Influence of Centrifugation and Shaking on the Self-Assembly of Lysozyme Fibrils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Solutions
2.2. Fibrilization
2.3. Dynamic Light Scattering (DLS)
2.4. Atomic Force Microscopy (AFM)
2.5. Fourier Transform Infrared Spectroscopy (FTIR)
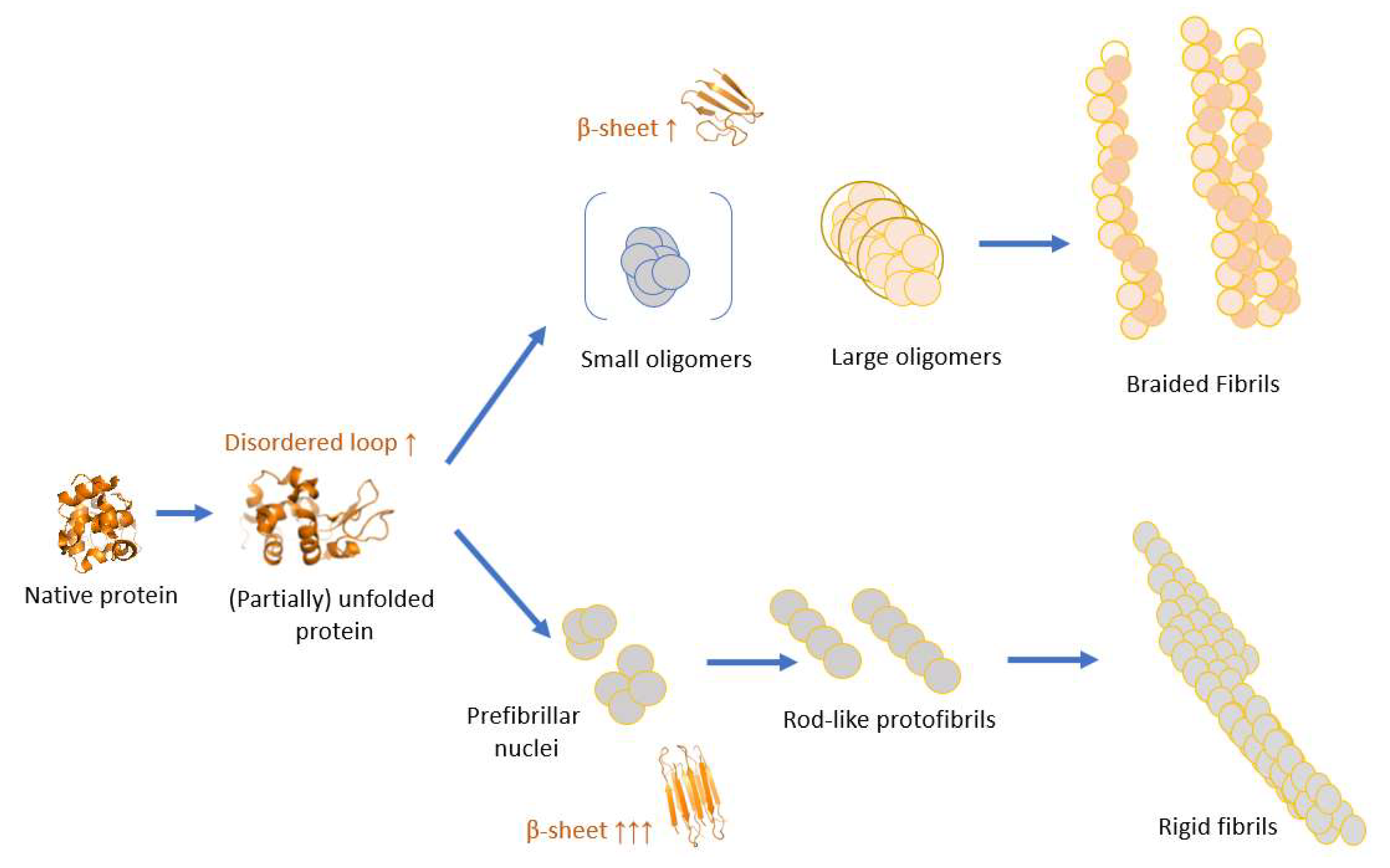
3. Results and Discussion
3.1. Shaking and an Increased Gravitational Field Triggers Fibrilization
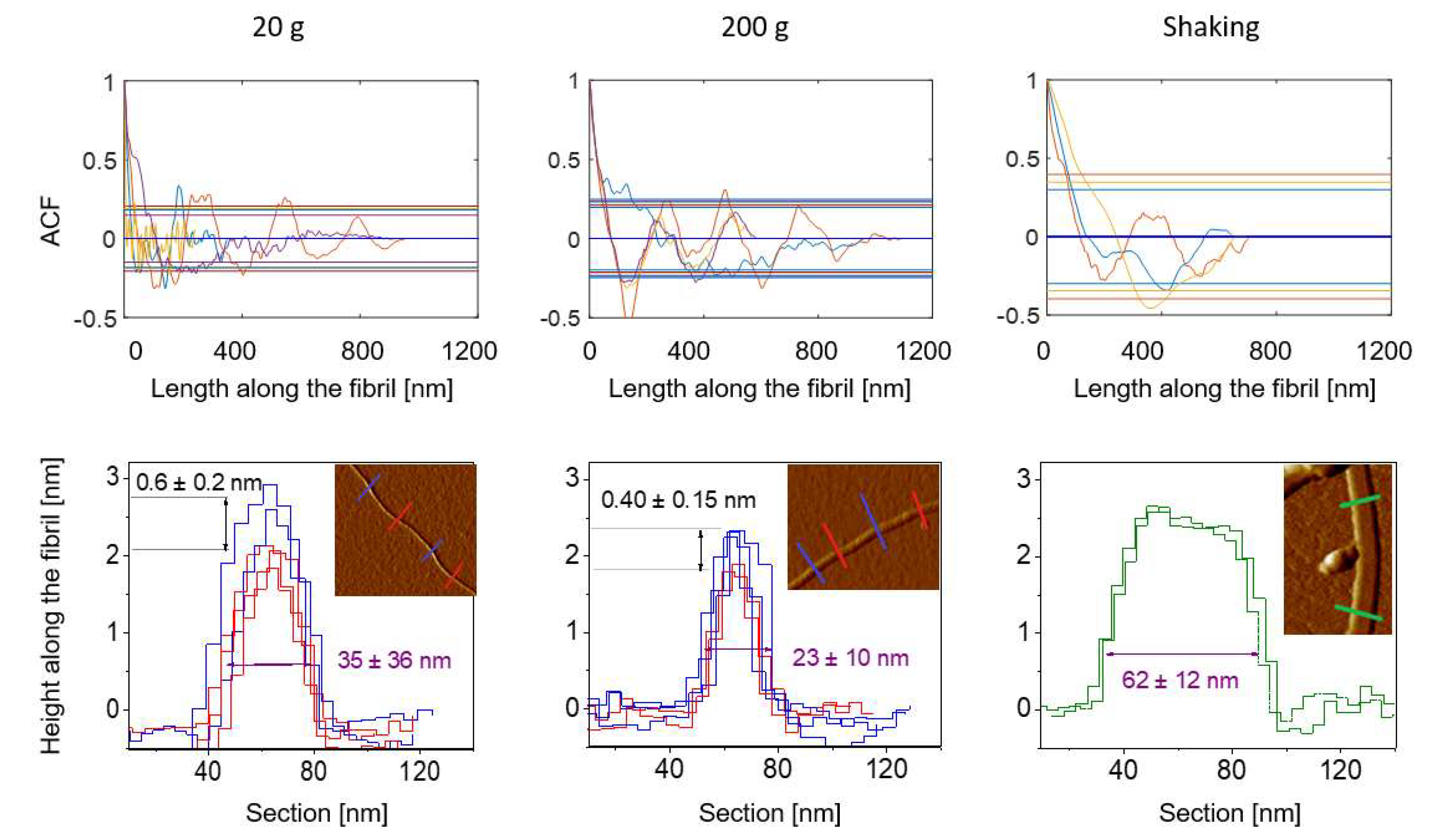
3.2. Influence of Mass Transfer Configuration on Fibril Properties
3.2.1. The Height and Length of the Fibrils
3.2.2. The Rigidity of the Fibrils
3.2.3. Fibril Morphology at the Protofilaments Level
3.3. Submolecular Structural Features of the Lysozyme Mixtures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiti, F.; Dobson, C.M. Protein Misfolding, Amyloid Formation, and Human Disease: A Summary of Progress Over the Last Decade. Annu. Rev. Biochem. 2017, 86, 27–68. [Google Scholar] [CrossRef] [PubMed]
- Vekilov, P.G.; Vorontsova, M.A. Nucleation precursors in protein crystallization. Acta Crystallogr. Sect. FStructural Biol. Commun. 2014, 70, 271–282. [Google Scholar] [CrossRef] [Green Version]
- Häggqvist, B.; Näslund, J.; Sletten, K.; Westermark, G.T.; Mucchiano, G.; Tjernberg, L.O.; Nordstedt, C.; Engström, U.; Westermark, P. Medin: An integral fragment of aortic smooth muscle cell-produced lactadherin forms the most common human amyloid. Proc. Natl. Acad. Sci. USA 1999, 96, 8669–8674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowler, D.M.; Koulov, A.V.; Alory-Jost, C.; Marks, M.S.; Balch, W.E.; Kelly, J.W. Functional Amyloid Formation within Mammalian Tissue. PLoS Biol. 2005, 4, e6. [Google Scholar] [CrossRef]
- Marinõ, L.; Pauwels, K.; Casasnovas, R.; Sanchis, P.; Vilanova, B.; Munõz, F.; Donoso, J.; Adrover, M. Ortho-methylated 3-hydroxypyridines hinder hen egg-white lysozyme fibrillogenesis. Sci. Rep. 2015, 5, 12052. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Ravi, V.K.; Swaminathan, R. How do surfactants and DTT affect the size, dynamics, activity and growth of soluble lysozyme aggregates? Biochem. J. 2008, 415, 275–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, S.B.; Macchi, F.; Raccosta, S.; Langkilde, A.E.; Giehm, L.; Kyrsting, A.; Svane, A.S.P.; Manno, M.; Christiansen, G.; Nielsen, N.C.; et al. Wildtype and A30P Mutant Alpha-Synuclein Form Different Fibril Structures. PLoS ONE 2013, 8, e67713. [Google Scholar] [CrossRef] [Green Version]
- Pansieri, J.; Halim, M.A.; Vendrely, C.; Dumoulin, M.; Legrand, F.; Sallanon, M.M.; Chierici, S.; Denti, S.; Dagany, X.; Dugourd, P.; et al. Mass and charge distributions of amyloid fibers involved in neurodegenerative diseases: Mapping heterogeneity and polymorphism. Chem. Sci. 2018, 9, 2791–2796. [Google Scholar] [CrossRef]
- Rousseau, F.; Schymkowitz, J.; Oliveberg, M. ALS precursor finally shaken into fibrils. Proc. Natl. Acad. Sci. USA 2008, 105, 18649–18650. [Google Scholar] [CrossRef] [Green Version]
- Bee, J.S.; Stevenson, J.L.; Mehta, B.; Svitel, J.; Pollastrini, J. Response of mAb to high shear. Biotechnol. Bioeng. 2010, 103, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Ouberai, M.M.; Dos Santos, A.L.G.; Kinna, S.; Madalli, S.; Hornigold, D.C.; Baker, D.; Naylor, J.; Sheldrake, L.; Corkill, D.J.; Hood, J.; et al. Controlling the bioactivity of a peptide hormone in vivo by reversible self-assembly. Nat. Commun. 2017, 8, 1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kummer, N.; Wu, T.; De France, K.J.; Zuber, F.; Ren, Q.; Fischer, P.; Campioni, S.; Nyström, G. Self-Assembly Pathways and Antimicrobial Properties of Lysozyme in Different Aggregation States. Biomacromolecules 2021, 22, 4327–4336. [Google Scholar] [CrossRef] [PubMed]
- Hoppenreijs, L.J.G.; Fitzner, L.; Ruhmlieb, T.; Heyn, T.R.; Schild, K.; van der Goot, A.J.; Boom, R.M.; Steffen-Heins, A.; Schwarz, K.; Keppler, J.K. Engineering amyloid and amyloid-like morphologies of β-lactoglobulin. Food Hydrocol. 2022, 124, 107301. [Google Scholar] [CrossRef]
- Lendel, C.; Solin, N. Protein nanofibrils and their use as building blocks of sustainable materials. RSC Adv. 2021, 11, 39188–39215. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Udgaonkar, J.B. Defining the pathway of worm-like amyloid fibril formation by the mouse prion protein by delineation of the productive and unproductive oligomerization reactions. Biochemistry 2011, 50, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Sabareesan, A.T.; Mathew, M.K.; Udgaonkar, J.B. Development of the structural core and of conformational heterogeneity during the conversion of oligomers of the mouse prion protein to worm-like amyloid fibrils. J. Mol. Biol. 2012, 423, 217–231. [Google Scholar] [CrossRef]
- Kurouski, D.; Lu, X.; Popova, L.; Wan, W.; Shanmugasundaram, M.; Stubbs, G.; Dukor, R.K.; Lednev, I.K.; Nafie, L.A. Is supramolecular filament chirality the underlying cause of major morphology differences in amyloid fibrils? J. Am. Chem. Soc. 2014, 136, 2302–2312. [Google Scholar] [CrossRef]
- Hasecke, F.; Miti, T.; Perez, C.; Barton, J.; Schölzel, D.; Schölzel, S.; Gremer, L.; Grüning, C.S.R.; Grüning, G.; Matthews, G.; et al. Origin of metastable oligomers and their effects on amyloid fibril self-assembly. Chem. Sci. 2018, 9, 5937–5948. [Google Scholar] [CrossRef] [Green Version]
- Perez, C.; Miti, T.; Hasecke, F.; Meisl, G.; Hoyer, W.; Muschol, M.; Ullah, G. Mechanism of Fibril and Soluble Oligomer Formation in Amyloid Beta and Hen Egg White Lysozyme Proteins. J. Phys. Chem. B 2019, 123, 5678–5689. [Google Scholar] [CrossRef]
- Barton, J.; Sebastian Arias, D.; Niyangoda, C.; Borjas, G.; Le, N.; Mohamed, S.; Muschol, M. Kinetic transition in amyloid assembly as a screening assay for oligomer-selective dyes. Biomolecules 2019, 9, 539. [Google Scholar] [CrossRef]
- Patke, S.; Srinivasan, S.; Maheshwari, R.; Srivastava, S.K.; Aguilera, J.J.; Colón, W.; Kane, R.S. Characterization of the Oligomerization and Aggregation of Human Serum Amyloid A. PLoS ONE 2013, 8, e64974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, R.; Akcan, M.; Khondker, A.; Rheinstädter, M.C.; Rheinstädter, R.; Jos´, J.; Bozelli, J.C.; Epand, R.M.; Huynh, V.; Wylie, R.G.; et al. Atomic resolution map of the soluble amyloid beta assembly toxic surfaces. Chem. Sci. 2019, 10, 6072–6082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubin, E.; Deroo, S.; Schierle, G.K.; Kaminski, C.; Serpell, L.; Subramaniam, V.; Van Nuland, N.; Broersen, K.; Raussens, V.; Sarroukh, R. Two distinct β-sheet structures in Italian-mutant amyloid-beta fibrils: A potential link to different clinical phenotypes. Cell. Mol. Life Sci. 2015, 72, 4899–4913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dear, A.J.; Meisl, G.; Šarić, A.; Michaels, T.C.T.; Kjaergaard, M.; Linse, S.; Knowles, T.P.J. Identification of on- And off-pathway oligomers in amyloid fibril formation. Chem. Sci. 2020, 11, 6236–6247. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Patke, S.; Wang, Y.; Ye, Z.; Litt, J.; Srivastava, S.K.; Lopez, M.M.; Kurouski, D.; Lednev, I.K.; Kane, R.S.; et al. Pathogenic serum amyloid A 1.1 shows a long oligomer-rich fibrillation lag phase contrary to the highly amyloidogenic non-pathogenic SAA2.2. J. Biol. Chem. 2013, 288, 2744–2755. [Google Scholar] [CrossRef] [Green Version]
- Muschol, M.; Hill, S.E.; Mulaj, M. Multiple Pathways of Lysozyme Aggregation. In Bio-Nanoimaging: Protein Misfolding and Aggregation; Elsevier: Amsterdam, The Netherlands, 2013; pp. 389–396. ISBN 9780123944313. [Google Scholar]
- Petkova, A.T.; Leapman, R.D.; Guo, Z.; Yau, W.M.; Mattson, M.P.; Tycko, R. Self-propagating, molecular-level polymorphism in Alzheimer’s β-amyloid fibrils. Science 2005, 307, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Miti, T.; Mulaj, M.; Schmit, J.D.; Muschol, M. Stable, metastable, and kinetically trapped amyloid aggregate phases. Biomacromolecules 2015, 16, 326–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, S.E.; Miti, T.; Richmond, T.; Muschol, M. Spatial extent of charge repulsion regulates assembly pathways for lysozyme amyloid fibrils. PLoS ONE 2011, 6, e18171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campioni, S.; Bagnani, M.; Pinotsi, D.; Lecinski, S.; Rodighiero, S.; Adamcik, J.; Mezzenga, R. Interfaces Determine the Fate of Seeded α-Synuclein Aggregation. Adv. Mater. Interfaces 2020, 11, 2000446. [Google Scholar] [CrossRef]
- Adachi, M.; So, M.; Sakurai, K.; Kardos, J.; Goto, Y. Supersaturation-limited and unlimited phase transitions compete to produce the pathway complexity in amyloid fibrillation. J. Biol. Chem. 2015, 290, 18134–18145. [Google Scholar] [CrossRef] [PubMed]
- Agopian, A.; Guo, Z. Structural origin of polymorphism of Alzheimer’s amyloid β-fibrils. Biochem. J. 2012, 447, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Dunstan, D.E.; Hamilton-Brown, P.; Asimakis, P.; Ducker, W.; Bertolini, J. Shear-induced structure and mechanics of β-lactoglobulin amyloid fibrils. Soft Matter 2009, 5, 5020–5028. [Google Scholar] [CrossRef]
- Trumbore, C.N. Shear-Induced Amyloid Formation in the Brain: I. Potential Vascular and Parenchymal Processes. J. Alzheimer’s Dis. 2016, 54, 457–470. [Google Scholar] [CrossRef] [Green Version]
- Dunstan, D.E.; Hamilton-Brown, P.; Asimakis, P.; Ducker, W.; Bertolini, J. Shear flow promotes amyloid-β fibrilization. Protein Eng. Des. Sel. 2009, 22, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.; Durrance, S.; Kirk, D.; Gutierrez, H.; Woodard, D.; Avendano, J.; Sargent, J.; Leite, C.; Saldana, B.; Melles, T.; et al. Self-Assembly of Protein Fibrils in Microgravity. Gravitational Sp. Res. 2018, 6, 10–26. [Google Scholar] [CrossRef]
- Zhou, J.; Ruggeri, F.S.; Zimmermann, M.R.; Meisl, G.; Longo, G.; Sekatskii, S.K.; Knowles, T.P.J.; Dietler, G. Effects of sedimentation, microgravity, hydrodynamic mixing and air-water interface on α-synuclein amyloid formation. Chem. Sci. 2020, 11, 3687–3693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brockwell, D.J.; Paci, E.; Zinober, R.C.; Beddard, G.S.; Olmsted, P.D.; Smith, D.A.; Perham, R.N.; Radford, S.E. Pulling geometry defines the mechanical resistance of a β-sheet protein. Nat. Struct. Biol. 2003, 10, 731–737. [Google Scholar] [CrossRef]
- Usov, I.; Mezzenga, R. FiberApp: An open-source software for tracking and analyzing polymers, filaments, biomacromolecules, and fibrous objects. Macromolecules 2015, 48, 1269–1280. [Google Scholar] [CrossRef]
- Vandenakker, C.C.; Engel, M.F.M.; Velikov, K.P.; Bonn, M.; Koenderink, G.H. Morphology and Persistence Length of Amyloid Fibrils are Correlated to Peptide Molecular Structure. J. Am. Chem. Soc. 2011, 133, 18030–18033. [Google Scholar] [CrossRef]
- Reilly, J.T.; Walsh, J.M.; Greenfield, M.L.; Donohue, M.D. Analysis of FT-IR spectroscopic data: The Voigt profile. Spectrochim. Acta Part A Mol. Spectrosc. 1992, 48, 1459–1479. [Google Scholar] [CrossRef]
- Sethuraman, A.; Belfort, G. Protein structural perturbation and aggregation on homogeneous surfaces. Biophys. J. 2005, 88, 1322–1333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaari, A.; Fahy, C.; Chevillot-Biraud, A.; Rholam, M. Insights into Kinetics of Agitation-Induced Aggregation of Hen Lysozyme under Heat and Acidic Conditions from Various Spectroscopic Methods. PLoS ONE 2015, 10, e0142095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frare, E.; Mossuto, M.F.; de Laureto, P.P.; Tolin, S.; Menzer, L.; Dumoulin, M.; Dobson, C.M.; Fontana, A. Characterization of Oligomeric Species on the Aggregation Pathway of Human Lysozyme. J. Mol. Biol. 2009, 387, 17–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, R.; Sörgjerd, K.; Nyström, S.; Nordigården, A.; Yu, Y.C.; Hammarström, P. Lysozyme Amyloidogenesis Is Accelerated by Specific Nicking and Fragmentation but Decelerated by Intact Protein Binding and Conversion. J. Mol. Biol. 2007, 366, 1029–1044. [Google Scholar] [CrossRef]
- Lara, C.; Usov, I.; Adamcik, J.; Mezzenga, R. Sub-Persistence-Length Complex Scaling Behavior in Lysozyme Amyloid Fibrils. Phys. Rev. Lett. 2011, 107, 238101. [Google Scholar] [CrossRef]
- Lonescu-Zanetti, C.; Khurana, R.; Gillespie, J.R.; Petrick, J.S.; Trabachino, L.C.; Minert, L.J.; Carter, S.A.; Fink, A.L. Monitoring the assembly of Ig light-chain amyloid fibrils by atomic force microscopy. Proc. Natl. Acad. Sci. USA 1999, 96, 13175–13179. [Google Scholar] [CrossRef] [Green Version]
- Arimon, M.; Díez-Pérez, I.; Kogan, M.J.; Durany, N.; Giralt, E.; Sanz, F.; Fernández-Busquets, X. Fine structure study of A 1-42 fibrillogenesis with atomic force microscopy. FASEB J. 2005, 19, 1344–1346. [Google Scholar] [CrossRef]
- Kodali, R.; Wetzel, R. Polymorphism in the intermediates and products of amyloid assembly. Curr. Opin. Struct. Biol. 2007, 17, 48–57. [Google Scholar] [CrossRef]
- Wu, C.; Bowers, M.T.; Shea, J.E. Molecular structures of quiescently grown and brain-derived polymorphic fibrils of the Alzheimer amyloid Aβ9-40 peptide: A comparison to agitated fibrils. PLoS Comput. Biol. 2010, 6, e1000693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, K.; Toda, H.; Yamaguchi, K.; So, M.; Ikenaka, K.; Mochizuki, H.; Goto, Y.; Ogi, H. Half-Time Heat Map Reveals Ultrasonic Effects on Morphology and Kinetics of Amyloidogenic Aggregation Reaction. ACS Chem. Neurosci. 2021, 12, 3456–3466. [Google Scholar] [CrossRef]
- Sarroukh, R.; Goormaghtigh, E.; Ruysschaert, J.M.; Raussens, V. ATR-FTIR: A “rejuvenated” tool to investigate amyloid proteins. Biochim. Biophys. Acta-Biomembr. 2013, 1828, 2328–2338. [Google Scholar] [CrossRef] [PubMed]
- Cerf, E.; Sarroukh, R.; Tamamizu-Kato, S.; Breydo, L.; Derclaye, S.; Dufrêne, Y.F.; Narayanaswami, V.; Goormaghtigh, E.; Ruysschaert, J.-M.; Raussens, V. Antiparallel β-sheet: A signature structure of the oligomeric amyloid β-peptide. Biochem. J. 2009, 421, 415–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, Y.; Li, Y.; Hao, W.; Hu, X.; Ma, G. Parallel β-Sheet Fibril and Antiparallel β-Sheet Oligomer: New Insights into Amyloid Formation of Hen Egg White Lysozyme under Heat and Acidic Condition from FTIR Spectroscopy. J. Phys. Chem. B 2013, 117, 4003–4013. [Google Scholar] [CrossRef] [PubMed]
- van Loon, J.J.; Folgering, E.H.; Bouten, C.V.; Veldhuijzen, J.P.; Smit, T.H. Inertial shear forces and the use of centrifuges in gravity research. What is the proper control? J. Biomech. Eng. 2003, 125, 342–346. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzek, M.; Stroobants, S.; Gelin, P.; De Malsche, W.; Maes, D. Influence of Centrifugation and Shaking on the Self-Assembly of Lysozyme Fibrils. Biomolecules 2022, 12, 1746. https://doi.org/10.3390/biom12121746
Krzek M, Stroobants S, Gelin P, De Malsche W, Maes D. Influence of Centrifugation and Shaking on the Self-Assembly of Lysozyme Fibrils. Biomolecules. 2022; 12(12):1746. https://doi.org/10.3390/biom12121746
Chicago/Turabian StyleKrzek, Marzena, Sander Stroobants, Pierre Gelin, Wim De Malsche, and Dominique Maes. 2022. "Influence of Centrifugation and Shaking on the Self-Assembly of Lysozyme Fibrils" Biomolecules 12, no. 12: 1746. https://doi.org/10.3390/biom12121746
APA StyleKrzek, M., Stroobants, S., Gelin, P., De Malsche, W., & Maes, D. (2022). Influence of Centrifugation and Shaking on the Self-Assembly of Lysozyme Fibrils. Biomolecules, 12(12), 1746. https://doi.org/10.3390/biom12121746







