IGFBP7 Concentration May Reflect Subclinical Myocardial Damage and Kidney Function in Patients with Stable Ischemic Heart Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Study Population
2.2. Biochemical Evaluation
2.3. Statistical Analysis
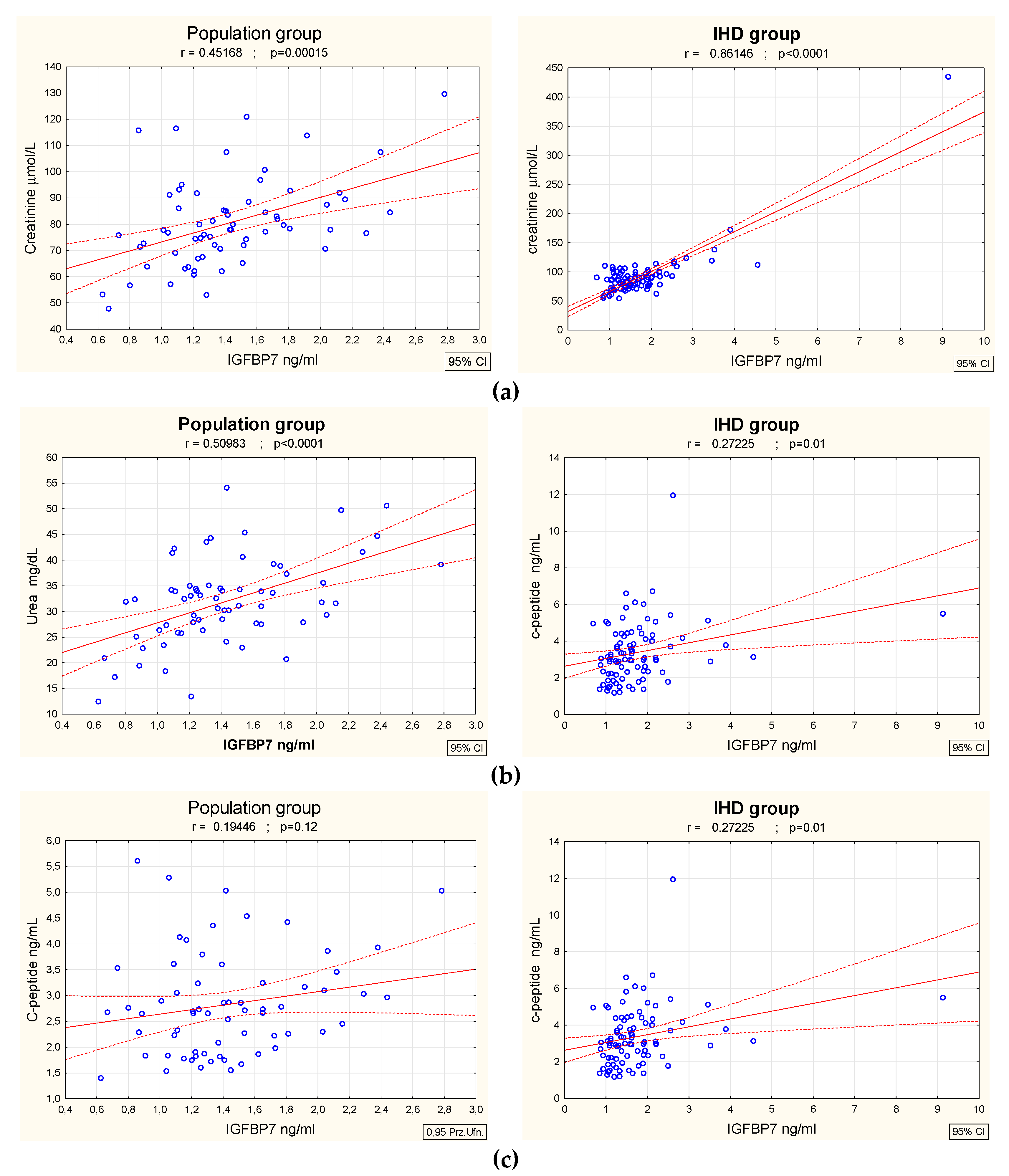
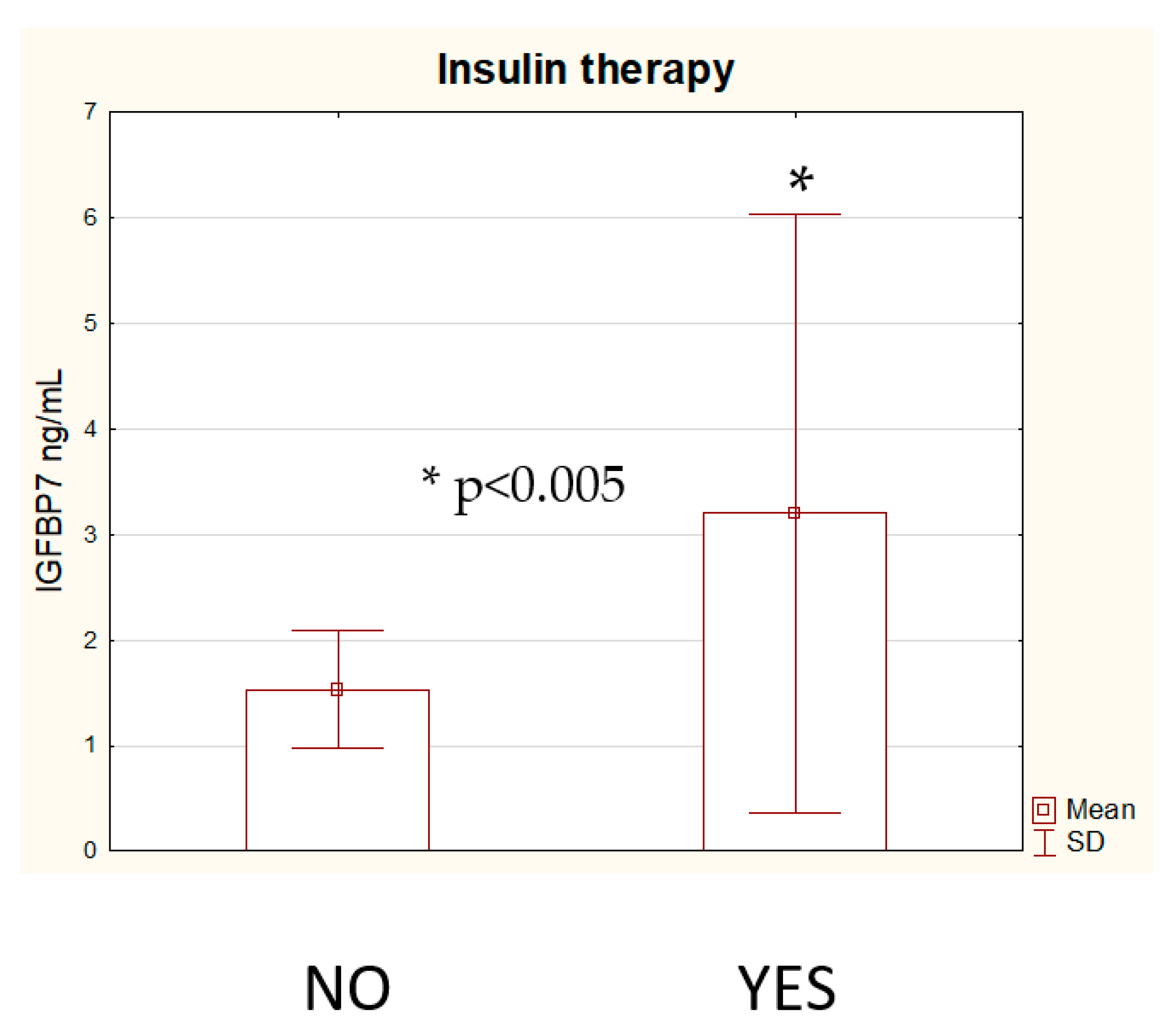
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Anuradha, L.; Akshay, S.D. The Role of Coronary Artery Disease in Heart Failure. Heart Fail. Clin. 2014, 10, 353–365. [Google Scholar] [CrossRef]
- Shahab, K.; Farshad, F.; John, O.; Majid, E.; Andrew, M. World-wide risk factors for heart failure: A systematic review and pooled analysis. Int. J. Cardiol. 2013, 168, 1186–1194. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; Al Suwaidi, S.K.B.M.; AlKatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.E.A.H.; Alzaabi, M.E.H.; Al Darmaki, R.S.; et al. Global Epidemiology of Ischemic Heart Disease: Results from the Global Burden of Disease Study. Cureus 2020, 12, e9349. [Google Scholar] [CrossRef]
- Xiaoming, J.; Wensheng, S.; Ron, C.H.; Vijay, N.; Kunihiro, M.; Aaron, R.F.; Gerardo, H.; David, J.C.; Scott, D.S.; Eric, B.; et al. High-Sensitivity Troponin I and Incident Coronary Events, Stroke, Heart Failure Hospitalization, and Mortality in the ARIC Study. Circulation 2019, 139, 2642–2653. [Google Scholar] [CrossRef]
- Peder, L.M.; Brian, C.; Christie, M.B.; Elizabeth, S.; Helge, R.; Torbjørn, O.; Scott, D.S.; Hicham, S.; Amil, M.S. Association Between Circulating Troponin Concentrations, Left Ventricular Systolic and Diastolic Functions, and Incident Heart Failure in Older Adults. JAMA Cardiol. 2019, 4, 997–1006. [Google Scholar] [CrossRef]
- Rasmus, R.; Pardeep, S.J.; Søren, L.K.; Akshay, S.D.; Lars, K.; Jean, L.R.; Scott, D.S.; Karl, S.; Michael, R.Z.; Milton, P.; et al. The prognostic value of troponin T and N-terminal pro B-type natriuretic peptide, alone and in combination, in heart failure patients with and without diabetes. Eur. J. Heart Fail. 2019, 21, 40–49. [Google Scholar] [CrossRef] [Green Version]
- Szyszkowska, A.; Knapp, M.; Kaminski, K.; Lisowska, A. Insulin-like growth factor-binding protein 7 (IGFBP7): Novel, independent marker of cardiometabolic diseases? Postepy Hig. Med. Dosw. 2019, 73, 735–740. [Google Scholar] [CrossRef]
- Chen, D.; Yoo, B.K.; Santhekadur, P.K.; Gredler, R.; Bhutia, S.K.; Das, S.K.; Fuller, C.; Su, Z.Z.; Fisher, P.B.; Sarkar, D. Insulin-like growth factor binding protein-7 (IGFBP-7) functions as a potential tumor suppressor in hepatocellular carcinoma (HCC). Clin. Cancer. Res. 2011, 17, 6693–6701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higashi, Y.; Gautam, S.; Delafontaine, P.; Sukhanov, S. IGF-1 and cardiovascular disease. Growth Horm. IGF Res. 2019, 45, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Ruidavets, J.B.; Luc, G.; Machez, E.; Genoux, A.L.; Kee, F.; Arveiler, D.; Morange, P.; Woodside, J.V.; Amouyel, P.; Evans, A.; et al. Effects of insulin-like growth factor 1 in preventing acute coronary syndromes: The PRIME study. Atherosclerosis 2011, 218, 464–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lisowska, A.; Święcki, P.; Knapp, M.; Gil, M.; Musiał, W.J.; Kamiński, K.; Hirnle, T.; Tycińska, A. Insulin-like growth factor-binding protein 7 (IGFBP 7) as a new biomarker in coronary heart disease. Adv. Med. Sci. 2019, 64, 195–201. [Google Scholar] [CrossRef] [PubMed]
- De Backer, G.; Jankowski, P.; Kotseva, K.; Mirrakhimov, E.; Reiner, Ž.; Rydén, L.; Tokgözoğlu, L.; Wood, D.; De Bacquer, D.; EUROASPIRE V collaborators. Management of dyslipidaemia in patients with coronary heart disease: Results from the ESC-EORP EUROASPIRE V survey in 27 countries. Atherosclerosis 2019, 285, 135–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paniczko, M.; Chlabicz, M.; Jamiołkowski, J.; Sowa, P.; Szpakowicz, M.; Łapińska, M.; Kondraciuk, M.; Ptaszyńska-Kopczyńska, K.; Raczkowski, A.; Szpakowicz, A.; et al. Impact of Pulse Wave Velocity and Parameters Reflecting Android Type Fat Distribution on Left Ventricular Diastolic Dysfunction in Patients with Chronic Coronary Syndromes. J. Clin. Med. 2020, 9, 3924. [Google Scholar] [CrossRef] [PubMed]
- Chlabicz, M.; Dubatówka, M.; Jamiołkowski, J.; Sowa, P.; Łapińska, M.; Raczkowski, A.; Łaguna, W.; Moniuszko-Malinowska, A.M.; Waszkiewicz, N.; Kowalska, I.; et al. Subjective well-being in non-obese individuals depends strongly on body composition. Sci. Rep. 2021, 11, 21797. [Google Scholar] [CrossRef] [PubMed]
- Januzzi, J.L.; Packer, M.; Claggett, B.; Liu, J.; Shah, A.M.; Zile, M.R.; Pieske, B.; Voors, A.; Gandhi, P.U.; Prescott, M.F.; et al. IGFBP7 (insulin-like growth factor–binding protein-7) and neprilysin inhibition in patients with heart failure. Circ. Heart Fail. 2018, 11, e005133. [Google Scholar] [CrossRef]
- Ruan, W.; Wu, M.; Shi, L.; Li, F.; Dong, L.; Qiu, Y.; Wu, X.; Ying, K. Serum levels ofIGFBP7 are elevated during acute exacerbation inCOPD patients. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 1775–1780. [Google Scholar] [CrossRef] [Green Version]
- Bolomsky, A.; Hose, D.; Schreder, M.; Seckinger, A.; Meißner, T.; Klein, B.; Heintel, D.; Pfeifer, S.; Ludwig, H.; Zojer, N. Insulinlike growth factor binding protein 7 (IGFBP7) is downregulated in multiple myeloma with consequneces for myeloma cell growth and bone disease. Blood 2012, 120, 3947. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, M.; Ling, J.; Cai, L.; Zhang, D.; Gu, H.F.; Wang, H.; Zhu, Y.; Lai, M. Serum IGFBP7 levels associate with insulin resistance and the risk of metabolic syndrome in a Chinese population. Sci. Rep. 2015, 5, 10227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aregger, F.; Uehlinger, D.E.; Witowski, J.; Brunisholz, R.A.; Hunziker, P.; Frey, F.J.; Jörres, A. Identification of IGFBP-7 by urinary proteomics as a novel prognostic marker in early acute kidney injury. Kidney Int. 2014, 85, 909–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, H.M.; Huang, L.F.; Zheng, Y.; Li, W.X. Prognostic value of cell cycle arrest biomarkers in patients at high risk for acute kidney injury: A systematic review and meta-analysis. Nephrology 2017, 22, 831–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schanz, M.; Shi, J.; Wasser, C.; Alscher, M.D.; Kimmel, M. Urinary [TIMP-2] × [IGFBP7] for risk prediction of acute kidney injury in decompensated heart failure. Clin. Cardiol. 2017, 40, 485–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandhi, P.U.; Chow, S.L.; Rector, T.S.; Krum, H.; Gaggin, H.K.; McMurray, J.J.; Zile, M.R.; Komajda, M.; McKelvie, R.S.; Carson, P.E.; et al. Prognostic value of insulin-like growth factor-binding protein 7 in patients with heart failure and preserved ejection fraction. J. Card. Fail. 2017, 23, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Barroso, M.C.; Kramer, F.; Greene, S.J.; Scheyer, D.; Köhler, T.; Karoff, M.; Seyfarth, M.; Gheorghiade, M.; Dinh, W. Serum insulin-like growth factor-1 and its binding protein-7: Potential novel biomarkers for heart failure with preserved ejection fraction. BMC Cardiovasc. Disord. 2016, 16, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, N.E.; Afilalo, M.; Chen-Tournoux, A.; Christenson, R.H.; Gaggin, H.K.; Hollander, J.E.; Kastner, P.; Levy, P.D.; Mang, A.; Masson, S.; et al. Diagnostic and prognostic utilities of insulin-like growth factor binding protein-7 in patients with dyspnea. J. Am. Coll. Cardiol. HF 2020, 8, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Kalayci, A.; Peacock, W.F.; Nagurney, J.T.; Hollander, J.E.; Phillip, D.; Levy, P.D. Echocardiographic assessment of insulin-like growth factor binding protein-7 and early identification of acute heart failure. ESC Heart Fail. 2020, 7, 1664–1675. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Wu, J.; Garami, M.; Gardner, D.G. Mechanical strain increases expression of the brain natriuretic peptidegene in rat cardiac myocytes. J. Biol. Chem. 1997, 272, 28050–28056. [Google Scholar] [CrossRef] [Green Version]
- Omland, T.; Rosjo, H.; Giannitsis, E.; Agewall, S. Troponins in heart failure. Clin. Chim. Acta 2015, 443, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Święcki, P.; Sawicki, R.; Knapp, M.; Kamiński, K.A.; Ptaszyńska-Kopczyńska, K.; Sobkowicz, B.; Lisowska, A. Galectin-3 as the Prognostic Factor of Adverse Cardiovascular Events in Long-Term Follow up in Patients after Myocardial Infarction—A Pilot Study. J. Clin. Med. 2020, 9, 1640. [Google Scholar] [CrossRef]

| Variables | IHD (n = 88) | Population Group (n = 65) | p |
|---|---|---|---|
| Age (years) | 63.2 (7.8) | 61.2 (8.2) | NS |
| Male gender (n, %) | 69 (78%) | 41 (61.2%) | NS |
| BMI | 29.8 (5.5) | 28.7 (4.1) | NS |
| IGFBP7 (ng/mL) | 1.76 (1.04) | 1.41 (0.45) | 0.019 |
| Creatinine (µmol/L) | 92.2 (41.4) | 80.5 (16.8) | <0.0001 |
| Urea (mg/dL) | 38 (15.2) | 31.9 (8.4) | <0.0001 |
| Glucose (mg/dL) | 114.7 (36.7) | 95.9 (14.2) | <0.0001 |
| Insulin (µU/mL) | 15.03 (9.5) | 12.8 (6.4) | <0.0001 |
| C-peptide (ng/mL) | 3.38 (1.6) | 2.83 (1) | NS |
| TnT (pg/mL) | 13.57 (13.6) | 7.5 (3.7) | <0.0001 |
| NT-proBNP (pg/mL) | 355.2 (538.7) | 98.2 (97.9) | <0.0001 |
| Multiple Regression Analysis R2 for the Multivariate Model 0.77 | Beta | p |
|---|---|---|
| Urea | 0.45 | <0.0001 |
| Creatinine | 0.23 | <0.0001 |
| TnT | 0.23 | <0.001 |
| NT-BNP | 0.1 | 0.055 |
| Ischemic heart disease | −0.02 | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisowska, A.; Szyszkowska, A.; Knapp, M.; Łapińska, M.; Kondraciuk, M.; Kamińska, I.; Hryszko, T.; Ptaszyńska-Kopczyńska, K.; Kamiński, K. IGFBP7 Concentration May Reflect Subclinical Myocardial Damage and Kidney Function in Patients with Stable Ischemic Heart Disease. Biomolecules 2022, 12, 274. https://doi.org/10.3390/biom12020274
Lisowska A, Szyszkowska A, Knapp M, Łapińska M, Kondraciuk M, Kamińska I, Hryszko T, Ptaszyńska-Kopczyńska K, Kamiński K. IGFBP7 Concentration May Reflect Subclinical Myocardial Damage and Kidney Function in Patients with Stable Ischemic Heart Disease. Biomolecules. 2022; 12(2):274. https://doi.org/10.3390/biom12020274
Chicago/Turabian StyleLisowska, Anna, Anna Szyszkowska, Małgorzata Knapp, Magda Łapińska, Marcin Kondraciuk, Inga Kamińska, Tomasz Hryszko, Katarzyna Ptaszyńska-Kopczyńska, and Karol Kamiński. 2022. "IGFBP7 Concentration May Reflect Subclinical Myocardial Damage and Kidney Function in Patients with Stable Ischemic Heart Disease" Biomolecules 12, no. 2: 274. https://doi.org/10.3390/biom12020274
APA StyleLisowska, A., Szyszkowska, A., Knapp, M., Łapińska, M., Kondraciuk, M., Kamińska, I., Hryszko, T., Ptaszyńska-Kopczyńska, K., & Kamiński, K. (2022). IGFBP7 Concentration May Reflect Subclinical Myocardial Damage and Kidney Function in Patients with Stable Ischemic Heart Disease. Biomolecules, 12(2), 274. https://doi.org/10.3390/biom12020274









