- Sandhya, V.; Ali, S.Z.; Venkateswarlu, B.; Reddy, G.; Grover, M. Effect of osmotic stress on plant growth promoting Pseudomonas spp. Arch. Microbiol. 2010, 192, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.Q.; Ye, X.S.; Kuc, J. Induction of chitinases in tobacco plants systemically protected against blue mold by peronospora-tabacina or tobacco mosaic-virus. Phytopathology 1992, 821, 119–123. [Google Scholar] [CrossRef]
- Park, C.J.; Kim, K.J.; Shin, R.; Park, J.M.; Shin, Y.C.; Paek, K.H. Pathogenesis-related protein 10 isolated from hot pepper functions as a ribonuclease in an antiviral pathway. Plant J. 2004, 37, 186–198. [Google Scholar] [CrossRef]
- Lee, W.S.; Fu, S.F.; Verchot-Lubicz, J.; Carr, J.P. Genetic modification of alternative respiration in Nicotiana benthamiana affects basal and salicylic acid-induced resistance to potato virus X. BMC Plant Biol. 2011, 11, 41. [Google Scholar] [CrossRef]
- Yang, X.; Lu, Y.; Zhao, X.; Jiang, L.; Xu, S.; Peng, J.; Zheng, H.; Lin, L.; Wu, Y.; MacFarlane, S.; et al. Downregulation of nuclear protein H2B induces salicylic acid mediated defense against PVX infection in Nicotiana benthamiana. Front. Microbiol. 2019, 10, 1000. [Google Scholar] [CrossRef]
- Makarova, S.; Makhotenko, A.; Spechenkova, N.; Love, A.J.; Kalinina, N.O.; Taliansky, M. Interactive responses of potato (Solanum tuberosum L.) plants to heat stress and infection with Potato virus Y. Front. Microbiol. 2018, 9, 2582. [Google Scholar] [CrossRef] [PubMed]
- Baebler, Š.; Witek, K.; Petek, M.; Stare, K.; Tušek-Žnidarič, M.; Pompe-Novak, M.; Renaut, J.; Szajko, K.; Strzelczyk-Żyta, D.; Marczewski, W.; et al. Salicylic acid is an indispensable component of the Ny-1 resistance-gene-mediated response against Potato virus Y infection in potato. J. Exp. Bot. 2014, 65, 1095–1109. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Xi, D.H.; Xu, F.; Wang, S.D.; Cao, S.; Xu, M.Y.; Zhao, P.P.; Wang, J.H.; Jia, S.D.; Zhang, Z.W.; et al. A broad-spectrum, efficient and nontransgenic approach to control plant viruses by application of salicylic acid and jasmonic acid. Planta 2011, 233, 299–308. [Google Scholar] [CrossRef]
- Baebler, S.; Krecic-Stres, H.; Rotter, A.; Kogovšek, P.; Cankar, K.; Kok, E.J. PVYNTN elicits a diverse gene expression response in different potato genotypes in the first 12 h after inoculation. Mol. Plant Pathol. 2009, 10, 263–275. [Google Scholar] [CrossRef] [PubMed]
- García-Marcos, A.; Pacheco, R.; Manzano, A.; Aguilar, E.; Tenllado, F. Oxylipin biosynthesis genes positively regulate programmed cell death during compatible infections with the synergistic pair potato virus X-potato virus Y and Tomato spotted wilt virus. J. Virol. 2013, 87, 5769–5783. [Google Scholar] [CrossRef]
- Alazem, M.; Lin, N.S. Antiviral roles of abscisic acid in plants. Front. Plant Sci. 2017, 8, 1760. [Google Scholar] [CrossRef]
- Kudoyarova, G.R.; Vysotskaya, L.B.; Arkhipova, T.N.; Yu, K.L.; Galimsyanova, N.F.; Sidorova, L.V.; Gabbasova, I.M.; Melentiev, A.I.; Veselov, S.Y. Effect of auxin producing and phosphate solubilizing bacteria on mobility of soil phosphorus, growth rate, and P acquisition by wheat plants. Acta Physiol. Plant. 2017, 39, 253. [Google Scholar] [CrossRef]
- Arkhipova, T.N.; Prinsen, E.; Veselov, S.U.; Martinenko, E.V.; Melentiev, A.I.; Kudoyarova, G.R. Cytokinin producing bacteria enhance plant growth in drying soil. Plant Soil 2007, 292, 305–315. [Google Scholar] [CrossRef]
- Cohen, A.C.; Travaglia, C.N.; Bottini, R.; Piccoli, P.N. Participation of abscisic acid and gibberellins produced by endophytic Azospirillum in the alleviation of drought effects in maize. Botany 2009, 87, 455–462. [Google Scholar] [CrossRef]
- Dermastia, M.; Ravnikar, M. Altered cytokinin pattern and enhanced tolerance to potato virus Y-NTN in the susceptible potato cultivar (Solanum tuberosum cv Igor) grown in vitro. Physiol. Mol. Plant Pathol. 1996, 48, 65–71. [Google Scholar] [CrossRef]
- Clarke, S.F.; McKenzie, M.J.; Burritt, D.J.; Guy, P.L.; Jameson, P.E. Influence of white clover mosaic potexvirus infection on the endogenous cytokinin content of bean. Plant Physiol. 1999, 120, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Kovač, M.; Müller, A.; Milovanovič Jarh, D.; Milavec, M.; Düchting, P.; Ravnikar, M. Multiple hormone analysis indicates involvement of jasmonate signalling in the early defence of potato to potato virus YNTN. Biol. Plant. 2009, 53, 195–199. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Benková, E. Cytokinin cross-talking during biotic and abiotic stress responses. Front. Plant Sci. 2013, 4, 451. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Mekureyaw, M.F.; Pandey, C.; Roitsch, T. Role of cytokinins for interactions of plants with microbial pathogens and pest insects. Front. Plant Sci. 2020, 10, 1777. [Google Scholar] [CrossRef]
- Pollini, C.P.; Masia, A.; Giunchedi, L. Free indole-3-acetic acid in sugar-beet root of rhizomania-susceptible and moderately resistant cultivars. Phytopathol. Mediterr. 1990, 29, 191–195. [Google Scholar]
- Qin, Q.; Li, G.; Jin, L.; Huang, Y.; Wang, Y.; Wei, C.; Xu, Z.; Yang, Z.; Wang, H.; Li, Y. Auxin response factors (ARFs) differentially regulate rice antiviral immune response against rice dwarf virus. PLoS Pathog. 2020, 16, e1009118. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Meng, D.; Wang, Y.; Wu, Y.; Lang, C.; Jin, T.; Zhou, X. The viral suppressor HCPro decreases DNA methylation and activates auxin biosynthesis genes. Virology 2020, 546, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Rudd, J.J.; Kanyuka, K. Virus induced gene silencing (VIGS) for functional analysis of wheat genes involved in Zymoseptoria tritici susceptibility and resistance. Fungal Genet. Biol. 2015, 79, 84–88. [Google Scholar] [CrossRef]
- Alazem, M.; Lin, K.Y.; Lin, N.S. The abscisic acid pathway has multifaceted effects on the accumulation of Bamboo mosaic virus. Mol. Plant-Microbe Int. 2014, 27, 177–189. [Google Scholar] [CrossRef]
- Alazem, M.; He, M.H.; Moffett, P.; Lin, N.S. Abscisic acid induces resistance against Bamboo mosaic virus through Argonaute2 and 3. Plant Physiol. 2017, 174, 339–355. [Google Scholar] [CrossRef]
- Jaubert, M.; Bhattacharjee, S.; Mello, A.F.; Perry, K.L.; Moffett, P. ARGONAUTE2 mediates RNA-silencing antiviral defenses against Potato virus X in Arabidopsis. Plant Physiol. 2011, 156, 1556–1564. [Google Scholar] [CrossRef] [PubMed]
- Alazem, M.; Lin, N.S. Roles of plant hormones in the regulation of host-virus interactions. Mol. Plant Pathol. 2015, 16, 529–540. [Google Scholar] [CrossRef] [PubMed]

| Strain | Endophyticity, CFU * 104/g of Fresh Weight | Ribonuclease Activity, Units/(mL of Liquid Medium per Min) | Phytohormone Level, µg/mL of Culture Medium | |||
|---|---|---|---|---|---|---|
| Shoot | Root | IAA | ABA | Cytokinins ** | ||
| B. subtilis 26D | 13.1 ± 1.43 a | 0.17 ± 0.03 a | 5.23 ± 0.25 a | 0.11 ± 0.02 a | 0.0 a | 0.15 ± 0.007 a |
| B. subtilis Ttl2 | 7.28 ± 1.22 b | 0.99 ± 0.06 b | 3.02 ± 0.16 b | 0.27 ± 0.01 b | 0.0 a | 0.08 ± 0.004 b |
| Variant of Treatment | Parameter | ||
|---|---|---|---|
| Shoot Height, cm | Fresh Weight of Shoot, g | Dry Weight of Shoot, g | |
| Cultivar Ural | |||
| Control | 25.9 ± 0.6 a | 8.3 ± 0.6 a | 0.9 ± 0.04 a |
| B. subtilis 26D | 32.2 ± 0.6 b | 11.7 ± 0.7 b | 1.5 ± 0.06 b |
| Bacillus sp. Ttl2 | 30.9 ± 0.7 b | 11.0 ± 0.6 b | 1.5 ± 0.05 b |
| Potato virus X(PVX) | 21.3 ± 0.8 c | 4.5 ± 0.3 c | 0.4 ± 0.03 c |
| B. subtilis 26D + PVX | 25.7 ± 0.9 a | 9.3 ± 0.5 a | 1.0 ± 0.05 a |
| Bacillus sp. Ttl2 + PVX | 25.0 ± 0.5 a | 9.6 ± 0.6 a | 1.1 ± 0.06 a |
| Potato virus Y(PVY) | 24.6 ± 0.7 a | 7.5 ± 0.4 a | 0.8 ± 0.05 a |
| B. subtilis 26D + PVY | 26.8 ± 0.8 a | 9.6 ± 0.5 a | 1.1 ± 0.05 a |
| Bacillus sp. Ttl2 + PVY | 25.4 ± 0.9 a | 8.8 ± 0.6 a | 1.0 ± 0.05 a |
| Cultivar Volovye Serdtse | |||
| Control | 19.8 ± 0.4 a | 6.3 ± 0.4 a | 0.7 ± 0.04 a |
| B. subtilis 26D | 23.5 ± 0.3 b | 6.7 ± 0.3 a | 0.8 ± 0.03 a |
| Bacillus sp. Ttl2 | 22.4 ± 0.8 b | 6.9 ± 0.5 a | 0.9 ± 0.04 a |
| Potato virus X(PVX) | 14.7 ± 0.9 c | 4.9 ± 0.3 b | 0.4 ± 0.02 b |
| B. subtilis 26D + PVX | 19.5 ± 0.8 a | 6.0 ± 0.4 a | 0.6 ± 0.03 a |
| Bacillus sp. Ttl2 + PVX | 18.8 ± 0.9 a | 6.0 ± 0.3 a | 0.7 ± 0.04 a |
| Potato virus Y(PVY) | 13.5 ± 0.9 c | 4.4 ± 0.2 b | 0.4 ± 0.02 b |
| B. subtilis 26D + PVY | 19.6 ± 0.9 a | 5.5 ± 0.4 ab | 0.6 ± 0.04 a |
| Bacillus sp. Ttl2 + PVY | 17.1 ± 1.1 a | 4.2 ± 0.2 b | 0.4 ± 0.02 b |
| Variant of Treatment | Parameter | |||
|---|---|---|---|---|
| Number of Fruits, n | Average Fruit Weight, g | Total Weight of Fruits per Bush, g | Yield, % of Control | |
| Cultivar Ural | ||||
| Control | 19.3 ± 0.9 a | 103.6 ± 5.6 a | 1995.0 ± 32.9 a | 100 |
| B. subtilis 26D | 22.0 ± 1.0 a | 143.6 ± 3.8 b | 3157.6 ± 66.7 b | 158.3 ± 3.3 |
| Bacillus sp. Ttl2 | 20.0 ± 0.6 a | 128.7 ± 2.4 ab | 2574.0 ± 99.0 c | 129.0 ± 5.0 |
| Potato virus X(PVX) | 0.0 ± 0.0 b | 0.0 ± 0.0 c | 0.0 ± 0.0 d | 0.0 ± 0.0 |
| B. subtilis 26D + PVX | 16.3 ± 0.9 c | 99.0 ± 2.1 a | 1613.3 ± 52.4 e | 80.1 ± 2.6 |
| Bacillus sp. Ttl2 + PVX | 14.0 ± 1.2 c | 82.7 ± 4.3 d | 1147.3 ± 35.4 f | 57.5 ± 1.8 |
| Potato virus Y(PVY) | 8.3 ± 0.9 d | 82.0 ± 6.1 d | 673.0 ± 23.5 g | 33.7 ± 1.2 |
| B. subtilis 26D + PVY | 17.0 ± 0.6 c | 124.3 ± 3.0 ab | 2111.3 ± 52.6 a | 105.8 ± 2.6 |
| Bacillus sp. Ttl2 + PVY | 12.0 ± 1.2 cd | 87.3 ± 3.5 d | 1040.0 ± 59.4 f | 52.1 ± 3.0 |
| Cultivar Volovye Serdtse | ||||
| Control | 11.7 ± 0.9 a | 142.7 ± 4.3 a | 1658.0 ± 89.8 a | 100 |
| B. subtilis 26D | 13.3 ± 0.9 a | 200.3 ± 5.8 b | 2661.0 ± 98.2 b | 160.5 ± 5.9 |
| Bacillus sp. Ttl2 | 13.3 ± 1.2 a | 162.3 ± 4.3 c | 2162.3 ± 190.8 c | 130.4 ± 11.5 |
| Potato virus X(PVX) | 0.0 ± 0.0 b | 0.0 ± 0.0 d | 0.0 ± 0.0 d | 0.0 ± 0.0 |
| B. subtilis 26D + PVX | 7.3 ± 1.2 c | 71.0 ± 2.1 e | 520.7 ± 85.4 e | 31.4 ± 5.2 |
| Bacillus sp. Ttl2 + PVX | 5.0 ± 0.6 c | 117.3 ± 9.3 f | 576.0 ± 24.0 e | 34.7 ± 1.5 |
| Potato virus Y(PVY) | 0.0 ± 0.0 b | 0.0 ± 0.0 d | 0.0 ± 0.0 d | 0.0 ± 0.0 |
| B. subtilis 26D + PVY | 11.0 ± 0.6 a | 79.3 ± 2.9 e | 874.0 ± 67.1 f | 52.7 ± 4.0 |
| Bacillus sp. Ttl2 + PVY | 6.0 ± 0.6 c | 95.0 ± 2.9 f | 566.7 ± 37.6 e | 34.2 ± 2.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veselova, S.V.; Sorokan, A.V.; Burkhanova, G.F.; Rumyantsev, S.D.; Cherepanova, E.A.; Alekseev, V.Y.; Sarvarova, E.R.; Kasimova, A.R.; Maksimov, I.V. By Modulating the Hormonal Balance and Ribonuclease Activity of Tomato Plants Bacillus subtilis Induces Defense Response against Potato Virus X and Potato Virus Y. Biomolecules 2022, 12, 288. https://doi.org/10.3390/biom12020288
Veselova SV, Sorokan AV, Burkhanova GF, Rumyantsev SD, Cherepanova EA, Alekseev VY, Sarvarova ER, Kasimova AR, Maksimov IV. By Modulating the Hormonal Balance and Ribonuclease Activity of Tomato Plants Bacillus subtilis Induces Defense Response against Potato Virus X and Potato Virus Y. Biomolecules. 2022; 12(2):288. https://doi.org/10.3390/biom12020288
Chicago/Turabian StyleVeselova, Svetlana V., Antonina V. Sorokan, Guzel F. Burkhanova, Sergey D. Rumyantsev, Ekaterina A. Cherepanova, Valentin Y. Alekseev, Elena R. Sarvarova, Albina R. Kasimova, and Igor V. Maksimov. 2022. "By Modulating the Hormonal Balance and Ribonuclease Activity of Tomato Plants Bacillus subtilis Induces Defense Response against Potato Virus X and Potato Virus Y" Biomolecules 12, no. 2: 288. https://doi.org/10.3390/biom12020288
APA StyleVeselova, S. V., Sorokan, A. V., Burkhanova, G. F., Rumyantsev, S. D., Cherepanova, E. A., Alekseev, V. Y., Sarvarova, E. R., Kasimova, A. R., & Maksimov, I. V. (2022). By Modulating the Hormonal Balance and Ribonuclease Activity of Tomato Plants Bacillus subtilis Induces Defense Response against Potato Virus X and Potato Virus Y. Biomolecules, 12(2), 288. https://doi.org/10.3390/biom12020288








