Unlocking the Memory Component of Alzheimer’s Disease: Biological Processes and Pathways across Brain Regions
Abstract
:1. Introduction
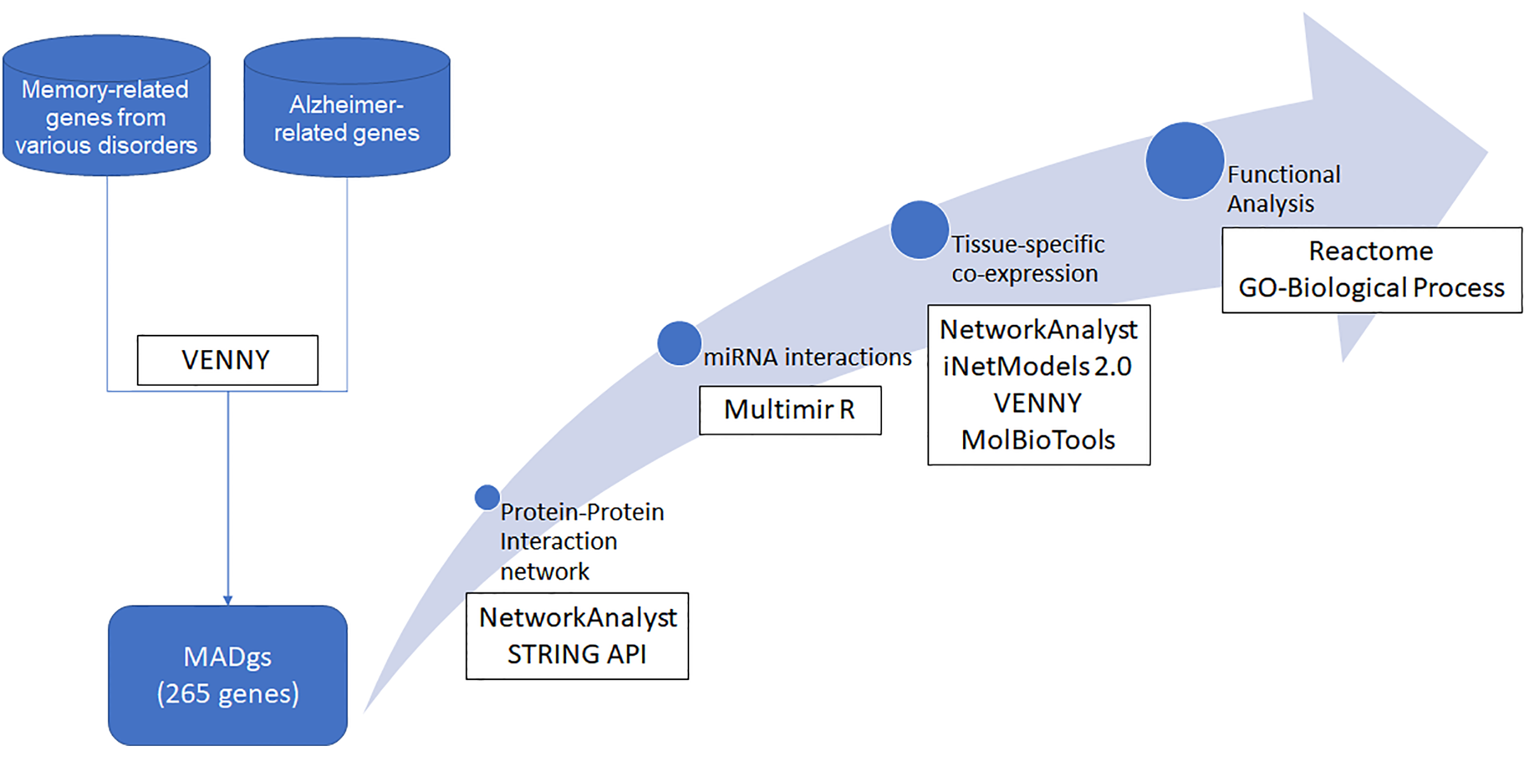
2. Methodology
2.1. Literature-Based Gene Retrieval
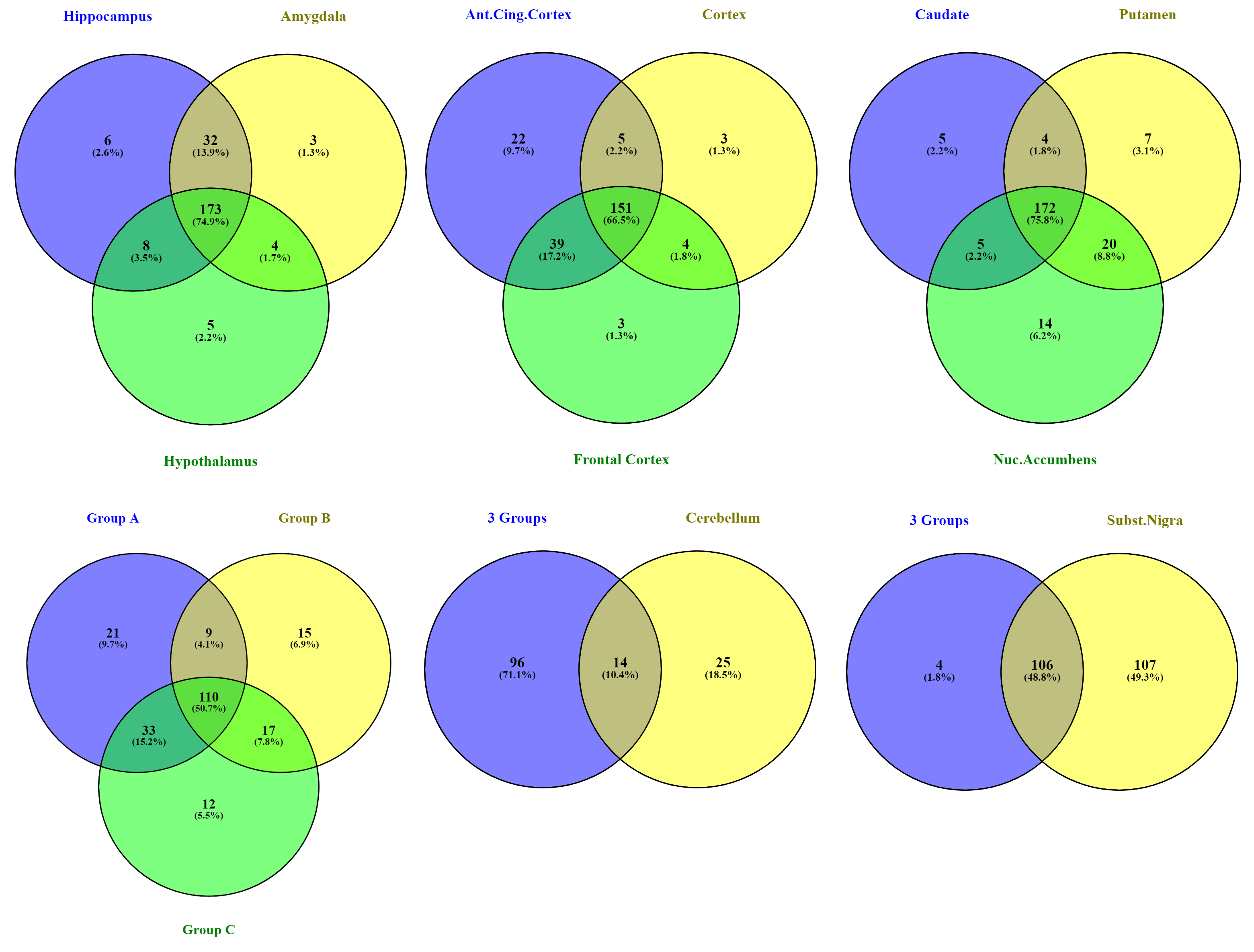
2.2. Brain Region Co-Expression
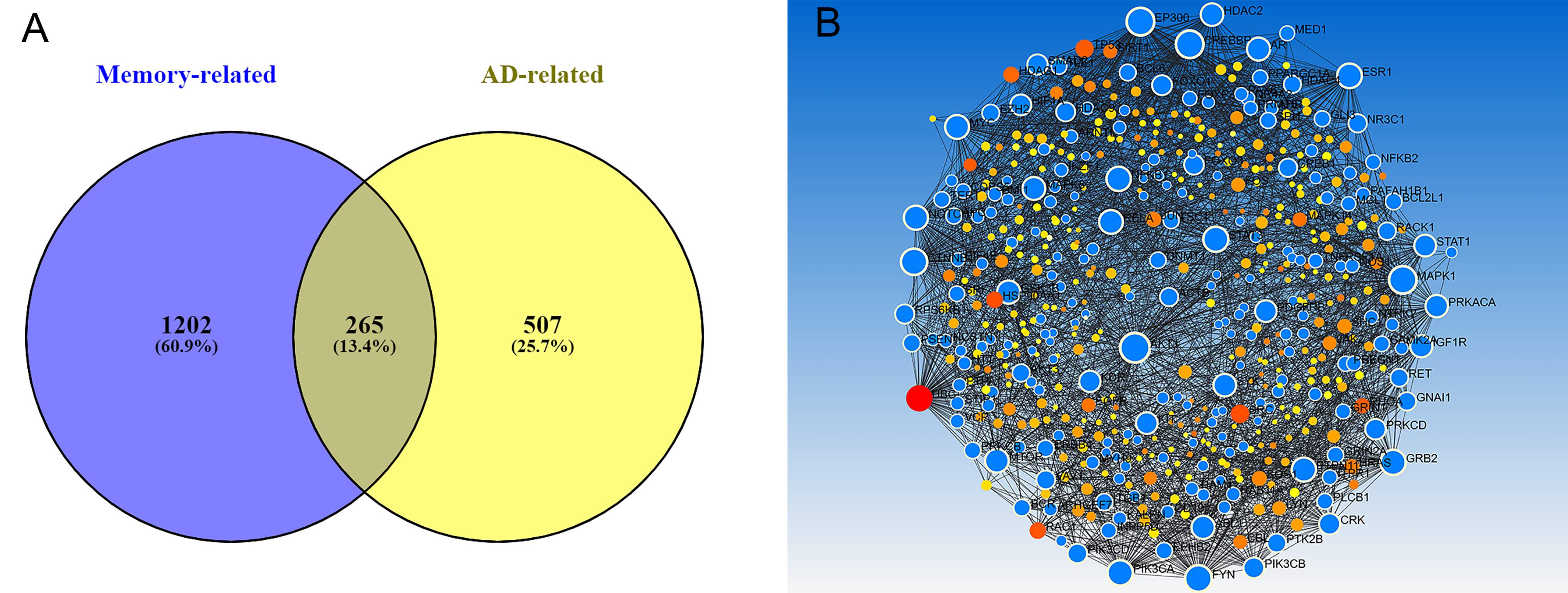
2.3. Protein–Protein Interaction Network
2.4. Functional Analysis
2.5. miRNA Analysis
3. Results
3.1. Genetic Background of Memory in AD
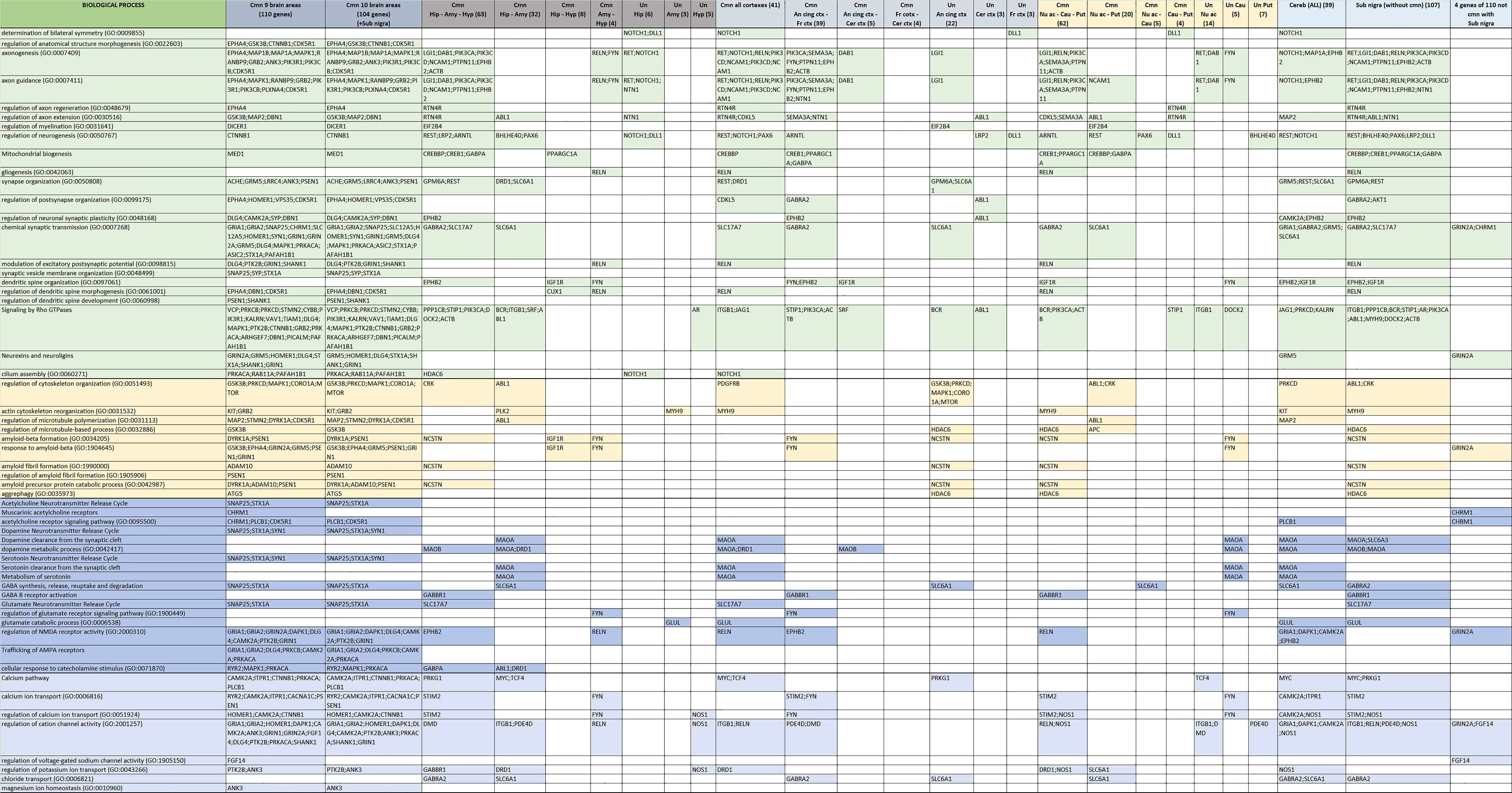
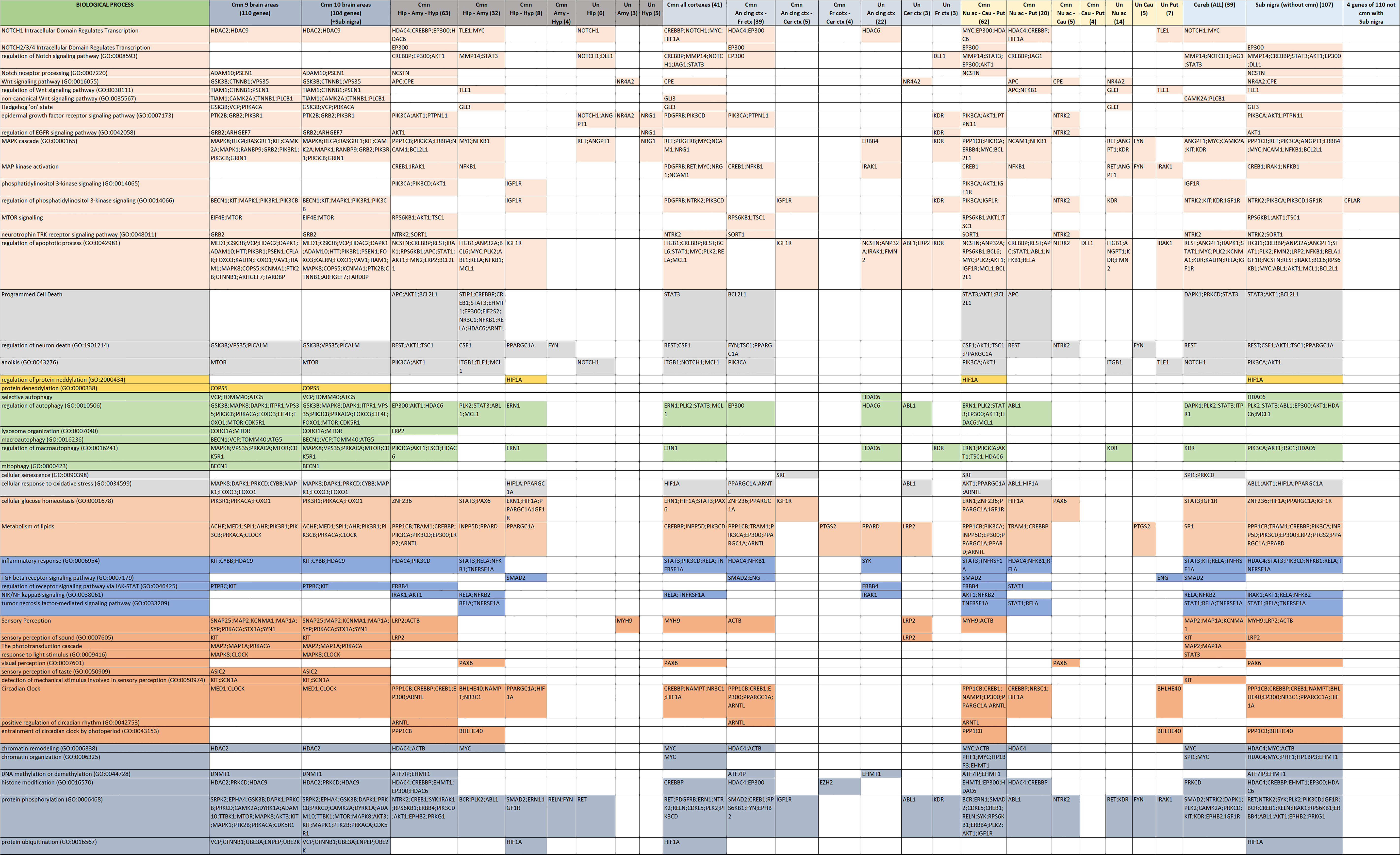
3.2. Functional Background of Memory in AD
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Cipriani, G.; Dolciotti, C.; Picchi, L.; Bonuccelli, U. Alzheimer and his disease: A brief history. Neurol. Sci. 2011, 32, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Dovrolis, N.; Kolios, G.; Spyrou, G.; Maroulakou, I. Laying in silico pipelines for drug repositioning: A paradigm in ensemble analysis for neurodegenerative diseases. Drug Discov. Today 2017, 22, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, L.G. Memory function in normal aging. Acta Neurol. Scand. 2003, 107, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Eichenbaum, H. Memory, Amnesia, and the Hippocampal System; MIT Press: Cambridge, MA, USA, 1993. [Google Scholar]
- Kensinger, E.A.; Schacter, D.L. Memory and emotion. In Handbook of Emotions; The Guilford Press: New York, NY, USA, 2008. [Google Scholar]
- Hartley, T.; Bird, C.M.; Chan, D.; Cipolotti, L.; Husain, M.; Vargha-Khadem, F.; Burgess, N. The hippocampus is required for short-term topographical memory in humans. Hippocampus 2007, 17, 34–48. [Google Scholar] [CrossRef] [Green Version]
- Chiu, Y.-C.; Algase, D.; Whall, A.; Liang, J.; Liu, H.-C.; Lin, K.-N.; Wang, P.-N. Getting lost: Directed attention and executive functions in early Alzheimer’s disease patients. Dement. Geriatr. Cogn. Disord. 2004, 17, 174–180. [Google Scholar] [CrossRef]
- Sozinova, E.; Kozlovskiy, S.; Vartanov, A.; Skvortsova, V.; Pirogov, Y.; Anisimov, N.; Kupriyanov, D. The role of hippocampal parts in verbal memory and activation processes. Int. J. Psychophysiol. 2008, 3, 312. [Google Scholar] [CrossRef]
- Gluck, M.A. Learning and Memory from Brain to Behavior; Worth Publishers: New York, NY, USA, 2008. [Google Scholar]
- McGaugh, J.L.; McIntyre, C.K.; Power, A.E. Amygdala modulation of memory consolidation: Interaction with other brain systems. Neurobiol. Learn. Mem. 2002, 78, 539–552. [Google Scholar] [CrossRef] [Green Version]
- Ressler, K.; Davis, M. Genetics of childhood disorders: L. Learning and memory, part 3: Fear conditioning. J. Am. Acad. Child Adolesc. Psychiatry 2003, 42, 612–615. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Stevenson, R.F.; Mander, B.A.; Mnatsakanyan, L.; Hsu, F.P.; Vadera, S.; Knight, R.T.; Yassa, M.A.; Lin, J.J. Multiplexing of theta and alpha rhythms in the amygdala-hippocampal circuit supports pattern separation of emotional information. Neuron 2019, 102, 887–898.e5. [Google Scholar] [CrossRef]
- Burdakov, D.; Peleg-Raibstein, D. The hypothalamus as a primary coordinator of memory updating. Physiol. Behav. 2020, 223, 112988. [Google Scholar] [CrossRef]
- Lin, L.; Wu, J.; Yuan, Y.; Sun, X.; Zhang, L. Working memory predicts hypothalamus-pituitary-adrenal axis response to psychosocial stress in males. Front. Psychiatry 2020, 11, 142. [Google Scholar] [CrossRef] [Green Version]
- Yeo, B.T.T.; Krienen, F.M.; Sepulcre, J.; Sabuncu, M.R.; Lashkari, D.; Hollinshead, M.; Roffman, J.L.; Smoller, J.W.; Zöllei, L.; Polimeni, J.R.; et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011, 106, 1125–1165. [Google Scholar]
- Guarino, A.; Favieri, F.; Boncompagni, I.; Agostini, F.; Cantone, M.; Casagrande, M. Executive functions in Alzheimer disease: A systematic review. Front. Aging Neurosci. 2019, 10, 437. [Google Scholar] [CrossRef]
- Duma, G.M.; Mento, G.; Cutini, S.; Sessa, P.; Baillet, S.; Brigadoi, S.; Dell’Acqua, R. Functional dissociation of anterior cingulate cortex and intraparietal sulcus in visual working memory. Cortex 2019, 121, 277–291. [Google Scholar] [CrossRef]
- Rolls, E.T. The cingulate cortex and limbic systems for emotion, action, and memory. Brain Struct. Funct. 2019, 224, 3001–3018. [Google Scholar] [CrossRef] [Green Version]
- Kerfoot, E.C.; Williams, C.L. Contributions of the nucleus accumbens shell in mediating the enhancement in memory following noradrenergic activation of either the amygdala or hippocampus. Front. Pharmacol. 2018, 9, 47. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Schmitz, T.W.; Mur, M.; Ferreira, C.S.; Anderson, M.C. A supramodal role of the basal ganglia in memory and motor inhibition: Meta-analytic evidence. Neuropsychologia 2018, 108, 117–134. [Google Scholar] [CrossRef]
- Amita, H.; Kim, H.F.; Smith, M.K.; Gopal, A.; Hikosaka, O. Neuronal connections of direct and indirect pathways for stable value memory in caudal basal ganglia. Eur. J. Neurosci. 2019, 49, 712–725. [Google Scholar] [CrossRef] [Green Version]
- Da Cunha, C.; Wietzikoski, S.; Wietzikoski, E.C.; Miyoshi, E.; Ferro, M.M.; Anselmo-Franci, J.A.; Canteras, N.S. Evidence for the substantia nigra pars compacta as an essential component of a memory system independent of the hippocampal memory system. Neurobiol. Learn. Mem. 2003, 79, 236–242. [Google Scholar] [CrossRef]
- Silveri, M.C.; Misciagna, S. Language, memory, and the cerebellum. J. Neurolinguistics 2000, 13, 129–143. [Google Scholar] [CrossRef]
- Amini, M.; Pedram, M.; Moradi, A.; Ouchani, M. Diagnosis of Alzheimer’s Disease Severity with fMRI Images Using Robust Multitask Feature Extraction Method and Convolutional Neural Network (CNN). Comput. Math. Methods Med. 2021, 2021, 5514839. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Adeli, E.; Chen, X.; Liu, M.; Shen, D. Multiview feature learning with multiatlas-based functional connectivity networks for MCI diagnosis. IEEE Trans. Cybern. 2020, 1–12. [Google Scholar] [CrossRef]
- Li, K.; Wang, J.; Li, S.; Yu, H.; Zhu, L.; Liu, J.; Wu, L. Feature extraction and identification of Alzheimer’s disease based on latent factor of multi-channel EEG. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 1557–1567. [Google Scholar] [CrossRef]
- Duan, F.; Huang, Z.; Sun, Z.; Zhang, Y.; Zhao, Q.; Cichocki, A.; Yang, Z.; Solé-Casals, J. Topological network analysis of early Alzheimer’s disease based on resting-state EEG. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 2164–2172. [Google Scholar] [CrossRef]
- Min, R.; Wu, G.; Cheng, J.; Wang, Q.; Shen, D.; Alzheimer’s Disease Neuroimaging Initiative. Multi-atlas based representations for Alzheimer’s disease diagnosis. Hum. Brain Mapp. 2014, 35, 5052–5070. [Google Scholar] [CrossRef] [Green Version]
- Long, Z.; Huang, J.; Li, B.; Li, Z.; Li, Z.; Chen, H.; Jing, B. A comparative atlas-based recognition of mild cognitive impairment with voxel-based morphometry. Front. Neurosci. 2018, 12, 916. [Google Scholar] [CrossRef] [Green Version]
- Piñero, J.; Bravo, À.; Queralt-Rosinach, N.; Gutiérrez-Sacristán, A.; Deu-Pons, J.; Centeno, E.; García-García, J.; Sanz, F.; Furlong, L.I. DisGeNET: A comprehensive platform integrating information on human disease-associated genes and variants. Nucleic Acids Res. 2016, 45, D833–D839. [Google Scholar] [CrossRef] [PubMed]
- Stalker, J.; Gibbins, B.; Meidl, P.; Smith, J.; Spooner, W.; Hotz, R.-H.; Cox, A.V. The Ensembl Web site: Mechanics of a genome browser. Genome Res. 2004, 14, 951–955. [Google Scholar] [CrossRef] [Green Version]
- Ochoa, D.; Hercules, A.; Carmona, M.; Suveges, D.; Gonzalez-Uriarte, A.; Malangone, C.; Miranda, A.; Fumis, L.; Carvalho-Silva, D.; Spitzer, M.; et al. Open Targets Platform: Supporting systematic drug–target identification and prioritisation. Nucleic Acids Res. 2021, 49, D1302–D1310. [Google Scholar] [CrossRef] [PubMed]
- MacArthur, J.; Bowler, E.; Cerezo, M.; Gil, L.; Hall, P.; Hastings, E.; Junkins, H.; McMahon, A.; Milano, A.; Morales, J.; et al. The new NHGRI-EBI Catalog of published genome-wide association studies (GWAS Catalog). Nucleic Acids Res. 2017, 45, D896–D901. [Google Scholar] [CrossRef] [PubMed]
- Oliveros, J.; VENNY. An Interactive Tool for Comparing Lists with Venn Diagrams. 2007. Available online: https://bioinfogp.cnb.csic.es/tools/venny/index.html (accessed on 1 February 2022).
- Xia, J.; Gill, E.E.; Hancock, R.E. NetworkAnalyst for statistical, visual and network-based meta-analysis of gene expression data. Nat. Protoc. 2015, 10, 823. [Google Scholar] [CrossRef]
- Arif, M.; Zhang, C.; Li, X.; Güngör, C.; Çakmak, B.; Arslantürk, M.; Tebani, A.; Özcan, B.; Subaş, O.; Zhou, W. iNetModels 2.0: An interactive visualization and database of multi-omics data. Nucleic Acids Res. 2021, 49, W271–W276. [Google Scholar] [CrossRef]
- Vladimír Čermák. Molbiotools. Available online: https://molbiotools.com/index.html (accessed on 1 February 2022).
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P. The STRING database in 2017: Quality-controlled protein–protein association networks, made broadly accessible. Nucleic Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef]
- Joshi-Tope, G.; Gillespie, M.; Vastrik, I.; D’Eustachio, P.; Schmidt, E.; de Bono, B.; Jassal, B.; Gopinath, G.; Wu, G.; Matthews, L. Reactome: A knowledgebase of biological pathways. Nucleic Acids Res. 2005, 33, D428–D432. [Google Scholar] [CrossRef] [Green Version]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Ru, Y.; Kechris, K.J.; Tabakoff, B.; Hoffman, P.; Radcliffe, R.A.; Bowler, R.; Mahaffey, S.; Rossi, S.; Calin, G.A.; Bemis, L.; et al. The multiMiR R package and database: Integration of microRNA–target interactions along with their disease and drug associations. Nucleic Acids Res. 2014, 42, e133. [Google Scholar] [CrossRef]
- Xiao, F.; Zuo, Z.; Cai, G.; Kang, S.; Gao, X.; Li, T. miRecords: An integrated resource for microRNA–target interactions. Nucleic Acids Res. 2009, 37, D105–D110. [Google Scholar] [CrossRef]
- Huang, H.Y.; Lin, Y.-C.-D.; Li, J.; Huang, K.-Y.; Shrestha, S.; Hong, H.-C.; Tang, Y.; Chen, Y.-G.; Jin, C.-N.; Yu, Y.; et al. miRTarBase 2020: Updates to the experimentally validated microRNA–target interaction database. Nucleic Acids Res. 2020, 48, D148–D154. [Google Scholar] [CrossRef] [Green Version]
- Paraskevopoulou, M.D.; Vlachos, I.S.; Hatzigeorgiou, A.G. DIANA-TarBase and DIANA suite tools: Studying experimentally supported microRNA targets. Curr. Protoc. Bioinform. 2016, 55, 12.14.1–12.14.18. [Google Scholar] [CrossRef]
- Mathys, H.; Davila-Velderrain, J.; Peng, Z.; Gao, F.; Mohammadi, S.; Young, J.Z.; Menon, M.; He, L.; Abdurrob, F.; Jiang, X.; et al. Single-cell transcriptomic analysis of Alzheimer’s disease. Nature 2019, 570, 332–337. [Google Scholar] [CrossRef]
- Piras, I.S.; Bleul, C.; Talboom, J.S.; De Both, M.D.; Schrauwen, I.; Halliday, G.; Myers, A.J.; Serrano, G.E.; Beach, T.G.; Huentelman, M.J. ESHRD: Deconvolution of brain homogenate RNA expression data to identify cell-type-specific alterations in Alzheimer’s disease. Aging 2020, 12, 4124. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jiang, Y.; Huang, Z. Loss of C9orf72 in Microglia Drives Neuronal Injury by Enhancing Synaptic Pruning in Aged and Alzheimer’s Disease Mice. Neurosci. Bull. 2021, 1–4, preprint. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, C.; Qi, R.; Fu, H.; Ma, Q. scREAD: A single-cell RNA-Seq database for Alzheimer’s Disease. iScience 2020, 23, 101769. [Google Scholar] [CrossRef] [PubMed]
- Chew, G.; Petretto, E. Transcriptional networks of microglia in Alzheimer’s disease and insights into pathogenesis. Genes 2019, 10, 798. [Google Scholar] [CrossRef] [Green Version]
- Marx, V. Method of the Year: Spatially resolved transcriptomics. Nat. Methods 2021, 18, 9–14. [Google Scholar] [CrossRef]
- Chen, W.-T.; Lu, A.; Craessaerts, K.; Pavie, B.; Frigerio, C.S.; Mancuso, R.; Qian, X.; Lalakova, J.; Kühnemund, M.; Voytyuk, I. Spatial and temporal transcriptomics reveal microglia-astroglia crosstalk in the amyloid-β plaque cell niche of Alzheimer’s disease. BioRxiv 2019, 719930. [Google Scholar]
- McNamara, N.B.; Miron, V.E. Replenishing our mind orchards: Enhancing myelin renewal to rescue cognition in Alzheimer’s disease. Neuron 2021, 109, 2204–2206. [Google Scholar] [CrossRef]
- Swarbrick, S.; Wragg, N.; Ghosh, S.; Stolzing, A. Systematic review of miRNA as biomarkers in Alzheimer’s disease. Mol. Neurobiol. 2019, 56, 6156–6167. [Google Scholar] [CrossRef] [Green Version]
- Leidinger, P.; Backes, C.; Deutscher, S.; Schmitt, K.; Mueller, S.C.; Frese, K.; Haas, J.; Ruprecht, K.; Paul, F.; Stähler, C.; et al. A blood based 12-miRNA signature of Alzheimer disease patients. Genome Biol. 2013, 14, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Dezso, Z.; MacKenzie, C.; Oestreicher, J.; Agoulnik, S.; Byrne, M.; Bernier, F.; Yanagimachi, M.; Aoshima, K.; Oda, Y. Circulating miRNA biomarkers for Alzheimer’s disease. PLoS ONE 2013, 8, e69807. [Google Scholar] [CrossRef]
- Turk, A.; Kunej, T.; Peterlin, B. MicroRNA-Target Interaction Regulatory Network in Alzheimer’s Disease. J. Pers. Med. 2021, 11, 1275. [Google Scholar] [CrossRef]
- Liu, F.; Qiu, F.; Chen, H. miR-124-3p Ameliorates Isoflurane-Induced Learning and Memory Impairment via Targeting STAT3 and Inhibiting Neuroinflammation. Neuroimmunomodulation 2021, 28, 1–7. [Google Scholar] [CrossRef]
- Ge, X.; Guo, M.; Hu, T.; Li, W.; Huang, S.; Yin, Z.; Li, Y.; Chen, F.; Zhu, L.; Kang, C.; et al. Increased microglial exosomal miR-124-3p alleviates neurodegeneration and improves cognitive outcome after rmTBI. Mol. Ther. 2020, 28, 503–522. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Zhou, N.; Yang, P.; Deng, L.; Liu, G. MicroRNA-27a-3p downregulation inhibits inflammatory response and hippocampal neuronal cell apoptosis by upregulating mitogen-activated protein kinase 4 (MAP2K4) expression in epilepsy: In vivo and in vitro studies. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 8499. [Google Scholar] [CrossRef]
- Frigerio, C.S.; Lau, P.; Salta, E.; Tournoy, J.; Bossers, K.; Vandenberghe, R.; Wallin, A.; Bjerke, M.; Zetterberg, H.; Blennow, K. Reduced expression of hsa-miR-27a-3p in CSF of patients with Alzheimer disease. Neurology 2013, 81, 2103–2106. [Google Scholar] [CrossRef]
- Hart, M.; Walch-Rückheim, B.; Friedmann, K.S.; Rheinheimer, S.; Tänzer, T.; Glombitza, B.; Sester, M.; Lenhof, H.-P.; Hoth, M.; Schwarz, E.C. miR-34a: A new player in the regulation of T cell function by modulation of NF-κB signaling. Cell Death Dis. 2019, 10, 46. [Google Scholar] [CrossRef] [Green Version]
- Zingale, V.D.; Gugliandolo, A.; Mazzon, E. MiR-155: An Important Regulator of Neuroinflammation. Int. J. Mol. Sci. 2022, 23, 90. [Google Scholar] [CrossRef]
- Vachon, P.H. Integrin signaling, cell survival, and anoikis: Distinctions, differences, and differentiation. J. Signal Transduct. 2011, 2011, 738137. [Google Scholar] [CrossRef] [Green Version]
- Cai, C.-Z.; Yang, C.; Zhuang, X.-X.; Yuan, N.-N.; Wu, M.-Y.; Tan, J.-Q.; Song, J.-X.; Cheung, K.-H.; Su, H.; Wang, Y.-T.; et al. NRBF2 is a RAB7 effector required for autophagosome maturation and mediates the association of APP-CTFs with active form of RAB7 for degradation. Autophagy 2021, 17, 1112–1130. [Google Scholar] [CrossRef]
- Yang, C.; Cai, C.-Z.; Song, J.-X.; Tan, J.-Q.; Durairajan, S.S.K.; Iyaswamy, A.; Wu, M.-Y.; Chen, L.-L.; Yue, Z.; Li, M.; et al. NRBF2 is involved in the autophagic degradation process of APP-CTFs in Alzheimer disease models. Autophagy 2017, 13, 2028–2040. [Google Scholar] [CrossRef]
- Di Meco, A.; Curtis, M.E.; Lauretti, E.; Praticò, D. Autophagy dysfunction in Alzheimer’s disease: Mechanistic insights and new therapeutic opportunities. Biol. Psychiatry 2020, 87, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Jegga, A.G.; Schneider, L.; Ouyang, X.; Zhang, J. Systems biology of the autophagy-lysosomal pathway. Autophagy 2011, 7, 477–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.; Zhu, A.; Feng, J.; Wang, X. Role of neddylation in neurological development and diseases. Biotechnol. Appl. Biochem. 2021. preprint. [Google Scholar] [CrossRef] [PubMed]
- Winner, B.; Winkler, J. Adult neurogenesis in neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2015, 7, a021287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boros, B.D.; Greathouse, K.M.; Gearing, M.; Herskowitz, J.H. Dendritic spine remodeling accompanies Alzheimer’s disease pathology and genetic susceptibility in cognitively normal aging. Neurobiol. Aging 2019, 73, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.-O.; Ip, N.Y. Recent advances in understanding the roles of Cdk5 in synaptic plasticity. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2009, 1792, 741–745. [Google Scholar] [CrossRef] [Green Version]
- Karunakaran, K.B.; Chaparala, S.; Lo, C.W.; Ganapathiraju, M.K. Cilia interactome with predicted protein–protein interactions reveals connections to Alzheimer’s disease, aging and other neuropsychiatric processes. Sci. Rep. 2020, 10, 15629. [Google Scholar] [CrossRef]
- Bolognin, S.; Lorenzetto, E.; Diana, G.; Buffelli, M. The potential role of rho GTPases in Alzheimer’s disease pathogenesis. Mol. Neurobiol. 2014, 50, 406–422. [Google Scholar] [CrossRef]
- Aguilar, B.J.; Zhu, Y.; Lu, Q. Rho GTPases as therapeutic targets in Alzheimer’s disease. Alzheimers Res. Ther. 2017, 9, 74. [Google Scholar] [CrossRef] [Green Version]
- Wheway, G.; Nazlamova, L.; Hancock, J.T. Signaling through the primary cilium. Front. Cell Dev. Biol. 2018, 6, 8. [Google Scholar] [CrossRef]
- Pugacheva, E.N.; Jablonski, S.A.; Hartman, T.R.; Henske, E.P.; Golemis, E.A. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell 2007, 129, 1351–1363. [Google Scholar] [CrossRef] [Green Version]
- Ma, D.K.; Bonaguidi, M.A.; Ming, G.L.; Song, H. Adult neural stem cells in the mammalian central nervous system. Cell Res. 2009, 19, 672–682. [Google Scholar] [CrossRef] [Green Version]
- Duncan, G.J.; Simkins, T.J.; Emery, B. Neuron-oligodendrocyte interactions in the structure and integrity of axons. Front. Cell Dev. Biol. 2021, 9, 460. [Google Scholar] [CrossRef]
- Kihara, T.; Shimohama, S. Alzheimer’s disease and acetylcholine receptors. Acta Neurobiol. Exp. 2004, 64, 99–106. [Google Scholar]
- Geldenhuys, W.J.; Van der Schyf, C.J. Role of serotonin in Alzheimer’s disease. CNS Drugs 2011, 25, 765–781. [Google Scholar] [CrossRef]
- Cirrito, J.R.; Disabato, B.M.; Restivo, J.L.; Verges, D.K.; Goebel, W.D.; Sathyan, A.; Hayreh, D.; D’Angelo, G.; Benzinger, T.; Yoon, H.; et al. Serotonin signaling is associated with lower amyloid-β levels and plaques in transgenic mice and humans. Proc. Natl. Acad. Sci. USA 2011, 108, 14968–14973. [Google Scholar] [CrossRef] [Green Version]
- Südhof, T.C. Neuroligins and neurexins link synaptic function to cognitive disease. Nature 2008, 455, 903–911. [Google Scholar] [CrossRef] [Green Version]
- Coley, A.A.; Gao, W.-J. PSD95: A synaptic protein implicated in schizophrenia or autism? Prog. Neuro Psychopharmacol. Biol. Psychiatry 2018, 82, 187–194. [Google Scholar] [CrossRef]
- Hieronymus, F.; Lisinski, A.; Nilsson, S.; Eriksson, E. Efficacy of selective serotonin reuptake inhibitors in the absence of side effects: A mega-analysis of citalopram and paroxetine in adult depression. Mol. Psychiatry 2018, 23, 1731–1736. [Google Scholar] [CrossRef] [Green Version]
- Girardeau, G.; Inema, I.; Buzsáki, G. Reactivations of emotional memory in the hippocampus–amygdala system during sleep. Nat. Neurosci. 2017, 20, 1634–1642. [Google Scholar] [CrossRef]
- Gerstner, J.R.; Yin, J.C. Circadian rhythms and memory formation. Nat. Rev. Neurosci. 2010, 11, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Hut, R.; Van der Zee, E. The cholinergic system, circadian rhythmicity, and time memory. Behav. Brain Res. 2011, 221, 466–480. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Farrell, K.; Navabpour, S.; Narayanan, S.N.; Jarome, T.J. Epigenetic Mechanisms in Memory and Cognitive Decline Associated with Aging and Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 12280. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Huang, M.; Bushong, E.; Phan, S.; Uytiepo, M.; Beutter, E.; Boemer, D.; Tsui, K.; Ellisman, M.; Maximov, A. Class IIa HDACs regulate learning and memory through dynamic experience-dependent repression of transcription. Nat. Commun. 2019, 10, 3469. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dovrolis, N.; Nikou, M.; Gkrouzoudi, A.; Dimitriadis, N.; Maroulakou, I. Unlocking the Memory Component of Alzheimer’s Disease: Biological Processes and Pathways across Brain Regions. Biomolecules 2022, 12, 263. https://doi.org/10.3390/biom12020263
Dovrolis N, Nikou M, Gkrouzoudi A, Dimitriadis N, Maroulakou I. Unlocking the Memory Component of Alzheimer’s Disease: Biological Processes and Pathways across Brain Regions. Biomolecules. 2022; 12(2):263. https://doi.org/10.3390/biom12020263
Chicago/Turabian StyleDovrolis, Nikolas, Maria Nikou, Alexandra Gkrouzoudi, Nikolaos Dimitriadis, and Ioanna Maroulakou. 2022. "Unlocking the Memory Component of Alzheimer’s Disease: Biological Processes and Pathways across Brain Regions" Biomolecules 12, no. 2: 263. https://doi.org/10.3390/biom12020263
APA StyleDovrolis, N., Nikou, M., Gkrouzoudi, A., Dimitriadis, N., & Maroulakou, I. (2022). Unlocking the Memory Component of Alzheimer’s Disease: Biological Processes and Pathways across Brain Regions. Biomolecules, 12(2), 263. https://doi.org/10.3390/biom12020263






