Gut Microbiota Alterations in Trace Amine-Associated Receptor 9 (TAAR9) Knockout Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Public Transcriptome Data Analysis
2.2. Animals and Sample Collection
2.3. Gut Bacterial DNA Extraction and Sequencing
2.4. Sequencing Data Processing
2.5. Bioinformatics and Statistical Analysis of 16S rRNA Sequencing Data
3. Results
3.1. Analysis of TAAR9 Expression and TAAR9 Co-Expressed Gene Cluster in Colon Tissue
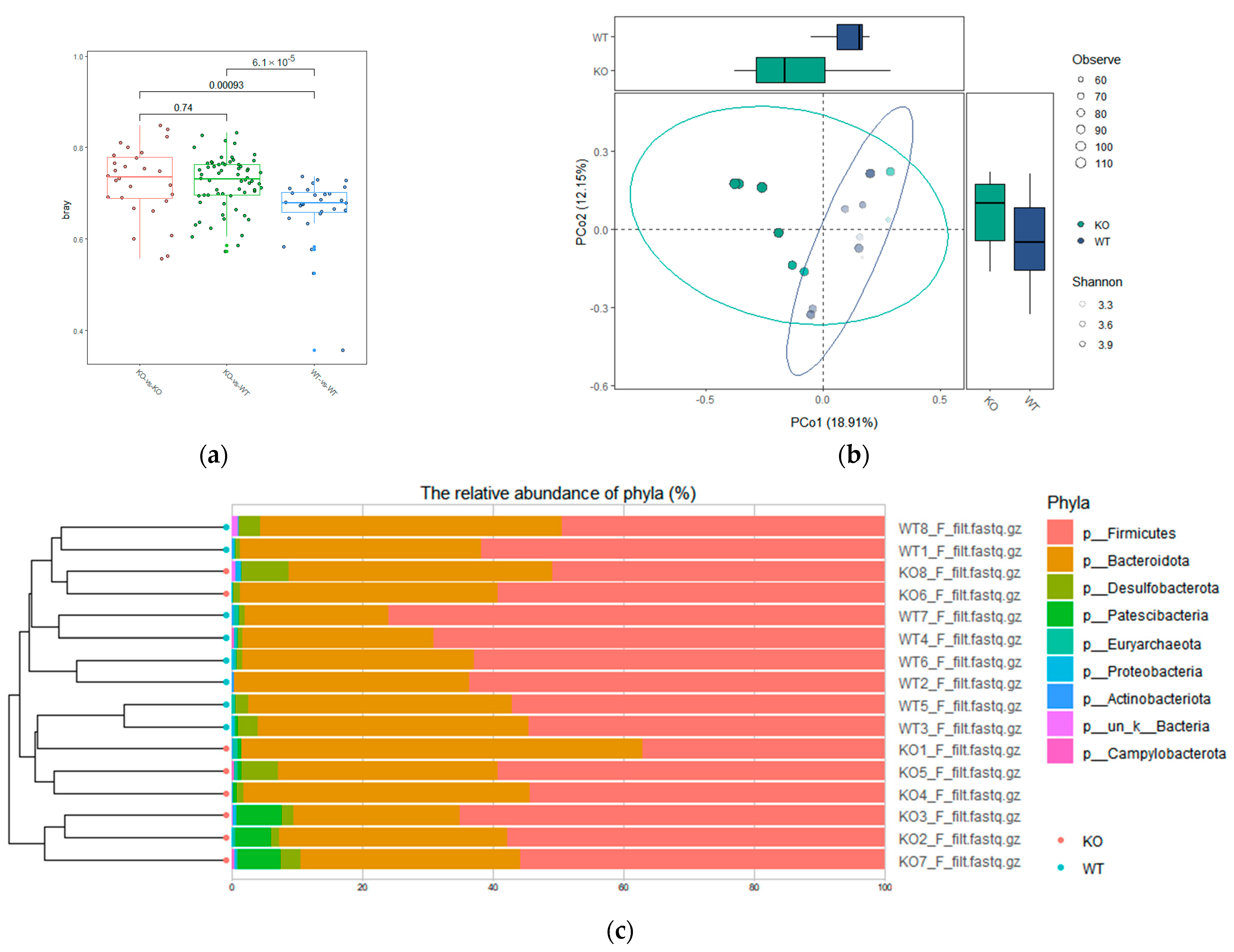
3.2. Fecal Microbiota α-Diversity in TAAR9-Ko Rats and Wild-Type Littermates
3.3. Difference in β-Diversity of Fecal Microbiota Species between TAAR9-Ko Rats and Wild-Type Littermates
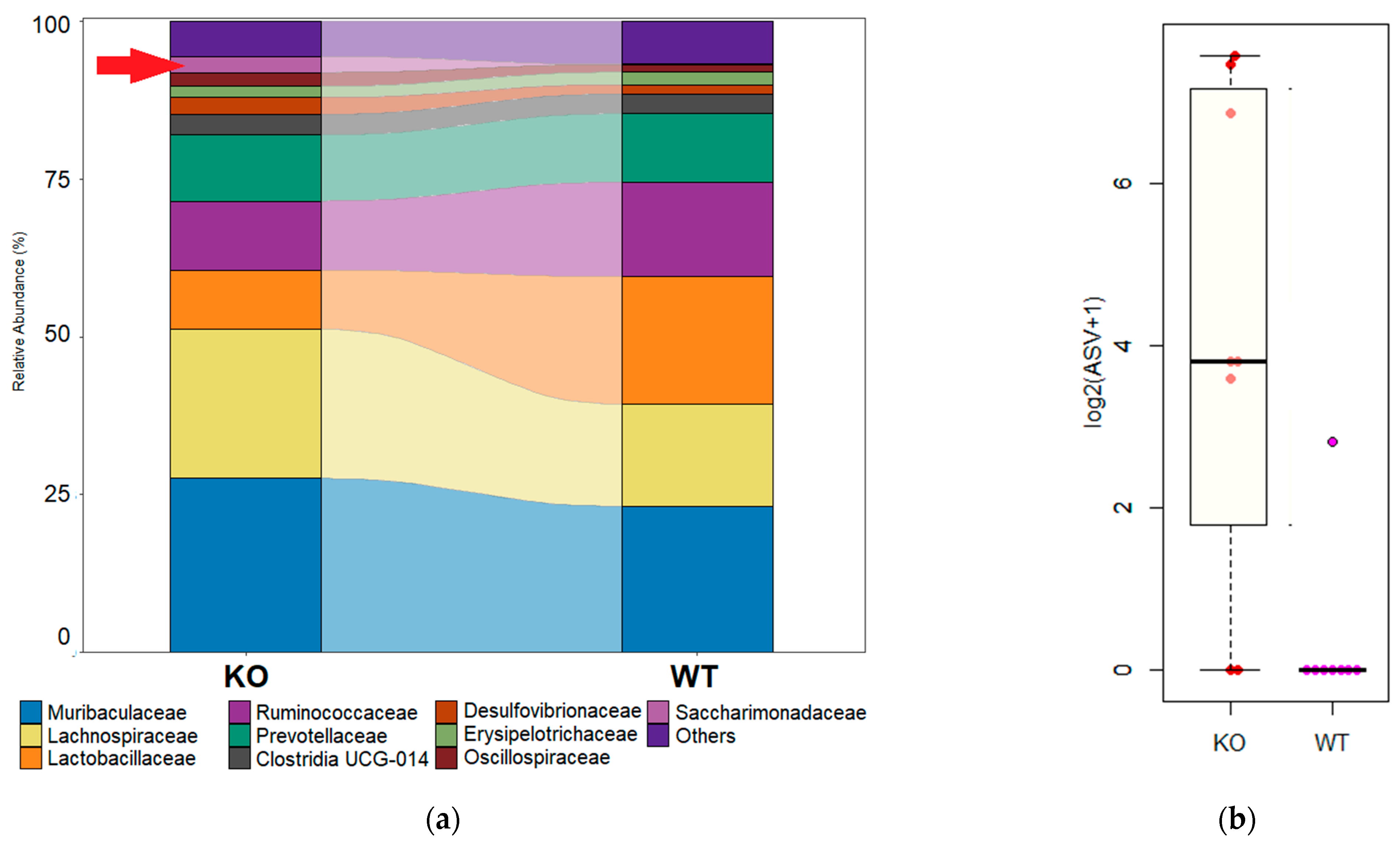
3.4. Differential Abundances of Bacterial Taxa
3.5. Asv—Amplicon Sequence Variant; KO—TAAR9-KO Group; Wt—Wild-Type Control Group
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gainetdinov, R.R.; Hoener, M.C.; Berry, M.D. Trace Amines and Their Receptors. Pharm. Rev. 2018, 70, 549–620. [Google Scholar] [CrossRef] [Green Version]
- Liberles, S.D. Trace Amine-Associated Receptors: Ligands, Neural Circuits, and Behaviors. Curr. Opin. Neurobiol. 2015, 34, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Lindemann, L.; Hoener, M.C. A Renaissance in Trace Amines Inspired by a Novel GPCR Family. Trends Pharm. Sci. 2005, 26, 274–281. [Google Scholar] [CrossRef]
- Berry, M.D.; Gainetdinov, R.R.; Hoener, M.C.; Shahid, M. Pharmacology of Human Trace Amine-Associated Receptors: Therapeutic Opportunities and Challenges. Pharmacol. Ther. 2017, 180, 161–180. [Google Scholar] [CrossRef]
- Bugda Gwilt, K.; González, D.P.; Olliffe, N.; Oller, H.; Hoffing, R.; Puzan, M.; El Aidy, S.; Miller, G.M. Actions of Trace Amines in the Brain-Gut-Microbiome Axis via Trace Amine-Associated Receptor-1 (TAAR1). Cell Mol. Neurobiol. 2020, 40, 191–201. [Google Scholar] [CrossRef]
- Gardini, F.; Özogul, Y.; Suzzi, G.; Tabanelli, G.; Özogul, F. Technological Factors Affecting Biogenic Amine Content in Foods: A Review. Front. Microbiol. 2016, 7, 1218. [Google Scholar] [CrossRef] [Green Version]
- Naila, A.; Flint, S.; Fletcher, G.; Bremer, P.; Meerdink, G. Control of Biogenic Amines in Food—Existing and Emerging Approaches. J. Food Sci. 2010, 75, R139–R150. [Google Scholar] [CrossRef] [Green Version]
- Pessione, E.; Cirrincione, S. Bioactive Molecules Released in Food by Lactic Acid Bacteria: Encrypted Peptides and Biogenic Amines. Front. Microbiol. 2016, 7, 876. [Google Scholar] [CrossRef] [Green Version]
- Toro-Funes, N.; Bosch-Fuste, J.; Latorre-Moratalla, M.L.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Biologically Active Amines in Fermented and Non-Fermented Commercial Soybean Products from the Spanish Market. Food Chem. 2015, 173, 1119–1124. [Google Scholar] [CrossRef]
- Cottus, R.T.; Baker, G.B.; Pasutto, F.M. Foodstuffs as Sources of Psychoactive Amines and Their Precursors: Content, Significance and Identification. Adv. Drug Res. 1986, 15, 169–232. [Google Scholar]
- Finberg, J.P.M.; Gillman, K. Selective Inhibitors of Monoamine Oxidase Type B and the “Cheese Effect”. In Monoamine Oxidase and their Inhibitors; International Review of Neurobiology; Youdim, M.B.H., Douce, P., Eds.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2011; Volume 100, pp. 169–190. [Google Scholar]
- Ito, J.; Ito, M.; Nambu, H.; Fujikawa, T.; Tanaka, K.; Iwaasa, H.; Tokita, S. Anatomical and Histological Profiling of Orphan G-Protein-Coupled Receptor Expression in Gastrointestinal Tract of C57BL/6J Mice. Cell Tissue Res. 2009, 338, 257–269. [Google Scholar] [CrossRef]
- Ohta, H.; Takebe, Y.; Murakami, Y.; Takahama, Y.; Morimura, S. Tyramine and β-Phenylethylamine, from Fermented Food Products, as Agonists for the Human Trace Amine-Associated Receptor 1 (HTAAR1) in the Stomach. Biosci. Biotechnol. Biochem. 2017, 81, 1002–1006. [Google Scholar] [CrossRef] [Green Version]
- Regard, J.B.; Sato, I.T.; Coughlin, S.R. Anatomical Profiling of G Protein-Coupled Receptor Expression. Cell 2008, 135, 561–571. [Google Scholar] [CrossRef] [Green Version]
- Babusyte, A.; Kotthoff, M.; Fiedler, J.; Krautwurst, D. Biogenic Amines Activate Blood Leukocytes via Trace Amine-Associated Receptors TAAR1 and TAAR2. J. Leukoc. Biol. 2013, 93, 387–394. [Google Scholar] [CrossRef]
- Zhukov, I.S.; Ptukha, M.A.; Zolotoverkhaja, E.A.; Sinitca, E.L.; Tissen, I.Y.; Karpova, I.V.; Volnova, A.B.; Gainetdinov, R.R. Evaluation of Approach to a Conspecific and Blood Biochemical Parameters in TAAR1 Knockout Mice. Brain Sci. 2022, 12, 614. [Google Scholar] [CrossRef]
- Apryatin, S.A.; Zhukov, I.S.; Manasyan, A.L.; Murtazina, R.Z.; Khunagov, T.A.; Lezina, A.I.; Klimenko, V.M. Effect of High-Fructose Diet on Physiological, Biochemical and Morphological Parameters of TAAR9 Knockout Female Rats. J. Evol. Biochem. Phys. 2021, 57, 1163–1173. [Google Scholar] [CrossRef]
- Murtazina, R.Z.; Zhukov, I.S.; Korenkova, O.M.; Popova, E.A.; Kuvarzin, S.R.; Efimova, E.V.; Kubarskaya, L.G.; Batotsyrenova, E.G.; Zolotoverkhaya, E.A.; Vaganova, A.N.; et al. Genetic Deletion of Trace-Amine Associated Receptor 9 (TAAR9) in Rats Leads to Decreased Blood Cholesterol Levels. Int. J. Mol. Sci. 2021, 22, 2942. [Google Scholar] [CrossRef]
- Ferrero, D.M.; Lemon, J.K.; Fluegge, D.; Pashkovski, S.L.; Korzan, W.J.; Datta, S.R.; Spehr, M.; Fendt, M.; Liberles, S.D. Detection and Avoidance of a Carnivore Odor by Prey. Proc. Natl. Acad. Sci. USA 2011, 108, 11235–11240. [Google Scholar] [CrossRef] [Green Version]
- Saraiva, L.R.; Kondoh, K.; Ye, X.; Yoon, K.-H.; Hernandez, M.; Buck, L.B. Combinatorial Effects of Odorants on Mouse Behavior. Proc. Natl. Acad. Sci. USA 2016, 113, E3300–E3306. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Guo, L.; Qian, X.; Yu, C.; Li, S.; Zhu, C.; Ma, X.; Li, H.; Zhu, G.; Zhou, H.; et al. Two Entry Tunnels in Mouse TAAR9 Suggest the Possibility of Multi-Entry Tunnels in Olfactory Receptors. Sci. Rep. 2022, 12, 2691. [Google Scholar] [CrossRef]
- Bekebrede, A.F.; Keijer, J.; Gerrits, W.J.J.; de Boer, V.C.J. The Molecular and Physiological Effects of Protein-Derived Polyamines in the Intestine. Nutrients 2020, 12, 197. [Google Scholar] [CrossRef]
- Ma, L.; Ni, Y.; Wang, Z.; Tu, W.; Ni, L.; Zhuge, F.; Zheng, A.; Hu, L.; Zhao, Y.; Zheng, L.; et al. Spermidine Improves Gut Barrier Integrity and Gut Microbiota Function in Diet-Induced Obese Mice. Gut Microbes 2020, 12, 1832857. [Google Scholar] [CrossRef]
- Ramos-Molina, B.; Queipo-Ortuño, M.I.; Lambertos, A.; Tinahones, F.J.; Peñafiel, R. Dietary and Gut Microbiota Polyamines in Obesity- and Age-Related Diseases. Front. Nutr. 2019, 6, 24. [Google Scholar] [CrossRef] [Green Version]
- Rao, J.N.; Xiao, L.; Wang, J.-Y. Polyamines in Gut Epithelial Renewal and Barrier Function. Physiology 2020, 35, 328–337. [Google Scholar] [CrossRef]
- Timmons, J.; Chang, E.T.; Wang, J.-Y.; Rao, J.N. Polyamines and Gut Mucosal Homeostasis. J. Gastrointest. Dig. Syst. 2012, 2, 1. [Google Scholar] [CrossRef]
- Tofalo, R.; Cocchi, S.; Suzzi, G. Polyamines and Gut Microbiota. Front. Nutr. 2019, 6, 16. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Ni, L.; Yang, T.; Mao, P.; Huang, X.; Luo, Y.; Jiang, Z.; Hu, L.; Zhao, Y.; Fu, Z.; et al. Preventive and Therapeutic Spermidine Treatment Attenuates Acute Colitis in Mice. J. Agric. Food Chem. 2021, 69, 1864–1876. [Google Scholar] [CrossRef]
- Matsumoto, M.; Kurihara, S.; Kibe, R.; Ashida, H.; Benno, Y. Longevity in Mice Is Promoted by Probiotic-Induced Suppression of Colonic Senescence Dependent on Upregulation of Gut Bacterial Polyamine Production. PLoS ONE 2011, 6, e23652. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, A.; Ooga, T.; Matsumoto, M. Intestinal Luminal Putrescine Is Produced by Collective Biosynthetic Pathways of the Commensal Microbiome. Gut Microbes 2019, 10, 159–171. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, A.; Kurihara, S.; Takahashi, D.; Ohashi, W.; Nakamura, Y.; Kimura, S.; Onuki, M.; Kume, A.; Sasazawa, Y.; Furusawa, Y.; et al. Symbiotic Polyamine Metabolism Regulates Epithelial Proliferation and Macrophage Differentiation in the Colon. Nat. Commun. 2021, 12, 2105. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut Microbiota, Metabolites and Host Immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Yang, Y.; Misra, B.B.; Liang, L.; Bi, D.; Weng, W.; Wu, W.; Cai, S.; Qin, H.; Goel, A.; Li, X.; et al. Integrated Microbiome and Metabolome Analysis Reveals a Novel Interplay between Commensal Bacteria and Metabolites in Colorectal Cancer. Theranostics 2019, 9, 4101. [Google Scholar] [CrossRef]
- del Rio, B.; Redruello, B.; Linares, D.M.; Ladero, V.; Ruas-Madiedo, P.; Fernandez, M.; Martin, M.C.; Alvarez, M.A. The Biogenic Amines Putrescine and Cadaverine Show in Vitro Cytotoxicity at Concentrations That Can Be Found in Foods. Sci. Rep. 2019, 9, 120. [Google Scholar] [CrossRef] [Green Version]
- Grosheva, I.; Zheng, D.; Levy, M.; Polansky, O.; Lichtenstein, A.; Golani, O.; Dori-Bachash, M.; Moresi, C.; Shapiro, H.; Del Mare-Roumani, S.; et al. High-Throughput Screen Identifies Host and Microbiota Regulators of Intestinal Barrier Function. Gastroenterology 2020, 159, 1807–1823. [Google Scholar] [CrossRef]
- Guerra, G.P.; Rubin, M.A.; Mello, C.F. Modulation of Learning and Memory by Natural Polyamines. Pharmacol. Res. 2016, 112, 99–118. [Google Scholar] [CrossRef]
- Papatheodorou, I.; Fonseca, N.A.; Keays, M.; Tang, Y.A.; Barrera, E.; Bazant, W.; Burke, M.; Füllgrabe, A.; Fuentes, A.M.-P.; George, N.; et al. Expression Atlas: Gene and Protein Expression across Multiple Studies and Organisms. Nucleic Acids Res. 2018, 46, D246–D251. [Google Scholar] [CrossRef]
- Foth, B.J.; Tsai, I.J.; Reid, A.J.; Bancroft, A.J.; Nichol, S.; Tracey, A.; Holroyd, N.; Cotton, J.A.; Stanley, E.J.; Zarowiecki, M.; et al. Whipworm Genome and Dual-Species Transcriptome Analyses Provide Molecular Insights into an Intimate Host-Parasite Interaction. Nat. Genet. 2014, 46, 693–700. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [Green Version]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A Graphical Gene-Set Enrichment Tool for Animals and Plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef]
- Gaidatzis, D.; Lerch, A.; Hahne, F.; Stadler, M.B. QuasR: Quantification and Annotation of Short Reads in R. Bioinformatics 2015, 31, 1130–1132. [Google Scholar] [CrossRef] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Xu, S.; Yu, G. Microbiota Process: A Comprehensive R Package for Managing and Analyzing Microbiome and Other Ecological Data within the Tidy Framework. R Package Version 1.8.1. Available online: https://github.com/YuLab-SMU/MicrobiotaProcess (accessed on 30 August 2022).
- Wickham, H. Ggplot2 Elegant Graphics for Data Analysis, 2nd ed.; Springer: Cham, Switzerland; New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Oksanen, J. Vegan: Community Ecology Package. R Package Version 1.8-5. 2007. Available online: http://www.cran.r-project.org (accessed on 2 August 2022).
- Wei, T.; Simko, V.; Levy, M.; Xie, Y.; Jin, Y.; Zemla, J.; Freidank, M.; Cai, J.; Protivinsky, T. Corrplot: Visualization of a Correlation Matrix. Available online: https://CRAN.R-project.org/package=corrplot (accessed on 30 August 2022).
- López-Kleine, L.; Leal, L.; López, C. Biostatistical Approaches for the Reconstruction of Gene Co-Expression Networks Based on Transcriptomic Data. Brief. Funct. Genom. 2013, 12, 457–467. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Xia, S.; Arand, B.; Zhu, H.; Machiraju, R.; Huang, K.; Ji, H.; Qian, J. Single-Cell Co-Expression Analysis Reveals Distinct Functional Modules, Co-Regulation Mechanisms and Clinical Outcomes. PLoS Comput. Biol. 2016, 12, e1004892. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, N.; Lu, H. Correlation between Gene Expression Profiles and Protein–Protein Interactions within and across Genomes. Bioinformatics 2005, 21, 2730–2738. [Google Scholar] [CrossRef] [Green Version]
- Dhekne, H.S.; Pylypenko, O.; Overeem, A.W.; Ferreira, R.J.; van der Velde, K.J.; Rings, E.H.H.M.; Posovszky, C.; Swertz, M.A.; Houdusse, A.; van IJzendoorn, S.C.D. MYO5B, STX3, and STXBP2 Mutations Reveal a Common Disease Mechanism That Unifies a Subset of Congenital Diarrheal Disorders: A Mutation Update. Hum. Mutat. 2018, 39, 333–344. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, S.E.; Stacey, H.M.; Nahaei, Y.; Hale, S.J.; Hardy, A.B.; Reimann, F.; Gribble, F.M.; Larraufie, P.; Gaisano, H.Y.; Brubaker, P.L. The SNARE Protein Syntaxin-1a Plays an Essential Role in Biphasic Exocytosis of the Incretin Hormone Glucagon-Like Peptide 1. Diabetes 2017, 66, 2327–2338. [Google Scholar] [CrossRef] [Green Version]
- Mansbach, C.M.; Siddiqi, S. Control of Chylomicron Export from the Intestine. Am. J. Physiol.-Gastrointest. Liver Physiol. 2016, 310, G659–G668. [Google Scholar] [CrossRef] [Green Version]
- Nejsum, L.N.; Nelson, W.J. Epithelial Cell Surface Polarity: The Early Steps. Front Biosci. 2009, 14, 1088–1098. [Google Scholar] [CrossRef] [Green Version]
- Cosín-Roger, J.; Ortiz-Masia, D.; Barrachina, M.D.; Calatayud, S. Metabolite Sensing GPCRs: Promising Therapeutic Targets for Cancer Treatment? Cells 2020, 9, 2345. [Google Scholar] [CrossRef]
- Espinoza, S.; Salahpour, A.; Masri, B.; Sotnikova, T.D.; Messa, M.; Barak, L.S.; Caron, M.G.; Gainetdinov, R.R. Functional Interaction between Trace Amine-Associated Receptor 1 and Dopamine D2 Receptor. Mol. Pharmacol. 2011, 80, 416–425. [Google Scholar] [CrossRef] [Green Version]
- Espinoza, S.; Ghisi, V.; Emanuele, M.; Leo, D.; Sukhanov, I.; Sotnikova, T.D.; Chieregatti, E.; Gainetdinov, R.R. Postsynaptic D2 Dopamine Receptor Supersensitivity in the Striatum of Mice Lacking TAAR1. Neuropharmacology 2015, 93, 308–313. [Google Scholar] [CrossRef]
- Harmeier, A.; Obermueller, S.; Meyer, C.A.; Revel, F.G.; Buchy, D.; Chaboz, S.; Dernick, G.; Wettstein, J.G.; Iglesias, A.; Rolink, A.; et al. Trace Amine-Associated Receptor 1 Activation Silences GSK3β Signaling of TAAR1 and D2R Heteromers. Eur. Neuropsychopharmacol. 2015, 25, 2049–2061. [Google Scholar] [CrossRef]
- Pitts, M.S.; McShane, J.N.; Hoener, M.C.; Christian, S.L.; Berry, M.D. TAAR1 Levels and Sub-Cellular Distribution Are Cell Line but Not Breast Cancer Subtype Specific. Histochem. Cell Biol. 2019, 152, 155–166. [Google Scholar] [CrossRef]
- Efimova, E.V.; Kozlova, A.A.; Razenkova, V.; Katolikova, N.V.; Antonova, K.A.; Sotnikova, T.D.; Merkulyeva, N.S.; Veshchitskii, A.S.; Kalinina, D.S.; Korzhevskii, D.E.; et al. Increased Dopamine Transmission and Adult Neurogenesis in Trace Amine-Associated Receptor 5 (TAAR5) Knockout Mice. Neuropharmacology 2021, 182, 108373. [Google Scholar] [CrossRef]
- Aleksandrov, A.A.; Knyazeva, V.M.; Volnova, A.B.; Dmitrieva, E.S.; Korenkova, O.; Espinoza, S.; Gerasimov, A.; Gainetdinov, R.R. Identification of TAAR5 Agonist Activity of Alpha-NETA and Its Effect on Mismatch Negativity Amplitude in Awake Rats. Neurotox Res. 2018, 34, 442–451. [Google Scholar] [CrossRef]
- Aleksandrov, A.A.; Polyakova, N.V.; Vinogradova, E.P.; Gainetdinov, R.R.; Knyazeva, V.M. The TAAR5 Agonist α-NETA Causes Dyskinesia in Mice. Neurosci. Lett. 2019, 704, 208–211. [Google Scholar] [CrossRef]
- Aleksandrov, A.A.; Knyazeva, V.M.; Volnova, A.B.; Dmitrieva, E.S.; Polyakova, N.V. Putative TAAR5 Agonist Alpha-NETA Affects Event-Related Potentials in Oddball Paradigm in Awake Mice. Brain Res. Bull. 2020, 158, 116–121. [Google Scholar] [CrossRef]
- Maggi, S.; Bon, C.; Gustincich, S.; Tucci, V.; Gainetdinov, R.R.; Espinoza, S. Improved Cognitive Performance in Trace Amine-Associated Receptor 5 (TAAR5) Knock-out Mice. Sci. Rep. 2022, 12, 14708. [Google Scholar] [CrossRef]
- Yu, S.; Peng, H.; Zhang, Y.; Yin, Y.; Zhou, J. Central Dopaminergic Control of Cell Proliferation in the Colonic Epithelium. Neurosci. Res. 2022, 180, 72–82. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Liu, S.; Sun, Q.; Zhu, J.-X. Dopamine Receptors in the Gastrointestinal Tract. In Dopamine in the Gut; Zhu, J.-X., Ed.; Springer: Singapore, 2021; pp. 53–85. ISBN 978-981-336-586-5. [Google Scholar]
- Hertli, S.; Zimmermann, P. Molecular Interactions between the Intestinal Microbiota and the Host. Mol. Microbiol. 2022, 117, 1297–1307. [Google Scholar] [CrossRef]
- Huang, W.; Fang, Q.; Fan, L.; Hong, T.; Tan, H.; Nie, S. Pectin with Various Degrees of Esterification Differentially Alters Gut Microbiota and Metabolome of Healthy Adults. eFood 2022, 3, e5. [Google Scholar] [CrossRef]
- Yang, W.; Cong, Y. Gut Microbiota-Derived Metabolites in the Regulation of Host Immune Responses and Immune-Related Inflammatory Diseases. Cell Mol. Immunol. 2021, 18, 866–877. [Google Scholar] [CrossRef]
- Kurihara, S. Polyamine Metabolism and Transport in Gut Microbes. Biosci. Biotechnol. Biochem. 2022, 86, 957–966. [Google Scholar] [CrossRef]
- Fiori, J.; Turroni, S.; Candela, M.; Gotti, R. Assessment of Gut Microbiota Fecal Metabolites by Chromatographic Targeted Approaches. J. Pharm. Biomed. Anal. 2020, 177, 112867. [Google Scholar] [CrossRef]
- Igarashi, K.; Kashiwagi, K. The Functional Role of Polyamines in Eukaryotic Cells. Int. J. Biochem. Cell Biol. 2019, 107, 104–115. [Google Scholar] [CrossRef]
- Hirano, R.; Shirasawa, H.; Kurihara, S. Health-Promoting Effects of Dietary Polyamines. Med. Sci. 2021, 9, 8. [Google Scholar] [CrossRef]
- Proietti, E.; Rossini, S.; Grohmann, U.; Mondanelli, G. Polyamines and Kynurenines at the Intersection of Immune Modulation. Trends Immunol. 2020, 41, 1037–1050. [Google Scholar] [CrossRef]
- Di Martino, M.L.; Campilongo, R.; Casalino, M.; Micheli, G.; Colonna, B.; Prosseda, G. Polyamines: Emerging Players in Bacteria–Host Interactions. Int. J. Med. Microbiol. 2013, 303, 484–491. [Google Scholar] [CrossRef]
- McCann, K.S. The Diversity–Stability Debate. Nature 2000, 405, 228–233. [Google Scholar] [CrossRef]
- McLean, J.S.; Bor, B.; Kerns, K.A.; Liu, Q.; To, T.T.; Solden, L.; Hendrickson, E.L.; Wrighton, K.; Shi, W.; He, X. Acquisition and Adaptation of Ultra-Small Parasitic Reduced Genome Bacteria to Mammalian Hosts. Cell Rep. 2020, 32, 107939. [Google Scholar] [CrossRef]
- Lemos, L.N.; Medeiros, J.D.; Dini-Andreote, F.; Fernandes, G.R.; Varani, A.M.; Oliveira, G.; Pylro, V.S. Genomic Signatures and Co-Occurrence Patterns of the Ultra-Small Saccharimonadia (Phylum CPR/Patescibacteria) Suggest a Symbiotic Lifestyle. Mol. Ecol. 2019, 28, 4259–4271. [Google Scholar] [CrossRef]
- Kantor, R.S.; Wrighton, K.C.; Handley, K.M.; Sharon, I.; Hug, L.A.; Castelle, C.J.; Thomas, B.C.; Banfield, J.F. Small Genomes and Sparse Metabolisms of Sediment-Associated Bacteria from Four Candidate Phyla. mBio 2013, 4, e00708-13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kindaichi, T.; Yamaoka, S.; Uehara, R.; Ozaki, N.; Ohashi, A.; Albertsen, M.; Nielsen, P.H.; Nielsen, J.L. Phylogenetic Diversity and Ecophysiology of Candidate Phylum Saccharibacteria in Activated Sludge. FEMS Microbiol. Ecol. 2016, 92, fiw078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starr, E.P.; Shi, S.; Blazewicz, S.J.; Probst, A.J.; Herman, D.J.; Firestone, M.K.; Banfield, J.F. Stable Isotope Informed Genome-Resolved Metagenomics Reveals That Saccharibacteria Utilize Microbially-Processed Plant-Derived Carbon. Microbiome 2018, 6, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.; Li, Q.; Jiang, S.; Xu, Z.; Jiang, Y.; Liu, L.; Jiang, J.; Tong, Y.; Wang, P. Crocetin Ameliorates Chronic Restraint Stress-Induced Depression-like Behaviors in Mice by Regulating MEK/ERK Pathways and Gut Microbiota. J. Ethnopharmacol. 2021, 268, 113608. [Google Scholar] [CrossRef]
- Loubet Filho, P.S.; Baseggio, A.M.; Vuolo, M.M.; Reguengo, L.M.; Telles Biasoto, A.C.; Correa, L.C.; Junior, S.B.; Alves Cagnon, V.H.; Betim Cazarin, C.B.; Maróstica Júnior, M.R. Gut Microbiota Modulation by Jabuticaba Peel and Its Effect on Glucose Metabolism via Inflammatory Signaling. Curr. Res. Food Sci. 2022, 5, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yan, Y.; Zhou, W.; Chen, D.; Huang, K.; Yu, S.; Mi, J.; Lu, L.; Zeng, X.; Cao, Y. Effects of Polysaccharides from Bee Collected Pollen of Chinese Wolfberry on Immune Response and Gut Microbiota Composition in Cyclophosphamide-Treated Mice. J. Funct. Foods 2020, 72, 104057. [Google Scholar] [CrossRef]
- Ma, F.; Sun, M.; Song, Y.; Wang, A.; Jiang, S.; Qian, F.; Mu, G.; Tuo, Y. Lactiplantibacillus plantarum-12 Alleviates Inflammation and Colon Cancer Symptoms in AOM/DSS-Treated Mice through Modulating the Intestinal Microbiome and Metabolome. Nutrients 2022, 14, 1916. [Google Scholar] [CrossRef]
- Du, D.; Tang, W.; Zhou, C.; Sun, X.; Wei, Z.; Zhong, J.; Huang, Z. Fecal Microbiota Transplantation Is a Promising Method to Restore Gut Microbiota Dysbiosis and Relieve Neurological Deficits after Traumatic Brain Injury. Oxidative Med. Cell. Longev. 2021, 2021, e5816837. [Google Scholar] [CrossRef]
- Ortega-Hernández, A.; Martínez-Martínez, E.; Gómez-Gordo, R.; López-Andrés, N.; Fernández-Celis, A.; Gutiérrrez-Miranda, B.; Nieto, M.L.; Alarcón, T.; Alba, C.; Gómez-Garre, D.; et al. The Interaction between Mitochondrial Oxidative Stress and Gut Microbiota in the Cardiometabolic Consequences in Diet-Induced Obese Rats. Antioxidants 2020, 9, 640. [Google Scholar] [CrossRef]
- Longo, L.; Rampelotto, P.H.; Filippi-Chiela, E.; de Souza, V.E.G.; Salvati, F.; Cerski, C.T.; da Silveira, T.R.; Oliveira, C.P.; Uribe-Cruz, C.; Álvares-da-Silva, M.R. Gut Dysbiosis and Systemic Inflammation Promote Cardiomyocyte Abnormalities in an Experimental Model of Steatohepatitis. World J. Hepatol. 2021, 13, 2052–2070. [Google Scholar] [CrossRef] [PubMed]
- Bailén, M.; Bressa, C.; Martínez-López, S.; González-Soltero, R.; Montalvo Lominchar, M.G.; San Juan, C.; Larrosa, M. Microbiota Features Associated with a High-Fat/Low-Fiber Diet in Healthy Adults. Front. Nutr. 2020, 7, 583608. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Chen, X.; Yan, X.; Wang, J.; Yu, G.; Ma, W.; Xiao, B.; Quinones, S.; Tian, X.; Ren, X. Gut Microbiota Perturbations and Neurodevelopmental Impacts in Offspring Rats Concurrently Exposure to Inorganic Arsenic and Fluoride. Environ. Int. 2020, 140, 105763. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-D.; Guo, Y.-S.; Huang, J.-S.; Gao, Y.-F.; Peng, F.; Xu, R.-Y.; Su, H.-H.; Zhang, P.-J. Isomaltulose Exhibits Prebiotic Activity, and Modulates Gut Microbiota, the Production of Short Chain Fatty Acids, and Secondary Bile Acids in Rats. Molecules 2021, 26, 2464. [Google Scholar] [CrossRef]
- Cruz, B.C.D.S.; da Conceição, L.L.; Mendes, T.A.d.O.; Ferreira, C.L.d.L.F.; Gonçalves, R.V.; Peluzio, M.d.C.G. Use of the Synbiotic VSL#3 and Yacon-Based Concentrate Attenuates Intestinal Damage and Reduces the Abundance of Candidatus Saccharimonas in a Colitis-Associated Carcinogenesis Model. Food Res. Int. 2020, 137, 109721. [Google Scholar] [CrossRef]
- Dong, Y.-J.; Lin, M.-Q.; Fang, X.; Xie, Z.-Y.; Luo, R.; Teng, X.; Li, B.; Li, B.; Li, L.-Z.; Jin, H.-Y.; et al. Modulating Effects of a Functional Food Containing Dendrobium Officinale on Immune Response and Gut Microbiota in Mice Treated with Cyclophosphamide. J. Funct. Foods 2022, 94, 105102. [Google Scholar] [CrossRef]
- John, O.D.; Mouatt, P.; Majzoub, M.E.; Thomas, T.; Panchal, S.K.; Brown, L. Physiological and Metabolic Effects of Yellow Mangosteen (Garcinia dulcis) Rind in Rats with Diet-Induced Metabolic Syndrome. Int. J. Mol. Sci. 2020, 21, 272. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Bai, R.; Zhou, W.; Yao, Z.; Liu, Y.; Tang, S.; Ge, X.; Luo, L.; Luo, C.; Hu, G.; et al. Angiogenin Maintains Gut Microbe Homeostasis by Balancing α-Proteobacteria and Lachnospiraceae. Gut 2021, 70, 666–676. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, H.; Song, N.; Zhang, L.; Cao, Y.; Tai, J. Long-Term Hexavalent Chromium Exposure Facilitates Colorectal Cancer in Mice Associated with Changes in Gut Microbiota Composition. Food Chem. Toxicol. 2020, 138, 111237. [Google Scholar] [CrossRef]
- Yang, Y.-C.S.H.; Chang, H.-W.; Lin, I.-H.; Chien, L.-N.; Wu, M.-J.; Liu, Y.-R.; Chu, P.G.; Xie, G.; Dong, F.; Jia, W.; et al. Long-Term Proton Pump Inhibitor Administration Caused Physiological and Microbiota Changes in Rats. Sci. Rep. 2020, 10, 866. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-F.; Hsieh, A.-H.; Wang, L.-C.; Huang, Y.-J.; Tsai, Y.-C.; Tseng, W.-Y.; Kuo, Y.-L.; Luo, S.-F.; Yu, K.-H.; Kuo, C.-F. Fecal Microbiota Changes in NZB/W F1 Mice after Induction of Lupus Disease. Sci. Rep. 2021, 11, 22953. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.T.; Roesch, L.F.W.; Ördberg, M.; Ilonen, J.; Atkinson, M.A.; Schatz, D.A.; Triplett, E.W.; Ludvigsson, J. Genetic Risk for Autoimmunity Is Associated with Distinct Changes in the Human Gut Microbiome. Nat. Commun. 2019, 10, 3621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balasubramanian, B.; Soundharrajan, I.; Al-Dhabi, N.A.; Vijayaraghavan, P.; Balasubramanian, K.; Valan Arasu, M.; Choi, K.C. Probiotic Characteristics of Ligilactobacillus Salivarius AS22 Isolated from Sheep Dung and Its Application in Corn-Fox Tail Millet Silage. Appl. Sci. 2021, 11, 9447. [Google Scholar] [CrossRef]
- Guerrero Sanchez, M.; Passot, S.; Campoy, S.; Olivares, M.; Fonseca, F. Ligilactobacillus Salivarius Functionalities, Applications, and Manufacturing Challenges. Appl. Microbiol. Biotechnol. 2022, 106, 57–80. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Pattanaik, A.K.; Singh, A.; Sharma, S.; Jadhav, S.E.; Kumar, A.; Verma, A.K. Functional and Probiotic Characterization of Ligilactobacillus Salivarius CPN60 Isolated from Calf Faeces and Its Appraisal in Rats. J. Biosci. Bioeng. 2021, 132, 575–584. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A Taxonomic Note on the Genus Lactobacillus: Description of 23 Novel Genera, Emended Description of the Genus Lactobacillus Beijerinck 1901, and Union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Ludwig, W.; Schleifer, K.-H.; Whitman, W.B. Revised Road Map to the Phylum Firmicutes. In Bergey’s Manual® of Systematic Bacteriology: Volume Three the Firmicutes; De Vos, P., Garrity, G.M., Jones, D., Krieg, N.R., Ludwig, W., Rainey, F.A., Schleifer, K.-H., et al., Eds.; Springer: New York, NY, USA, 2009; pp. 1–13. ISBN 978-0-387-68489-5. [Google Scholar]
- Yutin, N.; Galperin, M.Y. A Genomic Update on Clostridial Phylogeny: Gram-Negative Spore-Formers and Other Misplaced Clostridia. Environ. Microbiol. 2013, 15, 2631–2641. [Google Scholar] [CrossRef]
- Grant, C.V.; Loman, B.R.; Bailey, M.T.; Pyter, L.M. Manipulations of the Gut Microbiome Alter Chemotherapy-Induced Inflammation and Behavioral Side Effects in Female Mice. Brain Behav. Immun. 2021, 95, 401–412. [Google Scholar] [CrossRef]
- Van Andel, R.A.; Franklin, C.L.; Besch-Williford, C.L.; Hook, R.R.; Rilly, L.K.Y. 2000 Prolonged Perturbations of Tumour Necrosis Factor-α and Interferon-γ in Mice Inoculated with Clostridium Piliforme. J. Med. Microbiol. 2000, 49, 557–563. [Google Scholar] [CrossRef] [Green Version]
- Efimova, E.V.; Kuvarzin, S.R.; Mor, M.S.; Katolikova, N.V.; Shemiakova, T.S.; Razenkova, V.; Ptukha, M.; Kozlova, A.A.; Murtazina, R.Z.; Smirnova, D.; et al. Trace Amine-Associated Receptor 2 Is Expressed in the Limbic Brain Areas and Is Involved in Dopamine Regulation and Adult Neurogenesis. Front. Behav. Neurosci. 2022, 16, 847410. [Google Scholar] [CrossRef]
- Dinter, J.; Mühlhaus, J.; Jacobi, S.F.; Wienchol, C.L.; Cöster, M.; Meister, J.; Hoefig, C.S.; Müller, A.; Köhrle, J.; Grüters, A.; et al. 3-Iodothyronamine Differentially Modulates α-2A-Adrenergic Receptor-Mediated Signaling. J. Mol. Endocrinol. 2015, 54, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Zhukov, I.S.; Karpova, I.V.; Krotova, N.A.; Tissen, I.Y.; Demin, K.A.; Shabanov, P.D.; Budygin, E.A.; Kalueff, A.V.; Gainetdinov, R.R. Enhanced Aggression, Reduced Self-Grooming Behavior and Altered 5-HT Regulation in the Frontal Cortex in Mice Lacking Trace Amine-Associated Receptor 1 (TAAR1). Int. J. Mol. Sci. 2022, 23, 14066. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhukov, I.S.; Vaganova, A.N.; Murtazina, R.Z.; Alferova, L.S.; Ermolenko, E.I.; Gainetdinov, R.R. Gut Microbiota Alterations in Trace Amine-Associated Receptor 9 (TAAR9) Knockout Rats. Biomolecules 2022, 12, 1823. https://doi.org/10.3390/biom12121823
Zhukov IS, Vaganova AN, Murtazina RZ, Alferova LS, Ermolenko EI, Gainetdinov RR. Gut Microbiota Alterations in Trace Amine-Associated Receptor 9 (TAAR9) Knockout Rats. Biomolecules. 2022; 12(12):1823. https://doi.org/10.3390/biom12121823
Chicago/Turabian StyleZhukov, Ilya S., Anastasia N. Vaganova, Ramilya Z. Murtazina, Lyubov S. Alferova, Elena I. Ermolenko, and Raul R. Gainetdinov. 2022. "Gut Microbiota Alterations in Trace Amine-Associated Receptor 9 (TAAR9) Knockout Rats" Biomolecules 12, no. 12: 1823. https://doi.org/10.3390/biom12121823
APA StyleZhukov, I. S., Vaganova, A. N., Murtazina, R. Z., Alferova, L. S., Ermolenko, E. I., & Gainetdinov, R. R. (2022). Gut Microbiota Alterations in Trace Amine-Associated Receptor 9 (TAAR9) Knockout Rats. Biomolecules, 12(12), 1823. https://doi.org/10.3390/biom12121823







