SNARE Modulators and SNARE Mimetic Peptides
Abstract
:1. Introduction
1.1. Overview of Intracellular Membrane Fusion
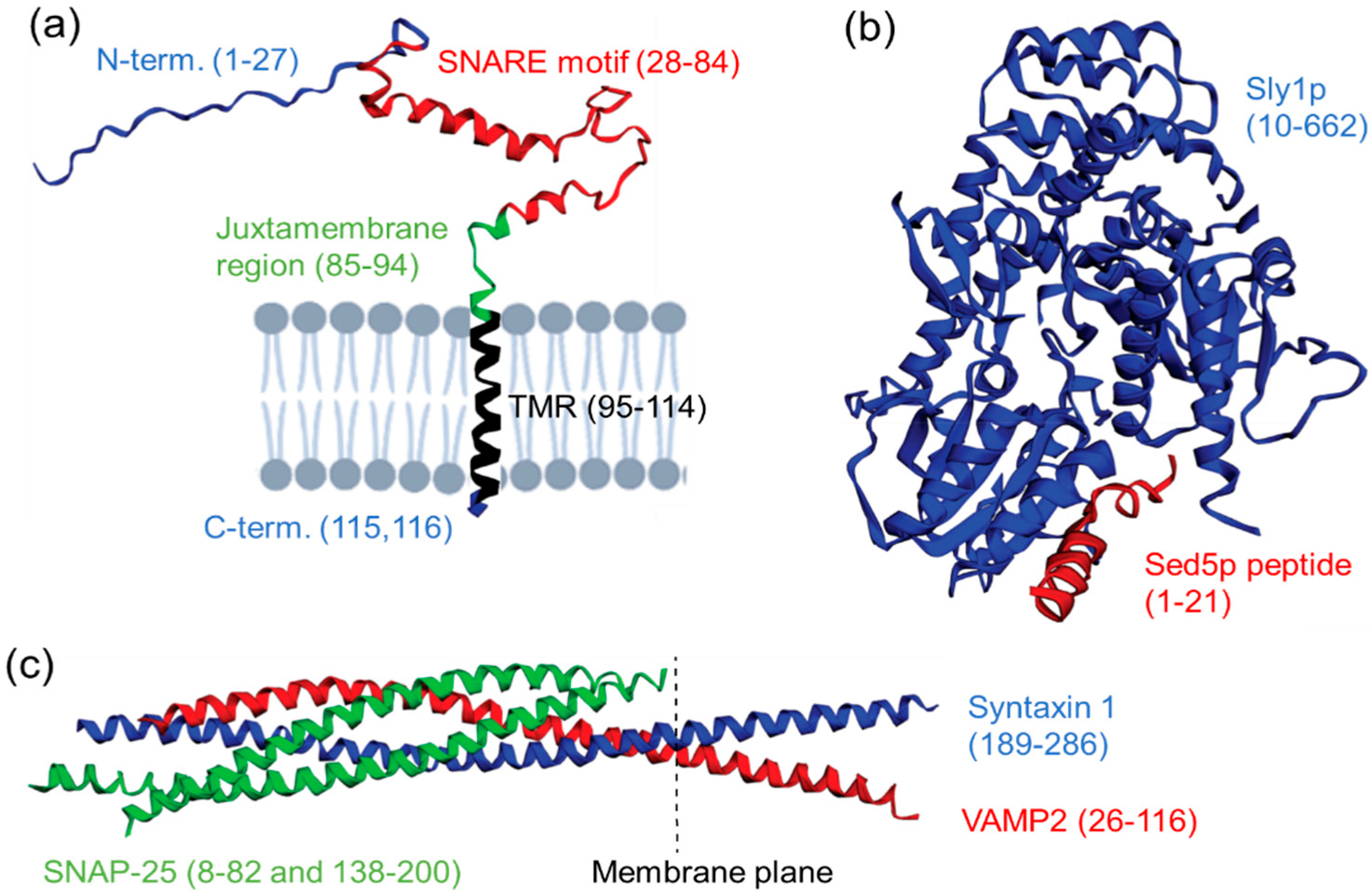
1.2. Native SNARE Protein Structure and Function
1.3. Molecular Manipulations of SNARE Proteins
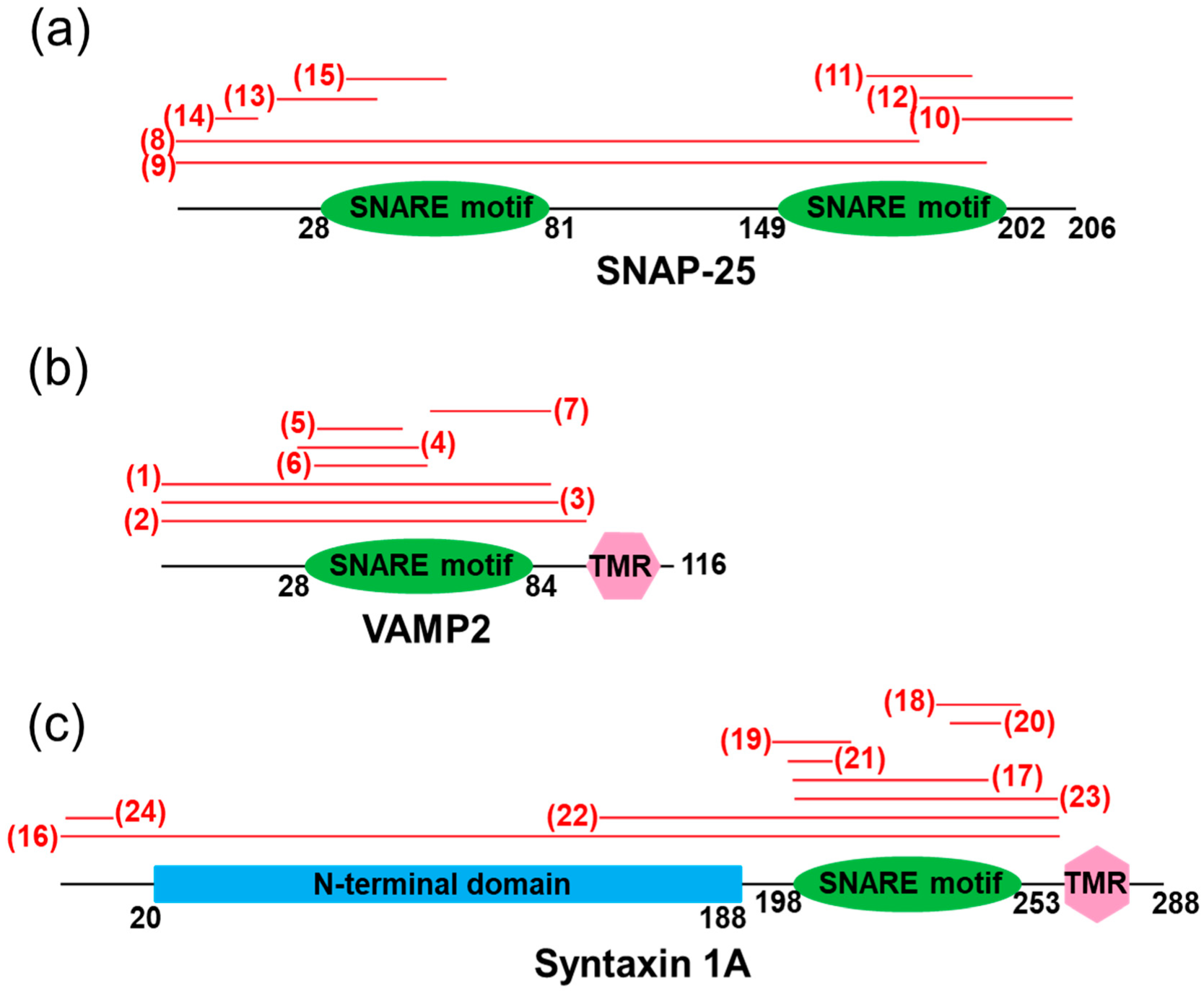
2. Peptides Modifying SNARE-Protein Functional Interfaces
2.1. SNARE Complex
2.2. SNAREs/SM Proteins
2.3. Other Functional Interfaces in SNARE Proteins Involved in Membrane Fusion
2.4. SNAREs/ion Channels and Transporters
3. SNARE-Mimetics, Functional Peptides Mediating Membrane Fusion
3.1. Overview of SNARE-Mimicry-based Fusogenic Systems
3.2. Peptide-nucleic acid (PNA) Fusogens
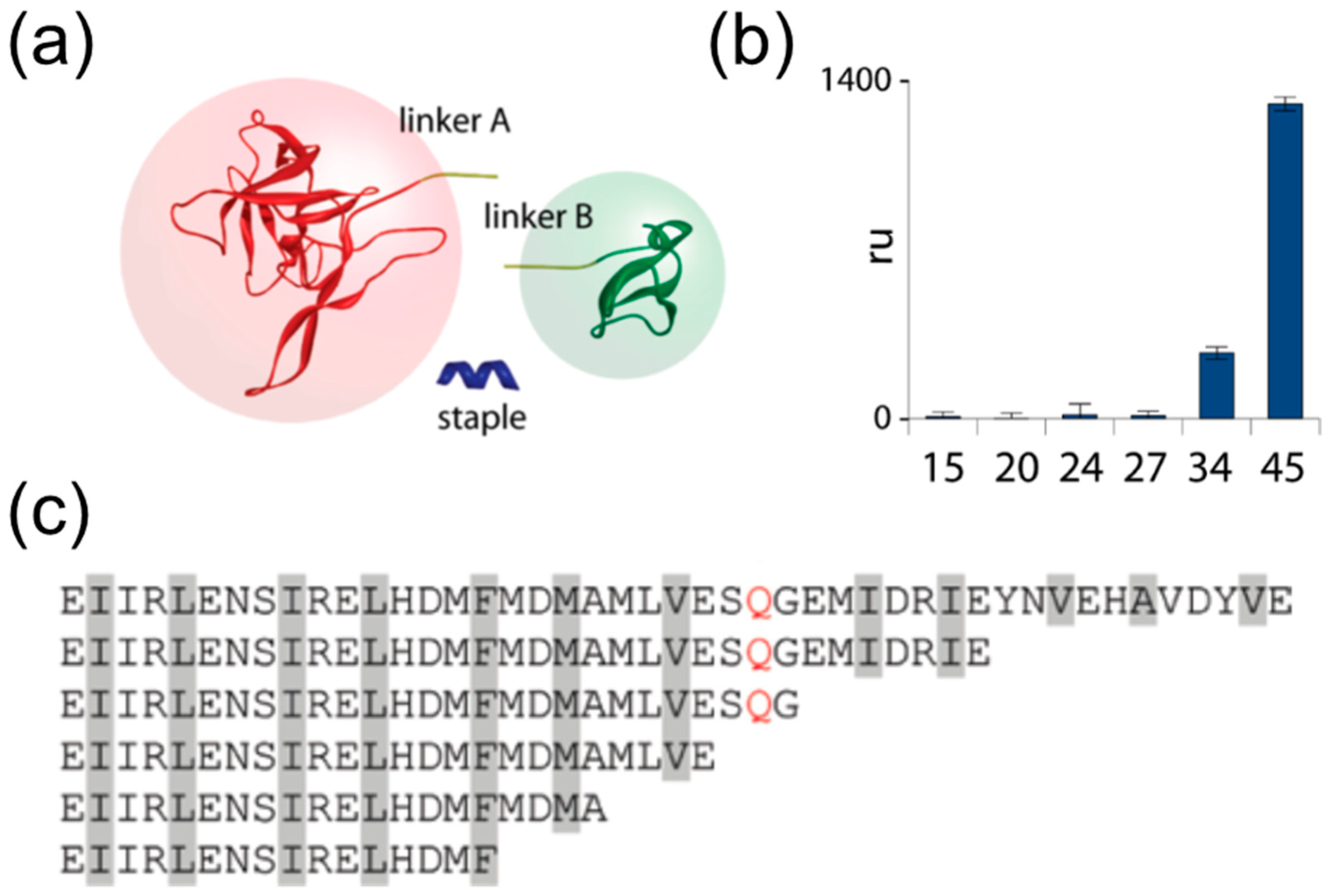
3.3. Coiled coil Peptides, True SNARE-Mimetics
4. SNARE-Inspired Polypeptide-based Binary Protein Assembly Systems
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Brunger, A.T.; Choi, U.B.; Lai, Y.; Leitz, J.; Zhou, Q. Molecular Mechanisms of Fast Neurotransmitter Release. Annu. Rev. Biophys. 2018, 47, 469–497. [Google Scholar] [CrossRef] [PubMed]
- Chernomordik, L.V.; Kozlov, M.M. Protein-Lipid Interplay in Fusion and Fission of Biological Membranes. Annu. Rev. Biochem. 2003, 72, 175–207. [Google Scholar] [CrossRef]
- Jahn, R.; Scheller, R.H. SNAREs--engines for membrane fusion. Nat. Rev. Mol. Cell. Biol. 2006, 7, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Dulubova, I.; Min, S.W.; Chen, X.; Rizo, J.; Südhof, T.C. Sly1 binds to Golgi and ER syntaxins via a conserved N-terminal peptide motif. Dev. Cell. 2002, 2, 295–305. [Google Scholar] [CrossRef] [Green Version]
- Sauvola, C.W.; Littleton, J.T. SNARE Regulatory Proteins in Synaptic Vesicle Fusion and Recycling. Front. Mol. Neurosci. 2021, 14, 733138. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.W.; Hughson, F.M. Chaperoning SNARE assembly and disassembly. Nat. Rev. Mol. Cell. Biol. 2016, 17, 465–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunger, A.T.; Weninger, K.; Bowen, M.; Chu, S. Single-molecule studies of the neuronal SNARE fusion machinery. Annu. Rev. Biochem. 2009, 78, 903–928. [Google Scholar] [CrossRef] [Green Version]
- Mohrmann, R.; de Wit, H.; Verhage, M.; Neher, E.; Sørensen, J.B. Fast vesicle fusion in living cells requires at least three SNARE complexes. Science 2010, 330, 502–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha, R.; Ahmed, S.; Jahn, R.; Klingauf, J. Two synaptobrevin molecules are sufficient for vesicle fusion in central nervous system synapses. Proc. Natl. Acad. Sci. USA 2011, 108, 14318–14323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kweon, D.H.; Kim, C.S.; Shin, Y.K. Insertion of the membrane-proximal region of the neuronal SNARE coiled coil into the membrane. J. Biol. Chem. 2003, 278, 12367–12373. [Google Scholar] [CrossRef]
- Van Komen, J.S.; Bai, X.; Rodkey, T.L.; Schaub, J.; McNew, J.A. The polybasic juxtamembrane region of Sso1p is required for SNARE function in vivo. Eukaryot. Cell. 2005, 4, 2017–2028. [Google Scholar] [CrossRef] [Green Version]
- Dhara, M.; Yarzagaray, A.; Makke, M.; Schindeldecker, B.; Schwarz, Y.; Shaaban, A.; Sharma, S.; Böckmann, R.A.; Lindau, M.; Mohrmann, R.; et al. v-SNARE transmembrane domains function as catalysts for vesicle fusion. Elife 2016, 5, e17571. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.W.; Peplowska, K.; Rohde, J.; Poschner, B.C.; Ungermann, C.; Langosch, D. Self-interaction of a SNARE transmembrane domain promotes the hemifusion-to-fusion transition. J. Mol. Biol. 2006, 364, 1048–1060. [Google Scholar] [CrossRef]
- Sharma, S.; Lindau, M. t-SNARE Transmembrane Domain Clustering Modulates Lipid Organization and Membrane Curvature. J. Am. Chem. Soc. 2017, 139, 18440–18443. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Chan, C.; Weir, N.R.; Denic, V. The Get1/2 transmembrane complex is an endoplasmic-reticulum membrane protein insertase. Nature 2014, 512, 441–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.W.; Chiang, C.W.; Gaffaney, J.D.; Chapman, E.R.; Jackson, M.B. Lipid-anchored Synaptobrevin Provides Little or No Support for Exocytosis or Liposome Fusion. J. Biol. Chem. 2016, 291, 2848–2857. [Google Scholar] [CrossRef] [Green Version]
- Zhou, P.; Bacaj, T.; Yang, X.; Pang, Z.P.; Südhof, T.C. Lipid-anchored SNAREs lacking transmembrane regions fully support membrane fusion during neurotransmitter release. Neuron 2013, 80, 470–483. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Zick, M.; Wickner, W.T.; Jun, Y. A lipid-anchored SNARE supports membrane fusion. Proc. Natl. Acad. Sci. USA 2011, 108, 17325–17330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daste, F.; Galli, T.; Tareste, D. Structure and function of longin SNAREs. J. Cell. Sci. 2015, 128, 4263–4272. [Google Scholar] [CrossRef] [Green Version]
- Rizo, J. Molecular Mechanisms Underlying Neurotransmitter Release. Annu. Rev. Biophys. 2022, 51, 377–408. [Google Scholar] [CrossRef]
- Dulubova, I.; Yamaguchi, T.; Arac, D.; Li, H.; Huryeva, I.; Min, S.W.; Rizo, J.; Sudhof, T.C. Convergence and divergence in the mechanism of SNARE binding by Sec1/Munc18-like proteins. Proc. Natl. Acad. Sci. USA 2003, 100, 32–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dulubova, I.; Sugita, S.; Hill, S.; Hosaka, M.; Fernandez, I.; Südhof, T.C.; Rizo, J. A conformational switch in syntaxin during exocytosis: Role of munc18. Embo J. 1999, 18, 4372–4382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rickman, C.; Jiménez, J.L.; Graham, M.E.; Archer, D.A.; Soloviev, M.; Burgoyne, R.D.; Davletov, B. Conserved prefusion protein assembly in regulated exocytosis. Mol. Biol. Cell 2006, 17, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Atlas, D. Functional and physical coupling of voltage-sensitive calcium channels with exocytotic proteins: Ramifications for the secretion mechanism. J. Neurochem. 2001, 77, 972–985. [Google Scholar] [CrossRef] [Green Version]
- Jarvis, S.E.; Zamponi, G.W. Interactions between presynaptic Ca2+ channels, cytoplasmic messengers and proteins of the synaptic vesicle release complex. Trends. Pharmacol. Sci. 2001, 22, 519–525. [Google Scholar] [CrossRef]
- Verhage, M.; Sørensen, J.B. SNAREopathies: Diversity in Mechanisms and Symptoms. Neuron 2020, 107, 22–37. [Google Scholar] [CrossRef]
- Melland, H.; Carr, E.M.; Gordon, S.L. Disorders of synaptic vesicle fusion machinery. J. Neurochem. 2021, 157, 130–164. [Google Scholar] [CrossRef]
- Margiotta, A. Role of SNAREs in Neurodegenerative Diseases. Cells 2021, 10, 991. [Google Scholar] [CrossRef]
- Tang, B.L. SNAREs and developmental disorders. J. Cell. Physiol. 2021, 236, 2482–2504. [Google Scholar] [CrossRef]
- Pirazzini, M.; Montecucco, C.; Rossetto, O. Toxicology and pharmacology of botulinum and tetanus neurotoxins: An update. Arch. Toxicol. 2022, 96, 1521–1539. [Google Scholar] [CrossRef]
- Arsenault, J.; Ferrari, E.; Niranjan, D.; Cuijpers, S.A.; Gu, C.; Vallis, Y.; O’Brien, J.; Davletov, B. Stapling of the botulinum type A protease to growth factors and neuropeptides allows selective targeting of neuroendocrine cells. J. Neurochem. 2013, 126, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Riley, L.G.; Roufogalis, B.D.; Li, G.Q.; Weiss, A.S. A radioassay for synaptic core complex assembly: Screening of herbal extracts for effectors. Anal. Biochem. 2006, 357, 50–57. [Google Scholar] [CrossRef]
- Heo, P.; Yang, Y.; Han, K.Y.; Kong, B.; Shin, J.H.; Jung, Y.; Jeong, C.; Shin, J.; Shin, Y.K.; Ha, T.; et al. A Chemical Controller of SNARE-Driven Membrane Fusion That Primes Vesicles for Ca2+-Triggered Millisecond Exocytosis. J. Am. Chem. Soc. 2016, 138, 4512–4521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Shin, J.Y.; Oh, J.M.; Jung, C.H.; Hwang, Y.; Kim, S.; Kim, J.S.; Yoon, K.J.; Ryu, J.Y.; Shin, J.; et al. Dissection of SNARE-driven membrane fusion and neuroexocytosis by wedging small hydrophobic molecules into the SNARE zipper. Proc. Natl. Acad. Sci. USA 2010, 107, 22145–22150. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Kim, S.H.; Heo, P.; Kong, B.; Shin, J.; Jung, Y.H.; Yoon, K.; Chung, W.J.; Shin, Y.K.; Kweon, D.H. SNARE zippering is hindered by polyphenols in the neuron. Biochem. Biophys. Res. Commun. 2014, 450, 831–836. [Google Scholar] [CrossRef] [Green Version]
- Nagele, P.; Mendel, J.B.; Placzek, W.J.; Scott, B.A.; D’Avignon, D.A.; Crowder, C.M. Volatile anesthetics bind rat synaptic snare proteins. Anesthesiology 2005, 103, 768–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Swinderen, B.; Saifee, O.; Shebester, L.; Roberson, R.; Nonet, M.L.; Crowder, C.M. A neomorphic syntaxin mutation blocks volatile-anesthetic action in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 1999, 96, 2479–2484. [Google Scholar] [CrossRef] [Green Version]
- Herring, B.E.; Xie, Z.; Marks, J.; Fox, A.P. Isoflurane inhibits the neurotransmitter release machinery. J. Neurophysiol. 2009, 102, 1265–1273. [Google Scholar] [CrossRef] [Green Version]
- Davletov, B.; Connell, E.; Darios, F. Regulation of SNARE fusion machinery by fatty acids. Cell. Mol. Life Sci. 2007, 64, 1597–1608. [Google Scholar] [CrossRef]
- Darios, F.; Wasser, C.; Shakirzyanova, A.; Giniatullin, A.; Goodman, K.; Munoz-Bravo, J.L.; Raingo, J.; Jorgacevski, J.; Kreft, M.; Zorec, R.; et al. Sphingosine facilitates SNARE complex assembly and activates synaptic vesicle exocytosis. Neuron 2009, 62, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Lauwers, E.; Goodchild, R.; Verstreken, P. Membrane Lipids in Presynaptic Function and Disease. Neuron 2016, 90, 11–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, P.; Guha, S.; Diederichsen, U. SNARE protein analog-mediated membrane fusion. J. Pept. Sci. 2015, 21, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Apland, J.P.; Adler, M.; Oyler, G.A. Inhibition of neurotransmitter release by peptides that mimic the N-terminal domain of SNAP-25. J. Protein. Chem. 2003, 22, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wheeler, M.B.; Kang, Y.H.; Sheu, L.; Lukacs, G.L.; Trimble, W.S.; Gaisano, H.Y. Truncated SNAP-25 (1-197), like botulinum neurotoxin A, can inhibit insulin secretion from HIT-T15 insulinoma cells. Mol. Endocrinol. 1998, 12, 1060–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.A.; Scales, S.J.; Jagath, J.R.; Scheller, R.H. A discontinuous SNAP-25 C-terminal coil supports exocytosis. J. Biol. Chem. 2001, 276, 28503–28508. [Google Scholar] [CrossRef] [Green Version]
- Cornille, F.; Deloye, F.; Fournié-Zaluski, M.C.; Roques, B.P.; Poulain, B. Inhibition of neurotransmitter release by synthetic proline-rich peptides shows that the N-terminal domain of vesicle-associated membrane protein/synaptobrevin is critical for neuro-exocytosis. J. Biol. Chem. 1995, 270, 16826–16832. [Google Scholar] [CrossRef] [Green Version]
- Guček, A.; Jorgačevski, J.; Singh, P.; Geisler, C.; Lisjak, M.; Vardjan, N.; Kreft, M.; Egner, A.; Zorec, R. Dominant negative SNARE peptides stabilize the fusion pore in a narrow, release-unproductive state. Cell. Mol. Life Sci. 2016, 73, 3719–3731. [Google Scholar] [CrossRef]
- Weber, T.; Zemelman, B.V.; McNew, J.A.; Westermann, B.; Gmachl, M.; Parlati, F.; Söllner, T.H.; Rothman, J.E. SNAREpins: Minimal machinery for membrane fusion. Cell 1998, 92, 759–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arsenault, J.; Cuijpers, S.A.; Ferrari, E.; Niranjan, D.; Rust, A.; Leese, C.; O’Brien, J.A.; Binz, T.; Davletov, B. Botulinum protease-cleaved SNARE fragments induce cytotoxicity in neuroblastoma cells. J. Neurochem. 2014, 129, 781–791. [Google Scholar] [CrossRef] [Green Version]
- Fournier, K.M.; Robinson, M.B. A dominant-negative variant of SNAP-23 decreases the cell surface expression of the neuronal glutamate transporter EAAC1 by slowing constitutive delivery. Neurochem. Int. 2006, 48, 596–603. [Google Scholar] [CrossRef]
- Gutiérrez, L.M.; Cànaves, J.M.; Ferrer-Montiel, A.V.; Reig, J.A.; Montal, M.; Viniegra, S. A peptide that mimics the carboxy-terminal domain of SNAP-25 blocks Ca2+-dependent exocytosis in chromaffin cells. FEBS Lett. 1995, 372, 39–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrer-Montiel, A.V.; Gutiérrez, L.M.; Apland, J.P.; Canaves, J.M.; Gil, A.; Viniegra, S.; Biser, J.A.; Adler, M.; Montal, M. The 26-mer peptide released from SNAP-25 cleavage by botulinum neurotoxin E inhibits vesicle docking. FEBS Lett. 1998, 435, 84–88. [Google Scholar] [CrossRef] [Green Version]
- Apland, J.P.; Biser, J.A.; Adler, M.; Ferrer-Montiel, A.V.; Montal, M.; Canaves, J.M.; Filbert, M.G. Peptides that mimic the carboxy-terminal domain of SNAP-25 block acetylcholine release at an Aplysia synapse. J. Appl. Toxicol. 1999, 19 (Suppl. 1), S23–S26. [Google Scholar] [CrossRef]
- Blanes-Mira, C.; Merino, J.M.; Valera, E.; Fernández-Ballester, G.; Gutiérrez, L.M.; Viniegra, S.; Pérez-Payá, E.; Ferrer-Montiel, A. Small peptides patterned after the N-terminus domain of SNAP25 inhibit SNARE complex assembly and regulated exocytosis. J. Neurochem. 2004, 88, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Blanes-Mira, C.; Clemente, J.; Jodas, G.; Gil, A.; Fernández-Ballester, G.; Ponsati, B.; Gutierrez, L.; Pérez-Payá, E.; Ferrer-Montiel, A. A synthetic hexapeptide (Argireline) with antiwrinkle activity. Int. J. Cosmet. Sci. 2002, 24, 303–310. [Google Scholar] [CrossRef]
- Martin, F.; Salinas, E.; Vazquez, J.; Soria, B.; Reig, J.A. Inhibition of insulin release by synthetic peptides shows that the H3 region at the C-terminal domain of syntaxin-1 is crucial for Ca2+- but not for guanosine 5’-[γ-thio]triphosphate-induced secretion. Biochem. J. 1996, 320, 201–205. [Google Scholar] [CrossRef]
- Martin, F.; Salinas, E.; Barahona, F.; Vázquez, J.; Soria, B.; Reig, J.A. Engineered peptides corresponding to segments of the H3 domain of syntaxin inhibit insulin release both in intact and permeabilized mouse pancreatic β cells. Biochem. Biophys. Res. Commun. 1998, 248, 83–86. [Google Scholar] [CrossRef]
- Mishima, T.; Fujiwara, T.; Akagawa, K. Reduction of neurotransmitter release by the exogenous H3 domain peptide of HPC-1/syntaxin 1A in cultured rat hippocampal neurons. Neurosci. Lett. 2002, 329, 273–276. [Google Scholar] [CrossRef]
- Ohara-Imaizumi, M.; Nakamichi, Y.; Nishiwaki, C.; Nagamatsu, S. Transduction of MIN6 β cells with TAT-syntaxin SNARE motif inhibits insulin exocytosis in biphasic insulin release in a distinct mechanism analyzed by evanescent wave microscopy. J. Biol. Chem. 2002, 277, 50805–50811. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, T.; Yamamori, T.; Akagawa, K. Suppression of transmitter release by Tat HPC-1/syntaxin 1A fusion protein. Biochim. Biophys. Acta. 2001, 1539, 225–232. [Google Scholar] [CrossRef]
- Yang, Y.; Kong, B.; Jung, Y.; Park, J.B.; Oh, J.M.; Hwang, J.; Cho, J.Y.; Kweon, D.H. Soluble N-Ethylmaleimide-Sensitive Factor Attachment Protein Receptor-Derived Peptides for Regulation of Mast Cell Degranulation. Front. Immunol. 2018, 9, 725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melia, T.J.; Weber, T.; McNew, J.A.; Fisher, L.E.; Johnston, R.J.; Parlati, F.; Mahal, L.K.; Sollner, T.H.; Rothman, J.E. Regulation of membrane fusion by the membrane-proximal coil of the t-SNARE during zippering of SNAREpins. J. Cell. Biol. 2002, 158, 929–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanes-Mira, C.; Pastor, M.T.; Valera, E.; Fernández-Ballester, G.; Merino, J.M.; Gutierrez, L.M.; Perez-Payá, E.; Ferrer-Montiel, A. Identification of SNARE complex modulators that inhibit exocytosis from an α-helix-constrained combinatorial library. Biochem. J. 2003, 375, 159–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerst, J.E. SNARE regulators: Matchmakers and matchbreakers. Biochim. Biophys. Acta. 2003, 1641, 99–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizo, J.; Südhof, T.C. The membrane fusion enigma: SNAREs, Sec1/Munc18 proteins, and their accomplices--guilty as charged? Annu. Rev. Cell. Dev. Biol. 2012, 28, 279–308. [Google Scholar] [CrossRef]
- Zhang, Y.; Hughson, F.M. Chaperoning SNARE Folding and Assembly. Annu. Rev. Biochem. 2021, 90, 581–603. [Google Scholar] [CrossRef]
- Johnson, J.R.; Ferdek, P.; Lian, L.Y.; Barclay, J.W.; Burgoyne, R.D.; Morgan, A. Binding of UNC-18 to the N-terminus of syntaxin is essential for neurotransmission in Caenorhabditis elegans. Biochem. J. 2009, 418, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Bin, N.R.; Rajah, M.; Kim, B.; Chou, T.C.; Kang, S.Y.; Sugita, K.; Parsaud, L.; Smith, M.; Monnier, P.P.; et al. Conformational states of syntaxin-1 govern the necessity of N-peptide binding in exocytosis of PC12 cells and Caenorhabditis elegans. Mol. Biol. Cell. 2016, 27, 669–685. [Google Scholar] [CrossRef]
- Vardar, G.; Salazar-Lázaro, A.; Brockmann, M.; Weber-Boyvat, M.; Zobel, S.; Kumbol, V.W.; Trimbuch, T.; Rosenmund, C. Reexamination of N-terminal domains of syntaxin-1 in vesicle fusion from central murine synapses. Elife 2021, 10, e69498. [Google Scholar] [CrossRef]
- Khvotchev, M.; Dulubova, I.; Sun, J.; Dai, H.; Rizo, J.; Südhof, T.C. Dual modes of Munc18-1/SNARE interactions are coupled by functionally critical binding to syntaxin-1 N terminus. J. Neurosci. 2007, 27, 12147–12155. [Google Scholar] [CrossRef]
- Khvotchev, M.; Südhof, T.C. Proteolytic processing of amyloid-β precursor protein by secretases does not require cell surface transport. J. Biol. Chem. 2004, 279, 47101–47108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Shen, C.; Liu, Y.; Menasche, B.L.; Ouyang, Y.; Stowell, M.H.B.; Shen, J. SNARE zippering requires activation by SNARE-like peptides in Sec1/Munc18 proteins. Proc. Natl. Acad. Sci. USA 2018, 115, E8421–E8429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathore, S.S.; Liu, Y.; Yu, H.; Wan, C.; Lee, M.; Yin, Q.; Stowell, M.H.B.; Shen, J. Intracellular Vesicle Fusion Requires a Membrane-Destabilizing Peptide Located at the Juxtamembrane Region of the v-SNARE. Cell. Rep. 2019, 29, 4583–4592.e4583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Abdul-Wahid, M.S.; Demill, C.M.; Serwin, M.B.; Prosser, R.S.; Stewart, B.A. Effect of juxtamembrane tryptophans on the immersion depth of Synaptobrevin, an integral vesicle membrane protein. Biochim. Biophys. Acta 2012, 1818, 2994–2999. [Google Scholar] [CrossRef] [Green Version]
- Tarafdar, P.K.; Chakraborty, H.; Bruno, M.J.; Lentz, B.R. Phosphatidylserine-Dependent Catalysis of Stalk and Pore Formation by Synaptobrevin JMR-TMD Peptide. Biophys. J. 2015, 109, 1863–1872. [Google Scholar] [CrossRef] [Green Version]
- Matsushita, K.; Morrell, C.N.; Lowenstein, C.J. A novel class of fusion polypeptides inhibits exocytosis. Mol. Pharmacol. 2005, 67, 1137–1144. [Google Scholar] [CrossRef]
- Burré, J.; Sharma, M.; Tsetsenis, T.; Buchman, V.; Etherton, M.R.; Südhof, T.C. A-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 2010, 329, 1663–1667. [Google Scholar] [CrossRef] [Green Version]
- Yoo, G.; Yeou, S.; Son, J.B.; Shin, Y.K.; Lee, N.K. Cooperative inhibition of SNARE-mediated vesicle fusion by α-synuclein monomers and oligomers. Sci. Rep. 2021, 11, 10955. [Google Scholar] [CrossRef]
- Chapman, E.R. A Ca2+ Sensor for Exocytosis. Trends. Neurosci. 2018, 41, 327–330. [Google Scholar] [CrossRef]
- Zhou, Q.; Lai, Y.; Bacaj, T.; Zhao, M.; Lyubimov, A.Y.; Uervirojnangkoorn, M.; Zeldin, O.B.; Brewster, A.S.; Sauter, N.K.; Cohen, A.E.; et al. Architecture of the synaptotagmin-SNARE machinery for neuronal exocytosis. Nature 2015, 525, 62–67. [Google Scholar] [CrossRef]
- Lai, Y.; Fois, G.; Flores, J.R.; Tuvim, M.J.; Zhou, Q.; Yang, K.; Leitz, J.; Peters, J.; Zhang, Y.; Pfuetzner, R.A.; et al. Inhibition of calcium-triggered secretion by hydrocarbon-stapled peptides. Nature 2022, 603, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Tuvim, M.J.; Leitz, J.; Peters, J.; Pfuetzner, R.A.; Esquivies, L.; Zhou, Q.; Czako, B.; Cross, J.B.; Jones, P.; et al. Screening of Hydrocarbon-Stapled Peptides for Inhibition of Calcium-Triggered Exocytosis. Front. Pharmacol. 2022, 13, 891041. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.K.; Calakos, N.; Scheller, R.H. Syntaxin: A synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science 1992, 257, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, A.; Oho, C.; Omori, A.; Kuwahara, R.; Ito, T.; Takahashi, M. HPC-1 is associated with synaptotagmin and omega-conotoxin receptor. J. Biol. Chem. 1992, 267, 24925–24928. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.H.; Rettig, J.; Takahashi, M.; Catterall, W.A. Identification of a syntaxin-binding site on N-type calcium channels. Neuron 1994, 13, 1303–1313. [Google Scholar] [CrossRef]
- Sheng, Z.H.; Rettig, J.; Cook, T.; Catterall, W.A. Calcium-dependent interaction of N-type calcium channels with the synaptic core complex. Nature 1996, 379, 451–454. [Google Scholar] [CrossRef]
- Kim, D.K.; Catterall, W.A. Ca2+-dependent and -independent interactions of the isoforms of the α1A subunit of brain Ca2+ channels with presynaptic SNARE proteins. Proc. Natl. Acad. Sci. USA 1997, 94, 14782–14786. [Google Scholar] [CrossRef] [Green Version]
- Mochida, S.; Sheng, Z.H.; Baker, C.; Kobayashi, H.; Catterall, W.A. Inhibition of neurotransmission by peptides containing the synaptic protein interaction site of N-type Ca2+ channels. Neuron 1996, 17, 781–788. [Google Scholar] [CrossRef] [Green Version]
- Rettig, J.; Heinemann, C.; Ashery, U.; Sheng, Z.H.; Yokoyama, C.T.; Catterall, W.A.; Neher, E. Alteration of Ca2+ dependence of neurotransmitter release by disruption of Ca2+ channel/syntaxin interaction. J. Neurosci. 1997, 17, 6647–6656. [Google Scholar] [CrossRef] [Green Version]
- Serra, S.A.; Cuenca-León, E.; Llobet, A.; Rubio-Moscardo, F.; Plata, C.; Carreño, O.; Fernàndez-Castillo, N.; Corominas, R.; Valverde, M.A.; Macaya, A.; et al. A mutation in the first intracellular loop of CACNA1A prevents P/Q channel modulation by SNARE proteins and lowers exocytosis. Proc. Natl. Acad. Sci. USA 2010, 107, 1672–1677. [Google Scholar] [CrossRef]
- Leung, Y.M.; Kang, Y.; Gao, X.; Xia, F.; Xie, H.; Sheu, L.; Tsuk, S.; Lotan, I.; Tsushima, R.G.; Gaisano, H.Y. Syntaxin 1A binds to the cytoplasmic C terminus of Kv2.1 to regulate channel gating and trafficking. J. Biol. Chem. 2003, 278, 17532–17538. [Google Scholar] [CrossRef] [Green Version]
- Singer-Lahat, D.; Chikvashvili, D.; Lotan, I. Direct interaction of endogenous Kv channels with syntaxin enhances exocytosis by neuroendocrine cells. PLoS ONE 2008, 3, e1381. [Google Scholar] [CrossRef] [Green Version]
- Tsuk, S.; Michaelevski, I.; Bentley, G.N.; Joho, R.H.; Chikvashvili, D.; Lotan, I. Kv2.1 channel activation and inactivation is influenced by physical interactions of both syntaxin 1A and the syntaxin 1A/soluble N-ethylmaleimide-sensitive factor-25 (t-SNARE) complex with the C terminus of the channel. Mol. Pharmacol. 2005, 67, 480–488. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, P.E.; Wang, G.; Tsuk, S.; Dodo, C.; Kang, Y.; Tang, L.; Wheeler, M.B.; Cattral, M.S.; Lakey, J.R.; Salapatek, A.M.; et al. Synaptosome-associated protein of 25 kilodaltons modulates Kv2.1 voltage-dependent K+ channels in neuroendocrine islet β-cells through an interaction with the channel N terminus. Mol. Endocrinol. 2002, 16, 2452–2461. [Google Scholar] [CrossRef]
- Zhuang, G.Q.; Wu, W.; Liu, F.; Ma, J.L.; Luo, Y.X.; Xiao, Z.X.; Liu, Y.; Wang, W.; He, Y. SNAP-25(1-180) enhances insulin secretion by blocking Kv2.1 channels in rat pancreatic islet β-cells. Biochem. Biophys. Res. Commun. 2009, 379, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Condliffe, S.B.; Carattino, M.D.; Frizzell, R.A.; Zhang, H. Syntaxin 1A regulates ENaC via domain-specific interactions. J. Biol. Chem. 2003, 278, 12796–12804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fletcher, S.; Bowden, S.E.; Marrion, N.V. False interaction of syntaxin 1A with a Ca2+-activated K+ channel revealed by co-immunoprecipitation and pull-down assays: Implications for identification of protein-protein interactions. Neuropharmacology 2003, 44, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Dolphin, A.C.; Lee, A. Presynaptic calcium channels: Specialized control of synaptic neurotransmitter release. Nat. Rev. Neurosci. 2020, 21, 213–229. [Google Scholar] [CrossRef]
- Söllner, T.H. Intracellular and viral membrane fusion: A uniting mechanism. Curr. Opin. Cell. Biol. 2004, 16, 429–435. [Google Scholar] [CrossRef]
- Hu, C.; Ahmed, M.; Melia, T.J.; Söllner, T.H.; Mayer, T.; Rothman, J.E. Fusion of cells by flipped SNAREs. Science 2003, 300, 1745–1749. [Google Scholar] [CrossRef]
- Parlati, F.; Weber, T.; McNew, J.A.; Westermann, B.; Söllner, T.H.; Rothman, J.E. Rapid and efficient fusion of phospholipid vesicles by the α-helical core of a SNARE complex in the absence of an N-terminal regulatory domain. Proc. Natl. Acad. Sci. USA 1999, 96, 12565–12570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wesolowski, J.; Paumet, F. SNARE motif: A common motif used by pathogens to manipulate membrane fusion. Virulence 2010, 1, 319–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, X.; Halder, P.; Yavuz, H.; Jahn, R.; Shuman, H.A. Direct targeting of membrane fusion by SNARE mimicry: Convergent evolution of Legionella effectors. Proc. Natl. Acad. Sci. USA 2016, 113, 8807–8812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, N.P.; Newton, P.; Schuelein, R.; Brown, D.L.; Petru, M.; Zarsky, V.; Dolezal, P.; Luo, L.; Bugarcic, A.; Stanley, A.C.; et al. Soluble NSF attachment protein receptor molecular mimicry by a Legionella pneumophila Dot/Icm effector. Cell. Microbiol. 2015, 17, 767–784. [Google Scholar] [CrossRef] [PubMed]
- Delevoye, C.; Nilges, M.; Dehoux, P.; Paumet, F.; Perrinet, S.; Dautry-Varsat, A.; Subtil, A. SNARE protein mimicry by an intracellular bacterium. PLoS Pathog. 2008, 4, e1000022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paumet, F.; Wesolowski, J.; Garcia-Diaz, A.; Delevoye, C.; Aulner, N.; Shuman, H.A.; Subtil, A.; Rothman, J.E. Intracellular bacteria encode inhibitory SNARE-like proteins. PLoS ONE 2009, 4, e7375. [Google Scholar] [CrossRef]
- Ma, M.; Bong, D. Controlled Fusion of Synthetic Lipid Membrane Vesicles. Acc. Chem. Res. 2013, 46, 2988–2997. [Google Scholar] [CrossRef]
- Sadek, M.; Berndt, D.; Milovanovic, D.; Jahn, R.; Diederichsen, U. Distance Regulated Vesicle Fusion and Docking Mediated by β-Peptide Nucleic Acid SNARE Protein Analogues. Chembiochem 2016, 17, 479–485. [Google Scholar] [CrossRef]
- Hubrich, B.E.; Kumar, P.; Neitz, H.; Grunwald, M.; Grothe, T.; Walla, P.J.; Jahn, R.; Diederichsen, U. PNA Hybrid Sequences as Recognition Units in SNARE-Protein-Mimicking Peptides. Angew. Chem. Int. Ed. Engl. 2018, 57, 14932–14936. [Google Scholar] [CrossRef]
- Lygina, A.S.; Meyenberg, K.; Jahn, R.; Diederichsen, U. Transmembrane domain peptide/peptide nucleic acid hybrid as a model of a SNARE protein in vesicle fusion. Angew. Chem. Int. Ed. Engl. 2011, 50, 8597–8601. [Google Scholar] [CrossRef]
- Langosch, D.; Crane, J.M.; Brosig, B.; Hellwig, A.; Tamm, L.K.; Reed, J. Peptide mimics of SNARE transmembrane segments drive membrane fusion depending on their conformational plasticity. J. Mol. Biol. 2001, 311, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Ollesch, J.; Poschner, B.C.; Nikolaus, J.; Hofmann, M.W.; Herrmann, A.; Gerwert, K.; Langosch, D. Secondary structure and distribution of fusogenic LV-peptides in lipid membranes. Eur. Biophys. J. 2008, 37, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Litowski, J.R.; Hodges, R.S. Designing heterodimeric two-stranded α-helical coiled-coils. Effects of hydrophobicity and α-helical propensity on protein folding, stability, and specificity. J. Biol. Chem. 2002, 277, 37272–37279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robson Marsden, H.; Elbers, N.A.; Bomans, P.H.; Sommerdijk, N.A.; Kros, A. A reduced SNARE model for membrane fusion. Angew. Chem. Int. Ed. Engl. 2009, 48, 2330–2333. [Google Scholar] [CrossRef]
- Versluis, F.; Dominguez, J.; Voskuhl, J.; Kros, A. Coiled-coil driven membrane fusion: Zipper-like vs. non-zipper-like peptide orientation. Faraday. Discuss. 2013, 166, 349–359. [Google Scholar] [CrossRef]
- Pähler, G.; Panse, C.; Diederichsen, U.; Janshoff, A. Coiled-coil formation on lipid bilayers--implications for docking and fusion efficiency. Biophys. J. 2012, 103, 2295–2303. [Google Scholar] [CrossRef] [Green Version]
- Rabe, M.; Aisenbrey, C.; Pluhackova, K.; de Wert, V.; Boyle, A.L.; Bruggeman, D.F.; Kirsch, S.A.; Böckmann, R.A.; Kros, A.; Raap, J.; et al. A Coiled-Coil Peptide Shaping Lipid Bilayers upon Fusion. Biophys. J. 2016, 111, 2162–2175. [Google Scholar] [CrossRef] [Green Version]
- Koukalová, A.; Pokorná, Š.; Boyle, A.L.; Lopez Mora, N.; Kros, A.; Hof, M.; Šachl, R. Distinct roles of SNARE-mimicking lipopeptides during initial steps of membrane fusion. Nanoscale 2018, 10, 19064–19073. [Google Scholar] [CrossRef] [Green Version]
- Wagle, S.; Georgiev, V.N.; Robinson, T.; Dimova, R.; Lipowsky, R.; Grafmüller, A. Interaction of SNARE Mimetic Peptides with Lipid bilayers: Effects of Secondary Structure, Bilayer Composition and Lipid Anchoring. Sci. Rep. 2019, 9, 7708. [Google Scholar] [CrossRef] [Green Version]
- Marsden, H.R.; Korobko, A.V.; Zheng, T.; Voskuhl, J.; Kros, A. Controlled liposome fusion mediated by SNARE protein mimics. Biomater. Sci. 2013, 1, 1046–1054. [Google Scholar] [CrossRef]
- Zheng, T.; Voskuhl, J.; Versluis, F.; Zope, H.R.; Tomatsu, I.; Marsden, H.R.; Kros, A. Controlling the rate of coiled coil driven membrane fusion. Chem. Commun. 2013, 49, 3649–3651. [Google Scholar] [CrossRef] [PubMed]
- Meyenberg, K.; Lygina, A.S.; van den Bogaart, G.; Jahn, R.; Diederichsen, U. SNARE derived peptide mimic inducing membrane fusion. Chem. Commun. 2011, 47, 9405–9407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daudey, G.A.; Schwieger, C.; Rabe, M.; Kros, A. Influence of Membrane-Fusogen Distance on the Secondary Structure of Fusogenic Coiled Coil Peptides. Langmuir 2019, 35, 5501–5508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crone, N.S.A.; Kros, A.; Boyle, A.L. Modulation of Coiled-Coil Binding Strength and Fusogenicity through Peptide Stapling. Bioconjug. Chem. 2020, 31, 834–843. [Google Scholar] [CrossRef]
- Versluis, F.; Voskuhl, J.; van Kolck, B.; Zope, H.; Bremmer, M.; Albregtse, T.; Kros, A. In Situ Modification of Plain Liposomes with Lipidated Coiled Coil Forming Peptides Induces Membrane Fusion. J. Am. Chem. Soc. 2013, 135, 8057–8062. [Google Scholar] [CrossRef] [PubMed]
- Mora, N.L.; Boyle, A.L.; Kolck, B.J.V.; Rossen, A.; Pokorná, Š.; Koukalová, A.; Šachl, R.; Hof, M.; Kros, A. Controlled Peptide-Mediated Vesicle Fusion Assessed by Simultaneous Dual-Colour Time-Lapsed Fluorescence Microscopy. Sci. Rep. 2020, 10, 3087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mora, N.L.; Bahreman, A.; Valkenier, H.; Li, H.; Sharp, T.H.; Sheppard, D.N.; Davis, A.P.; Kros, A. Targeted anion transporter delivery by coiled-coil driven membrane fusion. Chem. Sci. 2016, 7, 1768–1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daudey, G.A.; Shen, M.; Singhal, A.; van der Est, P.; Sevink, G.J.A.; Boyle, A.L.; Kros, A. Liposome fusion with orthogonal coiled coil peptides as fusogens: The efficacy of roleplaying peptides. Chem. Sci. 2021, 12, 13782–13792. [Google Scholar] [CrossRef] [PubMed]
- Fasshauer, D.; Margittai, M. A transient N-terminal interaction of SNAP-25 and syntaxin nucleates SNARE assembly. J. Biol. Chem. 2004, 279, 7613–7621. [Google Scholar] [CrossRef] [Green Version]
- Pobbati, A.V.; Stein, A.; Fasshauer, D. N- to C-terminal SNARE complex assembly promotes rapid membrane fusion. Science 2006, 313, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Südhof, T.C. Neurotransmitter release: The last millisecond in the life of a synaptic vesicle. Neuron 2013, 80, 675–690. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Rebane, A.A.; Ma, L.; Li, F.; Jiao, J.; Qu, H.; Pincet, F.; Rothman, J.E.; Zhang, Y. Stability, folding dynamics, and long-range conformational transition of the synaptic t-SNARE complex. Proc. Natl. Acad. Sci. USA 2016, 113, E8031–E8040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darios, F.; Niranjan, D.; Ferrari, E.; Zhang, F.; Soloviev, M.; Rummel, A.; Bigalke, H.; Suckling, J.; Ushkaryov, Y.; Naumenko, N.; et al. SNARE tagging allows stepwise assembly of a multimodular medicinal toxin. Proc. Natl. Acad. Sci. USA 2010, 107, 18197–18201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, E.; Gu, C.; Niranjan, D.; Restani, L.; Rasetti-Escargueil, C.; Obara, I.; Geranton, S.M.; Arsenault, J.; Goetze, T.A.; Harper, C.B.; et al. Synthetic self-assembling clostridial chimera for modulation of sensory functions. Bioconjug. Chem. 2013, 24, 1750–1759. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, E.; Maywood, E.S.; Restani, L.; Caleo, M.; Pirazzini, M.; Rossetto, O.; Hastings, M.H.; Niranjan, D.; Schiavo, G.; Davletov, B. Re-assembled botulinum neurotoxin inhibits CNS functions without systemic toxicity. Toxins 2011, 3, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Mangione, A.S.; Obara, I.; Maiarú, M.; Geranton, S.M.; Tassorelli, C.; Ferrari, E.; Leese, C.; Davletov, B.; Hunt, S.P. Nonparalytic botulinum molecules for the control of pain. Pain 2016, 157, 1045–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, E.; Soloviev, M.; Niranjan, D.; Arsenault, J.; Gu, C.; Vallis, Y.; O’Brien, J.; Davletov, B. Assembly of protein building blocks using a short synthetic peptide. Bioconjug. Chem. 2012, 23, 479–484. [Google Scholar] [CrossRef]
- Saccardo, A.; Soloviev, M.; Ferrari, E. A thermo-responsive, self-assembling biointerface for on demand release of surface-immobilised proteins. Biomater. Sci. 2020, 8, 2673–2681. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khvotchev, M.; Soloviev, M. SNARE Modulators and SNARE Mimetic Peptides. Biomolecules 2022, 12, 1779. https://doi.org/10.3390/biom12121779
Khvotchev M, Soloviev M. SNARE Modulators and SNARE Mimetic Peptides. Biomolecules. 2022; 12(12):1779. https://doi.org/10.3390/biom12121779
Chicago/Turabian StyleKhvotchev, Mikhail, and Mikhail Soloviev. 2022. "SNARE Modulators and SNARE Mimetic Peptides" Biomolecules 12, no. 12: 1779. https://doi.org/10.3390/biom12121779
APA StyleKhvotchev, M., & Soloviev, M. (2022). SNARE Modulators and SNARE Mimetic Peptides. Biomolecules, 12(12), 1779. https://doi.org/10.3390/biom12121779






