Isolation and Characterization of 1-Hydroxy-2,6,6-trimethyl-4-oxo-2-cyclohexene-1-acetic Acid, a Metabolite in Bacterial Transformation of Abscisic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. ABA and Its Tritium Labeled Analog
2.2. Cultivation of the Bacterium
2.3. Sample Extraction and Purification
2.4. HPLC-MS Analysis
2.5. Spectrophotometry
2.6. Polarimetry
2.7. Melting Point Measurement
2.8. FTIR Spectroscopic Measurements
2.9. NMR Spectroscopic Measurements and Experimental Conditions
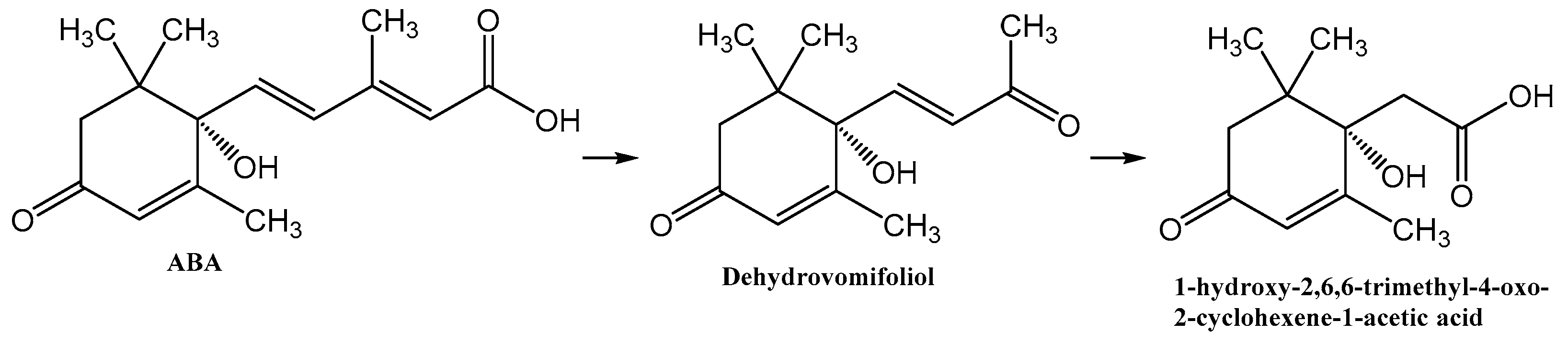
3. Results
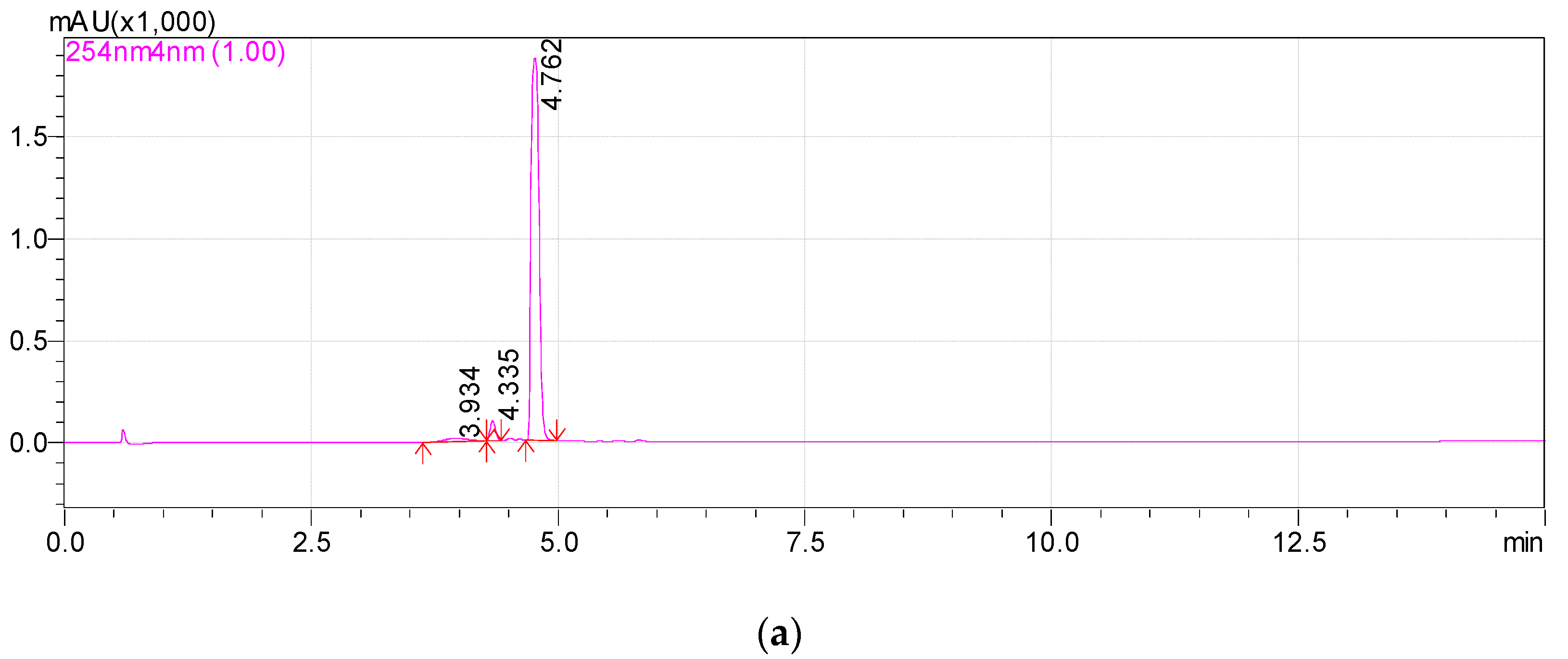
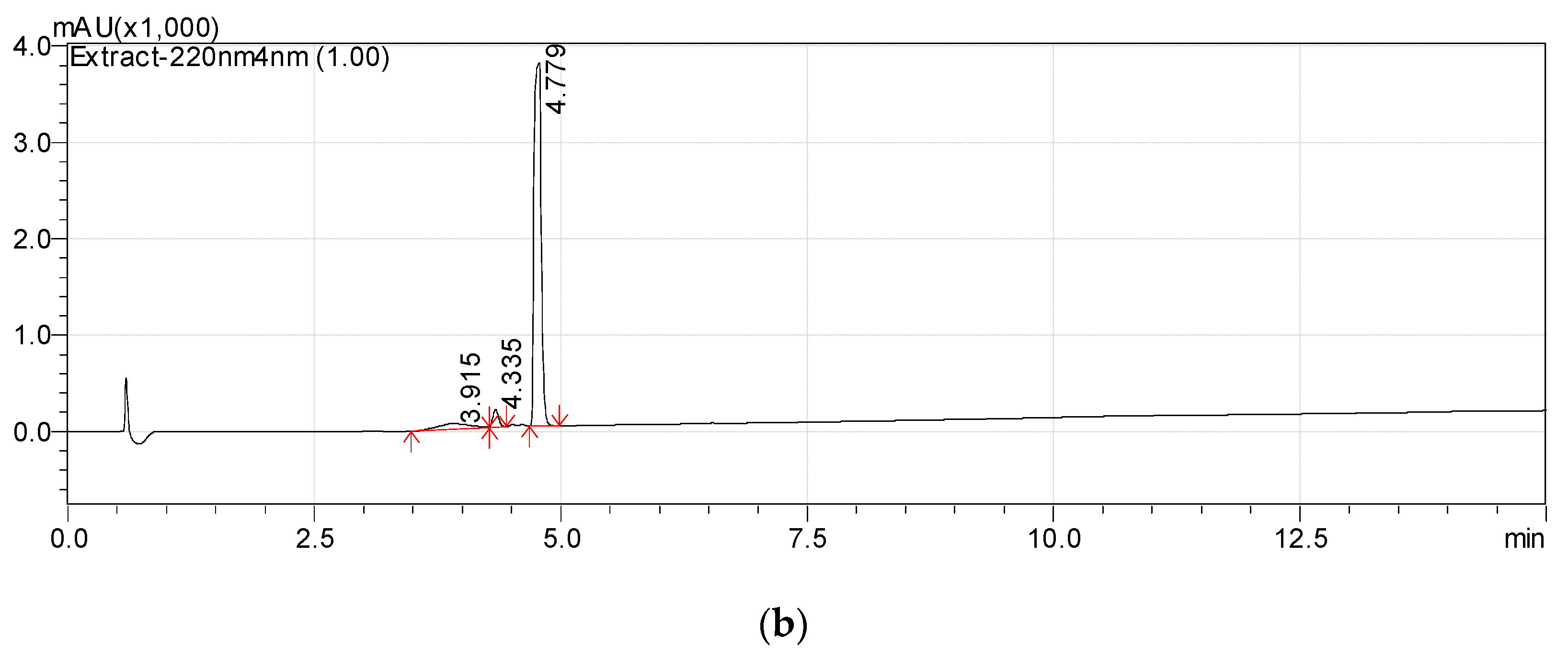
3.1. Isolation of Metabolite 2
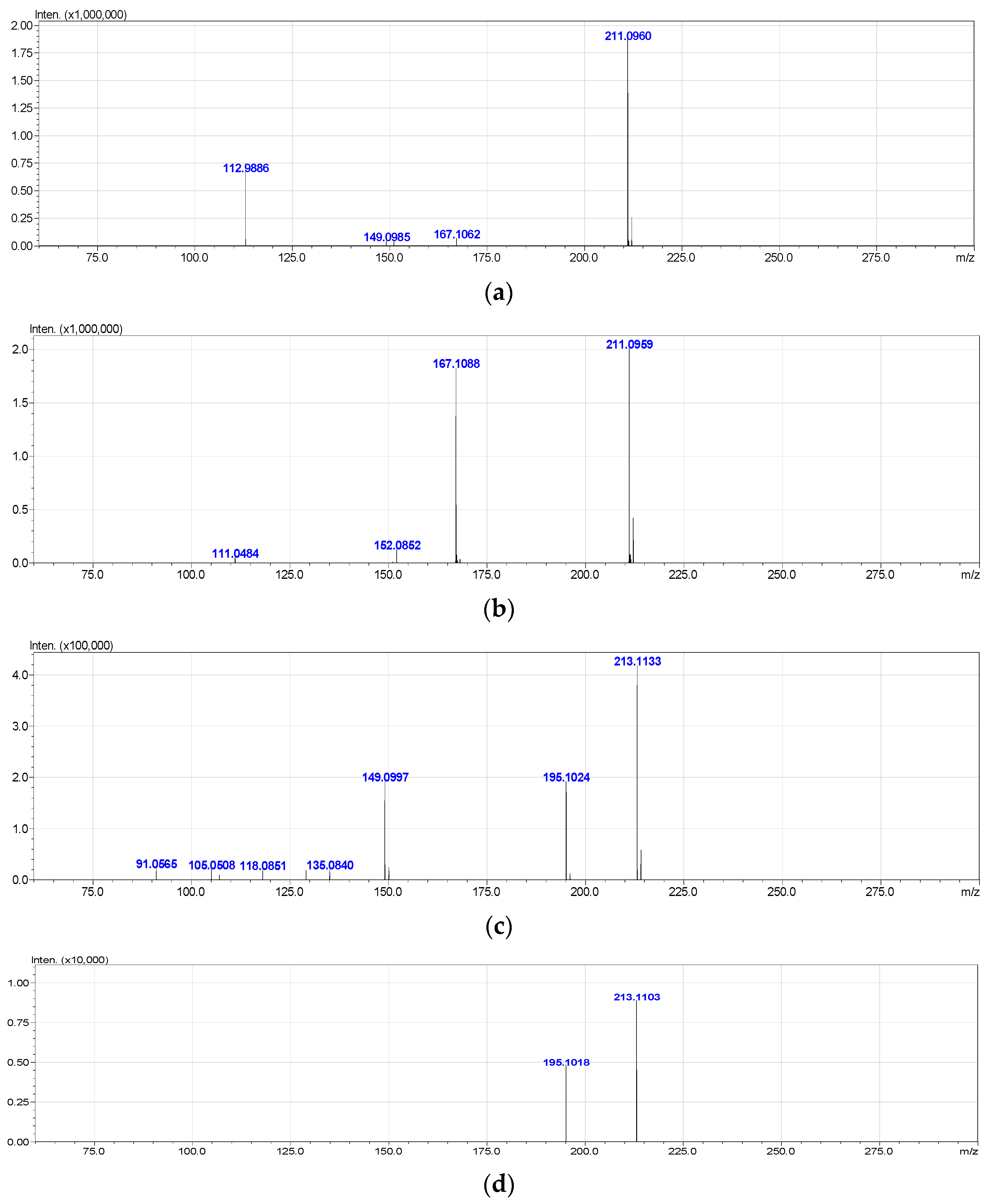
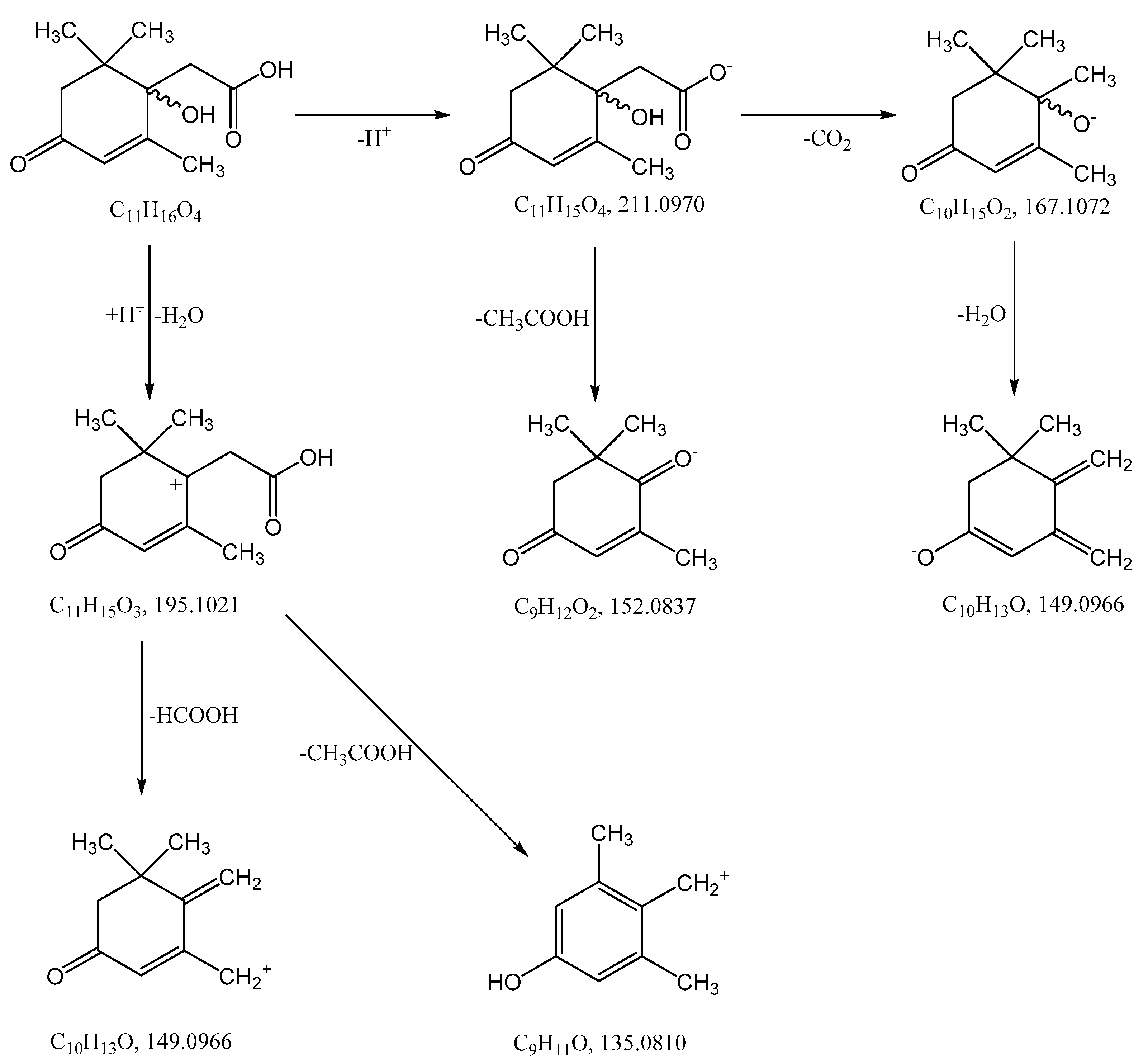
3.2. Mass Spectrometric Studies
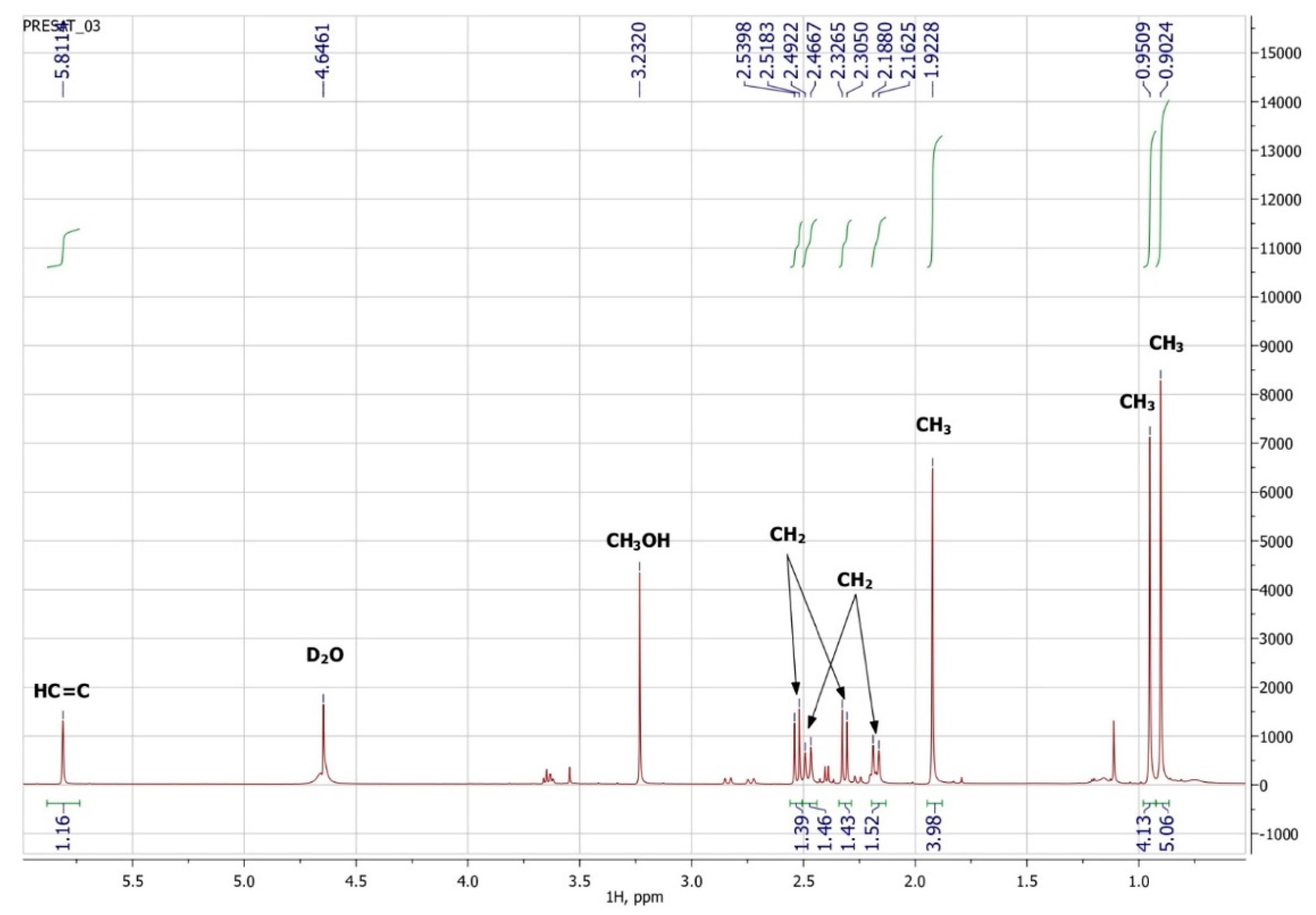
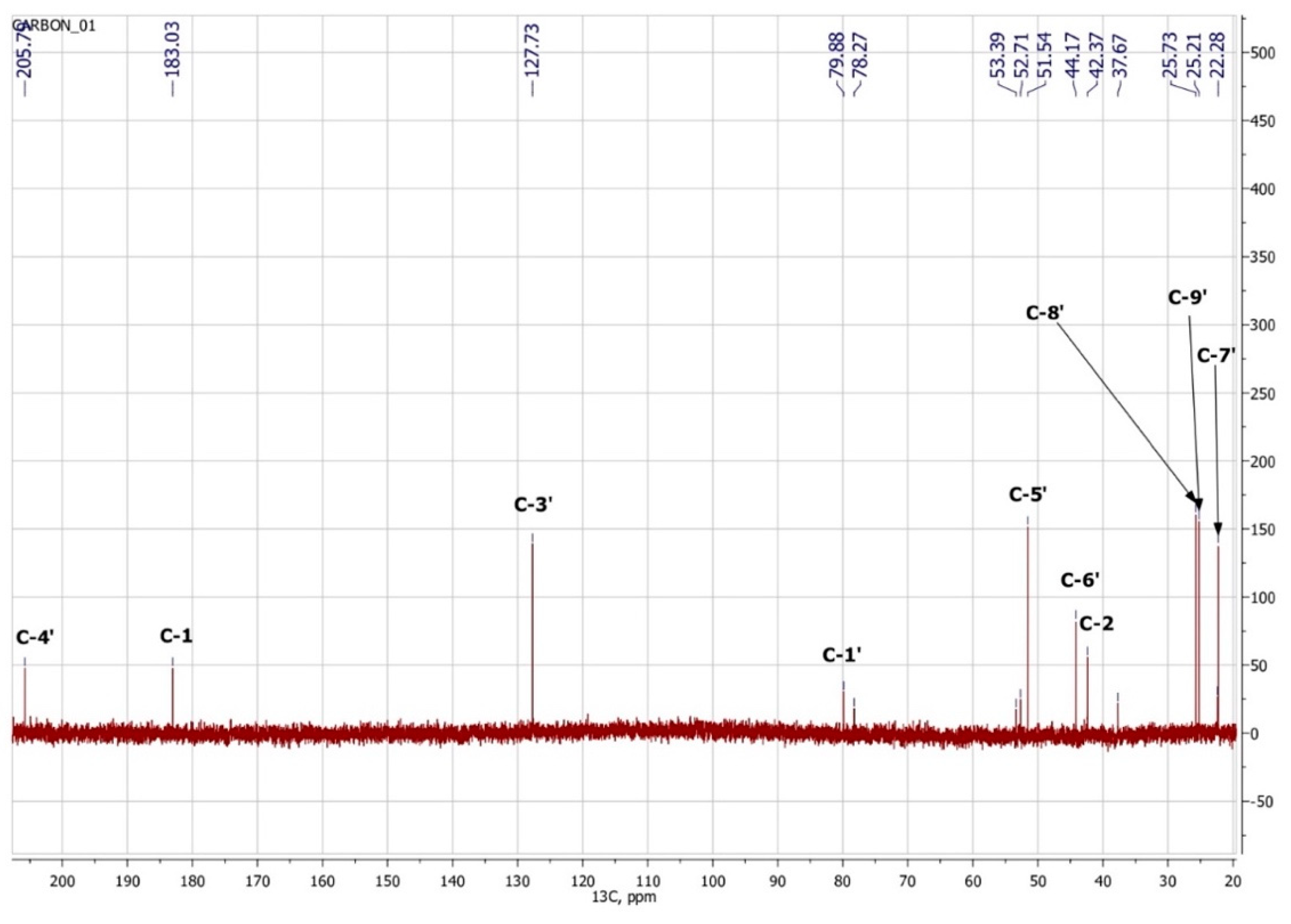
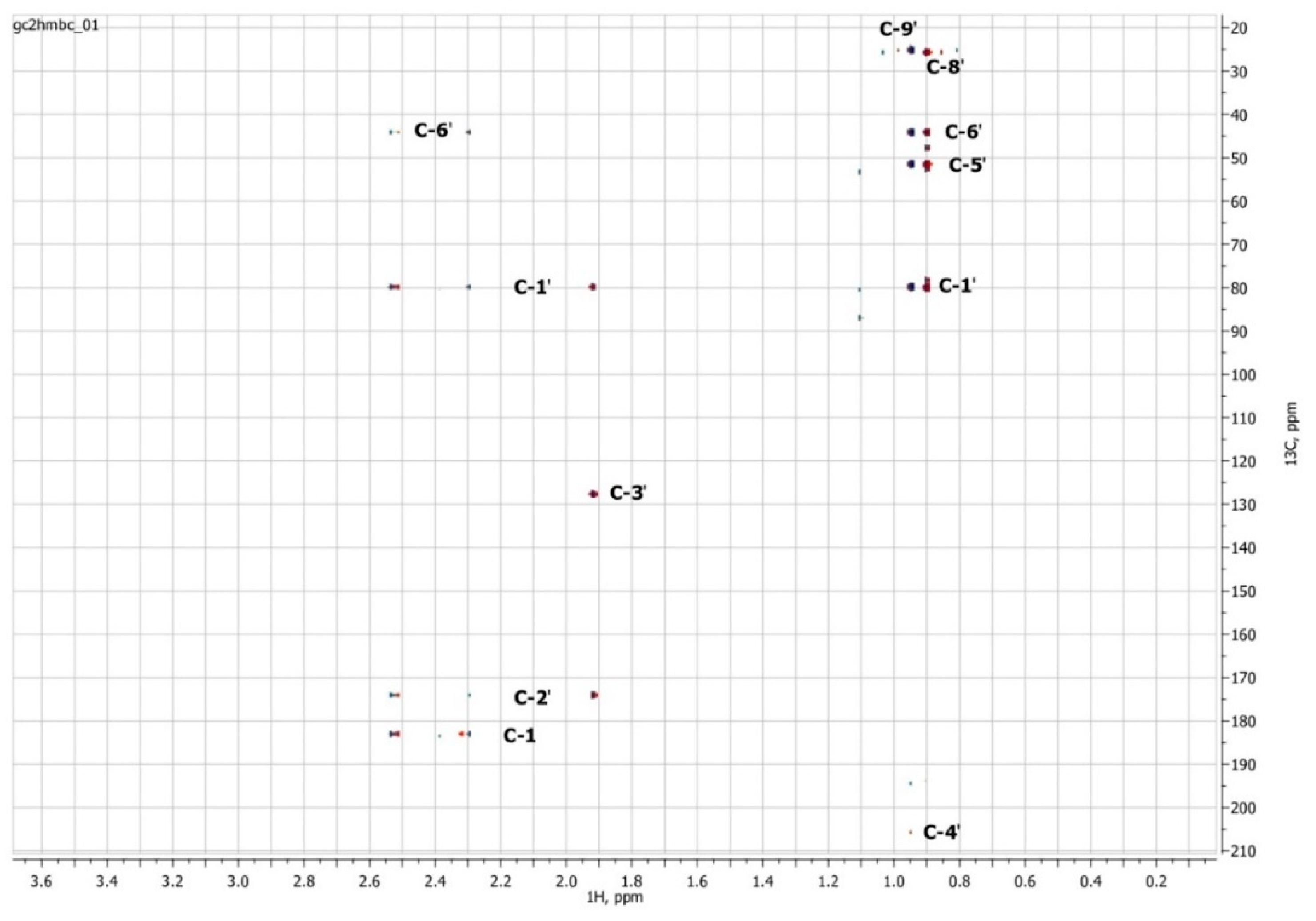
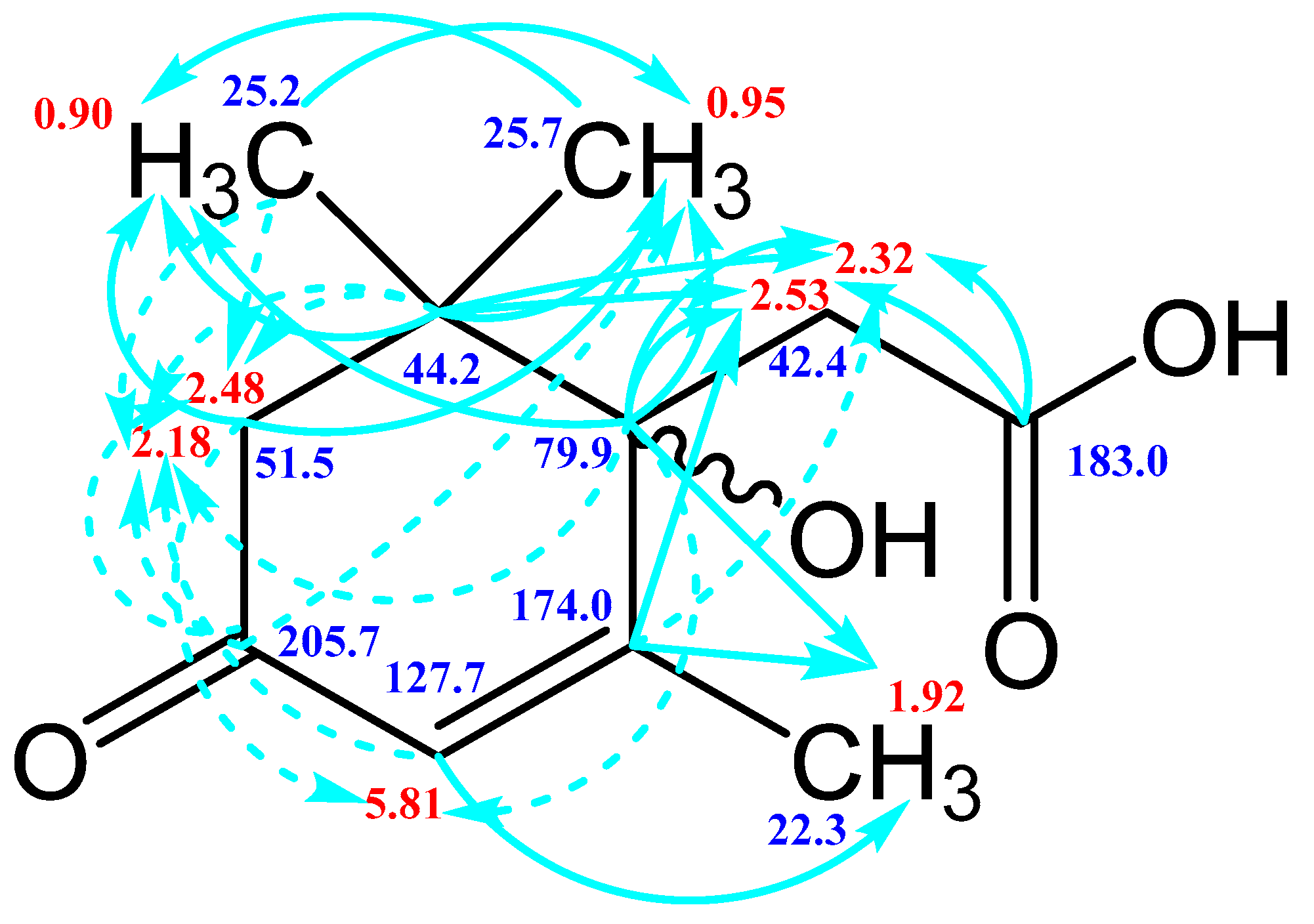
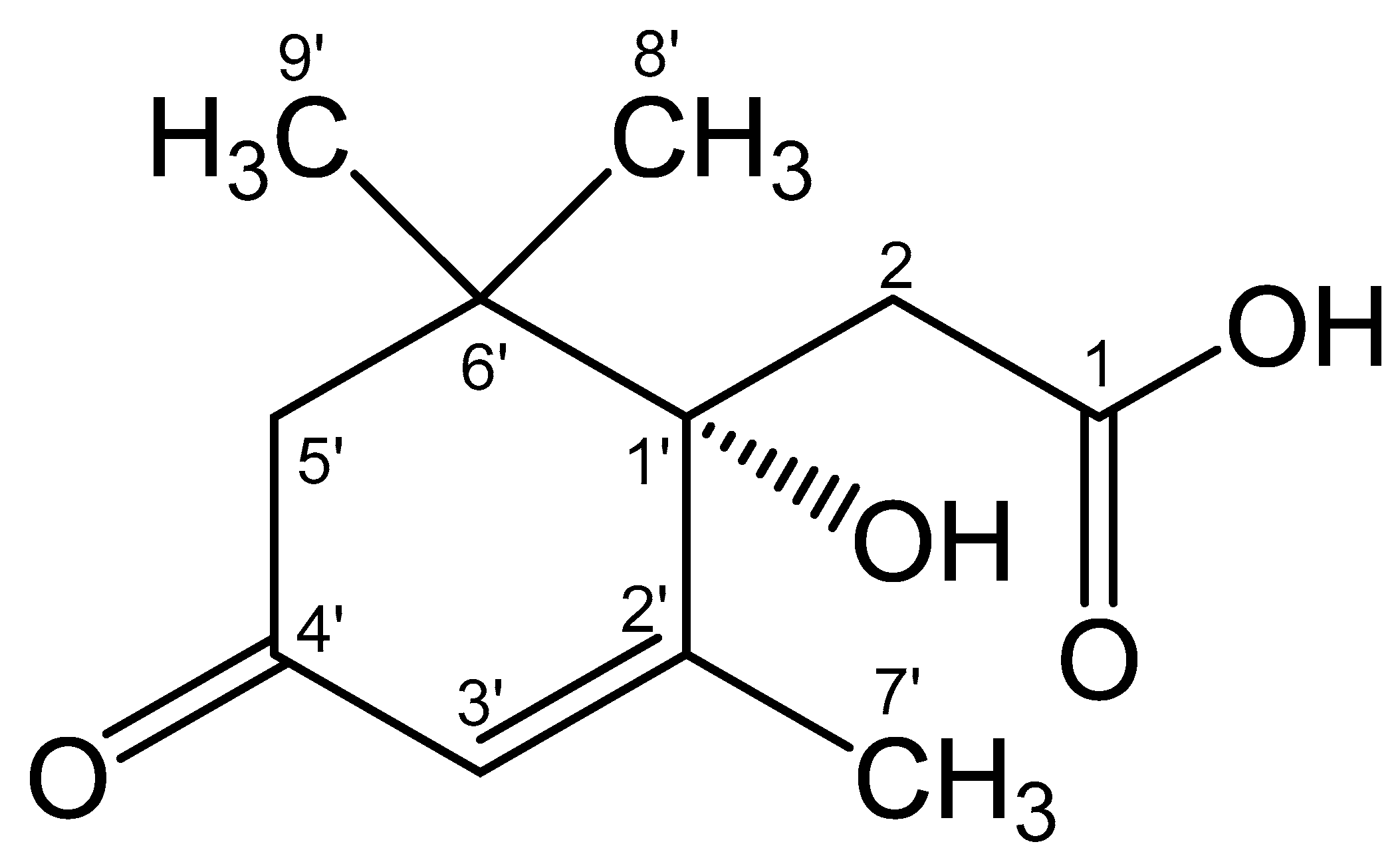
3.3. NMR Spectroscopy
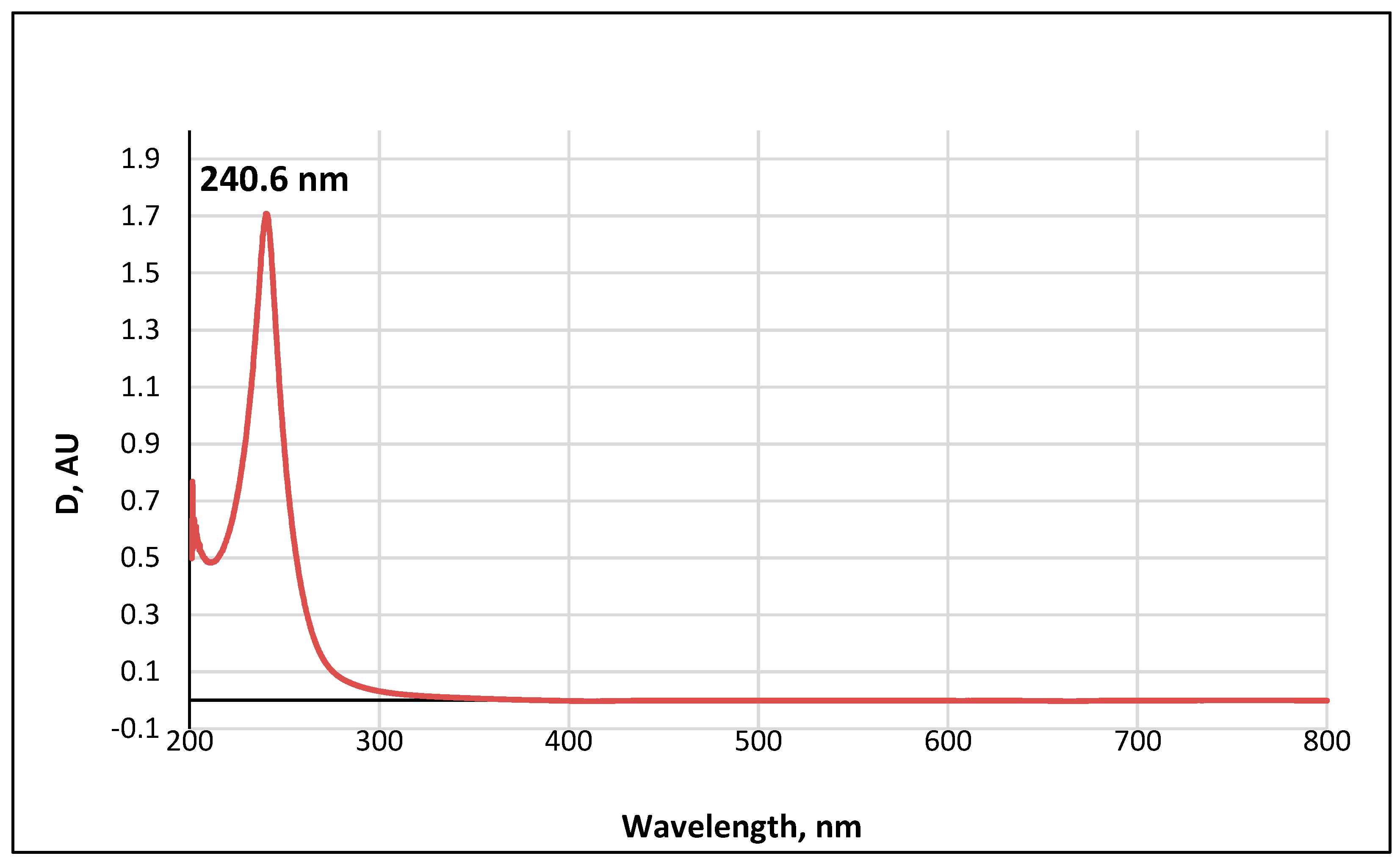
3.4. UV-Visible Absorption Spectrum of Metabolite 2
3.5. FTIR Spectroscopic Characterization
3.6. Polarimetry
3.7. Melting Point Measurement
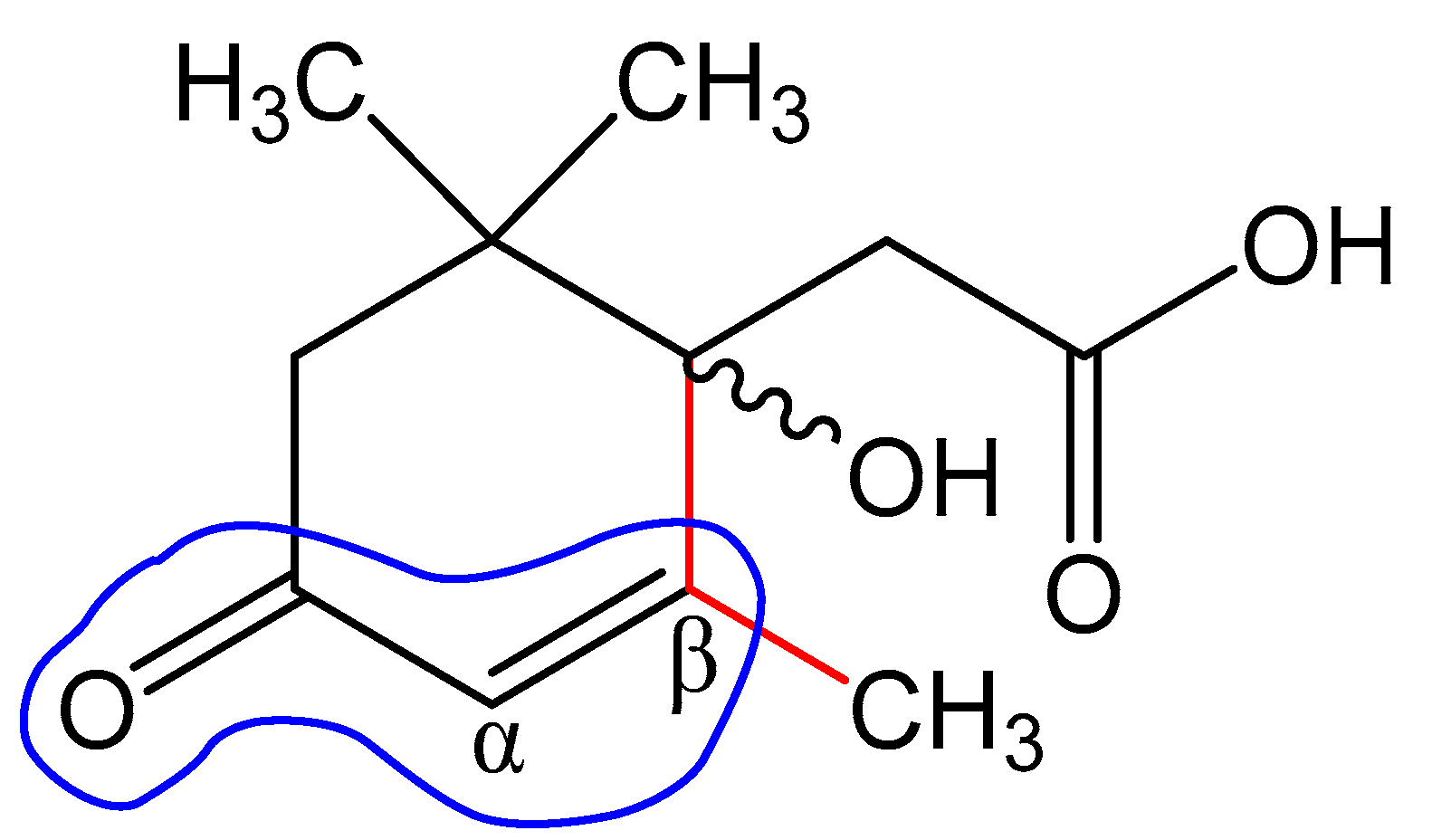
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeevaart, J.A.D.; Creelman, R.A. Metabolism and Physiology of Abscisic Acid. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1988, 39, 439–473. [Google Scholar] [CrossRef]
- LeNoble, M.E.; Spollen, W.G.; Sharp, R.E. Maintenance of shoot growth by endogenous ABA: Genetic assessment of the involvement of ethylene suppression. J. Exp. Bot. 2004, 55, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Ma, X.; Tan, H.; Zhou, J. Abscisic acid enhances resistance to Alternaria solani in tomato seedlings. Plant Physiol. Biochem. 2011, 49, 693–700. [Google Scholar] [CrossRef]
- Dodd, I.C.; Tan, L.P.; He, J. Do increases in xylem sap pH and/or ABA concentration mediate stomatal closure following nitrate deprivation? J. Exp. Bot. 2003, 54, 1281–1288. [Google Scholar] [CrossRef]
- Müller, M.D.; Deigele, C.; Ziegler, H. Hormonal interactions in the rhizosphere of maize (Zea mays L.) and their effects on plant development. Z. Für Pflanz. Und Bodenkd. 1989, 152, 247–254. [Google Scholar] [CrossRef]
- Hartung, W.; Sauter, A.; Turner, N.C.; Fillery, I.; Heilmeier, H. Abscisic acid in soils: What is its function and which factors and mechanisms influence its concentration? Plant Soil 1996, 184, 105–110. [Google Scholar] [CrossRef]
- Hartung, W.; Heilmeier, H.; Wartinger, A.; Kettemann, I.; Schulze, E. Ionic and abscisic acid relationships of Anastatica hierochuntica L. under arid conditions. Isr. J. Bot. 1990, 39, 373–382. [Google Scholar] [CrossRef]
- Frankenberger, W.T.; Arshad, M. Phytormones in Soils; Marcel Dekker Inc.: New York, NY, USA, 1995. [Google Scholar]
- Karssen, C.M. Uptake and effect of abscisic acid during induction and progress of radicle growth in seeds of Chenopodium album. Physiol. Plant. 1976, 36, 259–263. [Google Scholar] [CrossRef]
- Schopfer, P.; Bajracharya, D.; Plachy, C. Control of Seed Germination by Abscisic Acid: I. Time Course of Action in Sinapis alba L. Plant Physiol. 1979, 64, 822–827. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Forde, B.G.; Davies, W.J. The Biphasic Root Growth Response to Abscisic Acid in Arabidopsis Involves Interaction with Ethylene and Auxin Signalling Pathways. Front. Plant Sci. 2017, 8, 1493. [Google Scholar] [CrossRef]
- Sharp, R.E.; Davies, W.J. Solute Regulation and Growth by Roots and Shoots of Water-Stressed Maize Plants. Planta 1979, 147, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Watts, S.; Rodriguez, J.L.; Evans, S.E.; Davies, W.J. Root and Shoot Growth of Plants Treated with Abscisic-Acid. Ann. Bot. 1981, 47, 595–602. [Google Scholar] [CrossRef]
- Davies, W.J.; Zhang, J.H. Root Signals and the Regulation of Growth and Development of Plants in Drying Soil. Annu. Rev. Plant Phys. 1991, 42, 55–76. [Google Scholar] [CrossRef]
- Dodd, I.C.; Puertolas, J.; Huber, K.; Perez-Perez, J.G.; Wright, H.R.; Blackwell, M.S. The importance of soil drying and re-wetting in crop phytohormonal and nutritional responses to deficit irrigation. J. Exp. Bot. 2015, 66, 2239–2252. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Obata, T.; Feil, R.; Lunn, J.E.; Fujita, Y.; Yamaguchi-Shinozaki, K.; Fernie, A.R. The Role of Abscisic Acid Signaling in Maintaining the Metabolic Balance Required for Arabidopsis Growth under Nonstress Conditions. Plant Cell 2019, 31, 84–105. [Google Scholar] [CrossRef]
- Agehara, S.; Leskovar, D.I. Characterizing Concentration Effects of Exogenous Abscisic Acid on Gas Exchange, Water Relations, and Growth of Muskmelon Seedlings during Water Stress and Rehydration. J. Am. Soc. Hortic. Sci. 2012, 137, 400–410. [Google Scholar] [CrossRef]
- Henfling, J.W.D.M.; Bostock, R.; Kuc, J. Effect of Abscisic-Acid on Rishitin and Lubimin Accumulation and Resistance to Phytophthora infestans and Cladosporium cucumerinum in Potato-Tuber Tissue-Slices. Phytopathology 1980, 70, 1074–1078. [Google Scholar] [CrossRef]
- Mohr, P.G.; Cahill, D.M. Abscisic acid influences the susceptibility of Arabidopsis thaliana to Pseudomonas syringae pv. tomato and Peronospora parasitica. Funct. Plant Biol. 2003, 30, 461–469. [Google Scholar] [CrossRef]
- Ward, E.W.B.; Cahill, D.M.; Bhattacharyya, M.K. Abscisic-Acid Suppression of Phenylalanine Ammonia-Lyase Activity and mRNA, and Resistance of Soybeans to Phytophthora megasperma F-Sp glycinea. Plant Physiol. 1989, 91, 23–27. [Google Scholar] [CrossRef]
- Asselbergh, B.; Achuo, A.E.; Hofte, M.; Van Gijsegem, F. Abscisic acid deficiency leads to rapid activation of tomato defence responses upon infection with Erwinia chrysanthemi. Mol. Plant Pathol. 2008, 9, 11–24. [Google Scholar] [CrossRef]
- Lievens, L.; Pollier, J.; Goossens, A.; Beyaert, R.; Staal, J. Abscisic Acid as Pathogen Effector and Immune Regulator. Front. Plant Sci. 2017, 8, 587. [Google Scholar] [CrossRef] [PubMed]
- Forchetti, G.; Masciarelli, O.; Alemano, S.; Alvarez, D.; Abdala, G. Endophytic bacteria in sunflower (Helianthus annuus L.): Isolation, characterization, and production of jasmonates and abscisic acid in culture medium. Appl. Microbiol. Biotechnol. 2007, 76, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Sgroy, V.; Cassan, F.; Masciarelli, O.; Del Papa, M.F.; Lagares, A.; Luna, V. Isolation and characterization of endophytic plant growth-promoting (PGPB) or stress homeostasis-regulating (PSHB) bacteria associated to the halophyte Prosopis strombulifera. Appl. Microbiol. Biotechnol. 2009, 85, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.C.; Bottini, R.; Pontin, M.; Berli, F.J.; Moreno, D.; Boccanlandro, H.; Travaglia, C.N.; Piccoli, P.N. Azospirillum brasilense ameliorates the response of Arabidopsis thaliana to drought mainly via enhancement of ABA levels. Physiol. Plant. 2015, 153, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, R.; Khan, A.L.; Bilal, S.; Waqas, M.; Kang, S.M.; Lee, I.J. Inoculation of abscisic acid-producing endophytic bacteria enhances salinity stress tolerance in Oryza sativa. Environ. Exp. Bot. 2017, 136, 68–77. [Google Scholar] [CrossRef]
- Boiero, L.; Perrig, D.; Masciarelli, O.; Penna, C.; Cassan, F.; Luna, V. Phytohormone production by three strains of Bradyrhizobium japonicum and possible physiological and technological implications. Appl. Microbiol. Biotechnol. 2007, 74, 874–880. [Google Scholar] [CrossRef]
- Bano, A.; Batool, R.; Dazzo, F. Adaptation of chickpea to desiccation stress is enhanced by symbiotic rhizobia. Symbiosis 2010, 50, 129–133. [Google Scholar] [CrossRef]
- Suzuki, A.; Akune, M.; Kogiso, M.; Imagama, Y.; Osuki, K.; Uchiumi, T.; Higashi, S.; Han, S.Y.; Yoshida, S.; Asami, T.; et al. Control of nodule number by the phytohormone abscisic acid in the roots of two leguminous species. Plant Cell Physiol. 2004, 45, 914–922. [Google Scholar] [CrossRef]
- Syrova, D.S.; Makarova, N.M.; Gagkaeva, T.Y.; Khrapalova, I.A.; Emelyanov, V.V.; Gogolev, Y.V.; Gannibal, P.B.; Belimov, A.A. The ability of some species of phytopathogenic fungi to produce abscisic acid. Mycol. Phytopathol. 2019, 53, 301–310. [Google Scholar] [CrossRef]
- Hartung, W. The evolution of abscisic acid (ABA) and ABA function in lower plants, fungi and lichen. Funct. Plant Biol. 2010, 37, 806–812. [Google Scholar] [CrossRef]
- Spence, C.; Bais, H. Role of plant growth regulators as chemical signals in plant-microbe interactions: A double edged sword. Curr. Opin. Plant. Biol. 2015, 27, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Han, X.W.; Kahmann, R. Manipulation of Phytohormone Pathways by Effectors of Filamentous Plant Pathogens. Front. Plant Sci. 2019, 10, 822. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Yang, S.; Meng, L.; Wang, B.G. The plant hormone abscisic acid regulates the growth and metabolism of endophytic fungus Aspergillus nidulans. Sci. Rep. 2018, 8, 6504. [Google Scholar] [CrossRef] [PubMed]
- Bedini, A.; Mercy, L.; Schneider, C.; Franken, P.; Lucic-Mercy, E. Unraveling the Initial Plant Hormone Signaling, Metabolic Mechanisms and Plant Defense Triggering the Endomycorrhizal Symbiosis Behavior. Front. Plant Sci. 2018, 9, 1800. [Google Scholar] [CrossRef]
- Eichmann, R.; Richards, L.; Schafer, P. Hormones as go-betweens in plant microbiome assembly. Plant J. 2021, 105, 518–541. [Google Scholar] [CrossRef]
- Olds, C.L.; Glennon, E.K.K.; Luckhart, S. Abscisic acid: New perspectives on an ancient universal stress signaling molecule. Microbes Infect. 2018, 20, 484–492. [Google Scholar] [CrossRef]
- Magnone, M.; Ameri, P.; Salis, A.; Andraghetti, G.; Emionite, L.; Murialdo, G.; De Flora, A.; Zocchi, E. Microgram amounts of abscisic acid in fruit extracts improve glucose tolerance and reduce insulinemia in rats and in humans. FASEB J. 2015, 29, 4783–4793. [Google Scholar] [CrossRef]
- Magnone, M.; Sturla, L.; Guida, L.; Spinelli, S.; Begani, G.; Bruzzone, S.; Fresia, C.; Zocchi, E. Abscisic Acid: A Conserved Hormone in Plants and Humans and a Promising Aid to Combat Prediabetes and the Metabolic Syndrome. Nutrients 2020, 12, 1724. [Google Scholar] [CrossRef]
- Negri, P.M.; Maggi, M.D.; Ramirez, L.; De Feudis, L.; Szwarski, N.; Quintana, S.; Equaras, M.J.; Lamarttina, L. Abscisic acid enhances the immune response in Apis mellifera and contributes to the colony fitness. Apidologie 2015, 46, 542–557. [Google Scholar] [CrossRef]
- Khorasani, A.; Abbasnejad, M.; Esmaeili-Mahani, S. Phytohormone abscisic acid ameliorates cognitive impairments in streptozotocin-induced rat model of Alzheimer’s disease through PPARbeta/delta and PKA signaling. Int. J. Neurosci. 2019, 129, 1053–1065. [Google Scholar] [CrossRef]
- Hou, S.T.; Jiang, S.X.; Zaharia, L.I.; Han, X.; Benson, C.L.; Slinn, J.; Abrams, S.R. Phaseic Acid, an Endogenous and Reversible Inhibitor of Glutamate Receptors in Mouse Brain. J. Biol. Chem. 2016, 291, 27007–27022. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic Acid Signaling and Abiotic Stress Tolerance in Plants: A Review on Current Knowledge and Future Prospects. Front. Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Belimov, A.A.; Dodd, I.C.; Safronova, V.I.; Dumova, V.A.; Shaposhnikov, A.I.; Ladatko, A.G.; Davies, W.J. Abscisic acid metabolizing rhizobacteria decrease ABA concentrations in planta and alter plant growth. Plant Physiol. Biochem. 2014, 74, 84–91. [Google Scholar] [CrossRef]
- Yuzikhin, O.S.; Gogoleva, N.E.; Shaposhnikov, A.I.; Konnova, T.A.; Osipova, E.V.; Syrova, D.S.; Ermakova, E.A.; Shevchenko, V.P.; Nagaev, I.Y.; Shevchenko, K.V.; et al. Rhizosphere Bacterium Rhodococcus sp. P1Y Metabolizes Abscisic Acid to Form Dehydrovomifoliol. Biomolecules 2021, 11, 345. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, V.P.; Nagaev, I.Y.; Shaposhnikov, A.I.; Shevchenko, K.V.; Belimov, A.A.; Batasheva, S.N.; Gogoleva, N.E.; Gogolev, Y.V.; Myasoedov, N.F. Synthesis and Testing of Abscisic Acid with Predominant Replacement of Protium Atoms by Tritium in the Cyclohexene Moiety. Dokl. Chem. 2018, 483, 268–271. [Google Scholar] [CrossRef]
- Brown, D.W. Organic Spectroscopy; John and Wiley & Sons: Chichester, NY, USA, 1988. [Google Scholar]
- Bellamy, L.J. The Infra-Red Spectra of Complex Molecules; John and Wiley & Sons: Hoboken, NJ, USA, 1957. [Google Scholar]
- Zocchi, E.; Carpaneto, A.; Cerrano, C.; Bavestrello, G.; Giovine, M.; Bruzzone, S.; Guida, L.; Franco, L.; Usai, C. The temperature-signaling cascade in sponges involves a heat-gated cation channel, abscisic acid, and cyclic ADP-ribose. Proc. Natl. Acad. Sci. USA 2001, 98, 14859–14864. [Google Scholar] [CrossRef]
- Dong, T.; Park, Y.; Hwang, I. Abscisic acid: Biosynthesis, inactivation, homoeostasis and signalling. Essays Biochem. 2015, 58, 29–48. [Google Scholar] [CrossRef]
- Hirai, N.; Yoshida, R.; Todoroki, Y.; Ohigashi, H. Biosynthesis of abscisic acid by the non-mevalonate pathway in plants, and by the mevalonate pathway in fungi. Biosci. Biotechnol. Biochem. 2000, 64, 1448–1458. [Google Scholar] [CrossRef]
- Zhou, R.; Cutler, A.J.; Ambrose, S.J.; Galka, M.M.; Nelson, K.M.; Squires, T.M.; Loewen, M.K.; Jadhav, A.S.; Ross, A.R.; Taylor, D.C.; et al. A new abscisic acid catabolic pathway. Plant Physiol. 2004, 134, 361–369. [Google Scholar] [CrossRef]
- Nambara, E.; Marion-Poll, A. Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant Biol. 2005, 56, 165–185. [Google Scholar] [CrossRef] [PubMed]
- Hewage, K.A.H.; Yang, J.F.; Wang, D.; Hao, G.F.; Yang, G.F.; Zhu, J.K. Chemical Manipulation of Abscisic Acid Signaling: A New Approach to Abiotic and Biotic Stress Management in Agriculture. Adv. Sci. 2020, 7, 2001265. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Poling, S.M.; Mayer, V.P.; Bennett, R.D. Metabolism of abscisic acid: Bacterial conversion to dehydrovomifoliol and vomifoliol dehydrogenase activity. Phytochemistry 1984, 23, 2769–2771. [Google Scholar] [CrossRef]
- Gogoleva, N.E.; Konnova, T.A.; Ismailov, T.T.; Balkin, A.S.; Belimov, A.A.; Gogolev, Y.V. Dataset for transcriptome analysis of abscisic acid degrading bacterium Novosphingobium sp. P6W. Data Brief 2020, 28, 105001. [Google Scholar] [CrossRef]
- Le, T.N.; Mikolasch, A.; Klenk, H.P.; Schauer, F. Degradation of the multiple branched alkane 2,6,10,14-tetramethyl-pentadecane (pristane) in Rhodococcus ruber and Mycobacterium neoaurum. Int. Biodeterior. Biodegrad. 2004, 63, 201–207. [Google Scholar] [CrossRef]
- Le, T.N.; Mikolasch, A.; Awe, S.; Sheikhany, H.; Klenk, H.P.; Schauer, F. Oxidation of aliphatic, branched chain, and aromatic hydrocarbons by Nocardia cyriacigeorgica isolated from oil-polluted sand samples collected in the Saudi Arabian Desert. J. Basic Microbiol. 2010, 50, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Fürst, M.J.L.J.; Gran-Scheuch, A.; Aalbers, F.S.; Fraaije, M.W. Baeyer–Villiger Monooxygenases: Tunable Oxidative Biocatalysts. ACS Catal. 2019, 9, 11207–11241. [Google Scholar] [CrossRef]



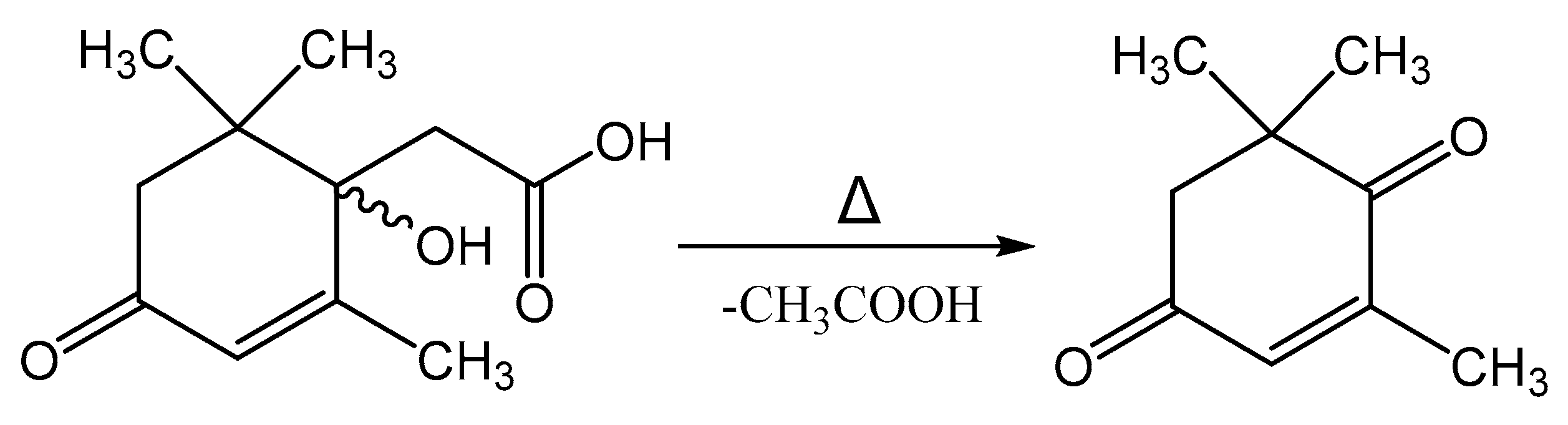
| No. of Fraction 1 | Starting Solvents Ratio, vol.% | Final Solvents Ratio, vol.% |
|---|---|---|
| 1 | A 100 | A 100 |
| 2 | A 100 | A-B 50:50 |
| 3 | A-B 50:50 | A-B 50:50 |
| 4 | A-B 50:50 | B 100 |
| 5–7 | B 100 | B 100 |
| 8 | B 100 | B-C 80:20 |
| 9–11 | B-C 80:20 | B-C 80:20 |
| 12 | B-C 80:20 | B-C 60:40 |
| 13 | B-C 60:40 | B-C 60:40 |
| 14 | B-C 60:40 | B-C 40:60 |
| 15–17 | B-C 40:60 | B-C 40:60 |
| 18 | B-C 40:60 | B-C 20:80 |
| 19 | B-C 20:80 | B-C 20:80 |
| 20 | B-C 20:80 | C 100 |
| 21, 22 | C 100 | C 100 |
| Atom Number | 1H | 13C | Group | ||||
|---|---|---|---|---|---|---|---|
| δ, ppm | J, Hz | m | nH | δ, ppm | nC | ||
| 4′ | 205.7 | 1 | C=O | ||||
| 1 | 183.0 | 1 | C(O)OH | ||||
| 2′ | 174.0 | 1 | Cq | ||||
| 3′ | 5.81 | s | 1 | 127.7 | 1 | HC=C | |
| 1′ | 79.9 | 1 | C–OH | ||||
| 5′a | 2.48 | 17.9 | d | 1 | 51.5 | 1 | CH2 |
| 5′b | 2.18 | 17.9 | d | 1 | |||
| 6′ | 44.2 | 1 | Cq | ||||
| 2a | 2.32 | 15.1 | d | 1 | 42.4 | 1 | CH2 |
| 2b | 2.53 | 15.1 | d | 1 | |||
| 8′ | 0.95 | s | 3 | 25.7 | CH3 | ||
| 9′ | 0.90 | s | 3 | 25.2 | 1 | CH3 | |
| 7′ | 1.92 | s | 3 | 22.3 | 1 | CH3 | |
| Band Maxima (Wavenumber Region), cm−1 | Assignments a,b |
|---|---|
| 3201, v.br. (~3600–3100) | ν(OH) in H-bonded hydroxo- and carboxylic groups |
| 3028 | ν(=C–H) at C3′ |
| 2963 | νas(CH3) |
| 2933 | νas(CH2), C2 |
| 2917 | νas(CH2), C5′ |
| 2876 | νs(CH3) |
| 2852 | νs(CH2) |
| 1707 | ν(C=O), C4′ |
| 1658 | ν(C=C) |
| 1593, br. | νas(COO), carboxylic group |
| 1454 | δas(CH3) |
| ~1465, sh. | δ(CH2), scissoring |
| 1399, br. | νs(COO), carboxylic group |
| ~1372, sh. | δs(CH3) |
| 1338 | δ(–CH=), C5′ |
| 1281 | δ(C–O–H), in-plane (carboxylic group) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuzikhin, O.S.; Shaposhnikov, A.I.; Konnova, T.A.; Syrova, D.S.; Hamo, H.; Ermekkaliev, T.S.; Shevchenko, V.P.; Shevchenko, K.V.; Gogoleva, N.E.; Nizhnikov, A.A.; et al. Isolation and Characterization of 1-Hydroxy-2,6,6-trimethyl-4-oxo-2-cyclohexene-1-acetic Acid, a Metabolite in Bacterial Transformation of Abscisic Acid. Biomolecules 2022, 12, 1508. https://doi.org/10.3390/biom12101508
Yuzikhin OS, Shaposhnikov AI, Konnova TA, Syrova DS, Hamo H, Ermekkaliev TS, Shevchenko VP, Shevchenko KV, Gogoleva NE, Nizhnikov AA, et al. Isolation and Characterization of 1-Hydroxy-2,6,6-trimethyl-4-oxo-2-cyclohexene-1-acetic Acid, a Metabolite in Bacterial Transformation of Abscisic Acid. Biomolecules. 2022; 12(10):1508. https://doi.org/10.3390/biom12101508
Chicago/Turabian StyleYuzikhin, Oleg S., Alexander I. Shaposhnikov, Tatyana A. Konnova, Darya S. Syrova, Hamza Hamo, Taras S. Ermekkaliev, Valerii P. Shevchenko, Konstantin V. Shevchenko, Natalia E. Gogoleva, Anton A. Nizhnikov, and et al. 2022. "Isolation and Characterization of 1-Hydroxy-2,6,6-trimethyl-4-oxo-2-cyclohexene-1-acetic Acid, a Metabolite in Bacterial Transformation of Abscisic Acid" Biomolecules 12, no. 10: 1508. https://doi.org/10.3390/biom12101508
APA StyleYuzikhin, O. S., Shaposhnikov, A. I., Konnova, T. A., Syrova, D. S., Hamo, H., Ermekkaliev, T. S., Shevchenko, V. P., Shevchenko, K. V., Gogoleva, N. E., Nizhnikov, A. A., Safronova, V. I., Kamnev, A. A., Belimov, A. A., & Gogolev, Y. V. (2022). Isolation and Characterization of 1-Hydroxy-2,6,6-trimethyl-4-oxo-2-cyclohexene-1-acetic Acid, a Metabolite in Bacterial Transformation of Abscisic Acid. Biomolecules, 12(10), 1508. https://doi.org/10.3390/biom12101508










