Gαs-Coupled CGRP Receptor Signaling Axis from the Trigeminal Ganglion Neuron to Odontoblast Negatively Regulates Dentin Mineralization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Solutions and Reagents
2.3. Dental Pulp Slice Preparation
2.4. Isolation of the TG Neurons
2.5. Immunofluorescence Analysis
2.6. Preparation of the Odontoblast–TG Neuron Co-Culture
2.7. Measurements of Intracellular cAMP- and/or Ca2+-Sensitive Dye Fluorescence
2.8. Mechanical Stimulation of the Single TG Neurons
2.9. Measurement of the Intercellular Distance and Size of the Stimulated TG Neurons
2.10. Mineralization Assay
2.11. Statistical Analysis
3. Results
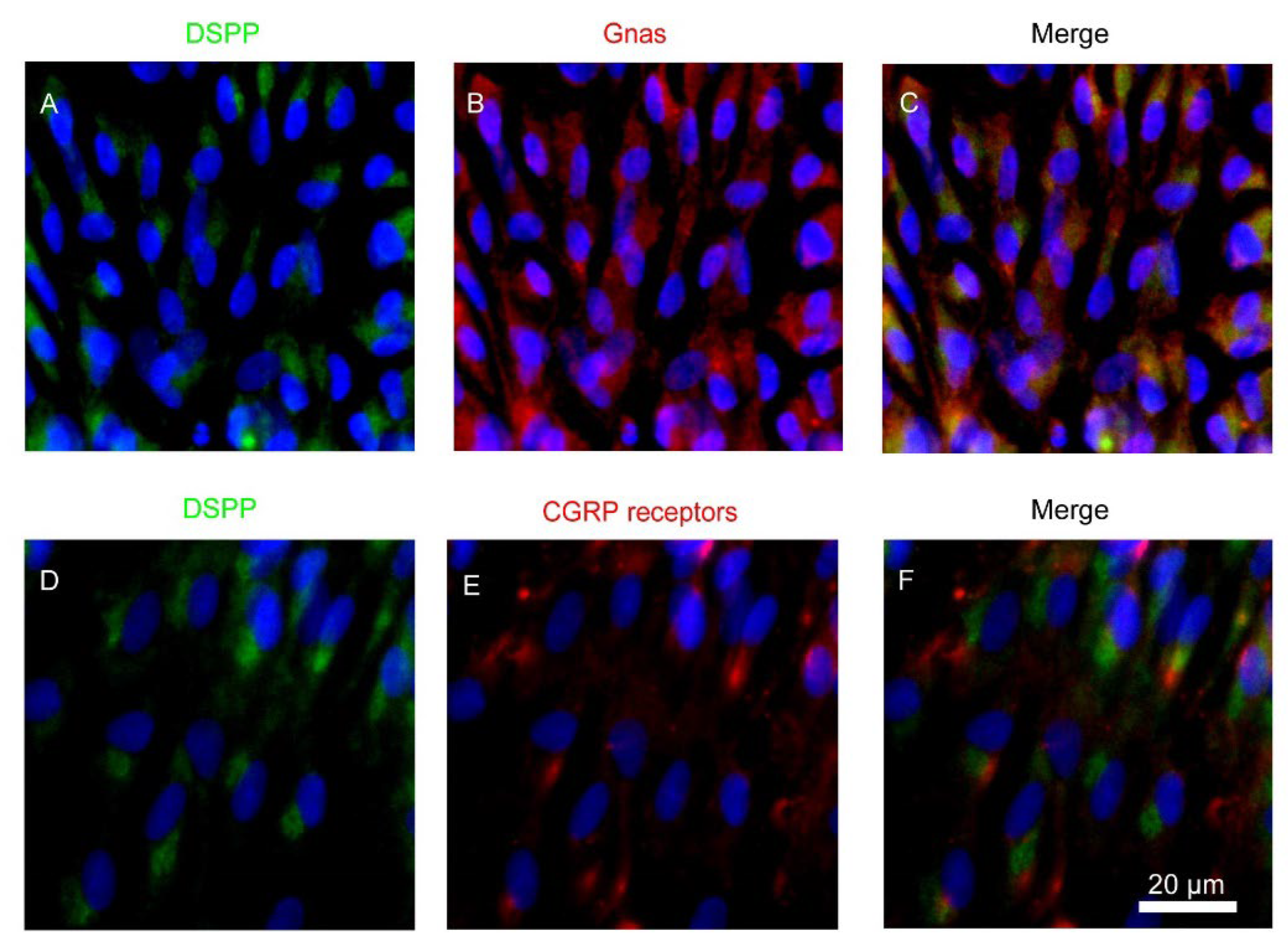
3.1. Expression of Heterotrimeric Gnas and CALCRL in Rat Odontoblasts
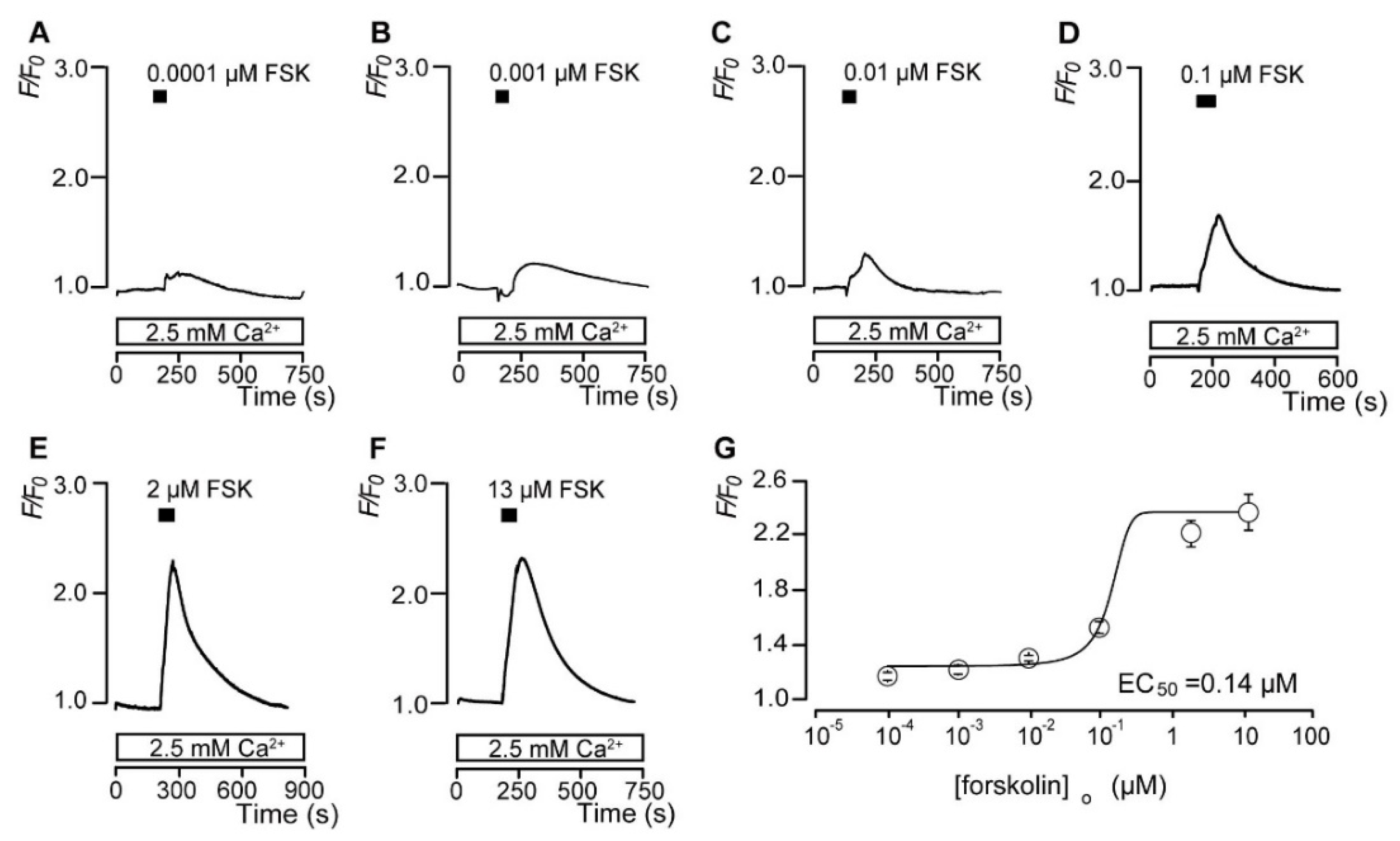
3.2. Forskolin Dose-Dependently Increases the Intracellular cAMP Level
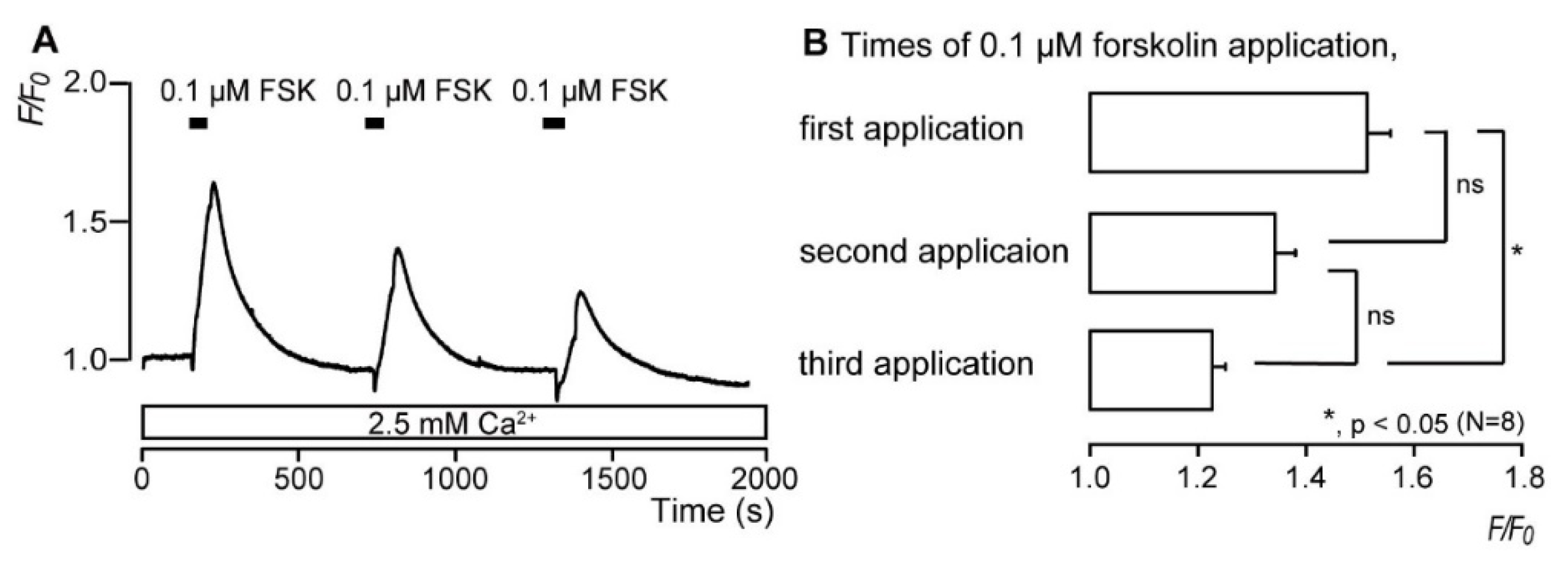
3.3. Repeated Application of Forskolin Has a Desensitizing Effect on the Increase in Intracellular cAMP Level
3.4. CGRP Increases the Intracellular cAMP Level
3.5. Direct Mechanical Stimulation of the TG Neurons Simultaneously Increases the [Ca2+]i in the Neurons and the [cAMP]i in the Odontoblasts Approximating the Stimulated Neurons
3.6. CGRP–CGRP Receptor Signaling Regulates Defensive Dentin Demineralization
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hilger, D.; Masureel, M.; Kobilka, B.K. Structure and dynamics of GPCR signaling complexes. Nat. Struct. Mol. Biol. 2018, 25, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Elnagdy, M.; Barve, S.; McClain, C.; Gobejishvili, L. CAMP signaling in pathobiology of alcohol associated liver disease. Biomolecules 2020, 10, 1433. [Google Scholar] [CrossRef]
- Qasim, H.; McConnell, B.K. AKAP12 Signaling Complex: Impacts of Compartmentalizing cAMP-Dependent Signaling Pathways in the Heart and Various Signaling Systems. J. Am. Heart Assoc. 2020, 9, e016615. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.K.; Kim, S. An insight into GPCR and G-proteins as cancer drivers. Cells 2021, 10, 3288. [Google Scholar] [CrossRef] [PubMed]
- Russell, F.A.; King, R.; Smillie, S.J.; Kodji, X.; Brain, S.D. Calcitonin gene-related peptide: Physiology and pathophysiology. Physiol. Rev. 2014, 94, 1099–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamuta, H.; Fukuda, Y.; Koida, M.; Fujii, N.; Otaka, A.; Funakoshi, S.; Yajima, H.; Mitsuyasu, N.; Orlowski, R.C. Binding sites of calcitonin gene-related peptide (CGRP): Abundant occurrence in visceral organs. Jpn. J. Pharmacol. 1986, 42, 175–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caviedes-Bucheli, J.; Muñoz, H.R.; Azuero-Holguín, M.M.; Ulate, E. Neuropeptides in dental pulp: The silent protagonists. J. Endod. 2008, 34, 773–788. [Google Scholar] [CrossRef] [PubMed]
- Taylor, F.R. CGRP, Amylin, immunology, and headache medicine. Headache 2019, 59, 131–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S. Neurovascular interactions in the dental pulp in health and inflammation. J. Endod. 1990, 16, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Berggreen, E.; Heyeraas, K.J. Effect of the sensory neuropeptide antagonists h-CGRP(8–37) and SR 140.33 on pulpal and gingival blood flow in ferrets. Arch. Oral Biol. 2000, 45, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Michot, B.; Casey, S.M.; Gibbs, J.L. Effects of calcitonin gene-related peptide on dental pulp stem cell viability, proliferation, and differentiation. J. Endod. 2020, 46, 950–956. [Google Scholar] [CrossRef]
- Shibukawa, Y.; Sato, M.; Kimura, M.; Sobhan, U.; Shimada, M.; Nishiyama, A.; Kawaguchi, A.; Soya, M.; Kuroda, H.; Katakura, A.; et al. Odontoblasts as sensory receptors: Transient receptor potential channels, pannexin-1, and ionotropic ATP receptors mediate intercellular odontoblast-neuron signal transduction. Pflug. Arch. 2015, 467, 843–863. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Ogura, K.; Kimura, M.; Nishi, K.; Ando, M.; Tazaki, M.; Shibukawa, Y. Activation of mechanosensitive transient receptor potential/piezo channels in odontoblasts generates action potentials in cocultured isolectin B4–negative medium-sized trigeminal ganglion neurons. J. Endod. 2018, 44, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Seamon, K.B.; Padgett, W.; Daly, J.W. Forskolin: Unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc. Natl. Acad. Sci. USA 1981, 78, 3363–3367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avanzato, D.; Genova, T.; Fiorio Pla, A.; Bernardini, M.; Bianco, S.; Bussolati, B.; Mancardi, D.; Giraudo, E.; Maione, F.; Cassoni, P.; et al. Activation of P2X7 and P2Y11 purinergic receptors inhibits migration and normalizes tumor-derived endothelial cells via cAMP signaling. Sci. Rep. 2016, 6, 32602. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, Y.; Pan, Z.; Huang, Z.; Chen, F. CAMP regulates 11β-Hydroxysteroid Dehydrogenase-2 and Sp1 expression in MLO-Y4/MC3T3-E1 cells. Exp. Ther. Med. 2020, 20, 2166–2172. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Wang, D.; Luo, E.; Yan, G.; Liu, B.; Hou, J.; Qiao, Y. Activation of inward rectifier K+ Channel 2.1 by PDGF-BB in rat vascular smooth muscle cells through protein kinase A. BioMed Res. Int. 2020, 2020, 4370832. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Shimatsu, A.; Murabe, H.; Mizuta, H.; Ihara, C.; Nakao, K. Calcitonin gene-related peptide as a GH secretagogue in human and rat pituitary somatotrophs. Brain Res. 1998, 807, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Doods, H.; Hallermayer, G.; Wu, D.; Entzeroth, M.; Rudolf, K.; Engel, W.; Eberlein, W. Pharmacological profile of BIBN4096BS, the first selective small molecule CGRP antagonist. Br. J. Pharmacol. 2000, 129, 420–423. [Google Scholar] [CrossRef] [Green Version]
- Shibukawa, Y.; Suzuki, T. Measurements of Cytosolic Free Ca2+ Concentrations in Odontoblasts. Bull. Tokyo Dent. Coll. 1997, 38, 177–185. [Google Scholar]
- Shibukawa, Y.; Suzuki, T. Ca2+ signaling mediated by IP3-dependent Ca2+ releasing and store-operated Ca2+ channels in rat odontoblasts. J. Bone Miner. Res. 2003, 18, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Nishi, K.; Higashikawa, A.; Ohyama, S.; Sakurai, K.; Tazaki, M.; Shibukawa, Y. High pH-sensitive store-operated Ca2+ entry mediated by Ca2+ release-activated Ca2+ channels in rat odontoblasts. Front. Physiol. 2018, 9, 443. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, H.; Shibukawa, Y.; Soya, M.; Masamura, A.; Kasahara, M.; Tazaki, M.; Ichinohe, T. Expression of P2X1 and P2X4 receptors in rat trigeminal ganglion neurons. Neuroreport 2012, 23, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, A.; Sato, M.; Kimura, M.; Ichinohe, T.; Tazaki, M.; Shibukawa, Y. Expression and function of purinergic P2Y12 receptors in rat trigeminal ganglion neurons. Neurosci. Res. 2015, 98, 17–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawaguchi, A.; Sato, M.; Kimura, M.; Yamazaki, T.; Yamamoto, H.; Tazaki, M.; Ichinohe, T.; Shibukawa, Y. Functional expression of bradykinin B1 and B2 receptors in neonatal rat trigeminal ganglion neurons. Front. Cell Neurosci. 2015, 9, 229. [Google Scholar] [CrossRef] [Green Version]
- Nishiyama, A.; Sato, M.; Kimura, M.; Katakura, A.; Tazaki, M.; Shibukawa, Y. Intercellular signal communication among odontoblasts and trigeminal ganglion neurons via glutamate. Cell Calcium 2016, 60, 341–355. [Google Scholar] [CrossRef]
- Krivanek, J.; Soldatov, R.A.; Kastriti, M.E.; Chontorotzea, T.; Herdina, A.N.; Petersen, J.; Szarowska, B.; Landova, M.; Matejova, V.K.; Holla, L.I.; et al. Dental Cell Type Atlas Reveals Stem and Differentiated Cell Types in Mouse and Human Teeth. Nat. Commun. 2020, 11, 4816. [Google Scholar] [CrossRef]
- Won, J.; Vang, H.; Kim, J.H.; Lee, P.R.; Kang, Y.; Oh, S.B. TRPM7 Mediates Mechanosensitivity in Adult Rat Odontoblasts. J. Dent. Res. 2018, 97, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Vandevska-Radunovic, V.; Fristad, I.; Wimalawansa, S.J.; Kvinnsland, I.H. CGRP1 and NK1 receptors in postnatal, developing rat dental tissues. Eur. J. Oral Sci. 2003, 111, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ono, K.; Inenaga, K. Electrophysiological and chemical properties in subclassified acutely dissociated cells of rat trigeminal ganglion by current signatures. J. Neurophysiol. 2010, 104, 3451–3461. [Google Scholar] [CrossRef] [Green Version]
- Ruscheweyh, R.; Forsthuber, L.; Schoffnegger, D.; Sandkühler, J. Modification of classical neurochemical markers in identified primary afferent neurons with Abeta-, Adelta-, and C-fibers after chronic constriction injury in mice. J. Comp. Neurol. 2007, 502, 325–336. [Google Scholar] [CrossRef]
- Ceruti, S.; Villa, G.; Fumagalli, M.; Colombo, L.; Magni, G.; Zanardelli, M.; Fabbretti, E.; Verderio, C.; van den Maagdenberg, A.M.J.M.; Nistri, A.; et al. Calcitonin gene-related peptide-mediated enhancement of purinergic neuron/glia communication by the algogenic factor bradykinin in mouse trigeminal ganglia from wild-type and R192Q Cav2.1 knock-in mice: Implications for basic mechanisms of migraine pain. J. Neurosci. 2011, 31, 3638–3649. [Google Scholar] [CrossRef] [Green Version]
- Fehrenbacher, J.C.; Sun, X.X.; Locke, E.E.; Henry, M.A.; Hargreaves, K.M. Capsaicin-Evoked ICGRP Release from Human Dental Pulp: A Model System for the Study of Peripheral Neuropeptide Secretion in Normal Healthy Tissue. Pain 2009, 144, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Caviedes-Bucheli, J.; Arenas, N.; Guiza, O.; Moncada, N.A.; Moreno, G.C.; Diaz, E.; Munoz, H.R. Calcitonin Gene-Related Peptide Receptor Expression in Healthy and Inflamed Human Pulp Tissue. Int. Endod. J. 2005, 38, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Della Pietra, A.; Mikhailov, N.; Giniatullin, R. The Emerging Role of Mechanosensitive Piezo Channels in Migraine Pain. Int. J. Mol. Sci. 2020, 21, 696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Won, J.; Vang, H.; Lee, P.R.; Kim, Y.H.; Kim, H.W.; Kang, Y.; Oh, S.B. Piezo2 expression in mechanosensitive dental primary afferent neurons. J. Dent. Res. 2017, 96, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.R.; Michot, B.; Erdogan, O.; Ba, A.; Gibbs, J.L.; Yang, Y. CGRP and shh mediate the dental pulp cell response to neuron stimulation. J. Dent. Res. 2022, 101, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Villa, I.; Mrak, E.; Rubinacci, A.; Ravasi, F.; Guidobono, F. CGRP Inhibits Osteoprotegerin Production in Human Osteoblast-like Cells via CAMP/PKA-Dependent Pathway. Am. J. Physiol. Cell Physiol. 2006, 291, C529–C537. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.-H.; Zhang, X.-B.; Lu, Y.-B.; Hu, Y.-C.; Chen, X.-Y.; Yu, D.-C.; Shi, J.-T.; Yuan, W.-H.; Wang, J.; Zhou, H.-Y. Calcitonin Gene-Related Peptide and Brain-Derived Serotonin Are Related to Bone Loss in Ovariectomized Rats. Brain Res. Bull. 2021, 176, 85–92. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, N.; Kimura, M.; Ouchi, T.; Ichinohe, T.; Shibukawa, Y. Gαs-Coupled CGRP Receptor Signaling Axis from the Trigeminal Ganglion Neuron to Odontoblast Negatively Regulates Dentin Mineralization. Biomolecules 2022, 12, 1747. https://doi.org/10.3390/biom12121747
Saito N, Kimura M, Ouchi T, Ichinohe T, Shibukawa Y. Gαs-Coupled CGRP Receptor Signaling Axis from the Trigeminal Ganglion Neuron to Odontoblast Negatively Regulates Dentin Mineralization. Biomolecules. 2022; 12(12):1747. https://doi.org/10.3390/biom12121747
Chicago/Turabian StyleSaito, Natsuki, Maki Kimura, Takehito Ouchi, Tatsuya Ichinohe, and Yoshiyuki Shibukawa. 2022. "Gαs-Coupled CGRP Receptor Signaling Axis from the Trigeminal Ganglion Neuron to Odontoblast Negatively Regulates Dentin Mineralization" Biomolecules 12, no. 12: 1747. https://doi.org/10.3390/biom12121747
APA StyleSaito, N., Kimura, M., Ouchi, T., Ichinohe, T., & Shibukawa, Y. (2022). Gαs-Coupled CGRP Receptor Signaling Axis from the Trigeminal Ganglion Neuron to Odontoblast Negatively Regulates Dentin Mineralization. Biomolecules, 12(12), 1747. https://doi.org/10.3390/biom12121747





