Macrophage-Conditioned Media Promotes Adipocyte Cancer Association, Which in Turn Stimulates Breast Cancer Proliferation and Migration
Abstract
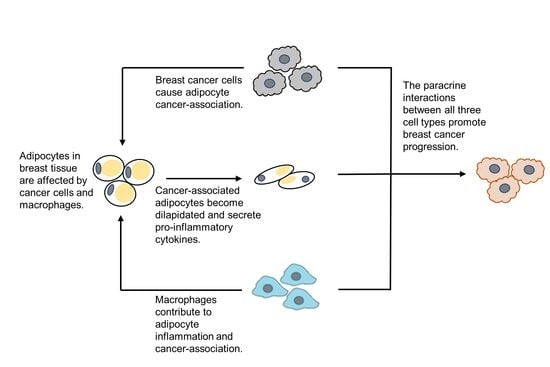
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Differentiation of hMSCs into Adipocytes
2.3. Differentiation of U937 Cells into Macrophages
2.4. Macrophage-Conditioned Media (CM) Preparation
2.5. Breast Cancer–Adipocyte Co-Culture
2.6. Breast Cancer Cell Counts
2.7. Wound Healing Assay
2.8. Real-Time Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR)
2.9. Statistical Analysis
3. Results
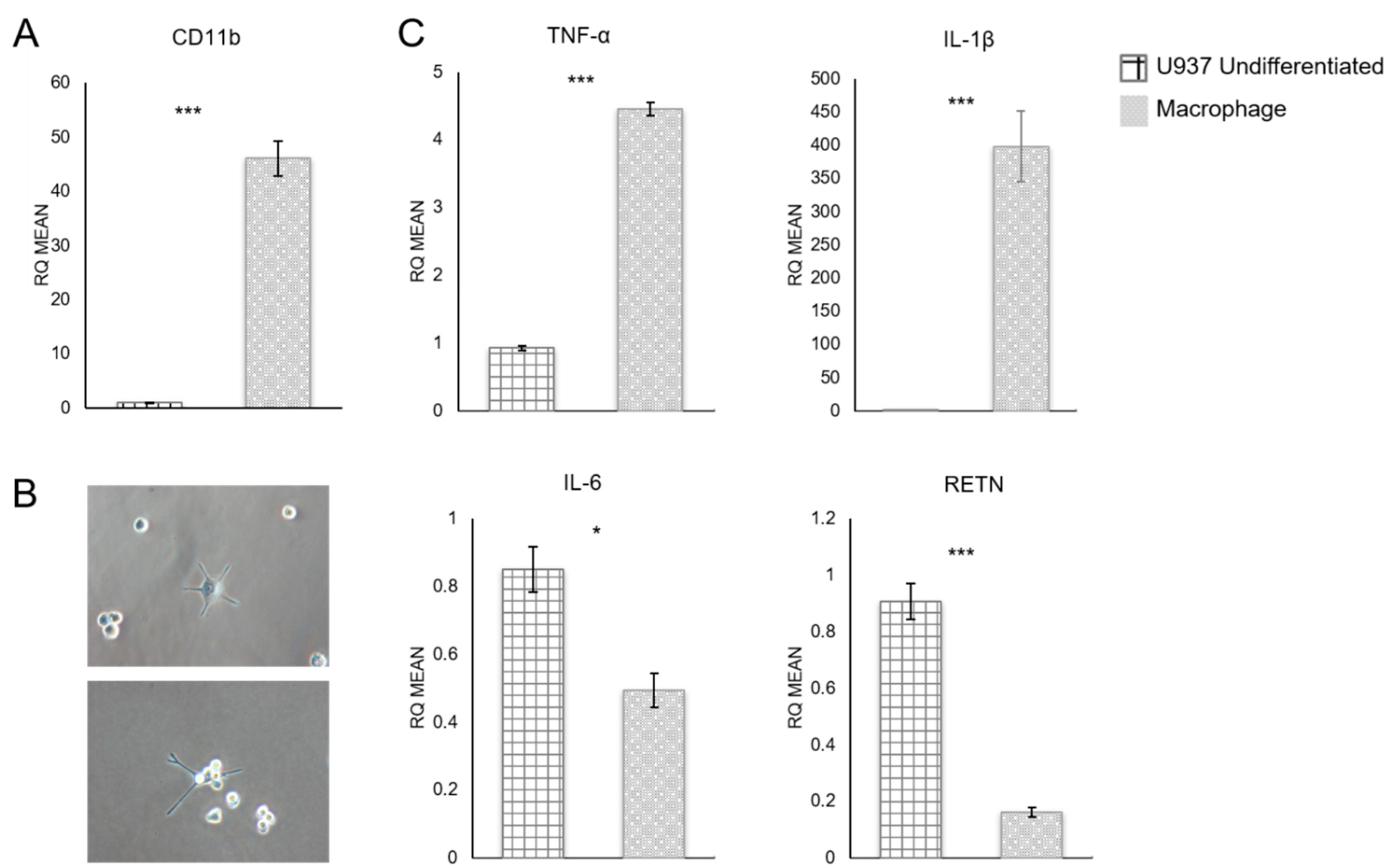
3.1. Quantitative RT-PCR Analysis of U937 Monocyte-Derived Macrophages
3.2. Breast Cancer Cell Proliferation and Migration
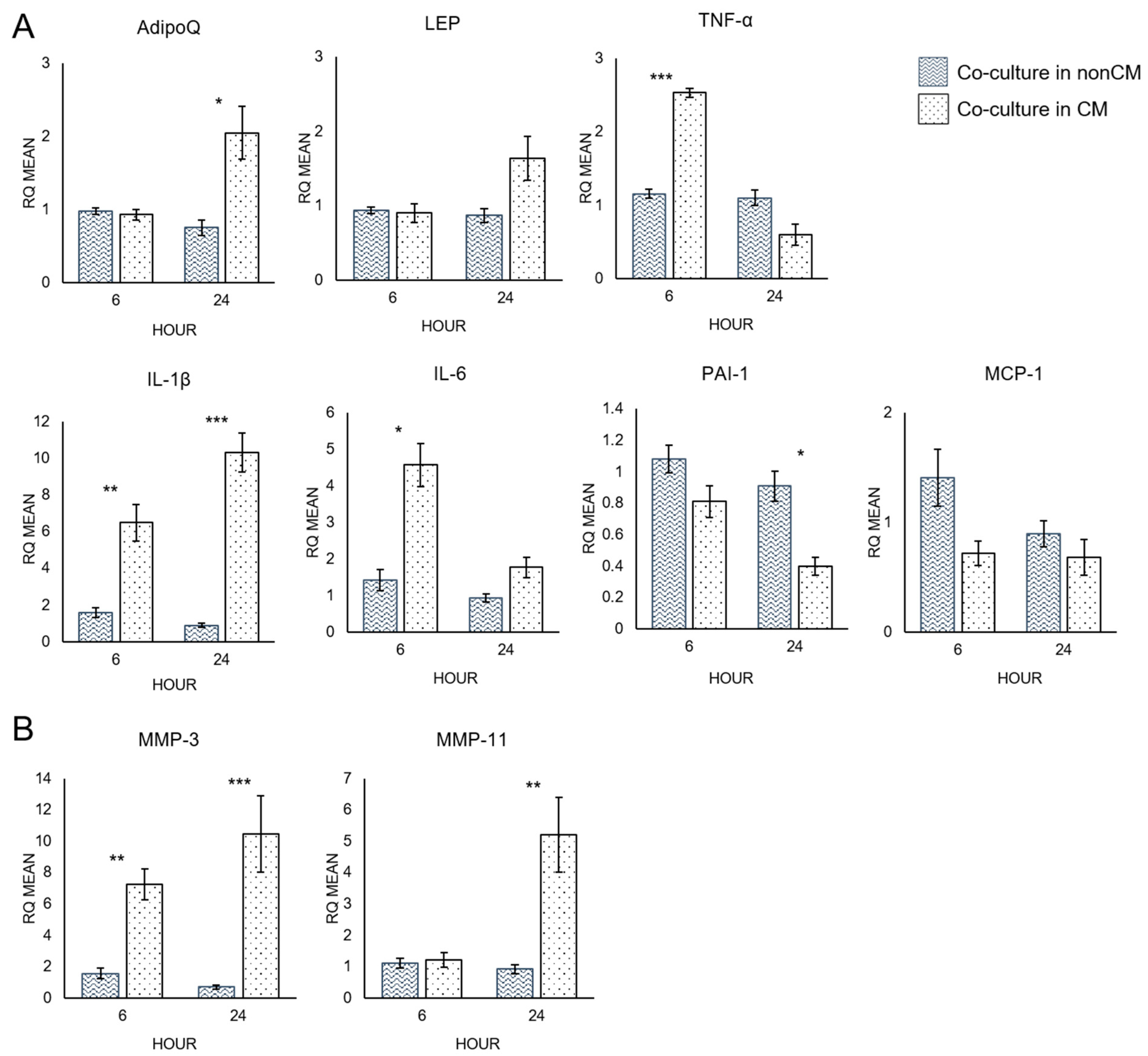
3.3. Quantitative RT-PCR Analysis of Adipocytes
4. Discussion
4.1. Macrophage-Conditioned Media Has Varying Effectiveness upon Different Types of Breast Cancer Cells
4.2. Macrophage-Conditioned Media Increases the Pro-Inflammatory Character of Adipocytes
4.3. Macrophage-Conditioned Media Increases Matrix Metalloproteinase Expression in Adipocytes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α-MEM | Minimum Essential Medium: Eagle Alpha Modification |
| AdipoQ | Adiponectin |
| BRCA | Breast cancer |
| CAA | Cancer-associated adipocytes |
| CD11b | Cluster of differentiation molecule 11B |
| CM | Conditioned media |
| ECM | Extracellular matrix |
| FBS | Fetal bovine serum |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| HMSCs | Human mesenchymal stem cells |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| LEP | Leptin |
| MMP-3 | Matrix metalloproteinase-3 |
| MMP-11 | Matrix metalloproteinase-11 |
| NonCM | Non-conditioned media |
| PAI-1 | Plasminogen activator inhibitor-1 |
| PMA | Phorbol myristate acetate |
| RETN | Resistin |
| RQ | Relative quantification |
| TNF-α | Tumor necrosis factor-α |
References
- World Health Organization. Obesity and Overweight—Fact Sheet (Updated February 2018). Available online: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 15 April 2019).
- Stierman, B.; Afful, J.; Carroll, M.D.; Chen, T.-C.; Davy, O.; Fink, S.; Fryar, C.D.; Gu, Q.; Hales, C.M.; Hughes, J.P.; et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2021.
- Bhaskaran, K.; Douglas, I.; Forbes, H.; dos-Santos-Silva, I.; Leon, D.A.; Smeeth, L. Body-mass index and risk of 22 specific cancers: A population-based cohort study of 5·24 million UK adults. Lancet 2014, 384, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Blair, C.K.; Wiggins, C.L.; Nibbe, A.M.; Storlie, C.B.; Prossnitz, E.R.; Royce, M.; Lomo, L.C.; Hill, D.A. Obesity and survival among a cohort of breast cancer patients is partially mediated by tumor characteristics. npj Breast Cancer 2019, 5, 33. [Google Scholar] [CrossRef]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of U.S. Adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef]
- Cao, Y.; Ma, J. Body Mass Index, Prostate Cancer–Specific Mortality, and Biochemical Recurrence: A Systematic Review and Meta-analysis. Cancer Prev. Res. 2011, 4, 486–501. [Google Scholar] [CrossRef]
- Dirat, B.; Bochet, L.; Dabek, M.; Daviaud, D.; Dauvillier, S.; Majed, B.; Wang, Y.Y.; Meulle, A.; Salles, B.; Le Gonidec, S.; et al. Cancer-Associated Adipocytes Exhibit an Activated Phenotype and Contribute to Breast Cancer Invasion. Cancer Res. 2011, 71, 2455–2465. [Google Scholar] [CrossRef]
- Duong, M.N.; Geneste, A.; Fallone, F.; Li, X.; Dumontet, C.; Muller, C. The fat and the bad: Mature adipocytes, key actors in tumor progression and resistance. Oncotarget 2017, 8, 57622–57641. [Google Scholar] [CrossRef]
- Carter, J.C.; Church, F.C. Mature breast adipocytes promote breast cancer cell motility. Exp. Mol. Pathol. 2012, 92, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Suganami, T.; Ogawa, Y. Adipose tissue macrophages: Their role in adipose tissue remodeling. J. Leukoc. Biol. 2010, 88, 33–39. [Google Scholar] [CrossRef]
- Qiu, S.Q.; Waaijer, S.J.; Zwager, M.C.; de Vries, E.G.; van der Vegt, B.; Schröder, C.P. Tumor-associated macrophages in breast cancer: Innocent bystander or important player? Cancer Treat. Rev. 2018, 70, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef]
- Sekiya, I.; Larson, B.L.; Vuoristo, J.T.; Cui, J.-G.; Prockop, D.J. Adipogenic Differentiation of Human Adult Stem Cells From Bone Marrow Stroma (MSCs). J. Bone Miner. Res. 2003, 19, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Bosco, D.B.; Kenworthy, R.; Zorio, D.A.R.; Sang, Q.-X.A. Human Mesenchymal Stem Cells Are Resistant to Paclitaxel by Adopting a Non-Proliferative Fibroblastic State. PLoS ONE 2015, 10, e0128511. [Google Scholar] [CrossRef] [PubMed]
- Sintiprungrat, K.; Singhto, N.; Sinchaikul, S.; Chen, S.-T.; Thongboonkerd, V. Alterations in cellular proteome and secretome upon differentiation from monocyte to macrophage by treatment with phorbol myristate acetate: Insights into biological processes. J. Proteom. 2010, 73, 602–618. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-G.; Ryoo, I.-G.; Choi, H.-Y.; Choi, B.-H.; Kim, S.-T.; Heo, T.-H.; Lee, J.Y.; Park, P.-H.; Kwak, M.-K. NRF2 Signaling Negatively Regulates Phorbol-12-Myristate-13-Acetate (PMA)-Induced Differentiation of Human Monocytic U937 Cells into Pro-Inflammatory Macrophages. PLoS ONE 2015, 10, e0134235. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Andarawewa, K.L.; Motrescu, E.R.; Chenard, M.-P.; Gansmuller, A.; Stoll, I.; Tomasetto, C.; Rio, M.-C. Stromelysin-3 Is a Potent Negative Regulator of Adipogenesis Participating to Cancer Cell-Adipocyte Interaction/Crosstalk at the Tumor Invasive Front. Cancer Res. 2005, 65, 10862–10871. [Google Scholar] [CrossRef]
- Lijnen, H.R.; Van, H.B.; Frederix, L.; Rio, M.C.; Collen, D. Adipocyte hypertrophy in stromelysin-3 deficient mice with nutritionally induced obesity. Thromb. Haemost. 2002, 87, 530–535. [Google Scholar]
- Maquoi, E.; Demeulemeester, D.; Vörös, G.; Collen, D.; Lijnen, H.R. Enhanced nutritionally induced adipose tissue development in mice with stromelysin-1 gene inactivation. Thromb. Haemost. 2003, 89, 696–704. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Lehuédé, C.; Laurent, V.; Dirat, B.; Dauvillier, S.; Bochet, L.; Le Gonidec, S.; Escourrou, G.; Valet, P.; Muller, C. Adipose tissue and breast epithelial cells: A dangerous dynamic duo in breast cancer. Cancer Lett. 2012, 324, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Solinas, G.; Germano, G.; Mantovani, A.; Allavena, P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J. Leukoc. Biol. 2009, 86, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Subbaramaiah, K.; Howe, L.R.; Bhardwaj, P.; Du, B.; Gravaghi, C.; Yantiss, R.K.; Zhou, X.K.; Blaho, V.A.; Hla, T.; Yang, P.; et al. Obesity Is Associated with Inflammation and Elevated Aromatase Expression in the Mouse Mammary Gland. Cancer Prev. Res. 2011, 4, 329–346. [Google Scholar] [CrossRef]
- Permana, P.A.; Menge, C.; Reaven, P.D. Macrophage-secreted factors induce adipocyte inflammation and insulin resistance. Biochem. Biophys. Res. Commun. 2006, 341, 507–514. [Google Scholar] [CrossRef]
- Booth, A.; Magnuson, A.; Fouts, J.; Foster, M. Adipose tissue, obesity and adipokines: Role in cancer promotion. Horm. Mol. Biol. Clin. Investig. 2015, 21, 57–74. [Google Scholar] [CrossRef]
- Hauner, H.; Petruschke, T.; Russ, M.; Röhrig, K.; Eckel, J. Effects of tumour necrosis factor alpha (TNFα) on glucose transport and lipid metabolism of newly-differentiated human fat cells in cell culture. Diabetologia 1995, 38, 764–771. [Google Scholar] [CrossRef]
- Petruschke, T.; Hauner, H. Tumor necrosis factor-alpha prevents the differentiation of human adipocyte precursor cells and causes delipidation of newly developed fat cells. J. Clin. Endocrinol. Metab. 1993, 76, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Hanby, A.; Dublin, E.; Poulsom, R.; Smith, P.; Barnes, D.; Rubens, R.; Anglard, P.; Hart, I. Stromelysin 3: An independent prognostic factor for relapse-free survival in node-positive breast cancer and demonstration of novel breast carcinoma cell expression. Am. J. Pathol. 1998, 152, 721–728. [Google Scholar]



| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| CD 11b | 5′-CTTCCAGGTTCTGGCTCCTTC-3′ | 5′-TCTCTTGGAAGGTCATTGCGTT-3′ |
| MCP-1 | 5′-TCTCAAACTGAAGCTCGCACT-3′ | 5′-GGGAATGAAGGTGGCTGCTA-3′ |
| IL-6 | 5′-CCTGCAGAAAAAGGCAAAGAATC-3′ | 5′-GAGTTGTCATGTCCTGCAGCC-3′ |
| RETN | 5′-TGCAGGATGAAAGCTCTCTGTCTC-3′ | 5′-CCTGGATCCTCTCATTGATGGC-3′ |
| TNF-α | 5′-TGGGATCATTGCCCTGTGAG-3′ | 5′-GGTGTCTGAAGGAGGGGGTA-3′ |
| IL-1β | 5′-CAGGCTGCTCTGGGATTCTC-3′ | 5′-AAGTCATCCTCATTGCCACTGT-3′ |
| AdipoQ | 5′-ATGGCCCCTGCACTACTCTA-3′ | 5′-CAGGGATGAGTTCGGCACTT-3′ |
| LEP | 5′-CCCTGGAGTGCAGTTTCCAA-3′ | 5′-TGCTCAGATGAACCCAACCC-3′ |
| PAI-1 | 5′-TTGCAGGATGGAACTACGGG-3′ | 5′-GTGGCAGGCAGTACAAGAGTGA-3′ |
| MMP-3 | 5′-CCATCTCTTCCTTCAGGCGT-3′ | 5′-ATGCCTCTTGGGTATCCAGC-3′ |
| MMP-11 | 5′-ATGAATTTGGCCACGTGCTG-3′ | 5′-CGAAAGGTGTAGAAGGCGGA-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vallega, K.A.; Bosco, D.B.; Ren, Y.; Sang, Q.-X.A. Macrophage-Conditioned Media Promotes Adipocyte Cancer Association, Which in Turn Stimulates Breast Cancer Proliferation and Migration. Biomolecules 2022, 12, 1757. https://doi.org/10.3390/biom12121757
Vallega KA, Bosco DB, Ren Y, Sang Q-XA. Macrophage-Conditioned Media Promotes Adipocyte Cancer Association, Which in Turn Stimulates Breast Cancer Proliferation and Migration. Biomolecules. 2022; 12(12):1757. https://doi.org/10.3390/biom12121757
Chicago/Turabian StyleVallega, Karin A., Dale B. Bosco, Yi Ren, and Qing-Xiang Amy Sang. 2022. "Macrophage-Conditioned Media Promotes Adipocyte Cancer Association, Which in Turn Stimulates Breast Cancer Proliferation and Migration" Biomolecules 12, no. 12: 1757. https://doi.org/10.3390/biom12121757
APA StyleVallega, K. A., Bosco, D. B., Ren, Y., & Sang, Q.-X. A. (2022). Macrophage-Conditioned Media Promotes Adipocyte Cancer Association, Which in Turn Stimulates Breast Cancer Proliferation and Migration. Biomolecules, 12(12), 1757. https://doi.org/10.3390/biom12121757