6-(Methylsulfonyl) Hexyl Isothiocyanate: A Chemopreventive Agent Inducing Autophagy in Leukemia Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. 6-MITC
2.3. Cell Culture and Treatment
2.3.1. Jurkat
2.3.2. HL-60
2.4. Flow Cytometry
2.4.1. Autophagy Analysis
2.4.2. ROS Analysis
2.5. Statistical Analysis
3. Results
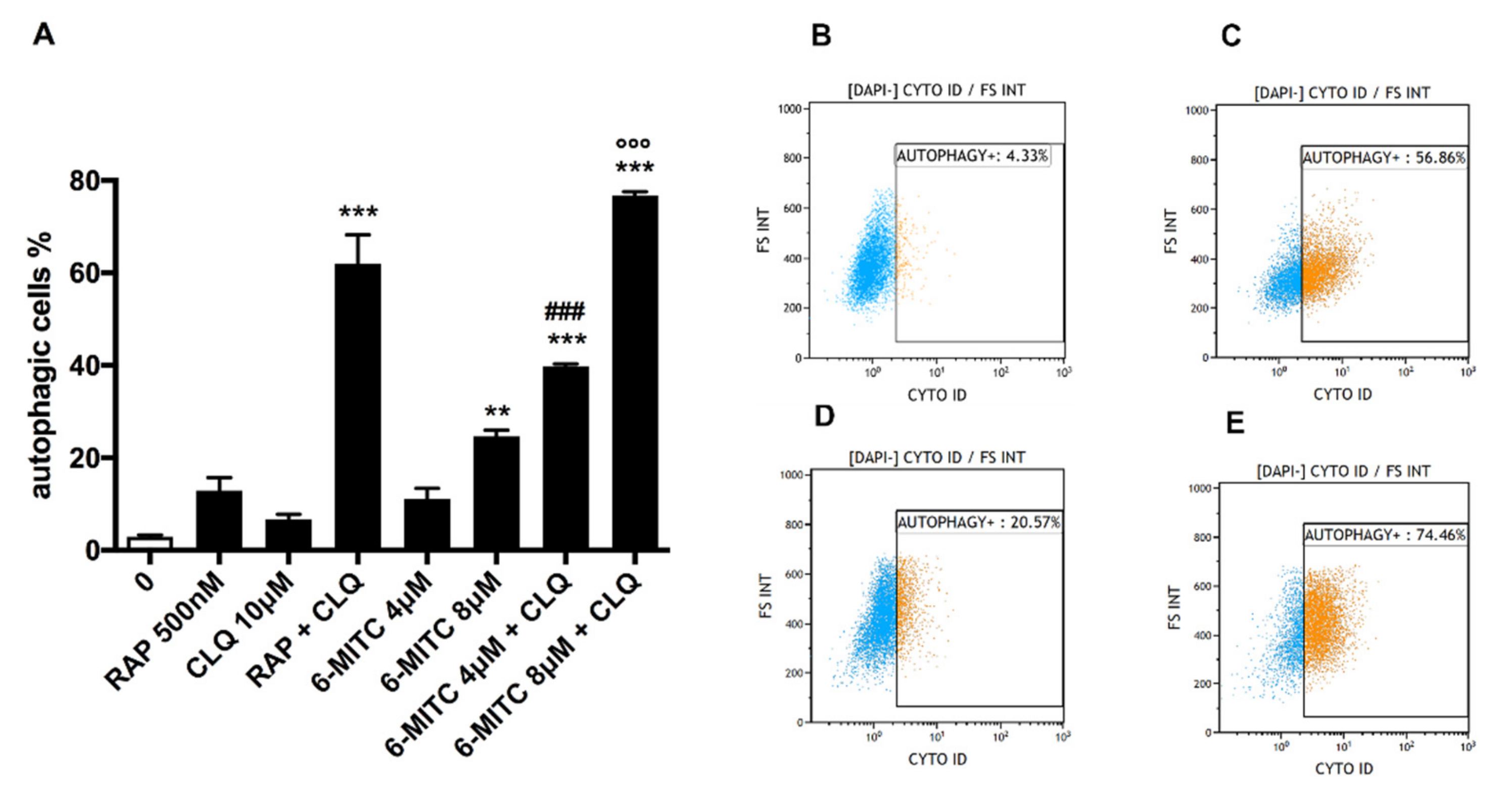
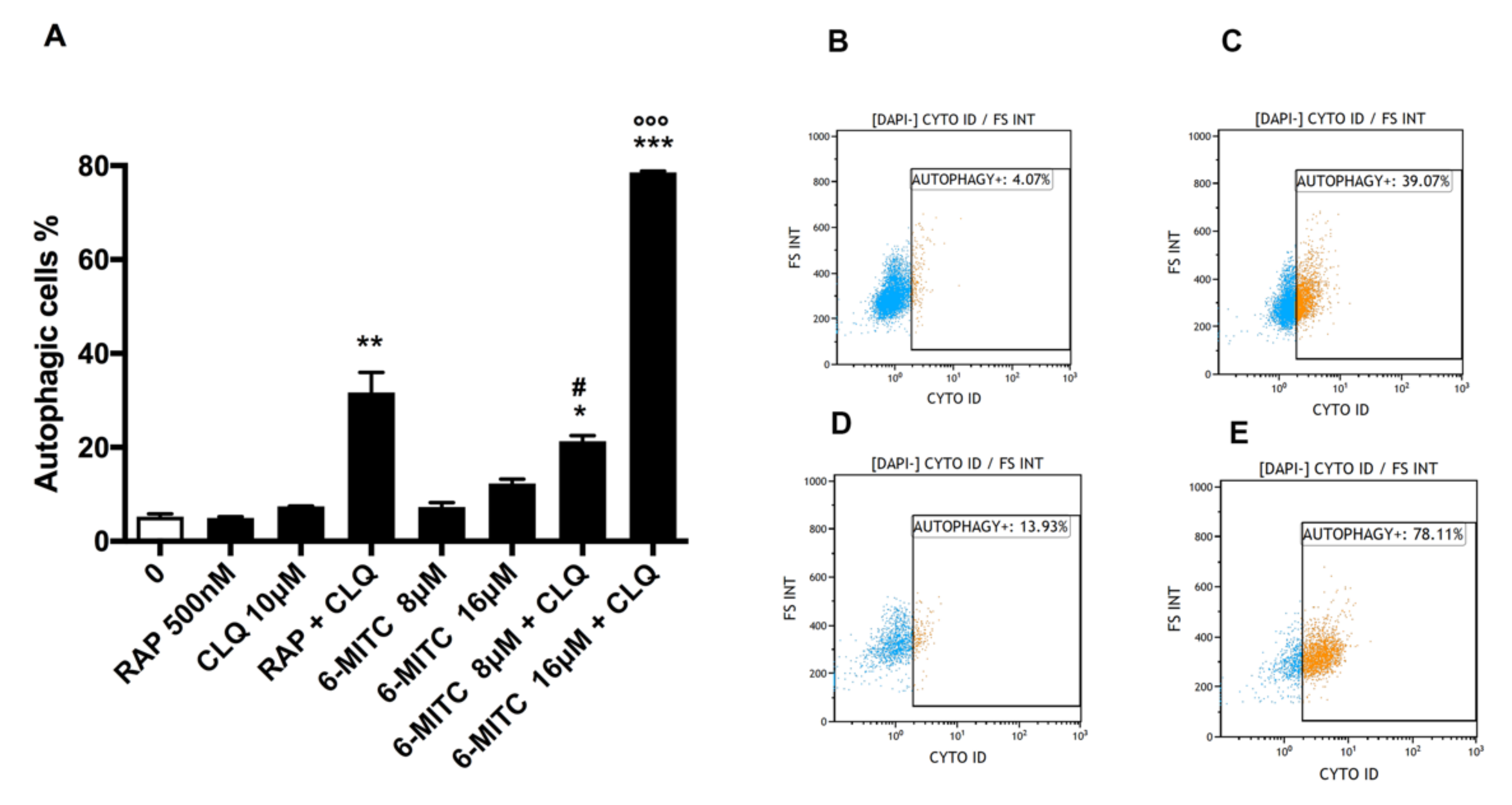
3.1. Autophagy Analysis
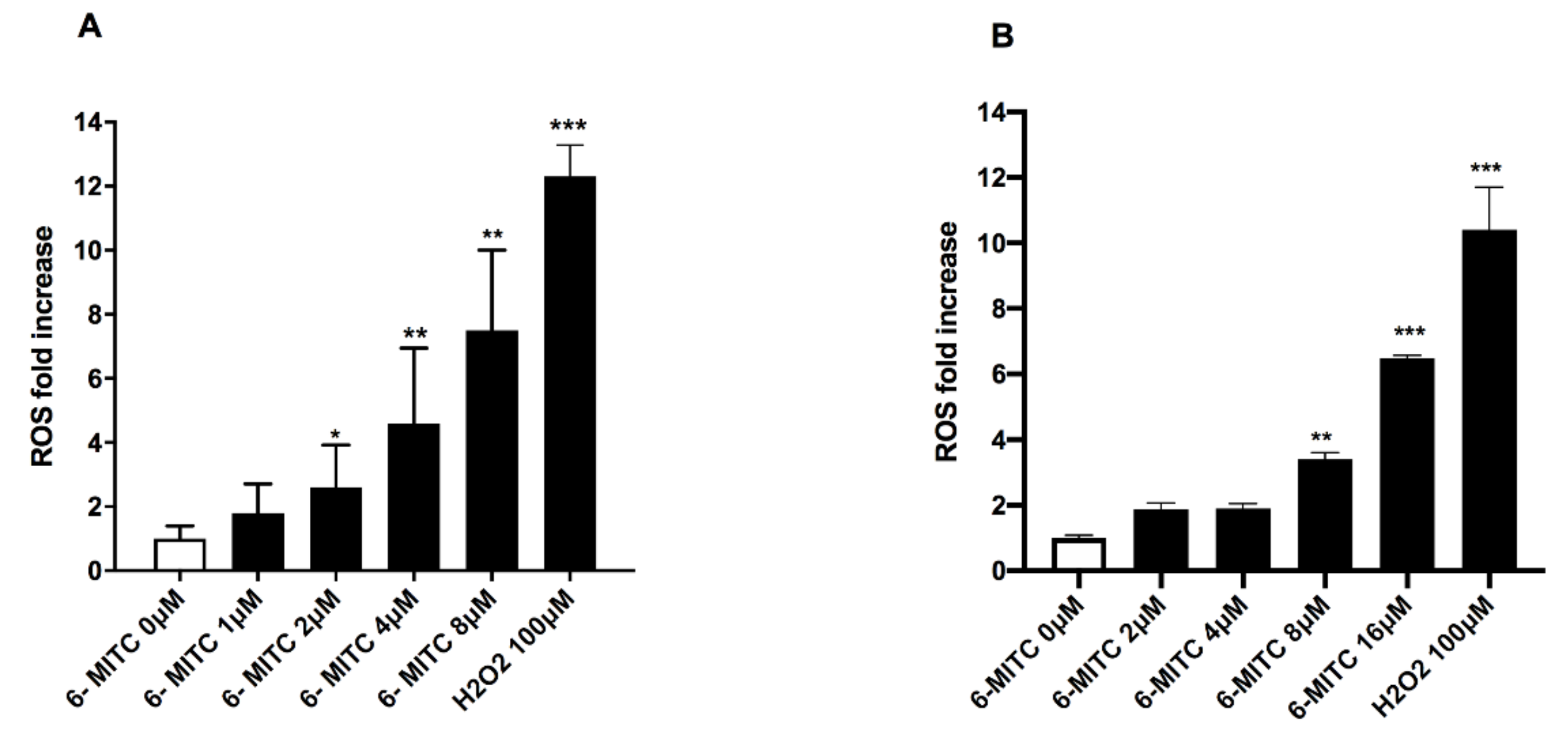
3.2. ROS Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and Molecular Mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef]
- Turek, K.; Jarocki, M.; Kulbacka, J.; Saczko, J. Dualistic Role of Autophagy in Cancer Progression. Adv. Clin. Exp. Med. 2021, 30, 1303–1314. [Google Scholar] [CrossRef]
- Yang, Z.; Klionsky, D.J. Mammalian Autophagy: Core Molecular Machinery and Signaling Regulation. Curr. Opin. Cell Biol. 2010, 22, 124–131. [Google Scholar] [CrossRef]
- Parzych, K.R.; Klionsky, D.J. An Overview of Autophagy: Morphology, Mechanism, and Regulation. Antioxid Redox Signal 2014, 20, 460–473. [Google Scholar] [CrossRef]
- Chen, C.; Gao, H.; Su, X. Autophagy-Related Signaling Pathways Are Involved in Cancer (Review). Exp. Ther. Med. 2021, 22, 710. [Google Scholar] [CrossRef]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of Cells and Tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef]
- Yorimitsu, T.; Klionsky, D.J. Autophagy: Molecular Machinery for Self-Eating. Cell Death Differ. 2005, 12 (Suppl. S2), 1542–1552. [Google Scholar] [CrossRef]
- Saha, S.; Panigrahi, D.P.; Patil, S.; Bhutia, S.K. Autophagy in Health and Disease: A Comprehensive Review. Biomed Pharm. 2018, 104, 485–495. [Google Scholar] [CrossRef]
- Wirawan, E.; Vanden Berghe, T.; Lippens, S.; Agostinis, P.; Vandenabeele, P. Autophagy: For Better or for Worse. Cell Res. 2012, 22, 43–61. [Google Scholar] [CrossRef]
- Levine, B.; Klionsky, D.J. Development by Self-Digestion: Molecular Mechanisms and Biological Functions of Autophagy. Dev. Cell 2004, 6, 463–477. [Google Scholar] [CrossRef]
- Radogna, F.; Dicato, M.; Diederich, M. Cancer-Type-Specific Crosstalk between Autophagy, Necroptosis and Apoptosis as a Pharmacological Target. Biochem. Pharmacol. 2015, 94, 1–11. [Google Scholar] [CrossRef]
- Lin, F.; Zhu, Y.-T.; Qin, Z.-H. Biomarkers of Autophagy. In Autophagy: Biology and Diseases: Technology and Methodology; Xie, Z., Ed.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2021; pp. 265–287. ISBN 9789811628306. [Google Scholar]
- Warnes, G. Flow Cytometric Assays for the Study of Autophagy. Methods 2015, 82, 21–28. [Google Scholar] [CrossRef]
- Xie, Z. (Ed.) Autophagy: Biology and Diseases: Technology and Methodology; Advances in Experimental Medicine and Biology; Springer: Singapore, 2021; Volume 1208, ISBN 9789811628290. [Google Scholar]
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Abudu, Y.P.; Acevedo-Arozena, A.; et al. Guidelines for the Use and Interpretation of Assays for Monitoring Autophagy (4th Edition). Autophagy 2021, 17, 1–382. [Google Scholar] [CrossRef]
- Ohsumi, Y. Historical Landmarks of Autophagy Research. Cell Res. 2014, 24, 9–23. [Google Scholar] [CrossRef]
- Jin, Y.; Hong, Y.; Park, C.Y.; Hong, Y. Molecular Interactions of Autophagy with the Immune System and Cancer. Int. J. Mol. Sci. 2017, 18, 1694. [Google Scholar] [CrossRef]
- Shintani, T.; Klionsky, D.J. Autophagy in Health and Disease: A Double-Edged Sword. Science 2004, 306, 990–995. [Google Scholar] [CrossRef]
- Poillet-Perez, L.; Despouy, G.; Delage-Mourroux, R.; Boyer-Guittaut, M. Interplay between ROS and Autophagy in Cancer Cells, from Tumor Initiation to Cancer Therapy. Redox Biol. 2015, 4, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Kocaturk, N.M.; Akkoc, Y.; Kig, C.; Bayraktar, O.; Gozuacik, D.; Kutlu, O. Autophagy as a Molecular Target for Cancer Treatment. Eur. J. Pharm. Sci. 2019, 134, 116–137. [Google Scholar] [CrossRef]
- Siegel, R.; Ward, E.; Brawley, O.; Jemal, A. Cancer Statistics, 2011: The Impact of Eliminating Socioeconomic and Racial Disparities on Premature Cancer Deaths. CA Cancer J. Clin. 2011, 61, 212–236. [Google Scholar] [CrossRef]
- Durko, L.; Malecka-Panas, E. Lifestyle Modifications and Colorectal Cancer. Curr. Colorectal. Cancer Rep. 2014, 10, 45–54. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. Engl. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Ramachandran, S.; Gupta, N.; Kaushik, I.; Wright, S.; Srivastava, S.; Das, H.; Srivastava, S.; Prasad, S.; Srivastava, S.K. Role of Phytochemicals in Cancer Prevention. Int. J. Mol. Sci. 2019, 20, 4981. [Google Scholar] [CrossRef]
- Nishino, H.; Murakoshi, M.; Mou, X.Y.; Wada, S.; Masuda, M.; Ohsaka, Y.; Satomi, Y.; Jinno, K. Cancer Prevention by Phytochemicals. Oncology 2005, 69 (Suppl. S1), 38–40. [Google Scholar] [CrossRef] [PubMed]
- Ismail, T.; Calcabrini, C.; Diaz, A.R.; Fimognari, C.; Turrini, E.; Catanzaro, E.; Akhtar, S.; Sestili, P. Ellagitannins in Cancer Chemoprevention and Therapy. Toxins 2016, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.-C.; Ho, C.-T.; Pan, M.-H. Recent Advances in Cancer Chemoprevention with Phytochemicals. J. Food Drug Anal. 2020, 28, 14–37. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, M.; Malaguti, M.; Cocchi, V.; Hrelia, S.; Hrelia, P. Castanea Sativa Mill. Bark Extract Exhibits Chemopreventive Properties Triggering Extrinsic Apoptotic Pathway in Jurkat Cells. BMC Complement. Altern. Med. 2017, 17, 251. [Google Scholar] [CrossRef] [PubMed]
- Fimognari, C.; Ferruzzi, L.; Turrini, E.; Carulli, G.; Lenzi, M.; Hrelia, P.; Cantelli-Forti, G. Metabolic and Toxicological Considerations of Botanicals in Anticancer Therapy. Expert Opin. Drug Metab. Toxicol. 2012, 8, 819–832. [Google Scholar] [CrossRef]
- Uto, T.; Hou, D.X.; Morinaga, O.; Shoyama, Y. Molecular Mechanisms Underlying Anti-Inflammatory Actions of 6-(Methylsulfinyl)Hexyl Isothiocyanate Derived from Wasabi (Wasabia Japonica). Adv. Pharmacol. Sci. 2012, 2012, 614046. [Google Scholar] [CrossRef]
- Jaafaru, M.S.; Abd Karim, N.A.; Enas, M.E.; Rollin, P.; Mazzon, E.; Abdull Razis, A.F. Protective Effect of Glucosinolates Hydrolytic Products in Neurodegenerative Diseases (NDDs). Nutrients 2018, 10, 580. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.-X.; Fukuda, M.; Fujii, M.; Fuke, Y. Transcriptional Regulation of Nicotinamide Adenine Dinucleotide Phosphate: Quinone Oxidoreductase in Murine Hepatoma Cells by 6-(Methylsufinyl)Hexyl Isothiocyanate, an Active Principle of Wasabi (Eutrema Wasabi Maxim). Cancer Lett. 2000, 161, 195–200. [Google Scholar] [CrossRef]
- Hou, D.-X.; Korenori, Y.; Tanigawa, S.; Yamada-Kato, T.; Nagai, M.; He, X.; He, J. Dynamics of Nrf2 and Keap1 in ARE-Mediated NQO1 Expression by Wasabi 6-(Methylsulfinyl)Hexyl Isothiocyanate. J. Agric. Food Chem. 2011, 59, 11975–11982. [Google Scholar] [CrossRef]
- Mizuno, K.; Kume, T.; Muto, C.; Takada-Takatori, Y.; Izumi, Y.; Sugimoto, H.; Akaike, A. Glutathione Biosynthesis via Activation of the Nuclear Factor E2–Related Factor 2 (Nrf2)–Antioxidant-Response Element (ARE) Pathway Is Essential for Neuroprotective Effects of Sulforaphane and 6-(Methylsulfinyl) Hexyl Isothiocyanate. J. Pharmacol. Sci. 2011, 115, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Trio, P.Z.; Fujisaki, S.; Tanigawa, S.; Hisanaga, A.; Sakao, K.; Hou, D.-X. DNA Microarray Highlights Nrf2-Mediated Neuron Protection Targeted by Wasabi-Derived Isothiocyanates in IMR-32 Cells. Gene Regul. Syst. Biol. 2016, 10, GRSB.S39440. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, N.; Matsumoto, Y.; Hoshino, A.; Iwashita, K. Comparison of Effects of Wasabia Japonica and Allyl Isothiocyanate on the Growth of Four Strains of Vibrio Parahaemolyticus in Lean and Fatty Tuna Meat Suspensions. Int. J. Food Microbiol. 1999, 49, 27–34. [Google Scholar] [CrossRef]
- Ko, M.-O.; Kim, M.-B.; Lim, S.-B. Relationship between Chemical Structure and Antimicrobial Activities of Isothiocyanates from Cruciferous Vegetables against Oral Pathogens. J. Microbiol. Biotechnol. 2016, 26, 2036–2042. [Google Scholar] [CrossRef]
- Kitakaze, T.; Yuan, S.; Inoue, M.; Yoshioka, Y.; Yamashita, Y.; Ashida, H. 6-(Methylsulfinyl)Hexyl Isothiocyanate Protects Acetaldehyde-Caused Cytotoxicity through the Induction of Aldehyde Dehydrogenase in Hepatocytes. Arch. Biochem. Biophys. 2020, 686, 108329. [Google Scholar] [CrossRef]
- Morimitsu, Y.; Hayashi, K.; Nakagawa, Y.; Fujii, H.; Horio, F.; Uchida, K.; Osawa, T. Antiplatelet and Anticancer Isothiocyanates in Japanese Domestic Horseradish, Wasabi. Mech. Ageing Dev. 2000, 116, 125–134. [Google Scholar] [CrossRef]
- Fuke, Y.; Haga, Y.; Ono, H.; Nomura, T.; Ryoyama, K. Anti-Carcinogenic Activity of 6-Methylsulfinylhexyl Isothiocyanate-, an Active Anti-Proliferative Principal of Wasabi (Eutrema Wasabi Maxim.). Cytotechnology 1997, 25, 197. [Google Scholar] [CrossRef]
- Hou, D.X.; Fukuda, M.; Fujii, M.; Fuke, Y. Induction of NADPH:Quinone Oxidoreductase in Murine Hepatoma Cells by Methylsulfinyl Isothiocyanates: Methyl Chain Length-Activity Study. Int. J. Mol. Med. 2000, 6, 441–445. [Google Scholar] [CrossRef]
- Nomura, T.; Shinoda, S.; Yamori, T.; Sawaki, S.; Nagata, I.; Ryoyama, K.; Fuke, Y. Selective Sensitivity to Wasabi-Derived 6-(Methylsulfinyl)Hexyl Isothiocyanate of Human Breast Cancer and Melanoma Cell Lines Studied in Vitro. Cancer Detect. Prev. 2005, 29, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Uehara, Y.; Kawajiri, H.; Ryoyama, K.; Yamori, T.; Fuke, Y. Alkyl Isothiocyanates Suppress Epidermal Growth Factor Receptor Kinase Activity but Augment Tyrosine Kinase Activity. Cancer Epidemiol. 2009, 33, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Huang, Y.-C.; Tsai, T.-H.; Liao, H.-F. Effect of Wasabi Component 6-(Methylsulfinyl)Hexyl Isothiocyanate and Derivatives on Human Pancreatic Cancer Cells. Evid.-Based Complement. Altern. Med. 2014, 2014, 494739. [Google Scholar] [CrossRef]
- Fuke, Y.; Hishinuma, M.; Namikawa, M.; Oishi, Y.; Matsuzaki, T. Wasabi-Derived 6-(Methylsulfinyl)Hexyl Isothiocyanate Induces Apoptosis in Human Breast Cancer by Possible Involvement of the NF-ΚB Pathways. Nutr. Cancer 2014, 66, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Hsuan, S.-W.; Chyau, C.-C.; Hung, H.-Y.; Chen, J.-H.; Chou, F.-P. The Induction of Apoptosis and Autophagy by Wasabia Japonica Extract in Colon Cancer. Eur. J. Nutr. 2016, 55, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Wu, S.; Sakao, K.; Hou, D.-X. Wasabi 6-(Methylsulfinyl)Hexyl Isothiocyanate Induces Apoptosis in Human Colorectal Cancer Cells through P53-Independent Mitochondrial Dysfunction Pathway. BioFactors 2018, 44, 361–368. [Google Scholar] [CrossRef]
- Lee, M.-J.; Tseng, W.-S.; Lai, J.C.-Y.; Shieh, H.-R.; Chi, C.-W.; Chen, Y.-J. Differential Pharmacological Activities of Oxygen Numbers on the Sulfoxide Moiety of Wasabi Compound 6-(Methylsulfinyl) Hexyl Isothiocyanate in Human Oral Cancer Cells. Molecules 2018, 23, 2427. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Wu, S.; Sakao, K.; Hou, D.-X. Involvement of ERK1/2-Mediated ELK1/CHOP/DR5 Pathway in 6-(Methylsulfinyl)Hexyl Isothiocyanate-Induced Apoptosis of Colorectal Cancer Cells. Biosci. Biotechnol. Biochem. 2019, 83, 960–969. [Google Scholar] [CrossRef]
- Wu, K.-M.; Liao, H.-F.; Chi, C.-W.; Kou, Y.R.; Chen, Y.-J. Wasabi Compound 6-(Methylsulfinyl) Hexyl Isothiocyanate Induces Cell Death with Coexisting Mitotic Arrest and Autophagy in Human Chronic Myelogenous Leukemia K562 Cells. Biomolecules 2019, 9, 774. [Google Scholar] [CrossRef]
- Fuke, Y.; Shinoda, S.; Nagata, I.; Sawaki, S.; Murata, M.; Ryoyama, K.; Koizumi, K.; Saiki, I.; Nomura, T. Preventive Effect of Oral Administration of 6-(Methylsulfinyl)Hexyl Isothiocyanate Derived from Wasabi (Wasabia Japonica Matsum) against Pulmonary Metastasis of B16-BL6 Mouse Melanoma Cells. Cancer Detect. Prev. 2006, 30, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, M.; Cocchi, V.; Malaguti, M.; Barbalace, M.C.; Marchionni, S.; Hrelia, S.; Hrelia, P. 6-(Methylsulfonyl) Hexyl Isothiocyanate as Potential Chemopreventive Agent: Molecular and Cellular Profile in Leukaemia Cell Lines. Oncotarget 2017, 8, 111697–111714. [Google Scholar] [CrossRef]
- Cocchi, V.; Hrelia, P.; Lenzi, M. Antimutagenic and Chemopreventive Properties of 6-(Methylsulfinyl) Hexyl Isothiocyanate on TK6 Human Cells by Flow Cytometry. Front. Pharmacol. 2020, 11, 1242. [Google Scholar] [CrossRef]
- CYTO-ID® Autophagy Detection Kit 2.0 Product Manual. Available online: https://www.enzolifesciences.com/fileadmin/files/manual/ENZ-KIT175_insert.pdf (accessed on 10 September 2022).
- O’Connor, J.-E.; Herrera, G.; Sala-de-Oyanguren, F.; Jávega, B.; Martínez-Romero, A. Cytomics of Oxidative Stress: Probes and Problems. In Single Cell Analysis: Contemporary Research and Clinical Applications; Robinson, J.P., Cossarizza, A., Eds.; Series in BioEngineering; Springer: Singapore, 2017; pp. 83–118. ISBN 978-981-10-4499-1. [Google Scholar]
- Chan, L.L.-Y.; Shen, D.; Wilkinson, A.R.; Patton, W.; Lai, N.; Chan, E.; Kuksin, D.; Lin, B.; Qiu, J. A Novel Image-Based Cytometry Method for Autophagy Detection in Living Cells. Autophagy 2012, 8, 1371–1382. [Google Scholar] [CrossRef] [PubMed]
- Manson, M.M.; Gescher, A.; Hudson, E.A.; Plummer, S.M.; Squires, M.S.; Prigent, S.A. Blocking and Suppressing Mechanisms of Chemoprevention by Dietary Constituents. Toxicol. Lett. 2000, 112–113, 499–505. [Google Scholar] [CrossRef]
- Maru, G.B.; Hudlikar, R.R.; Kumar, G.; Gandhi, K.; Mahimkar, M.B. Understanding the Molecular Mechanisms of Cancer Prevention by Dietary Phytochemicals: From Experimental Models to Clinical Trials. World J. Biol. Chem. 2016, 7, 88–99. [Google Scholar] [CrossRef]
- Lenzi, M.; Fimognari, C.; Hrelia, P. Sulforaphane as a Promising Molecule for Fighting Cancer. In Proceedings of the Advances in Nutrition and Cancer; Zappia, V., Panico, S., Russo, G.L., Budillon, A., Della Ragione, F., Eds.; Springer: Berlin, Heidelberg, 2014; pp. 207–223. [Google Scholar]
- Prata, C.; Facchini, C.; Leoncini, E.; Lenzi, M.; Maraldi, T.; Angeloni, C.; Zambonin, L.; Hrelia, S.; Fiorentini, D. Sulforaphane Modulates AQP8-Linked Redox Signalling in Leukemia Cells. Oxidative Med. Cell. Longev. 2018, 2018, e4125297. [Google Scholar] [CrossRef]
- Mangla, B.; Javed, S.; Sultan, M.H.; Kumar, P.; Kohli, K.; Najmi, A.; Alhazmi, H.A.; Al Bratty, M.; Ahsan, W. Sulforaphane: A Review of Its Therapeutic Potentials, Advances in Its Nanodelivery, Recent Patents, and Clinical Trials. Phytother. Res. 2021, 35, 5440–5458. [Google Scholar] [CrossRef]
- Słoczyńska, K.; Pękala, E.; Wajda, A.; Węgrzyn, G.; Marona, H. Evaluation of Mutagenic and Antimutagenic Properties of Some Bioactive Xanthone Derivatives Using Vibrio Harveyi Test. Lett. Appl. Microbiol. 2010, 50, 252–257. [Google Scholar] [CrossRef]
- Basu, A.K. DNA Damage, Mutagenesis, and Cancer. Int. J. Mol. Sci. 2018, 19, 970. [Google Scholar] [CrossRef]
- Navarro, M.; Bellmunt, J.; Balañá, C.; Colomer, R.; Jolis, L.; Campo, J.M. del Mitomycin-C and Vinblastine in Advanced Breast Cancer. OCL 1989, 46, 137–142. [Google Scholar] [CrossRef]
- Attaallah, A.; Lenzi, M.; Marchionni, S.; Bincoletto, G.; Cocchi, V.; Croco, E.; Hrelia, P.; Hrelia, S.; Sell, C.; Lorenzini, A. A pro Longevity Role for Cellular Senescence. GeroScience 2020, 42, 867–879. [Google Scholar] [CrossRef]
- Singh, S.S.; Vats, S.; Chia, A.Y.-Q.; Tan, T.Z.; Deng, S.; Ong, M.S.; Arfuso, F.; Yap, C.T.; Goh, B.C.; Sethi, G.; et al. Dual Role of Autophagy in Hallmarks of Cancer. Oncogene 2018, 37, 1142–1158. [Google Scholar] [CrossRef]
- Lim, S.M.; Mohamad Hanif, E.A.; Chin, S.-F. Is Targeting Autophagy Mechanism in Cancer a Good Approach? The Possible Double-Edge Sword Effect. Cell Biosci. 2021, 11, 56. [Google Scholar] [CrossRef]
- Kimura, T.; Takabatake, Y.; Takahashi, A.; Isaka, Y. Chloroquine in Cancer Therapy: A Double-Edged Sword of Autophagy. Cancer Res. 2013, 73, 3–7. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, X.; Wang, S.; Fan, J.; Wang, Z.; Song, P.; Mei, X.; Ju, D. Blocking Autophagy Enhanced Leukemia Cell Death Induced by Recombinant Human Arginase. Tumour. Biol. 2016, 37, 6627–6635. [Google Scholar] [CrossRef]
- Xia, T.; Wang, J.; Wang, Y.; Wang, Y.; Cai, J.; Wang, M.; Chen, Q.; Song, J.; Yu, Z.; Huang, W.; et al. Inhibition of Autophagy Potentiates Anticancer Property of 20(S)-Ginsenoside Rh2 by Promoting Mitochondria-Dependent Apoptosis in Human Acute Lymphoblastic Leukaemia Cells. Oncotarget 2016, 7, 27336–27349. [Google Scholar] [CrossRef] [PubMed]
- Amaravadi, R.K.; Kimmelman, A.C.; Debnath, J. Targeting Autophagy in Cancer: Recent Advances and Future Directions. Cancer Discov. 2019, 9, 1167–1181. [Google Scholar] [CrossRef]
- Gibellini, L.; De Biasi, S.; Pinti, M.; Nasi, M.; Riccio, M.; Carnevale, G.; Cavallini, G.M.; Sala de Oyanguren, F.J.; O’Connor, J.E.; Mussini, C.; et al. The Protease Inhibitor Atazanavir Triggers Autophagy and Mitophagy in Human Preadipocytes. AIDS 2012, 26, 2017–2026. [Google Scholar] [CrossRef]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of Cancer Cell Metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Loveless, J.; Shay, C.; Teng, Y. Targeting ROS-Mediated Crosstalk Between Autophagy and Apoptosis in Cancer. Adv. Exp. Med. Biol. 2020, 1260, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Booth, L.A.; Tavallai, S.; Hamed, H.A.; Cruickshanks, N.; Dent, P. The Role of Cell Signalling in the Crosstalk between Autophagy and Apoptosis. Cell Signal 2014, 26, 549–555. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cocchi, V.; Jávega, B.; Gasperini, S.; O’Connor, J.-E.; Lenzi, M.; Hrelia, P. 6-(Methylsulfonyl) Hexyl Isothiocyanate: A Chemopreventive Agent Inducing Autophagy in Leukemia Cell Lines. Biomolecules 2022, 12, 1485. https://doi.org/10.3390/biom12101485
Cocchi V, Jávega B, Gasperini S, O’Connor J-E, Lenzi M, Hrelia P. 6-(Methylsulfonyl) Hexyl Isothiocyanate: A Chemopreventive Agent Inducing Autophagy in Leukemia Cell Lines. Biomolecules. 2022; 12(10):1485. https://doi.org/10.3390/biom12101485
Chicago/Turabian StyleCocchi, Veronica, Beatriz Jávega, Sofia Gasperini, José-Enrique O’Connor, Monia Lenzi, and Patrizia Hrelia. 2022. "6-(Methylsulfonyl) Hexyl Isothiocyanate: A Chemopreventive Agent Inducing Autophagy in Leukemia Cell Lines" Biomolecules 12, no. 10: 1485. https://doi.org/10.3390/biom12101485
APA StyleCocchi, V., Jávega, B., Gasperini, S., O’Connor, J.-E., Lenzi, M., & Hrelia, P. (2022). 6-(Methylsulfonyl) Hexyl Isothiocyanate: A Chemopreventive Agent Inducing Autophagy in Leukemia Cell Lines. Biomolecules, 12(10), 1485. https://doi.org/10.3390/biom12101485






