Natural Sympathomimetic Drugs: From Pharmacology to Toxicology
Abstract
:1. Introduction
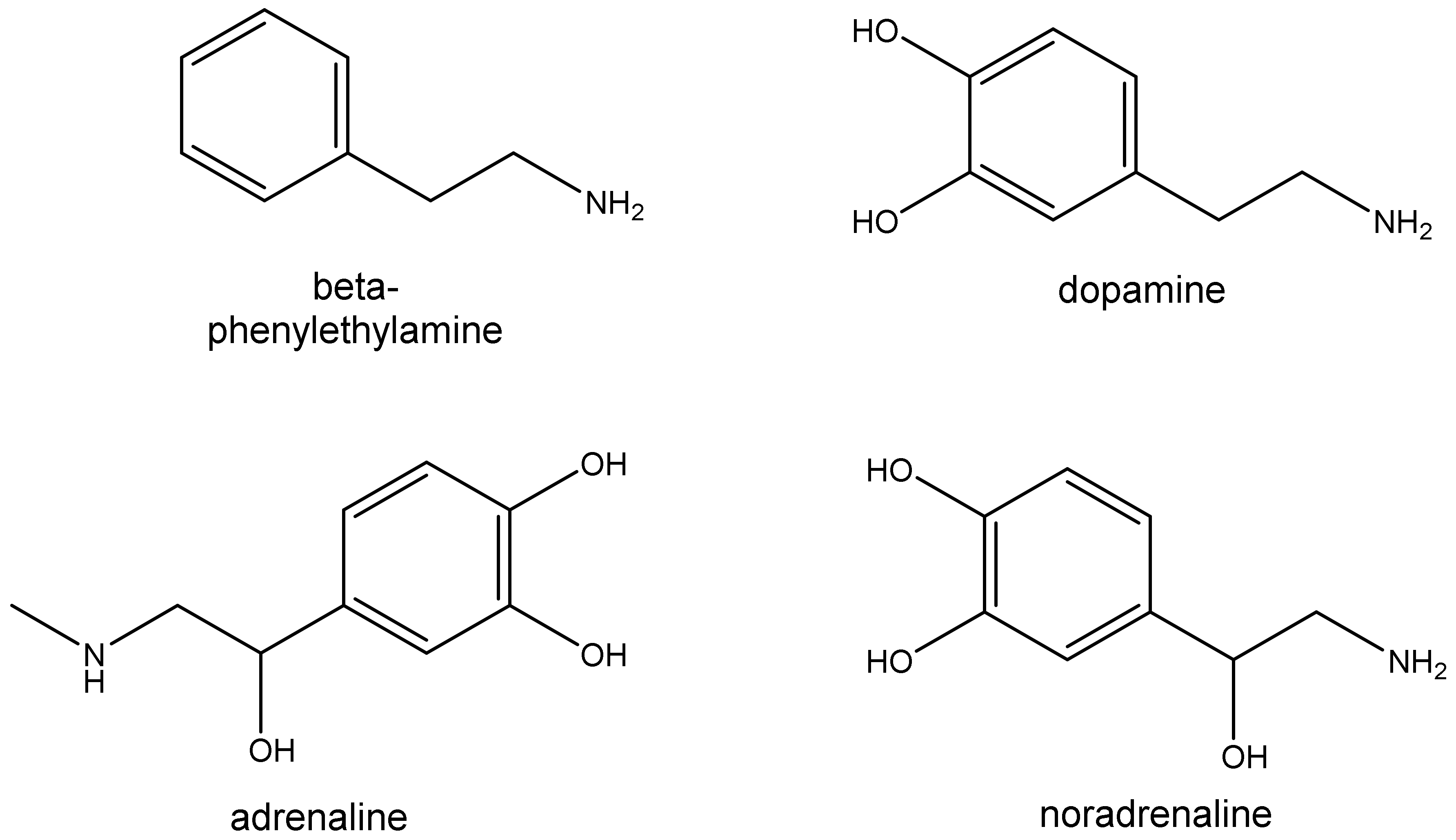
2. Trace Amines
2.1. Sources
2.2. Pharmacology
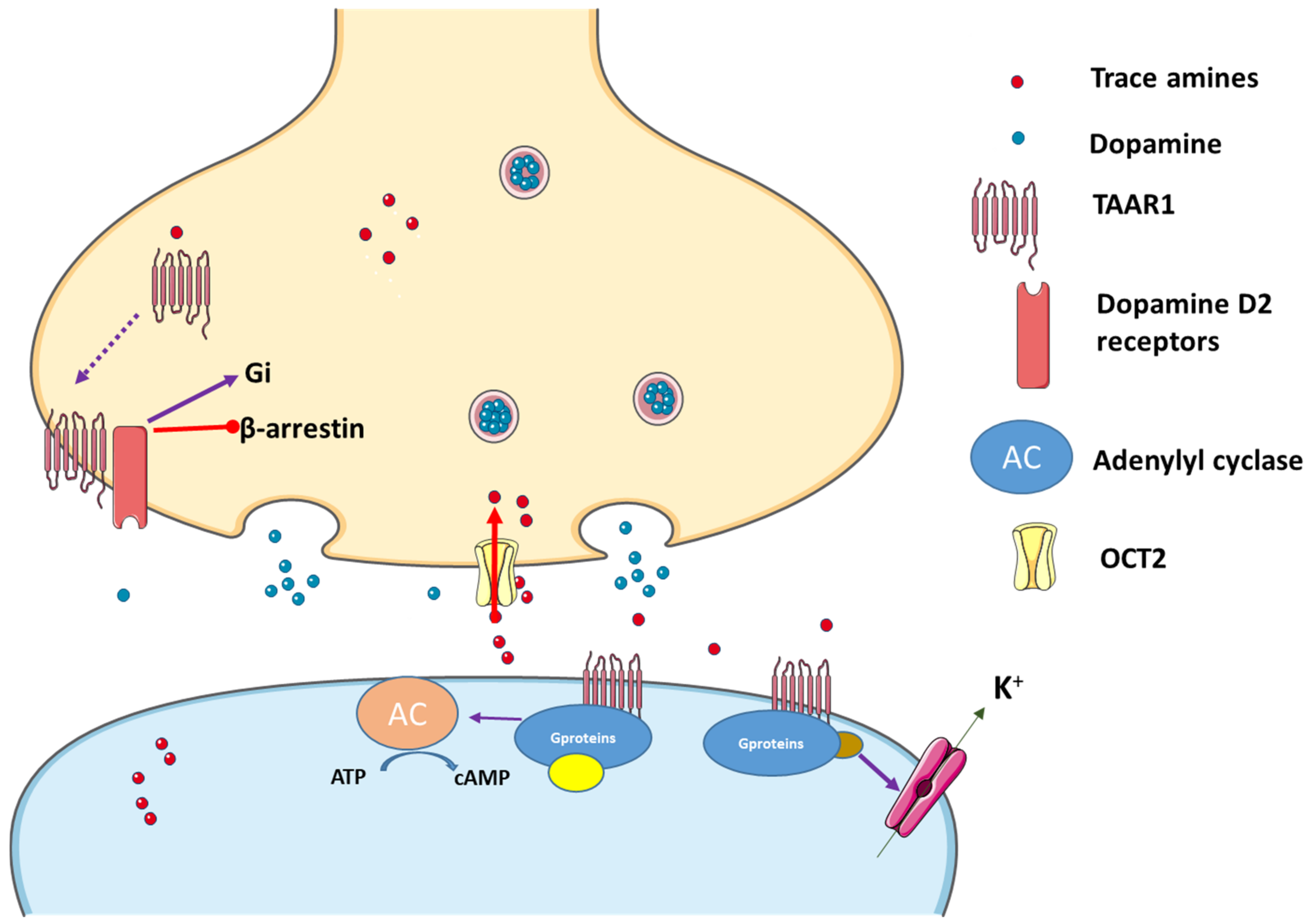
2.2.1. TAAR1 and Its Agonists
2.2.2. TAAR1 Localization
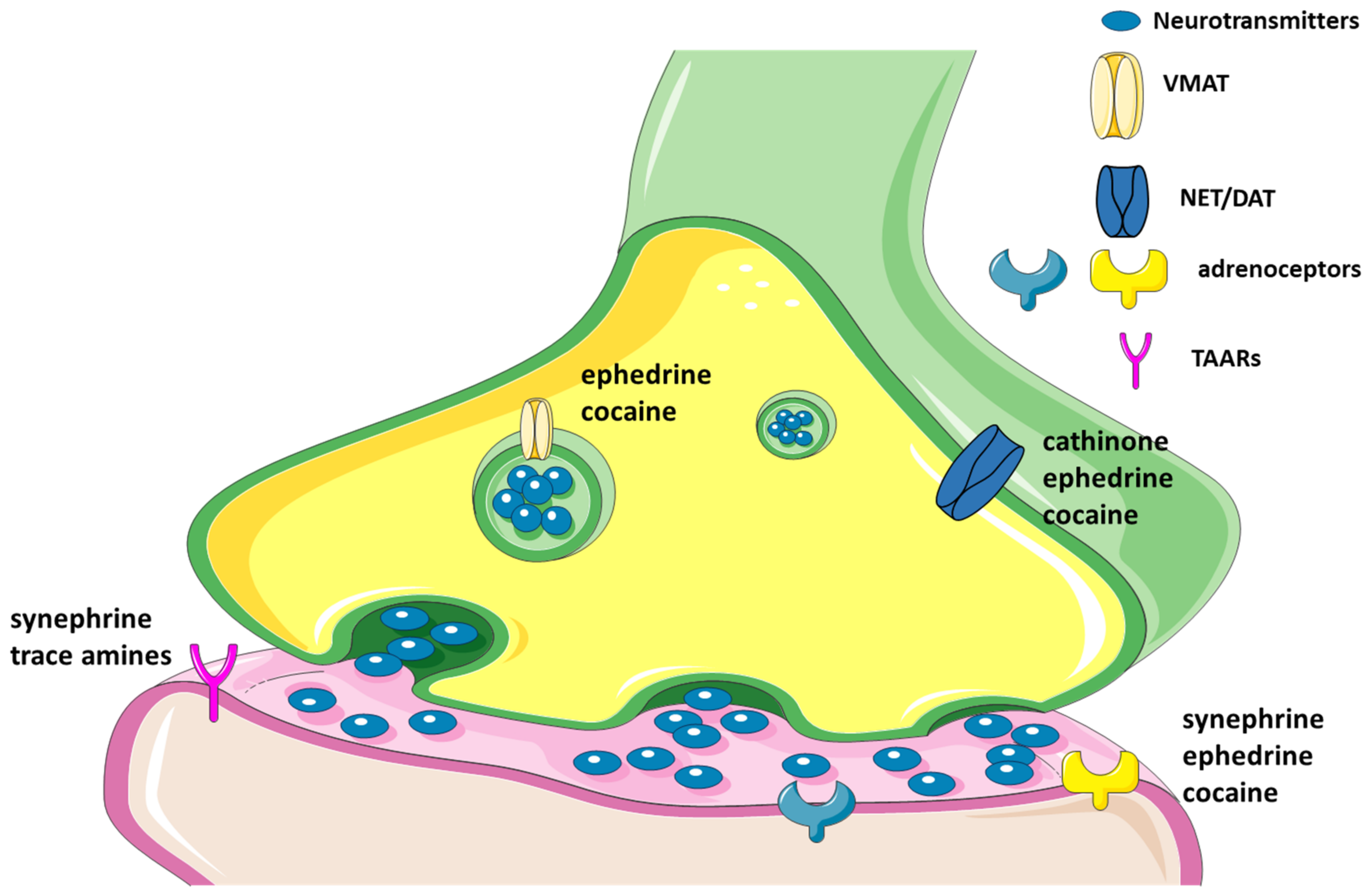
2.2.3. Trace Amines as Indirect Sympathomimetic Agents
2.2.4. Trace Amines Pharmacokinetics
2.3. Toxic Effects of Trace Amines
3. Synephrine
3.1. Sources and Amounts Found in Natural Sources
3.2. Pharmacological Effects
3.3. In Vitro and In Vivo Toxicological Effects
3.4. Toxicology and Clinical Cases
| Reference | Route of Administration | Toxidrome | Clinical History |
|---|---|---|---|
| [100] | Oral—Edita’s Skinny Pill 1 capsule daily containing 300 mg of C. aurantium, for one year. Other components: carnitine 250 mg, chromium 400 μg, chitosan 250 mg, herbal diuretic complex 200 mg, guarananine 30 mg, green tea 30 mg, calcium 150 mg. | Acute lateral-wall myocardial infarction. | The 55-year-old woman presented dull aching shoulder and chest pain after eating a Chinese food. An arteriogram revealed a lesion in the left main coronary artery (previously unknown), she reported smoking 1 ½ pack per day and high ingestion of caffeine. |
| [101] | Oral—Stacker 2 Ephedra Free 1 or 2 capsules daily, labelled as having 6 mg of synephrine and 200 mg of caffeine. Treatment underwent for 1 week. | Acute and subacute infarctions in the left thalamus and multiple infarctions in the left cerebellum. | The 38-year-old man presented recent onset of dizziness, difficulty in concentrating, memory loss, and unsteady gait. He had no major risk factors for cardiovascular disease, and had not been taking long-term medications. |
| [102] | Oral—CortiSlim 1 tablet twice daily. It contains: Leptiplex 125 mg (C. aurantium extract with 5% synephrine and Green tea leaf extract with 50% epigallocatechin. Other components: vitamin C 100 mg, calcium 100 mg, chromium 50 mg, insutrol 16.5 mg (Banaba leaf extract and vanadyl sulfate). | Variant angina involving the right coronary artery. | The 57-year-old man presented left-sided chest pressure with radiation to the jaw, shortness of breath, and diaphoresis while at rest. He had history of hypertriglyceridemia and gastroesophageal reflux disease. He had quit smoking 7 years yearlier and reported drinking 3 to 4 alcoholic beverages at night. Long-term medications used: fenofibrate, omeprazole, aspirin, a multivitamin, vitamin B complex, and vitamin E. |
| [103] | Oral—Lipo 6 twice daily for 3 months. The supplement contains: synephrine, caffeine, and yohimbine (concentrations were not available). | Severe rhabdomyolysis, complicated by acute renal failure and bilateral compartment syndrome. | The 22-year-old, previously healthy, obese (body mass index 31), man, with sickle cell trait presented fatigue, light-headedness, and myalgia that started while running. He was diagnosed with rhabdomyolysis and heat exhaustion, and was discharged after brief hospitalization. Several weeks later, he had another episode of rhabdomyolysis and heat exhaustion, in this case severe. He presented hypotension, tachycardia, tachypnea, and myalgias. Initial evaluation revealed combined lactic and respiratory acidosis with metabolic alkalosis stemming from muscle hypoxia and ischemia with imminent respiratory failure. He developed hypovolemic shock, respiratory failure, acute renal failure, and disseminated intravascular coagulation. The patient had no history of exercise-associated rhabdomyolysis when performing physical activities without dietary supplements. |
| [104] | Oral—Xenadrine-EFX was used for a few months. Posology was not detailed. | Left middle cerebral artery vasospasm and stroke. | The 36-year-old woman presented weakness in the right upper extremity, difficulty in speaking, right facial droop and severe headache. Exams revealed cerebral infarctions in the left frontal and opercular regions and decreased diameter of the left middle cerebral artery. She was previously healthy, with no history of migraine, tobacco use, thrombophilia, hyperlipidemia, or oral contraceptive use. |
| [105] | Oral—Hi-Tech Lipodrene 1 tablet twice a day in the first week and then 2 tablets twice a day for two weeks. | Ventricular fibrillation. | The 27-year-old active duty Air Force female was performing the routine physical training when suddenly stopped and lied inactive. She presented thread pulse and agonal breathing, and cardiopulmonary resuscitation was required. The electrocardiogram showed a right bundle branch block and a QTc prolongation. She had a five pack-per-year smoking history and was previously healthy. |
| [106] | Oral—Nutrex Lipo-6x 1 capsule twice a week for 3 weeks. The supplement contains synephrine, yohimbe, and phenylethylamine. He also reported the use of caffeine energy drinks. | ST-segment-elevation myocardial infarction (STEMI). | A previously healthy 24-year-old man presented acute-onset, “crushing,” mid-sternal chest pain that was accompanied by shortness of breath, ansiety, diaphoresis, and emesis. Emergent coronary angiography revealed extensive, diffuse thrombi in the left anterior descending coronary artery. The patient had no risk factors for coronary artery disease, and denied the use of drugs and alcohol. |
| [107] | Oral—Jillian Michaels’ Fat Burner (two pills every morning) and Calorie Control (two pills three times a day). She increased the dosages 4 days prior to the episode. | Severe psychosis. | The 52-year-old woman with a history of anxiety, depression, and hypothyroidism, presented a change in mental status, tachycardia, high blood pressure, and unsteady gait. She had abused phenylpropanolamine and over-the-counter diet pills 28 months prior, which led to a previous episode with similar symptoms. She was previously taking buspirone and levothyroxine regularly. She denied drug and alcohol abuse, but her urine drug screen was positive for amphetamines >1000 ng/mL. |
| [108] | Oral—She had been taking dietary supplements containing caffeine and synephrine during one week. Posology and product were not detailed. | Apical ballooning syndrome. | The 21-year-old female presented disturbed consciousness and seizure. Admission exams evidenced apical ballooning of the left ventricle due to akinesis from the apical to mid ventricular segments, hyperkinesis of the basal segments, with reduced left ventricular ejection fraction. She had no prior relevant medical history. |
| [109] | Oral—two doses of C4 pre-workout supplement (contains synephrine and caffeine) within one hour of symptom onset. He consumed the supplement and energy drinks chronically. | Ascending aortic dissection. | The 38-year-old male presented syncopal episode and hypotension. Exams revealed ascending aortic dissection extending to the left subclavian artery, tricuspid aortic valve with severe aortic regurgitation, moderate left ventricular dilatation with a mildly depressed ejection fraction, and severe pulmonary hypertension. He had no prior significant medical history. |
| [110] | Oral—Performix stim-free 1 capsule up to three times a day for one year. He drank 3 scoops of Performix SST for the first time on the afternoon of symptoms. | ST-segment-elevation myocardial infarction (STEMI) | The 22-year-old male presented acute onset of “pressure-like” substernal, non-radiating chest pain associated with shortness of breath and nausea while playing basketball. Angiography revealed acute dissection with thrombosis of the distal left main coronary artery leading into the proximal left anterior descending artery. He had no significant prior medical history and denied excessive caffeine use, smoking, and alcohol intake. |
4. Ephedrine
4.1. Sources and Amounts Found in Natural Sources
4.2. Pharmacological Effects
4.2.1. Activation of α1- and α2-Receptors
4.2.2. Activation of β1-, β2- and β3-Receptors
4.2.3. Hypoglycemic Effects
4.2.4. Nasal Vasoconstrictor Effects
4.3. Toxicological Effects
4.3.1. Cardiovascular Toxicity
4.3.2. Amphetamine-like Effects and CNS Toxicity
4.3.3. Hepatotoxicity
5. Cathinone
5.1. Definition and Sources
5.2. Pharmacology of Cathinone and Its Natural Derivatives
5.3. Toxicology of Cathinone and Its Natural Derivatives
6. Cocaine
6.1. Sources
6.2. Pharmacology of Cocaine and Its Metabolites
6.3. Toxicity of Cocaine
7. Caffeine and Other Anti-Thermogenic Drugs
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AADC | L- amino acid decarboxylase |
| CNS | Central nervous system |
| DAT | dopamine active transporter |
| GPCR | G protein-coupled receptors |
| EFSA | European Food Safety Authority |
| FDA | The U.S. Food and Drug Administration |
| MAO | monoamine oxidase |
| MAOI | monoamine oxidases inhibitor |
| TAARs | trace amine—associated receptors |
| VMAT 2 | vesicular monoamine transporter 2 |
References
- Westfall, T.C. Sympathomimetic Drugs and Adrenergic Receptor Antagonists. In Encyclopedia of Neuroscience; Squire, L.R., Ed.; Academic Press: Oxford, UK, 2009; pp. 685–695. [Google Scholar] [CrossRef]
- Goldstein, S.; Richards, J.R. Sympathomimetic Toxicity. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430757/ (accessed on 2 September 2021).
- Horowitz, A.J.; Smith, T.; Frey, D.; Denault, D. Sympathomimetics. Available online: https://www.ncbi.nlm.nih.gov/books/NBK546597/ (accessed on 2 September 2021).
- Costa, V.; Carvalho, F.; Bastos, M.; Carvalho, R.; Carvalho, M.; Remiao, F. Adrenaline and Noradrenaline: Partners and Actors in the Same Play. In Neurochemistry; Contreras, C.M., Ed.; Intech Open Access Publisher: London, UK, 2012; pp. 1–14. ISBN 978-953-51-0207-6. [Google Scholar]
- Costa, V.M.; Carvalho, F.; Bastos, M.L.; Carvalho, R.A.; Carvalho, M.; Remião, F. Contribution of catecholamine reactive intermediates and oxidative stress to the pathologic features of heart diseases. Curr. Med. Chem. 2011, 18, 2272–2314. [Google Scholar] [CrossRef]
- Williams, R.H.; Erickson, T.; Broussard, L.A. Evaluating Sympathomimetic Intoxication in an Emergency Setting. Laboratory. Medicine 2000, 31, 497–508. [Google Scholar] [CrossRef] [Green Version]
- Burchett, S.A.; Hicks, T.P. The mysterious trace amines: Protean neuromodulators of synaptic transmission in mammalian brain. Prog. Neurobiol. 2006, 79, 223–246. [Google Scholar] [CrossRef] [PubMed]
- Zucchi, R.; Chiellini, G.; Scanlan, T.S.; Grandy, D.K. Trace amine-associated receptors and their ligands. Br. J. Pharmacol. 2006, 149, 967–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, G.; Marcinek, P.; Sulzinger, N.; Schieberle, P.; Krautwurst, D. Food sources and biomolecular targets of tyramine. Nutr. Rev. 2019, 77, 107–115. [Google Scholar] [CrossRef]
- Gwilt, K.B.; Gonzalez, D.P.; Olliffe, N.; Oller, H.; Hoffing, R.; Puzan, M.; El Aidy, S.; Miller, G.M. Actions of Trace Amines in the Brain-Gut-Microbiome Axis via Trace Amine-Associated Receptor-1 (TAAR1). Cell. Mol. Neurobiol. 2020, 40, 191–201. [Google Scholar] [CrossRef]
- Roeder, T. Chapter 1—Trace Amines: An Overview. In Trace Amines and Neurological Disorders; Farooqui, T., Farooqui, A.A., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 3–9. [Google Scholar] [CrossRef]
- Broadley, K.J. The vascular effects of trace amines and amphetamines. Pharmacol. Ther. 2010, 125, 363–375. [Google Scholar] [CrossRef]
- Gainetdinov, R.R.; Hoener, M.C.; Berry, M.D. Trace Amines and Their Receptors. Pharmacol. Rev. 2018, 70, 549–620. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Herrero, A.M. Impact of Biogenic Amines on Food Quality and Safety. Foods 2019, 8, 62. [Google Scholar] [CrossRef] [Green Version]
- Ten, B.B.; Damink, C.; Joosten, H.M.L.J.; Huis in ’t Veld, J.H.J. Occurrence and formation of biologically active amines in foods. Int. J. Food Microbiol. 1990, 11, 73–84. [Google Scholar] [CrossRef]
- Doeun, D.; Davaatseren, M.; Chung, M.S. Biogenic amines in foods. Food Sci. Biotechnol. 2017, 26, 1463–1474. [Google Scholar] [CrossRef]
- Stratton, J.E.; Hutkins, R.W.; Taylor, S.L. Biogenic Amines in Cheese and other Fermented Foods: A Review. J. Food Prot. 1991, 54, 460–470. [Google Scholar] [CrossRef]
- Marcobal, A.; de las Rivas, B.; Landete, J.M.; Tabera, L.; Muñoz, R. Tyramine and Phenylethylamine Biosynthesis by Food Bacteria. Crit. Rev. Food Sci. Nutr. 2012, 52, 448–467. [Google Scholar] [CrossRef] [Green Version]
- Gardini, F.; Özogul, Y.; Suzzi, G.; Tabanelli, G.; Özogul, F. Technological Factors Affecting Biogenic Amine Content in Foods: A Review. Front. Microbiol. 2016, 7, 1218. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, I.M.; Pinho, O. Biogenic amines in Portuguese traditional foods and wines. J. Food Prot. 2006, 69, 2293–2303. [Google Scholar] [CrossRef]
- Majcherczyk, J.; Surówka, K. Effects of onion or caraway on the formation of biogenic amines during sauerkraut fermentation and refrigerated storage. Food Chem. 2019, 298, 125083. [Google Scholar] [CrossRef]
- Parente, E.; Martuscelli, M.; Gardini, F.; Grieco, S.; Crudele, M.A.; Suzzi, G. Evolution of microbial populations and biogenic amine production in dry sausages produced in Southern Italy. J. Appl. Microbiol. 2001, 90, 882–891. [Google Scholar] [CrossRef]
- Mayr, C.M.; Schieberle, P. Development of stable isotope dilution assays for the simultaneous quantitation of biogenic amines and polyamines in foods by LC-MS/MS. J. Agric. Food Chem. 2012, 60, 3026–3032. [Google Scholar] [CrossRef]
- Durlu-Özkaya, F.; Ayhan, K.; Vural, N. Biogenic amines produced by Enterobacteriaceae isolated from meat products. Meat Sci. 2001, 58, 163–166. [Google Scholar] [CrossRef]
- Restuccia, D.; Spizzirri, U.G.; Parisi, O.I.; Cirillo, G.; Picci, N. Brewing effect on levels of biogenic amines in different coffee samples as determined by LC-UV. Food Chem. 2015, 175, 143–150. [Google Scholar] [CrossRef]
- Martuscelli, M.; Arfelli, G.; Manetta, A.C.; Suzzi, G. Biogenic amines content as a measure of the quality of wines of Abruzzo (Italy). Food Chem. 2013, 140, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Zazzu, C.; Addis, M.; Caredda, M.; Scintu, M.F.; Piredda, G.; Sanna, G. Biogenic Amines in Traditional Fiore Sardo PDO Sheep Cheese: Assessment, Validation and Application of an RP-HPLC-DAD-UV Method. Separations 2019, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Bartkiene, E.; Krungleviciute, V.; Juodeikiene, G.; Vidmantiene, D.; Maknickiene, Z. Solid state fermentation with lactic acid bacteria to improve the nutritional quality of lupin and soya bean. J. Sci. Food Agric. 2015, 95, 1336–1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valsamaki, K.; Michaelidou, A.; Polychroniadou, A. Biogenic amine production in Feta cheese. Food Chem. 2000, 71, 259–266. [Google Scholar] [CrossRef]
- Lindemann, L.; Meyer, C.A.; Jeanneau, K.; Bradaia, A.; Ozmen, L.; Bluethmann, H.; Bettler, B.; Wettstein, J.G.; Borroni, E.; Moreau, J.L.; et al. Trace amine-associated receptor 1 modulates dopaminergic activity. J. Pharmacol. Exp. Ther. 2008, 324, 948–956. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.Z.; Nawaz, W. The emerging roles of human trace amines and human trace amine-associated receptors (hTAARs) in central nervous system. Biomed. Pharmacother. 2016, 83, 439–449. [Google Scholar] [CrossRef]
- Narang, D.; Tomlinson, S.; Holt, A.; Mousseau, D.D.; Baker, G.B. Trace Amines and Their Relevance to Psychiatry and Neurology: A Brief Overview. Bull. Clin. Psychopharmacol. 2011, 21, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Borowsky, B.; Adham, N.; Jones, K.A.; Raddatz, R.; Artymyshyn, R.; Ogozalek, K.L.; Durkin, M.M.; Lakhlani, P.P.; Bonini, J.A.; Pathirana, S.; et al. Trace amines: Identification of a family of mammalian G protein-coupled receptors. Proc. Natl. Acad. Sci. USA 2001, 98, 8966–8971. [Google Scholar] [CrossRef]
- Bunzow, J.R.; Sonders, M.S.; Arttamangkul, S.; Harrison, L.M.; Zhang, G.; Quigley, D.I.; Darland, T.; Suchland, K.L.; Pasumamula, S.; Kennedy, J.L.; et al. Amphetamine, 3,4-Methylenedioxymethamphetamine, Lysergic Acid Diethylamide, and Metabolites of the Catecholamine Neurotransmitters Are Agonists of a Rat Trace Amine Receptor. Mol. Pharmacol. 2001, 60, 1181–1188. [Google Scholar] [CrossRef] [Green Version]
- Kleinau, G.; Pratzka, J.; Nürnberg, D.; Grüters, A.; Führer-Sakel, D.; Krude, H.; Köhrle, J.; Schöneberg, T.; Biebermann, H. Differential modulation of Beta-adrenergic receptor signaling by trace amine-associated receptor 1 agonists. PLoS ONE 2011, 6, e27073. [Google Scholar] [CrossRef]
- Frascarelli, S.; Ghelardoni, S.; Chiellini, G.; Vargiu, R.; Ronca-Testoni, S.; Scanlan, T.S.; Grandy, D.K.; Zucchi, R. Cardiac effects of trace amines: Pharmacological characterization of trace amine-associated receptors. Eur. J. Pharmacol. 2008, 587, 231–236. [Google Scholar] [CrossRef]
- Liu, J.; Wu, R.; Li, J.X. TAAR1 and Psychostimulant Addiction. Cell. Mol. Neurobiol. 2020, 40, 229–238. [Google Scholar] [CrossRef]
- Pei, Y.; Asif-Malik, A.; Canales, J.J. Trace Amines and the Trace Amine-Associated Receptor 1: Pharmacology, Neurochemistry, and Clinical Implications. Front. Neurosci. 2016, 10, 148. [Google Scholar] [CrossRef] [Green Version]
- Babusyte, A.; Kotthoff, M.; Fiedler, J.; Krautwurst, D. Biogenic amines activate blood leukocytes via trace amine-associated receptors TAAR1 and TAAR2. J. Leukoc. Biol. 2013, 93, 387–394. [Google Scholar] [CrossRef]
- Christian, S.L.; Berry, M.D. Trace Amine-Associated Receptors as Novel Therapeutic Targets for Immunomodulatory Disorders. Front. Pharmacol. 2018, 9, 680. [Google Scholar] [CrossRef] [Green Version]
- Vitale, S.; Strisciuglio, C.; Pisapia, L.; Miele, E.; Barba, P.; Vitale, A.; Cenni, S.; Bassi, V.; Maglio, M.; Del Pozzo, G.; et al. Cytokine production profile in intestinal mucosa of paediatric inflammatory bowel disease. PLoS ONE 2017, 12, e0182313. [Google Scholar] [CrossRef] [Green Version]
- Latapy, C.; Beaulieu, J.M. β-Arrestins in the central nervous system. Prog. Mol. Biol. Transl. Sci. 2013, 118, 267–295. [Google Scholar] [CrossRef]
- Espinoza, S.; Masri, B.; Salahpour, A.; Gainetdinov, R.R. BRET approaches to characterize dopamine and TAAR1 receptor pharmacology and signaling. Methods Mol. Biol. 2013, 964, 107–122. [Google Scholar] [CrossRef]
- Kano, H.; Toyama, Y.; Imai, S.; Iwahashi, Y.; Mase, Y.; Yokogawa, M.; Osawa, M.; Shimada, I. Structural mechanism underlying G protein family-specific regulation of G protein-gated inwardly rectifying potassium channel. Nat. Commun. 2019, 10, 2008. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Miller, G.M. Trace Amine-Associated Receptor 1 Is a Modulator of the Dopamine Transporter. J. Pharmacol. Exp. Ther. 2007, 321, 128. [Google Scholar] [CrossRef]
- Xie, Z.; Westmoreland, S.V.; Bahn, M.E.; Chen, G.-L.; Yang, H.; Vallender, E.J.; Yao, W.-D.; Madras, B.K.; Miller, G.M. Rhesus Monkey Trace Amine-Associated Receptor 1 Signaling: Enhancement by Monoamine Transporters and Attenuation by the D2 Autoreceptor in Vitro. J. Pharmacol. Exp. Ther. 2007, 321, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grandy, D.K. Trace amine-associated receptor 1-Family archetype or iconoclast? Pharmacol. Ther. 2007, 116, 355–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herbert, A.A.; Kidd, E.J.; Broadley, K.J. Dietary trace amine-dependent vasoconstriction in porcine coronary artery. Br. J. Pharmacol. 2008, 155, 525–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, A.H.W.; Chess-Williams, R.; Lohning, A.E. Differential mechanisms of action of the trace amines octopamine, synephrine and tyramine on the porcine coronary and mesenteric artery. Sci. Rep. 2019, 9, 10925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broadley, K.J.; Akhtar Anwar, M.; Herbert, A.A.; Fehler, M.; Jones, E.M.; Davies, W.E.; Kidd, E.J.; Ford, W.R. Effects of dietary amines on the gut and its vasculature. Br. J. Nutr. 2009, 101, 1645–1652. [Google Scholar] [CrossRef] [Green Version]
- Collins, J.D.; Noerrung, B.; Budka, H.; Andreoletti, O.; Buncic, S.; Griffin, J.; Hald, T.; Havelaar, A.; Hope, J.; Klein, G.; et al. Scientific Opinion on risk based control of biogenic amine formation in fermented foods. EFSA J. 2011, 9, 2393. [Google Scholar] [CrossRef] [Green Version]
- Linares, D.M.; del Rio, B.; Redruello, B.; Ladero, V.; Martin, M.C.; Fernandez, M.; Ruas-Madiedo, P.; Alvarez, M.A. Comparative analysis of the in vitro cytotoxicity of the dietary biogenic amines tyramine and histamine. Food Chem. 2016, 197, 658–663. [Google Scholar] [CrossRef] [Green Version]
- Del Rio, B.; Redruello, B.; Linares, D.M.; Ladero, V.; Fernandez, M.; Martin, M.C.; Ruas-Madiedo, P.; Alvarez, M.A. The dietary biogenic amines tyramine and histamine show synergistic toxicity towards intestinal cells in culture. Food Chem. 2017, 218, 249–255. [Google Scholar] [CrossRef]
- Victor, L.; Marina, C.-E.; Maria, F.; Miguel, A.A. Toxicological Effects of Dietary Biogenic Amines. Curr. Nutr. Food Sci. 2010, 6, 145–156. [Google Scholar] [CrossRef]
- Ngo, A.S.; Ho, R.Y.; Olson, K.R. Phenelzine-induced myocardial injury: A case report. J. Med. Toxicol. 2010, 6, 431–434. [Google Scholar] [CrossRef] [Green Version]
- Costa, M.R.; Glória, M.B.A. Migraine and Diet. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 3940–3947. [Google Scholar] [CrossRef]
- Gillman, P.K. Monoamine Oxidase Inhibitors: A Review Concerning Dietary Tyramine and Drug Interactions. PsychoTrop. Comment. 2016, 16, 1–90. [Google Scholar]
- Watson, D.G.; Midgley, J.M.; Chen, R.N.; Huang, W.; Bain, G.M.; McDonald, N.M.; Reid, J.L.; McGhee, C.N. Analysis of biogenic amines and their metabolites in biological tissues and fluids by gas chromatography-negative ion chemical ionization mass spectrometry (GC-NICIMS). J. Pharm. Biomed. Anal. 1990, 8, 899–904. [Google Scholar] [CrossRef]
- Rossato, L.G.; de Pinho, P.G.; Silva, R.; Carmo, H.; Carvalho, F.; Bastos, M.e.L.; Costa, V.M.; Remião, F. Development and validation of a GC/IT-MS method for simultaneous quantitation of para and meta-synephrine in biological samples. J. Pharm. Biomed. Anal. 2010, 52, 721–726. [Google Scholar] [CrossRef]
- Rossato, L.G.; Costa, V.M.; Limberger, R.P.; Bastos, M.e.L.; Remião, F. Synephrine: From trace concentrations to massive consumption in weight-loss. Food Chem. Toxicol. 2011, 49, 8–16. [Google Scholar] [CrossRef]
- Stohs, S.J.; Badmaev, V. A Review of Natural Stimulant and Non-stimulant Thermogenic Agents. Phytother. Res. 2016, 30, 732–740. [Google Scholar] [CrossRef] [Green Version]
- Stohs, S.J.; Shara, M.; Ray, S.D. p-Synephrine, ephedrine, p-octopamine and m-synephrine: Comparative mechanistic, physiological and pharmacological properties. Phytother. Res. 2020, 34, 1838–1846. [Google Scholar] [CrossRef]
- Fugh-Berman, A.; Myers, A. Citrus aurantium, an ingredient of dietary supplements marketed for weight loss: Current status of clinical and basic research. Exp. Biol. Med. 2004, 229, 698–704. [Google Scholar] [CrossRef]
- Dragull, K.; Breksa, A.P.; Cain, B. Synephrine content of juice from Satsuma mandarins (Citrus unshiu Marcovitch). J. Agric. Food Chem. 2008, 56, 8874–8878. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Zhang, R.; Zhou, J.; Zhao, K.; Lu, Y.; Zheng, Y.; Wu, C.; Chen, F.; Mu, D.; Ding, Z.; et al. The levels of bioactive ingredients in Citrus aurantium L. at different harvest periods and antioxidant effects on H. J. Sci. Food Agric. 2021, 101, 1479–1490. [Google Scholar] [CrossRef] [PubMed]
- Avula, B.; Upparapalli, S.K.; Navarrete, A.; Khan, I.A. Simultaneous quantification of adrenergic amines and flavonoids in C. aurantium, various Citrus species, and dietary supplements by liquid chromatography. J. AOAC Int. 2005, 88, 1593–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hibino, T.; Yuzurihara, M.; Kase, Y.; Takeda, A. Synephrine, a component of Evodiae Fructus, constricts isolated rat aorta via adrenergic and serotonergic receptors. J. Pharmacol. Sci. 2009, 111, 73–81. [Google Scholar] [CrossRef]
- Arbo, M.D.; Larentis, E.R.; Linck, V.M.; Aboy, A.L.; Pimentel, A.L.; Henriques, A.T.; Dallegrave, E.; Garcia, S.C.; Leal, M.B.; Limberger, R.P. Concentrations of p-synephrine in fruits and leaves of Citrus species (Rutaceae) and the acute toxicity testing of Citrus aurantium extract and p-synephrine. Food Chem. Toxicol. 2008, 46, 2770–2775. [Google Scholar] [CrossRef]
- Pellati, F.; Benvenuti, S.; Melegari, M.; Firenzuoli, F. Determination of adrenergic agonists from extracts and herbal products of Citrus aurantium L. var. amara by LC. J. Pharm. Biomed. Anal. 2002, 29, 1113–1119. [Google Scholar] [CrossRef]
- Pellati, F.; Benvenuti, S.; Melegari, M. High-performance liquid chromatography methods for the analysis of adrenergic amines and flavanones in Citrus aurantium L. var. amara. Phytochem. Anal. 2004, 15, 220–225. [Google Scholar] [CrossRef]
- Allison, D.B.; Cutter, G.; Poehlman, E.T.; Moore, D.R.; Barnes, S. Exactly which synephrine alkaloids does Citrus aurantium (bitter orange) contain? Int. J. Obes. 2005, 29, 443–446. [Google Scholar] [CrossRef] [Green Version]
- Nelson, B.C.; Putzbach, K.; Sharpless, K.E.; Sander, L.C. Mass spectrometric determination of the predominant adrenergic protoalkaloids in bitter orange (Citrus aurantium). J. Agric. Food Chem. 2007, 55, 9769–9775. [Google Scholar] [CrossRef]
- Andrade, A.; Schmitt, G.; Rossato, L.G.; Russowsky, D.; Limberger, R.P. Gas Chromatographic Method for Analysis of p-Synephrine in Citrus aurantium L. Products. Chromatographia 2009, 69, 225–229. [Google Scholar] [CrossRef]
- Ma, G.; Bavadekar, S.A.; Schaneberg, B.T.; Khan, I.A.; Feller, D.R. Effects of synephrine and beta-phenethylamine on human alpha-adrenoceptor subtypes. Planta Med. 2010, 76, 981–986. [Google Scholar] [CrossRef]
- Penzak, S.R.; Jann, M.W.; Cold, J.A.; Hon, Y.Y.; Desai, H.D.; Gurley, B.J. Seville (sour) orange juice: Synephrine content and cardiovascular effects in normotensive adults. J. Clin. Pharmacol. 2001, 41, 1059–1063. [Google Scholar] [CrossRef]
- Bent, S.; Padula, A.; Neuhaus, J. Safety and efficacy of Citrus aurantium for weight loss. Am. J. Cardiol. 2004, 94, 1359–1361. [Google Scholar] [CrossRef]
- Pellati, F.; Benvenuti, S.; Melegari, M. Enantioselective LC analysis of synephrine in natural products on a protein-based chiral stationary phase. J. Pharm. Biomed. Anal. 2005, 37, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Mercolini, L.; Mandrioli, R.; Trerè, T.; Bugamelli, F.; Ferranti, A.; Raggi, M.A. Fast CE analysis of adrenergic amines in different parts of Citrus aurantium fruit and dietary supplements. J. Sep. Sci. 2010, 33, 2520–2527. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Chao, Y.; Liu, M.; Yang, Y.; Zhang, D.; Wang, K.; Tao, Y.; Zhang, J.; Li, Y.; Wei, M. Evaluation of dynamic changes in the bioactive components in Citri Reticulatae Pericarpium (Citrus reticulata ‘Chachi’) under different harvesting and drying conditions. J. Sci. Food Agric. 2021, 101, 3280–3289. [Google Scholar] [CrossRef] [PubMed]
- Tette, P.A.; Guidi, L.R.; Bastos, E.M.; Fernandes, C.; Gloria, M.B. Synephrine—A potential biomarker for orange honey authenticity. Food Chem. 2017, 229, 527–533. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, G.; Terrazzino, S.; Fortin, D.; Farruggio, A.; Rinaldi, L.; Leon, A. HPLC electrochemical detection of trace amines in human plasma and platelets and expression of mRNA transcripts of trace amine receptors in circulating leukocytes. Neurosci. Lett. 2003, 346, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, K.E.; Couch, M.W.; Williams, C.M.; Budd, M.B.; Yost, R.A.; Midgley, J.M. Quantitative measurement of octopamines and synephrines in urine using capillary column gas chromatography negative ion chemical ionization mass spectrometry. Anal. Chem. 1984, 56, 1695–1699. [Google Scholar] [CrossRef]
- Rang, H.P.; Dale, M.M.; Ritter, J.M.; Flower, R.J.; Henderson, G. Rang & Dale: Farmacologia, 7th ed.; Elsevier: Rio de Janeiro, Brazil, 2011. [Google Scholar]
- Hayat, K. Citrus: Molecular Phylogeny, Antioxidant Properties and Medicinal Uses; Nova Science Publishers: New York, NY, USA, 2014; Volume 1. [Google Scholar]
- Kim, K.W.; Kim, H.D.; Jung, J.S.; Woo, R.S.; Kim, H.S.; Suh, H.W.; Kim, Y.H.; Song, D.K. Characterization of antidepressant-like effects of p-synephrine stereoisomers. Naunyn-Schmiedeb. Arch. Pharmacol. 2001, 364, 21–26. [Google Scholar] [CrossRef]
- Song, D.K.; Suh, H.W.; Jung, J.S.; Wie, M.B.; Son, K.H.; Kim, Y.H. Antidepressant-like effects of p-synephrine in mouse models of immobility tests. Neurosci. Lett. 1996, 214, 107–110. [Google Scholar] [CrossRef]
- Miller, G.M. The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity. J. Neurochem. 2011, 116, 164–176. [Google Scholar] [CrossRef] [Green Version]
- D’Andrea, G.; D’Arrigo, A.; Carbonare, M.D.; Leon, A. Pathogenesis of migraine: Role of neuromodulators. Headache 2012, 52, 1155–1163. [Google Scholar] [CrossRef]
- Koncz, D.; Tóth, B.; Bahar, M.A.; Roza, O.; Csupor, D. The Safety and Efficacy of Citrus aurantium (Bitter Orange) Extracts and p-Synephrine: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 4019. [Google Scholar] [CrossRef]
- Pawar, R.S.; Grundel, E. Overview of regulation of dietary supplements in the USA and issues of adulteration with phenethylamines (PEAs). Drug Test. Anal. 2017, 9, 500–517. [Google Scholar] [CrossRef]
- Müller, L.S.; Moreira, A.P.L.; Muratt, D.T.; Viana, C.; de Carvalho, L.M. An Ultra-High Performance Liquid Chromatography-Electrospray Tandem Mass Spectrometric Method for Screening and Simultaneous Determination of Anorexic, Anxiolytic, Antidepressant, Diuretic, Laxative and Stimulant Drugs in Dietary Supplements Marketed for Weight Loss. J. Chromatogr. Sci. 2019, 57, 528–540. [Google Scholar] [CrossRef]
- Rossato, L.G.; Costa, V.M.; de Pinho, P.G.; Carvalho, F.; de Lourdes Bastos, M.; Remião, F. Structural isomerization of synephrine influences its uptake and ensuing glutathione depletion in rat-isolated cardiomyocytes. Arch. Toxicol. 2011, 85, 929–939. [Google Scholar] [CrossRef]
- Ribeiro, D.L.; Machado, A.R.T.; da Silva Machado, C.; Santos, P.W.D.S.; Aissa, A.F.; Barcelos, G.R.M.; Antunes, L.M.G. Analysis of the cytotoxic, genotoxic, mutagenic, and pro-oxidant effect of synephrine, a component of thermogenic supplements, in human hepatic cells in vitro. Toxicology 2019, 422, 25–34. [Google Scholar] [CrossRef]
- Arbo, M.D.; Schmitt, G.C.; Limberger, M.F.; Charão, M.F.; Moro, A.M.; Ribeiro, G.L.; Dallegrave, E.; Garcia, S.C.; Leal, M.B.; Limberger, R.P. Subchronic toxicity of Citrus aurantium L. (Rutaceae) extract and p-synephrine in mice. Regul. Toxicol. Pharmacol. 2009, 54, 114–117. [Google Scholar] [CrossRef]
- Suntar, I.; Khan, H.; Patel, S.; Celano, R.; Rastrelli, L. An Overview on Citrus aurantium L.: Its Functions as Food Ingredient and Therapeutic Agent. Oxid. Med. Cell. Longev. 2018, 2018, 7864269. [Google Scholar] [CrossRef] [Green Version]
- Koh, A.H.W.; Chess-Williams, R.; Lohning, A.E. Renal artery responses to trace amines: Multiple and differential mechanisms of action. Life Sci. 2021, 277, 119532. [Google Scholar] [CrossRef]
- NHPD. Guidelines for the Use of Synephrine in Natural Health Products. Canada. 2010. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/natural-non-prescription/legislation-guidelines/guidance-documents/notice-use-synephrine.html (accessed on 19 July 2020).
- Sawler, S. Synephrine, Octopamine and Caffeine Health Risk Assessment (HRA) Report, Canada. 2011. Available online: https://www.semanticscholar.org/paper/Synephrine-%2C-Octopamine-and-Caffeine-Health-Risk-(-Sawler/7c15c4a3ef32394284da0d29f412ba4b51b13bf6 (accessed on 18 July 2020).
- ANSES. French Agency for Food, Environmental and Occupational Health & Safety. Opinion of the French Agency for Food, Environmental and Occupational Health & Safety on the Risks Associated with the Presence in Food Supplements of P-Synephrine or Ingredients Obtained from Citrus spp. Fruits Containing This Substance France. 2014. Available online: https://www.anses.fr/en/content/opinion-french-agency-food-environmental-and-occupational-health-safety-risks-associated-1 (accessed on 18 July 2020).
- Nykamp, D.L.; Fackih, M.N.; Compton, A.L. Possible association of acute lateral-wall myocardial infarction and bitter orange supplement. Ann. Pharmacother. 2004, 38, 812–816. [Google Scholar] [CrossRef]
- Bouchard, N.C.; Howland, M.A.; Greller, H.A.; Hoffman, R.S.; Nelson, L.S. Ischemic stroke associated with use of an ephedra-free dietary supplement containing synephrine. Mayo Clin. Proc. 2005, 80, 541–545. [Google Scholar] [CrossRef] [Green Version]
- Gange, C.A.; Madias, C.; Felix-Getzik, E.M.; Weintraub, A.R.; Estes, N.A. Variant angina associated with bitter orange in a dietary supplement. Mayo Clin. Proc. 2006, 81, 545–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, J.; Seda, G.; Allen, D.; Knee, T.S. A case of severe exercise-induced rhabdomyolysis associated with a weight-loss dietary supplement. Mil. Med. 2007, 172, 656–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, R.O.; Tavee, J. Vasospasm and stroke attributable to ephedra-free xenadrine: Case report. Mil. Med. 2008, 173, 708–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephensen, T.A.; Sarlay, R. Ventricular fibrillation associated with use of synephrine containing dietary supplement. Mil. Med. 2009, 174, 1313–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, J.E.; Munir, J.A.; McIntyre, P.Z.; Ferguson, M.A. STEMI in a 24-year-old man after use of a synephrine-containing dietary supplement: A case report and review of the literature. Tex. Heart Inst. J. 2009, 36, 586–590. [Google Scholar]
- Retamero, C.; Rivera, T.; Murphy, K. “Ephedra-free” diet pill-induced psychosis. Psychosomatics 2011, 52, 579–582. [Google Scholar] [CrossRef]
- Chung, H.; Kwon, S.W.; Kim, T.H.; Yoon, J.H.; Ma, D.W.; Park, Y.M.; Hong, B.K. Synephrine-containing dietary supplement precipitating apical ballooning syndrome in a young female. Korean J. Intern. Med. 2013, 28, 356–360. [Google Scholar] [CrossRef]
- Doctorian, T.; Do, B. Ascending aortic dissection in a young patient using a synephrine-containing workout supplement. J. Cardiol. Cases 2017, 15, 150–152. [Google Scholar] [CrossRef]
- Unnikrishnan, D.; Annam, R.; Jacob, A.; Thyagarajan, B.; Farrugia, P. STEMI in a Young Male after Use of Synephrine-Containing Dietary Supplement. Case Rep. Cardiol. 2018, 2018, 7074104. [Google Scholar] [CrossRef]
- González-Juárez, D.E.; Escobedo-Moratilla, A.; Flores, J.; Hidalgo-Figueroa, S.; Martínez-Tagüeña, N.; Morales-Jiménez, J.; Muñiz-Ramírez, A.; Pastor-Palacios, G.; Pérez-Miranda, S.; Ramírez-Hernández, A.; et al. A review of the ephedra genus: Distribution, ecology, ethnobotany, phytochemistry and pharmacological properties. Molecules 2020, 25, 3283. [Google Scholar] [CrossRef]
- Abourashed, E.A.; El-Alfy, A.T.; Khan, I.A.; Walker, L. Ephedra in perspective-a current review. Phytother. Res. 2003, 17, 703–712. [Google Scholar] [CrossRef]
- Palamar, J. How ephedrine escaped regulation in the United States: A historical review of misuse and associated policy. Health Policy 2011, 99, 1–9. [Google Scholar] [CrossRef]
- Miao, S.M.; Zhang, Q.; Bi, X.B.; Cui, J.L.; Wang, M.L. A review of the phytochemistry and pharmacological activities of Ephedra herb. Chin. J. Nat. Med. 2020, 18, 321–344. [Google Scholar] [CrossRef]
- Lv, M.; Sun, J.; Wang, M.; Huang, W.; Fan, H.; Xu, F.; Zhang, Z. GC-MS based metabolomics study of stems and roots of Ephedra sinica. J. Pharm. Biomed. Anal. 2015, 114, 49–52. [Google Scholar] [CrossRef]
- Ma, G.; Bavadekar, S.A.; Davis, Y.M.; Lalchandani, S.G.; Nagmani, R.; Schaneberg, B.T.; Khan, I.A.; Feller, D.R. Pharmacological Effects of Ephedrine Alkaloids on Human α1- and α2-Adrenergic Receptor Subtypes. J. Pharmacol. Exp. Ther. 2007, 322, 214–221. [Google Scholar] [CrossRef]
- Alsufyani, H.A.; Docherty, J.R. Direct and indirect effects of ephedrine on heart rate and blood pressure in vehicle-treated and sympathectomised male rats. Eur. J. Pharmacol. 2018, 825, 34–38. [Google Scholar] [CrossRef]
- Li, Q.; Bian, L.; Zhao, X.; Gao, X.; Zheng, J.; Li, Z.; Zhang, Y.; Jiang, R.; Zheng, X. Immobilised histidine tagged β2-adrenoceptor oriented by a diazonium salt reaction and its application in exploring drug-protein interaction using ephedrine and pseudoephedrine as probes. PLoS ONE 2014, 9, e94955. [Google Scholar] [CrossRef] [Green Version]
- Vansal, S.S.; Feller, D.R. Direct effects of ephedrine isomers on human beta-adrenergic receptor subtypes. Biochem. Pharmacol. 1999, 58, 807–810. [Google Scholar] [CrossRef]
- De Matteis, R.; Arch, J.R.; Petroni, M.L.; Ferrari, D.; Cinti, S.; Stock, M.J. Immunohistochemical identification of the beta(3)-adrenoceptor in intact human adipocytes and ventricular myocardium: Effect of obesity and treatment with ephedrine and caffeine. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 1442–1450. [Google Scholar] [CrossRef]
- Bogacka, I.; Gettys, T.W.; de Jonge, L.; Nguyen, T.; Smith, J.M.; Xie, H.; Greenway, F.; Smith, S.R. The Effect of β-Adrenergic and Peroxisome Proliferator—Activated Receptor-γ Stimulation on Target Genes Related to Lipid Metabolism in Human Subcutaneous Adipose Tissue. Diabetes Care 2007, 30, 1179–1186. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.W.; Nam, D.; Kim, K.H.; Huh, J.-E.; Lee, J.-D. Effect of Gambisan on the Inhibition of Adipogenesis in 3T3-L1 Adipocytes. Evid. Based Complement. Altern. Med. 2013, 2013, 789067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rufino, A.T.; Costa, V.M.; Carvalho, F.; Fernandes, E. Flavonoids as antiobesity agents: A review. Med. Res. Rev. 2021, 41, 556–585. [Google Scholar] [CrossRef] [PubMed]
- Xiu, L.M.; Miura, A.B.; Yamamoto, K.; Kobayashi, T.; Song, Q.H.; Kitamura, H.; Cyong, J.C. Pancreatic islet regeneration by ephedrine in mice with streptozotocin-induced diabetes. Am. J. Chin. Med. 2001, 29, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-W.; Yang, J.-Y.; Lee, H.-S. Quinoline-2-carboxylic acid isolated from Ephedra pachyclada and its structural derivatives show inhibitory effects against α-glucosidase and α-amylase. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 441–444. [Google Scholar] [CrossRef] [Green Version]
- Han, H.Y.; Huh, J.I.; Han, S.R.; Kang, M.G.; Yoon, S.; Han, J.S.; Lee, B.S.; Kim, J.A.; Min, B.S. Assessing the safety of an Ephedrae Herba aqueous extract in rats: A repeat dose toxicity study. Regul. Toxicol. Pharmacol. 2018, 94, 144–151. [Google Scholar] [CrossRef]
- Laccourreye, O.; Werner, A.; Giroud, J.P.; Couloigner, V.; Bonfils, P.; Bondon-Guitton, E. Benefits, limits and danger of ephedrine and pseudoephedrine as nasal decongestants. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2015, 132, 31–34. [Google Scholar] [CrossRef] [Green Version]
- Dhar, R.; Stout, C.W.; Link, M.S.; Homoud, M.K.; Weinstock, J.; Estes, N.A.M., III. Cardiovascular toxicities of performance-enhancing substances in sports. Mayo Clin. Proc. 2005, 80, 1307–1315. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, R.; Nyska, A.; Dunnick, J.; Ramot, Y. The toxicologic pathology aspects of selected natural herbal products and related compounds. J. Toxicol. Pathol. 2021, 34, 181–211. [Google Scholar] [CrossRef]
- Van Mieghem, W.; Stevens, E.; Cosemans, J. Ephedrine-induced cardiopathy. Br. Med. J. 1978, 1, 816. [Google Scholar] [CrossRef]
- Schier, J.G.; Traub, S.J.; Hoffman, R.S.; Nelson, L.S. Ephedrine-induced cardiac ischemia: Exposure confirmed with a serum level. J. Toxicol. Clin. Toxicol. 2003, 41, 849–853. [Google Scholar] [CrossRef]
- Enders, J.M.; Dobesh, P.P.; Ellison, J.N. Acute myocardial infarction induced by ephedrine alkaloids. Pharmacotherapy 2003, 23, 1645–1651. [Google Scholar] [CrossRef]
- Rhidian, R. Running a risk? Sport supplement toxicity with ephedrine in an amateur marathon runner, with subsequent rhabdomyolysis. BMJ Case Rep. 2011, 2011, bcr1120115093. [Google Scholar] [CrossRef] [Green Version]
- Bowyer, J.F.; Newport, G.D.; Slikker, W., Jr.; Gough, B.; Ferguson, S.A.; Tor-Agbidye, J. An evaluation of l-ephedrine neurotoxicity with respect to hyperthermia and caudate/putamen microdialysate levels of ephedrine, dopamine, serotonin, and glutamate. Toxicol. Sci. 2000, 55, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Munhall, A.C.; Johnson, S.W. Dopamine-mediated actions of ephedrine in the rat substantia nigra. Brain Res. 2006, 1069, 96–103. [Google Scholar] [CrossRef]
- Ellis, J.D.; German, C.L.; Birdsall, E.; Hanson, J.E.; Crosby, M.A.; Rowley, S.D.; Sawada, N.A.; West, J.N.; Hanson, G.R.; Fleckenstein, A.E. Ephedrine decreases vesicular monoamine transporter-2 function. Synapse 2011, 65, 449–451. [Google Scholar] [CrossRef] [Green Version]
- Duan, S.; Xie, L.; Zheng, L.; Huang, J.; Guo, R.; Sun, Z.; Xie, Y.; Lv, J.; Lin, Z.; Ma, S. Long-term exposure to ephedrine leads to neurotoxicity and neurobehavioral disorders accompanied by up-regulation of CRF in prefrontal cortex and hippocampus in Rhesus macaques. Behav. Brain Res. 2020, 393, 112796. [Google Scholar] [CrossRef]
- Zheng, E.X.; Navarro, V.J. Liver injury from herbal, dietary, and weight loss supplements: A review. J. Clin. Transl. Hepatol. 2015, 3, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Wen, S.; Liao, T. Ephedrine causes liver toxicity in SD rats via oxidative stress and inflammatory responses. Hum. Exp. Toxicol. 2021, 40, 16–24. [Google Scholar] [CrossRef]
- Al-Hebshi, N.N.; Skaug, N. Khat (Catha edulis)—An updated review. Addict. Biol. 2005, 10, 299–307. [Google Scholar] [CrossRef]
- Alles, G.A.; Fairchild, M.D.; Jensen, M. Chemical pharmacology of Catha edulis. J. Med. Pharm. Chem. 1961, 3, 323–352. [Google Scholar] [CrossRef]
- Getasetegn, M. Chemical composition of Catha edulis (khat): A review. Phytochem. Rev. 2016, 15, 907–920. [Google Scholar] [CrossRef]
- Krikorian, A.D. Kat and its use: An historical perspective. J. Ethnopharmacol. 1984, 12, 115–178. [Google Scholar] [CrossRef] [PubMed]
- Odenwald, M.; Klein, A.; Warfa, N. Khat Addiction. In Textbook of Addiction Treatment: International Perspectives; El-Guebaly, N., Carrà, G., Galanter, M., Eds.; Springer: Milan, Italy, 2015; pp. 455–466. [Google Scholar] [CrossRef]
- United Nations Division of Narcotic Drugs. Studies on the Chemical Composition of Khat. III. In Investigations on the Phenylalkylamine Fraction; United Nations Document MNAR/11/75; United Nations Division of Narcotic Drugs: Vienna, Austria, 1975. [Google Scholar]
- Brenneisen, R.; Geisshüsler, S.; Schorno, X. Metabolism of cathinone to (−)-norephedrine and (−)-norpseudoephedrine. J. Pharm. Pharmacol. 1986, 38, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Pendl, E.; Pauritsch, U.; Kollroser, M.; Schmid, M.G. Determination of cathinone and cathine in Khat plant material by LC-MS/MS: Fresh vs. dried leaves. Forensic Sci. Int. 2021, 319, 110658. [Google Scholar] [CrossRef] [PubMed]
- Kalix, P.; Braenden, O. Pharmacological aspects of the chewing of khat leaves. Pharmacol. Rev. 1985, 37, 149. [Google Scholar]
- Abebe, M.; Kindie, S.; Adane, K. Adverse health effects of khat: A review. Fam. Med. Med. Sci. Res. 2015, 4, 154. [Google Scholar] [CrossRef]
- Kelly, J.P. Cathinone derivatives: A review of their chemistry, pharmacology and toxicology. Drug Test. Anal. 2011, 3, 439–453. [Google Scholar] [CrossRef]
- Kalix, P. Cathinone, a natural amphetamine. Pharmacol. Toxicol. 1992, 70, 77–86. [Google Scholar] [CrossRef]
- Mathys, K.; Brenneisen, R. Determination of (S)-(−)-cathinone and its metabolites (R,S)-(−)-norephedrine and (R,R)-(−)-norpseudoephedrine in urine by high-performance liquid chromatography with photodiode-array detection. J. Chromatogr. 1992, 593, 79–85. [Google Scholar] [CrossRef]
- Engidawork, E. Pharmacological and Toxicological Effects of Catha edulis F. (Khat). Phytother. Res. 2017, 31, 1019–1028. [Google Scholar] [CrossRef]
- Toennes, S.W.; Kauert, G.F. Excretion and Detection of Cathinone, Cathine, and Phenylpropanolamine in Urine after Kath Chewing. Clin. Chem. 2002, 48, 1715–1719. [Google Scholar] [CrossRef] [Green Version]
- Widler, P.; Mathys, K.; Brenneisen, R.; Kalix, P.; Fisch, H.U. Pharmacodynamics and pharmacokinetics of khat: A controlled study. Clin. Pharmacol. Ther. 1994, 55, 556–562. [Google Scholar] [CrossRef]
- Toennes, S.W.; Harder, S.; Schramm, M.; Niess, C.; Kauert, G.F. Pharmacokinetics of cathinone, cathine and norephedrine after the chewing of khat leaves. Br. J. Clin. Pharmacol. 2003, 56, 125–130. [Google Scholar] [CrossRef] [Green Version]
- Soares, J.; Costa, V.M.; Bastos, M.L.; Carvalho, F.; Capela, J.P. An updated review on synthetic cathinones. Arch. Toxicol. 2021, 95, 2895–2940. [Google Scholar] [CrossRef]
- Brenneisen, R.; Mathys, K. Catha. In Hagers Handbuch der Pharmazeutischen Praxis, 5th ed.; Hänsel, R., Keller, K., Rimpler, H., Schneider, G., Abel, G., Bader, G., Baumann, B., Bertram, B., Beyer, G., Bodesheim, U., et al., Eds.; Springer: Berlin/Heidelberg, Germany, 1992; Volume 4, pp. 730–740. [Google Scholar]
- Kalix, P. Cathinone, an alkaloid from khat leaves with an amphetamine-like releasing effect. Psychopharmacology 1981, 74, 269–270. [Google Scholar] [CrossRef]
- Kalix, P.; Glennon, R.A. Further evidence for an amphetamine-like mechanism of action of the alkaloid cathinone. Biochem. Pharmacol. 1986, 35, 3015–3019. [Google Scholar] [CrossRef]
- Kalix, P. A constituent of khat leaves with amphetamine-like releasing properties. Eur. J. Pharmacol. 1980, 68, 213–215. [Google Scholar] [CrossRef]
- Kalix, P. The amphetamine-like releasing effect of the alkaloid (−)cathinone on rat nucleus accumbens and rabbit caudate nucleus. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 1982, 6, 43–49. [Google Scholar] [CrossRef]
- Kalix, P. Effect of the alkaloid (−) cathinone on the release of radioactivity from rabbit atria prelabelled with 3H-norepinephrine. Life Sci. 1983, 32, 801–807. [Google Scholar] [CrossRef]
- Kalix, P. Effect of the alkaloid (−)-cathinone on the release of radioactivity from rat striatal tissue prelabelled with 3H-serotonin. Neuropsychobiology 1984, 12, 127–129. [Google Scholar] [CrossRef]
- Kalix, P. Hyperthermic response to (−)-cathinone, an alkaloid of Catha edulis (khat). J. Pharm. Pharmacol. 1980, 32, 662–663. [Google Scholar] [CrossRef] [PubMed]
- Pehek, E.A.; Schechter, M.D.; Yamamoto, B.K. Effects of cathinone and amphetamine on the neurochemistry of dopamine in vivo. Neuropharmacology 1990, 29, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Simmler, L.D.; Buser, T.A.; Donzelli, M.; Schramm, Y.; Dieu, L.H.; Huwyler, J.; Chaboz, S.; Hoener, M.C.; Liechti, M.E. Pharmacological characterization of designer cathinones in vitro. Br. J. Pharmacol. 2013, 168, 458–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, G.C.; Preston, K.; Ricaurte, G.A.; Schuster, C.R.; Seiden, L.S. Neurochemical similarities between d,l-cathinone and d-amphetamine. Drug Alcohol Depend. 1982, 9, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Zelger, J.L.; Carlini, E.A. Influence of cathinone (α-aminopropiophenone) and cathine (phenylpropanolamine) on circling behavior and on the uptake and release of [3H]dopamine in striatal slices of rats. Neuropharmacology 1981, 20, 839–843. [Google Scholar] [CrossRef]
- Hutsell, B.A.; Baumann, M.H.; Partilla, J.S.; Banks, M.L.; Vekariya, R.; Glennon, R.A.; Negus, S.S. Abuse-related neurochemical and behavioral effects of cathinone and 4-methylcathinone stereoisomers in rats. Eur. Neuropsychopharmacol. 2016, 26, 288–297. [Google Scholar] [CrossRef] [Green Version]
- Cleary, L.; Docherty, J.R. Actions of amphetamine derivatives and cathinone at the noradrenaline transporter. Eur. J. Pharmacol. 2003, 476, 31–34. [Google Scholar] [CrossRef]
- Nencini, P.; Amiconi, G.; Befani, O.; Abdullahi, M.A.; Anania, M.C. Possible involvement of amine oxidase inhibition in the sympathetic activation induced by khat (Catha edulis) chewing in humans. J. Ethnopharmacol. 1984, 11, 79–86. [Google Scholar] [CrossRef]
- Osorio-Olivares, M.; Rezende, M.C.; Sepúlveda-Boza, S.; Cassels, B.K.; Fierro, A. MAO inhibition by aryl isopropylamines: The effect of oxygen substituents at the beta-position. Bioorg. Med. Chem. 2004, 12, 4055–4066. [Google Scholar] [CrossRef]
- Freund-Michel, V.C.; Birrell, M.A.; Patel, H.J.; Murray-Lyon, I.M.; Belvisi, M.G. Modulation of cholinergic contractions of airway smooth muscle by cathinone: Potential beneficial effects in airway diseases. Eur. Respir. J. 2008, 32, 579–584. [Google Scholar] [CrossRef] [Green Version]
- Odenwald, M.; al’Absi, M. Khat use and related addiction, mental health and physical disorders: The need to address a growing risk. East. Mediterr. Health J. 2017, 23, 236–244. [Google Scholar] [CrossRef]
- Jones, S.; Fileccia, E.L.; Murphy, M.; Fowler, M.J.; King, M.V.; Shortall, S.E.; Wigmore, P.M.; Green, A.R.; Fone, K.C.F.; Ebling, F.J.P. Cathinone increases body temperature, enhances locomotor activity, and induces striatal c-fos expression in the Siberian hamster. Neurosci. Lett. 2014, 559, 34–38. [Google Scholar] [CrossRef]
- Woolverton, W.L.; Johanson, C.E. Preference in rhesus monkeys given a choice between cocaine and d,l-cathinone. J. Exp. Anal. Behav. 1984, 41, 35–43. [Google Scholar] [CrossRef]
- LaHoste, G.J.; Yu, J.; Marshall, J.F. Striatal Fos expression is indicative of dopamine D1/D2 synergism and receptor supersensitivity. Proc. Natl. Acad. Sci. USA 1993, 90, 7451–7455. [Google Scholar] [CrossRef] [Green Version]
- Al-Motarreb, A.L.; Broadley, K.J. Coronary and aortic vasoconstriction by cathinone, the active constituent of khat. Auton. Autacoid Pharmacol. 2003, 23, 319–326. [Google Scholar] [CrossRef]
- Alsufyani, H.A.; Docherty, J.R. Direct and indirect cardiovascular actions of cathinone and MDMA in the anaesthetized rat. Eur. J. Pharmacol. 2015, 758, 142–146. [Google Scholar] [CrossRef]
- Cleary, L.; Buber, R.; Docherty, J.R. Effects of amphetamine derivatives and cathinone on noradrenaline-evoked contractions of rat right ventricle. Eur. J. Pharmacol. 2002, 451, 303–308. [Google Scholar] [CrossRef]
- Tesfaye, F.; Byass, P.; Wall, S.; Berhane, Y.; Bonita, R. Association of smoking and khat (Catha edulis Forsk) use with high blood pressure among adults in Addis Ababa, Ethiopia, 2006. Prev. Chronic Dis. 2008, 5, A89. [Google Scholar]
- Al-Motarreb, A.; Briancon, S.; Al-Jaber, N.; Al-Adhi, B.; Al-Jailani, F.; Salek, M.S.; Broadley, K.J. Khat chewing is a risk factor for acute myocardial infarction: A case-control study. Br. J. Clin. Pharmacol. 2005, 59, 574–581. [Google Scholar] [CrossRef] [Green Version]
- Alkadi, H.O.; Noman, M.A.; Al-Thobhani, A.K.; Al-Mekhlafi, F.S.; Raja’a, Y.A. Clinical and experimental evaluation of the effect of Khat-induced myocardial infarction. Saudi Med. J. 2002, 23, 1195–1198. [Google Scholar]
- Kalix, P. Hypermotility of the amphetamine type induced by a constituent of khat leaves. Br. J. Pharmacol. 1980, 68, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Nyongesa, A.W.; Oduma, J.A.; Nakajima, M.; Odongo, H.O.; Adoyo, P.A.; al’Absi, M. Dose-response inhibitory effects of purified cathinone from khat (Catha edulis) on cortisol and prolactin release in vervet monkeys (Chlorocebus aethiops). Metab. Brain Dis. 2014, 29, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.; Soares, J.; Rocha-Pereira, C.; Mladěnka, P.; Remião, F.; on behalf of the Oemonom Researchers. Khat, a Cultural Chewing Drug: A Toxicokinetic and Toxicodynamic Summary. Toxins 2022, 14, 71. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, J.L. Cocaine intoxication. Crit. Care Clin. 2012, 28, 517–526. [Google Scholar] [CrossRef]
- Richards, J.R.; Le, J.K. Cocaine Toxicity. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430976/ (accessed on 13 December 2021).
- Roque Bravo, R.; Faria, A.C.; Brito-da-Costa, A.M.; Carmo, H.; Mladěnka, P.; Dias da Silva, D.; Remião, F.; on behalf of the Oemonom Researchers. Cocaine: An Updated Overview on Chemistry, Detection, Biokinetics, and Pharmacotoxicological Aspects including Abuse Pattern. Toxins 2022, 14, 278. [Google Scholar] [CrossRef] [PubMed]
- Han, D.D.; Gu, H.H. Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacol. 2006, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, R.A.; DesLauriers, C.; Burda, A.; Johnson-Arbor, K. Cocaine: History, social implications, and toxicity: A review. Semin. Diagn. Pathol. 2009, 26, 10–17. [Google Scholar] [CrossRef]
- Perry, A.N.; Westenbroek, C.; Jagannathan, L.; Becker, J.B. The Roles of Dopamine and α1-Adrenergic Receptors in Cocaine Preferences in Female and Male Rats. Neuropsychopharmacology 2015, 40, 2696–2704. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.M.; Hanson, G.R.; Fleckenstein, A.E. Regulation of the vesicular monoamine transporter-2: A novel mechanism for cocaine and other psychostimulants. J. Pharmacol. Exp. Ther. 2001, 296, 762–767. [Google Scholar]
- Pei, Y.; Lee, J.; Leo, D.; Gainetdinov, R.R.; Hoener, M.C.; Canales, J.J. Activation of the Trace Amine-Associated Receptor 1 Prevents Relapse to Cocaine Seeking. Neuropsychopharmacology 2014, 39, 2299–2308. [Google Scholar] [CrossRef] [Green Version]
- Asif-Malik, A.; Hoener, M.C.; Canales, J.J. Interaction Between the Trace Amine-Associated Receptor 1 and the Dopamine D2 Receptor Controls Cocaine’s Neurochemical Actions. Sci. Rep. 2017, 7, 13901. [Google Scholar] [CrossRef] [Green Version]
- Mladěnka, P.; Applová, L.; Patočka, J.; Costa, V.M.; Remiao, F.; Pourová, J.; Mladěnka, A.; Karlíčková, J.; Jahodář, L.; Vopršalová, M.; et al. Comprehensive review of cardiovascular toxicity of drugs and related agents. Med. Res. Rev. 2018, 38, 1332–1403. [Google Scholar] [CrossRef]
- Tsatsakis, A.; Docea, A.O.; Calina, D.; Tsarouhas, K.; Zamfira, L.M.; Mitrut, R.; Sharifi-Rad, J.; Kovatsi, L.; Siokas, V.; Dardiotis, E.; et al. A Mechanistic and Pathophysiological Approach for Stroke Associated with Drugs of Abuse. J. Clin. Med. 2019, 8, 1295. [Google Scholar] [CrossRef] [Green Version]
- Lange, R.A.; Cigarroa, R.G.; Yancy, C.W., Jr.; Willard, J.E.; Popma, J.J.; Sills, M.N.; McBride, W.; Kim, A.S.; Hillis, L.D. Cocaine-induced coronary-artery vasoconstriction. N. Engl. J. Med. 1989, 321, 1557–1562. [Google Scholar] [CrossRef]
- Moliterno, D.J.; Willard, J.E.; Lange, R.A.; Negus, B.H.; Boehrer, J.D.; Glamann, D.B.; Landau, C.; Rossen, J.D.; Winniford, M.D.; Hillis, L.D. Coronary-artery vasoconstriction induced by cocaine, cigarette smoking, or both. N. Engl. J. Med. 1994, 330, 454–459. [Google Scholar] [CrossRef]
- Pradhan, L.; Mondal, D.; Chandra, S.; Ali, M.; Agrawal, K.C. Molecular analysis of cocaine-induced endothelial dysfunction: Role of endothelin-1 and nitric oxide. Cardiovasc. Toxicol. 2008, 8, 161–171. [Google Scholar] [CrossRef]
- Mo, W.; Singh, A.K.; Arruda, J.A.L.; Dunea, G. Role of Nitric Oxide in Cocaine-Induced Acute Hypertension. Am. J. Hypertens. 1998, 11, 708–714. [Google Scholar] [CrossRef]
- Vongpatanasin, W.; Mansour, Y.; Chavoshan, B.; Arbique, D.; Victor, R.G. Cocaine stimulates the human cardiovascular system via a central mechanism of action. Circulation 1999, 100, 497–502. [Google Scholar] [CrossRef] [Green Version]
- Fowler, J.S.; Ding, Y.S.; Volkow, N.D.; Martin, T.; MacGregor, R.R.; Dewey, S.; King, P.; Pappas, N.; Alexoff, D.; Shea, C.; et al. PET studies of cocaine inhibition of myocardial norepinephrine uptake. Synapse 1994, 16, 312–317. [Google Scholar] [CrossRef]
- Heesch, C.M.; Wilhelm, C.R.; Ristich, J.; Adnane, J.; Bontempo, F.A.; Wagner, W.R. Cocaine activates platelets and increases the formation of circulating platelet containing microaggregates in humans. Heart 2000, 83, 688–695. [Google Scholar] [CrossRef] [Green Version]
- McCord, J.; Jneid, H.; Hollander, J.E.; de Lemos, J.A.; Cercek, B.; Hsue, P.; Gibler, W.B.; Ohman, E.M.; Drew, B.; Philippides, G.; et al. Management of cocaine-associated chest pain and myocardial infarction: A scientific statement from the American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology. Circulation 2008, 117, 1897–1907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hobbs, W.E.; Moore, E.E.; Penkala, R.A.; Bolgiano, D.D.; López, J.A. Cocaine and specific cocaine metabolites induce von Willebrand factor release from endothelial cells in a tissue-specific manner. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Arner, P. Catecholamine-induced lipolysis in obesity. Int. J. Obes. Relat. Metab. Disord. 1999, 23 (Suppl. 1), 10–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diepvens, K.; Westerterp, K.R.; Westerterp-Plantenga, M.S. Obesity and thermogenesis related to the consumption of caffeine, ephedrine, capsaicin, and green tea. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R77–R85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, D.; Frölich, J.C.; Carr, R.K.; Watson, J.T.; Hollifield, J.W.; Shand, D.G.; Oates, J.A. Effects of caffeine on plasma renin activity, catecholamines and blood pressure. N. Engl. J. Med. 1978, 298, 181–186. [Google Scholar] [CrossRef]
- Dulloo, A.G.; Duret, C.; Rohrer, D.; Girardier, L.; Mensi, N.; Fathi, M.; Chantre, P.; Vandermander, J. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am. J. Clin. Nutr. 1999, 70, 1040–1045. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.M.; Wang, S.W.; Ho, S.C.; Tang, Y.L. Protective effect of green tea (−)-epigallocatechin-3-gallate against the monoamine oxidase B enzyme activity increase in adult rat brains. Nutrition 2010, 26, 1195–1200. [Google Scholar] [CrossRef]
- Hou, W.C.; Lin, R.D.; Chen, C.T.; Lee, M.H. Monoamine oxidase B (MAO-B) inhibition by active principles from Uncaria rhynchophylla. J. Ethnopharmacol. 2005, 100, 216–220. [Google Scholar] [CrossRef]
- Borchardt, R.T.; Huber, J.A. Catechol O-methyltransferase. 5. Structure-activity relationships for inhibition by flavonoids. J. Med. Chem. 1975, 18, 120–122. [Google Scholar] [CrossRef]
- Ludy, M.-J.; Moore, G.E.; Mattes, R.D. The effects of capsaicin and capsiate on energy balance: Critical review and meta-analyses of studies in humans. Chem. Senses 2012, 37, 103–121. [Google Scholar] [CrossRef] [Green Version]
- Tremblay, A.; Arguin, H.; Panahi, S. Capsaicinoids: A spicy solution to the management of obesity? Int. J. Obes. 2016, 40, 1198–1204. [Google Scholar] [CrossRef]
- Watanabe, T.; Kawada, T.; Kato, T.; Harada, T.; Iwai, K. Effects of capsaicin analogs on adrenal catecholamine secretion in rats. Life Sci. 1994, 54, 369–374. [Google Scholar] [CrossRef]
- Watanabe, T.; Kawada, T.; Iwai, K. Effect of capsaicin pretreatment on capsaicin-induced catecholamine secretion from the adrenal medulla in rats. Proc. Soc. Exp. Biol. Med. 1988, 187, 370–374. [Google Scholar] [CrossRef]
- Osaka, T.; Lee, T.H.; Kobayashi, A.; Inoue, S.; Kimura, S. Thermogenesis mediated by a capsaicin-sensitive area in the ventrolateral medulla. Neuroreport 2000, 11, 2425–2428. [Google Scholar] [CrossRef]
- Tsatsakis, A.M.; Docea, A.O.; Tsitsimpikou, C. New challenges in risk assessment of chemicals when simulating real exposure scenarios; simultaneous multi-chemicals’ low dose exposure. Food Chem. Toxicol. 2016, 96, 174–176. [Google Scholar] [CrossRef]
- Georgiadis, N.; Tsarouhas, K.; Dorne, J.-L.C.M.; Kass, G.E.N.; Laspa, P.; Toutouzas, K.; Koulaouzidou, E.A.; Kouretas, D.; Tsitsimpikou, C. Cardiotoxicity of Chemical Substances: An Emerging Hazard Class. J. Cardiovasc. Dev. Dis. 2022, 9, 226. [Google Scholar] [CrossRef]
- Poyatos, L.; Torres, A.; Papaseit, E.; Pérez-Mañá, C.; Hladun, O.; Núñez-Montero, M.; de la Rosa, G.; Torrens, M.; Fuster, D.; Muga, R.; et al. Abuse Potential of Cathinones in Humans: A Systematic Review. J. Clin. Med. 2022, 11, 1004. [Google Scholar] [CrossRef]

| Sample | Trace Amines (mg/kg) | Reference | ||
|---|---|---|---|---|
| TYR | PEA | TRYP | ||
| Soppresata | 8.12–511.44 | 10.56–19.90 | ND | [22] |
| Sausages | 10.33–338.85 | ND | ND | [22] |
| Salami | 77.14 | 3.2 | 1.63 | [23] |
| Hamburger | 1.5–27.0 | ND | 9.3–13.5 | [24] |
| Chocolate | 3.11 | 2.67 | 1.43 | [23] |
| Coffee | 1.26–16.41 | / | / | [9,25] |
| Fermented cabbage | 60.66 | 0.73 | 0.18 | [23] |
| Azeitão cheese 30 days of ripening | 122 | / | ≅ 65 | [20] |
| Parmigiano cheese | 3.75 | 0.2 | 0.07 | [23] |
| Yogurt | ND | 0.001 | ND | [23] |
| Leerdamer | ND | 0.005 | 0.04 | [23] |
| Red wine | 1.93 | 0.61 | 0.03 | [23] |
| Red wine Abruzzo (Chieti, Italy) | 11.94 ± 7.79 mg L−1, mean ± s.d | ND | / | [26] |
| Rosé wine Abruzzo (Chieti, Italy) | 0.27 ± 0.23 mg L−1, mean ± s.d. | 0.16 | / | [26] |
| White wine Abruzzo (Chieti, Italy) | ND | ND | / | [26] |
| Fiore Sardo Sheep Cheese (Sardinia, Italy) | 350 ± 300 mg/kg, mean ± s.d | / | / | [27] |
| Lupin Luteus Spontaneously fermented | 32.6 | 128.8 | / | [28] |
| Lupin Albus Spontaneously fermented | 69.1 | 144.1 | / | [28] |
| Soybean Rudoji Spontaneously fermented | 30.1 ± 3.1 | 230.1 ± 8.6 | / | [28] |
| Soybean Progress Spontaneously fermented | 27.8 ± 3.2 | 234.5 ± 12.3 | / | [28] |
| Feta cheese | 0–246 | 0.77–4.94 | 2.18–6.24 | [29] |
| Reference | Sample | Synephrine Content |
|---|---|---|
| [75] | Seville orange (C. aurantium) juice | 56.9 ± 0.52 μg/mL |
| [66] | Dried fruits of Citrus species | 0.11–2.0 mg/g dry weight |
| [68] | Unripe fruits and leaves of Citrus species | Fruits: 0.037–0.197% Leaves: 0.006–0.087% |
| [77] | C. aurantium fruits and peels | Fruits: 0.99 ± 0.05 mg/g Peels: 1.14 ± 0.02 mg/g |
| [64] | Juices of peeled Citrus unshiu fruits | 73.3–158.1 mg/L |
| [78] | C. aurantium fruit extracts and fruit parts | Whole fruit extract: 600 µg/g |
| Exocarp: 1100 µg/g | ||
| Mesocarp: 580 µg/g | ||
| Endocarp + juice: 94 µg/g | ||
| [79] | Citrus reticulata ‘Chachi’ pericarp harvested at different stages | Early harvest: 1.90 ± 0.26 g/kg |
| Middle harvest: 1.77 ± 0.27 g/kg | ||
| Late harvest: 1.50 ± 0.20 g/kg | ||
| [65] | C. aurantium fruits, leaves, stems, and roots in different harvest periods (June–September) | Fruits |
| June: 3.36 ± 0.64 g/kg | ||
| September: 0.41 ± 0.04 g/kg | ||
| Leaves | ||
| June: 1.86 ± 0.31 g/kg | ||
| September: 1.08 ± 0.15 g/kg | ||
| Stems | ||
| June: 0.10 ± 0.00 g/kg | ||
| September: 0.49 ± 0.23 g/kg | ||
| Roots | ||
| June: 0.03 ± 0.01 g/kg | ||
| September: ≤ 0.01 g/kg |
| Reference | Route of Administration | Concentrations | Toxidrome | Clinical History |
|---|---|---|---|---|
| [130] | Oral—cough mixture containing ephedrine. | Consumption of more than 400 mg of ephedrine a day. Ephedrine plasma concentration was not evaluated. | Cardiomiopathy | The 35-year-old man presented with general fatigue and shortness of breath. An electrocardiogram showed sinus tachycardia of 110 beats per min and left ventricular hypertrophy. Chest radiographs showed generalised cardiomegaly. Depressed left ventricular function with a corrected left ventricular ejection time of 65%. The man had had exercise-induced and hyperventilation asthma since the age of 14. In 1972 and 1973 he was treated for cardiac failure. |
| [131] | Oral—Tablets containing 10 mg ephedrine and 100 mg caffeine. Morning of the presentation day: 20 mg ephedrine 200 mg caffeine Afternoon of the presentation day: 30 mg ephedrine 300 mg caffeine The day before presentation (2 pills morning and afternoon): 40 mg ephedrine 400 mg caffeine | Ephedrine plasma concentration of 150 ng/mL. | Cardiac Ischemia | The 22-year old woman presented with palpitations, nausea, tremulousness, abdominal pain, and vomiting. The initial electrocardiogram revealed a ST segment depression of 1 mm in leads V3 and V4, along with inverted T waves in leads V1–V4. |
| [132] | Oral—Two tablets (one in the morning and one at lunch) making up a daily ingestion of 24 mg ephedrine and 80 mg caffeine for one month. | Ephedrine plasma concentration was not evaluated. | Myocardial infarction | The 45-year-old woman presented complaining of a 2-h substernal chest pressure with the pain radiating down into both forearms. An electrocardiogram showed a T-wave inversion and nonspecific ST changes suggestive of possible ischemia. The patient smoked 30 packs of cigarettes/year. Her family history was positive for premature coronary artery disease (her father underwent coronary artery bypass grafting at age of 63). Cardiac enzyme measurements revealed an elevated troponin level of 9.6 ng/mL. Cardiac catheterization was performed and no atherosclerotic disease was found in the coronary vessels, thus leading to the conclusion that vasospasm induced by ephedrine alkaloids was the cause of the patient’s acute myocardial infarction. |
| [133] | Oral— ephedrine 30 mg, as well as aspirin 30 mg, caffeine 120 mg and narnegin 80 mg. | Ephedrine plasma concentration was not evaluated | Collapse | Male with collapse after running a half-marathon. On hospital arrival, a Glasgow coma score fluctuating between 12 and 13, hypoxic, and a temperature of 39 °C. Tachycardia and bilateral mydriasis. After being resuscitated, became hypoglycaemic and suffered two seizures. After intubation, he was admitted to the critical care unit. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, V.M.; Grando, L.G.R.; Milandri, E.; Nardi, J.; Teixeira, P.; Mladěnka, P.; Remião, F.; on behalf of The OEMONOM. Natural Sympathomimetic Drugs: From Pharmacology to Toxicology. Biomolecules 2022, 12, 1793. https://doi.org/10.3390/biom12121793
Costa VM, Grando LGR, Milandri E, Nardi J, Teixeira P, Mladěnka P, Remião F, on behalf of The OEMONOM. Natural Sympathomimetic Drugs: From Pharmacology to Toxicology. Biomolecules. 2022; 12(12):1793. https://doi.org/10.3390/biom12121793
Chicago/Turabian StyleCosta, Vera Marisa, Luciana Grazziotin Rossato Grando, Elisa Milandri, Jessica Nardi, Patrícia Teixeira, Přemysl Mladěnka, Fernando Remião, and on behalf of The OEMONOM. 2022. "Natural Sympathomimetic Drugs: From Pharmacology to Toxicology" Biomolecules 12, no. 12: 1793. https://doi.org/10.3390/biom12121793






