Comparative Genomic Analysis Uncovers the Chloroplast Genome Variation and Phylogenetic Relationships of Camellia Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. DNA Sequencing and Chloroplast Genome Assembly
2.3. Genome Annotation and Visualization
2.4. Sequence Divergence Analysis
2.5. Selection Pressure Analysis
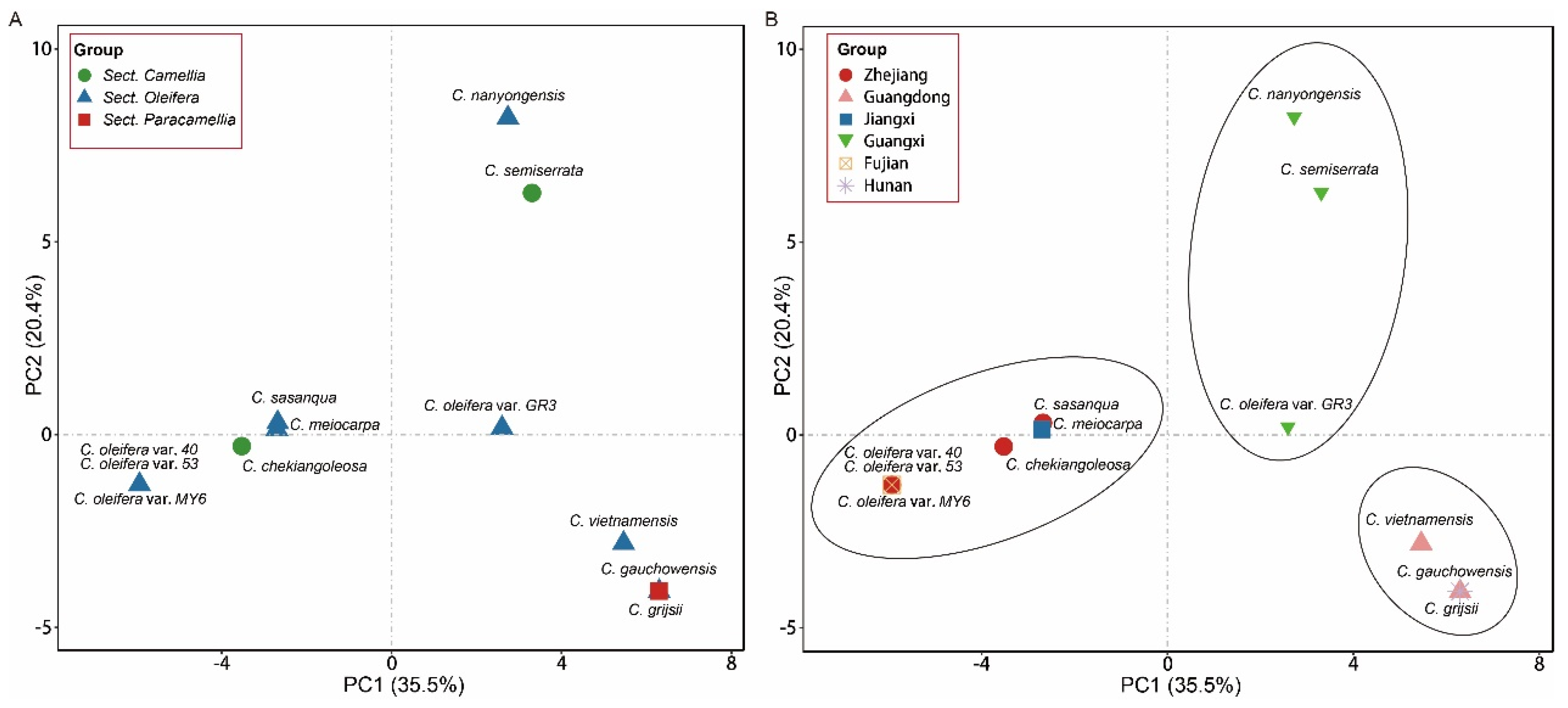
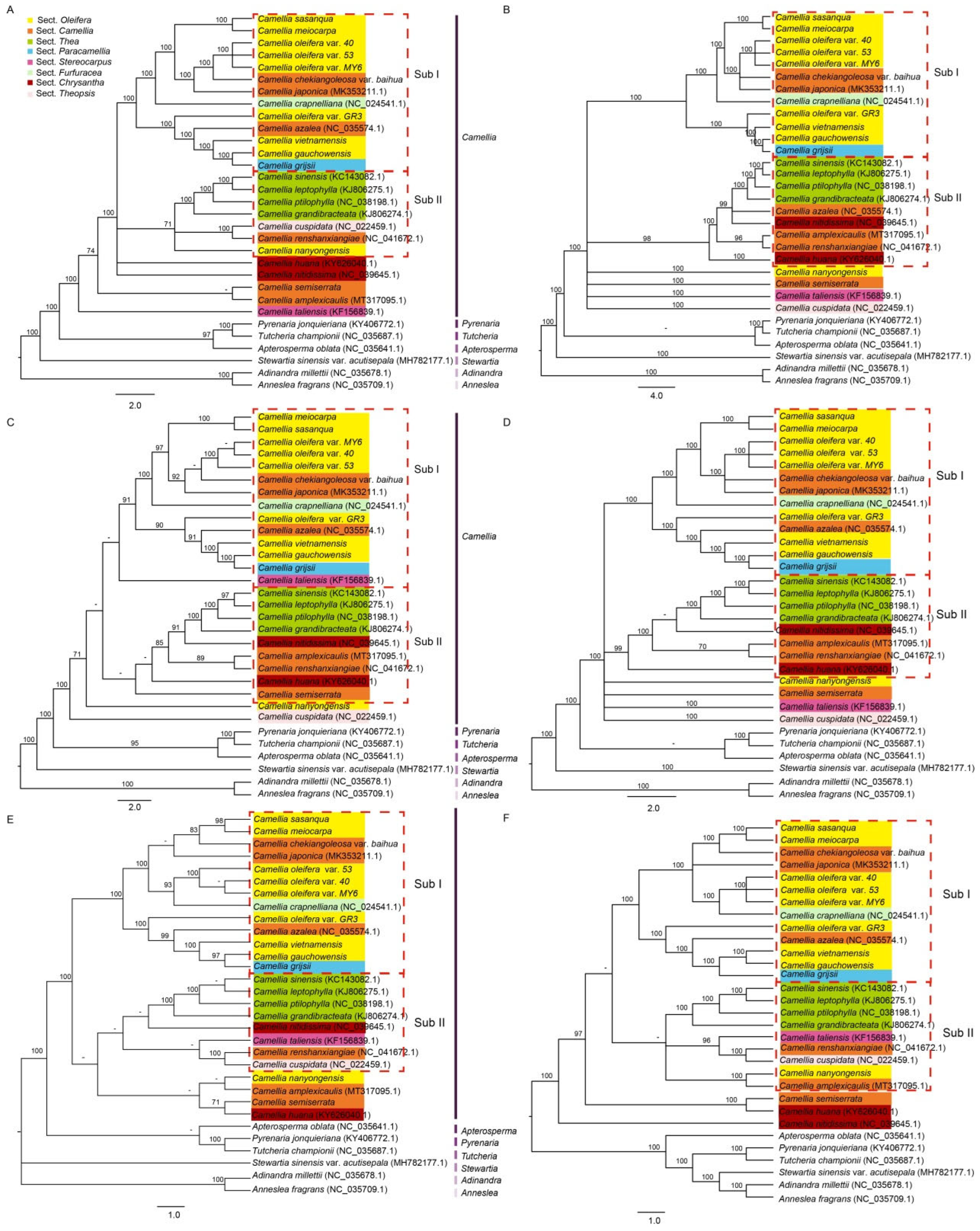
2.6. Principal Component Analysis (PCA) and Phylogenetic Analysis
3. Results
3.1. Chloroplast Genome Sequencing and Assembly
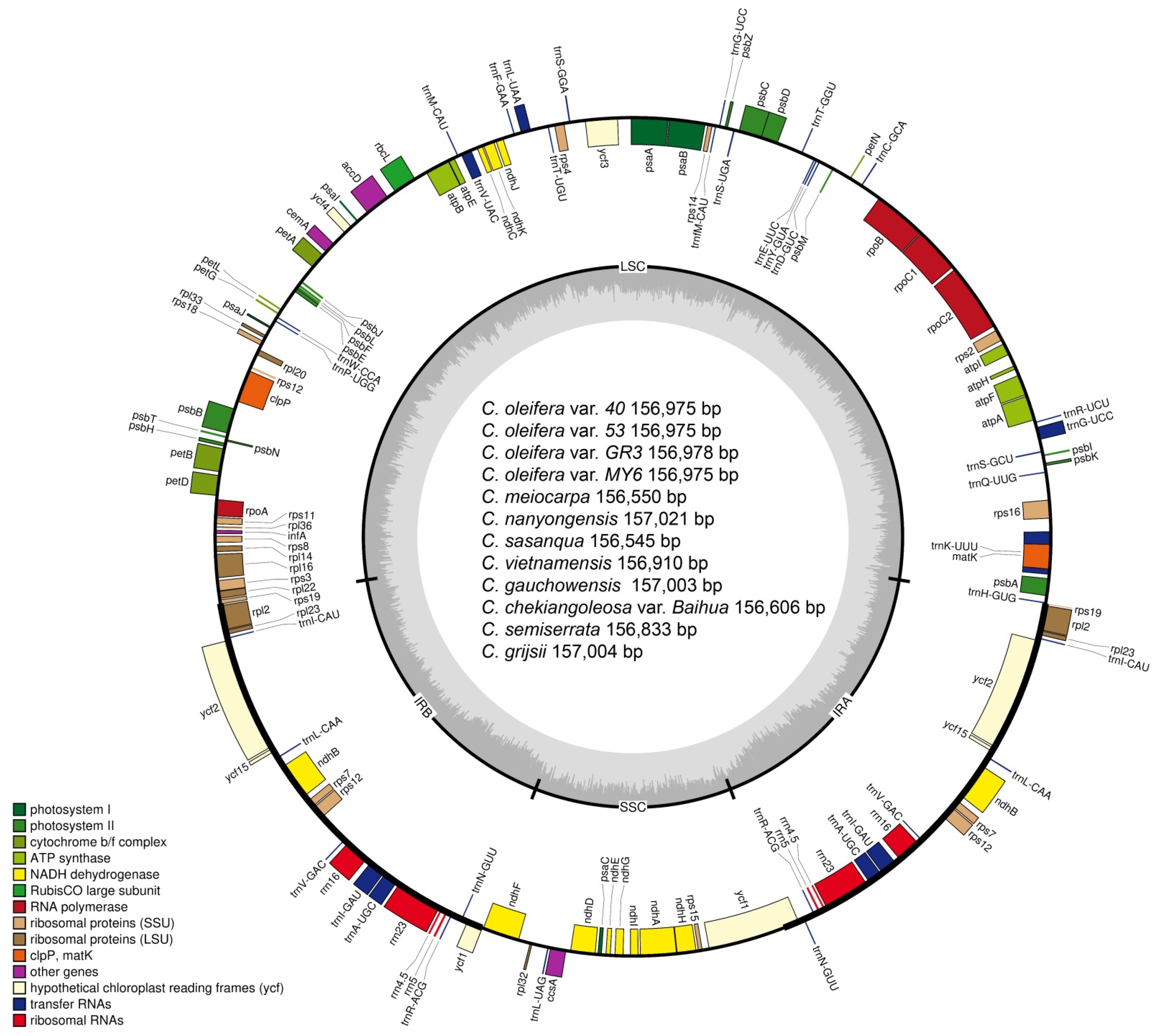
3.2. Characterization of Chloroplast Genomes of Selected Camellia Species
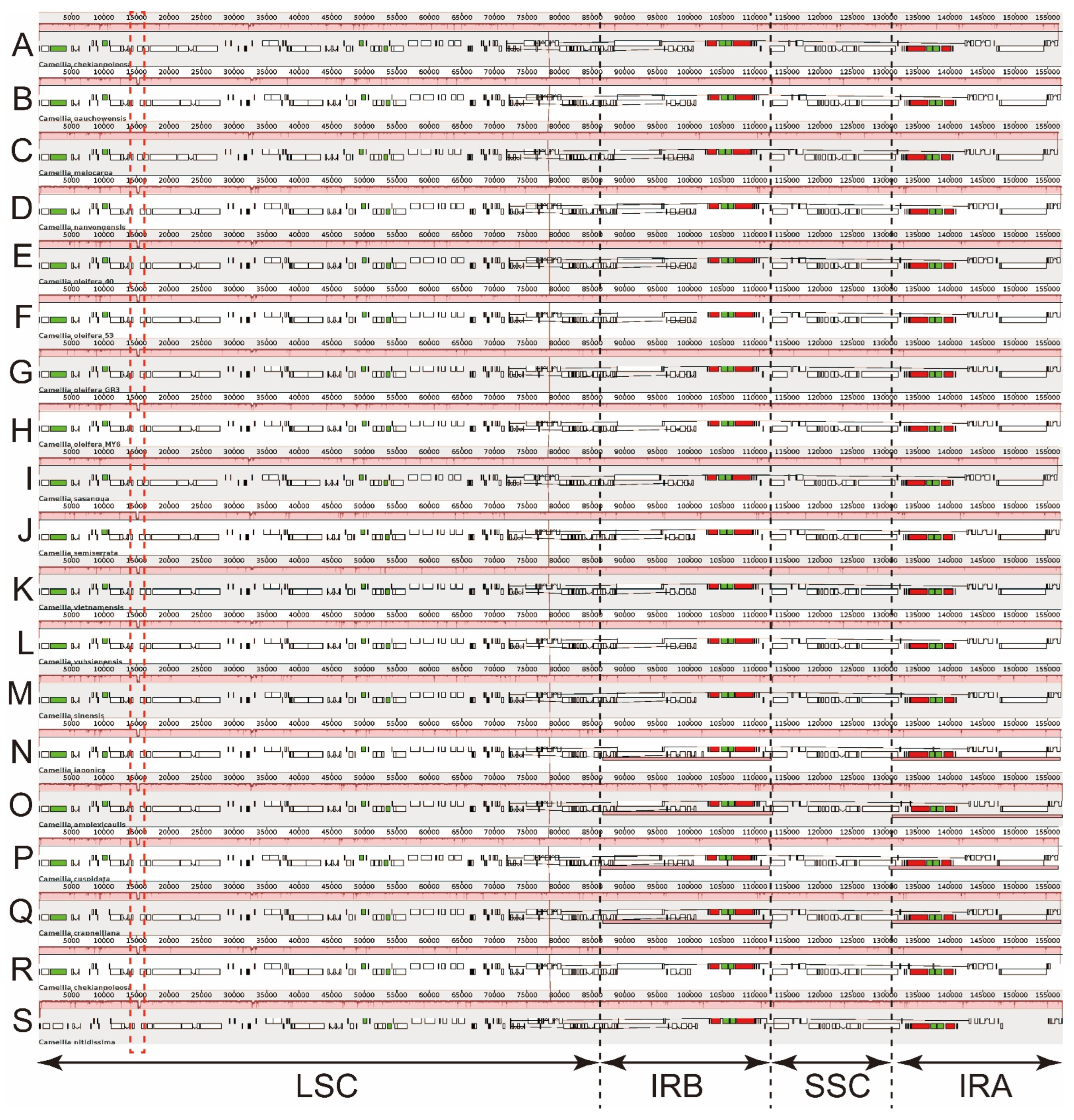
3.3. Expansion and Contraction of the Border Regions
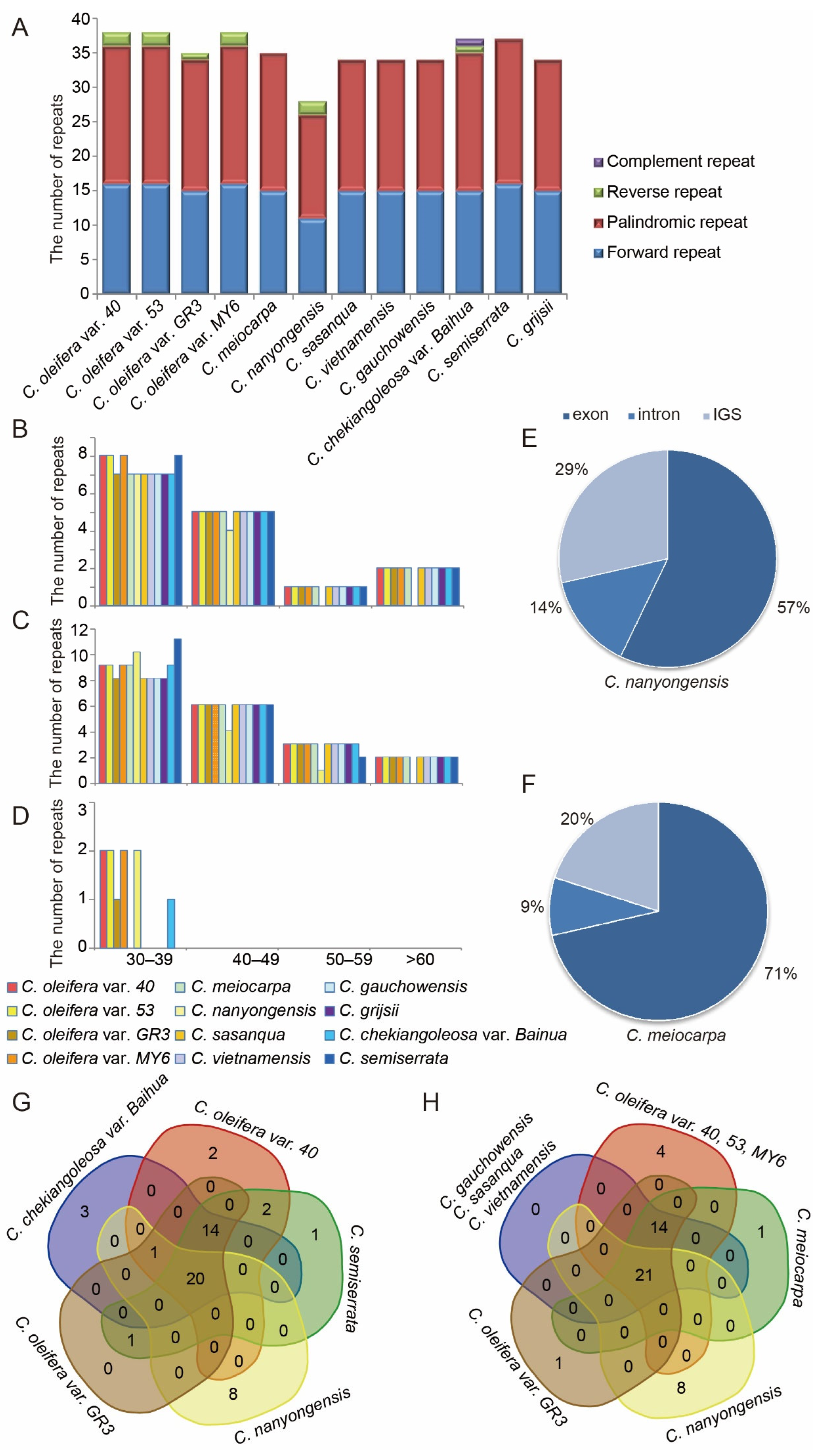
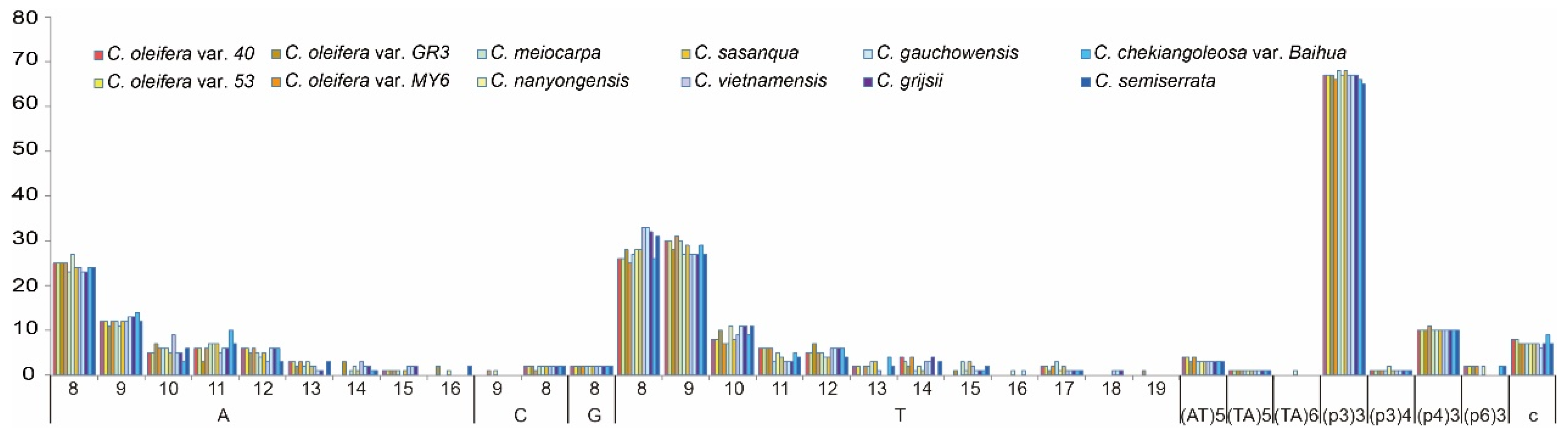
3.4. Repeat Sequences and Microsatellite Analyses
3.5. SNP and InDel Variations
3.6. Sequence Divergence and Hotspots
3.7. Estimation of the Selection Pressure of the Camellia Cp Genomes
3.8. PCA and Phylogenetic Analysis of Camellia Species
4. Discussion
4.1. Materials
4.2. Genome Organization
4.3. Sequence Divergence
4.4. Genome Divergent Hotspot Regions
4.5. Inference of Phylogenetic Relationships
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Min, T.; Bruce, B. Flora of China; Science Press: Beijing, China, 2010. [Google Scholar]
- Vijayan, K.; Zhang, W.J.; Tsou, C.H. Molecular taxonomy of Camellia (Theaceae) inferred from nrITS sequences. Am. J. Bot. 2009, 96, 1348–1360. [Google Scholar] [CrossRef]
- Xia, E.H.; Zhang, H.B.; Sheng, J.; Li, K.; Zhang, Q.J.; Kim, C.; Zhang, Y.; Liu, y.; Zhu, T.; Li, W.; et al. The tea tree genome provides insights into tea flavor and independent evolution of caffeine biosynthesis. Mol. Plant. 2017, 10, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Min, T.L.; Zhang, W.J. The evolution and distribution of genus Camellia. Acta Bot. Yunnanica. 1996, 18, 1–13. [Google Scholar]
- Zhang, H.; Ren, S. Theaceae. Flora China 2008, 49, 1–91. [Google Scholar]
- Huang, H.; Shi, C.; Liu, Y.; Mao, S.; Gao, L. Thirteen Camellia chloroplast genome sequences determined by high-throughput sequencing: Genome structure and phylogenetic relationships. BMC Evol. Biol. 2014, 14, 151–167. [Google Scholar] [CrossRef]
- Balasaravanan, T.; Pius, P.K.; Kumar, R.R.; Muraleedharan, N.; Shasany, A.K. Genetic diversity among south Indian tea germplasm (Camellia sinensis, C. assamica and C. assamica spp. lasiocalyx) using AFLP markers. Plant Sci. 2003, 165, 365–372. [Google Scholar]
- Wang, L.Y.; Liu, B.Y.; Jiang, Y.H.; Duan, Y.S.; Cheng, H.; Zhou, J.; Tang, Y.C. Phylogenetic analysis of interspecies in section thea through SSR markers. J. Tea Sci. 2009, 29, 341–346. [Google Scholar]
- Chen, L.; Yamaguchi, S.; Wang, P.; Xu, M.; Song, W.; Tong, Q. Genetic polymorphism and molecular phylogeny analysis of section Thea based on RAPD markers. J. Tea Sci. 2002, 22, 19–24. [Google Scholar]
- Tian, M.; Li, J.; Ni, S.; Fan, Z.; Li, X. Phylogenetic study on section Camellia based on ITS sequences data. Acta Hortic. Sin. 2008, 35, 1685–1688. [Google Scholar]
- Yang, J.B.; Yang, S.X.; Li, H.T.; Yang, J.; Li, D.Z. Comparative chloroplast genomes of Camellia species. PLoS ONE 2013, 8, e73053. [Google Scholar] [CrossRef]
- Wang, G.; Luo, Y.; Hou, N.; Deng, L. The complete chloroplast genomes of three rare and endangered camellias (Camellia huana, C. liberofilamenta and C. luteoflora) endemic to Southwest China. Conserv. Genet. Resour. 2017, 9, 583–585. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, G. The complete chloroplast genome of Camellia gauchowensis and its phylogenetic analysis. Mitochondrial. DNA B Resour. 2020, 5, 2299–2300. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, N.; Okubo, H.; Ozaki, Y. Chloroplast DNA phylogeography in the genus Camellia in Japan. Acta Hortic. 2010, 885, 367–373. [Google Scholar] [CrossRef]
- Jansen, R.K.; Cai, Z.; Raubeson, L.A.; Daniell, H.; Boore, J.L. Analysis of 81 genes from 64 plastid genomes resolves relationships in angiosperms and identifies genome-scale evolutionary patterns. Proc. Natl. Acad. Sci. USA 2008, 104, 19369–19374. [Google Scholar] [CrossRef]
- Parks, M.; Cronn, R.; Liston, A. Increasing phylogenetic resolution at low taxonomic levels using massively parallel sequencing of chloroplast genomes. BMC Biol. 2009, 7, 84. [Google Scholar] [CrossRef]
- Moore, M.J.; Soltis, P.S.; Bell, C.D.; Burleigh, J.G.; Donoghue, S. Phylogenetic analysis of 83 plastid genes further resolves the early diversification of eudicots. Proc. Natl. Acad. Sci. USA 2010, 107, 4623–4628. [Google Scholar] [CrossRef] [PubMed]
- McCauley, D.E.; Stevens, J.E. The spatial distribution of chloroplast DNA and allozyme polymorphisms within a population of Silene alba (Caryophyllaceae). Am. J. Bot. 1996, 83, 727–731. [Google Scholar] [CrossRef]
- Small, R.L.; Cronn, R.C.; Wendel, J.F. Use of nuclear genes for phylogeny reconstruction in plants. Aust. Syst. Bot. 2004, 17, 145–170. [Google Scholar] [CrossRef]
- Kim, G.B.; Lim, C.E.; Kim, J.S.; Kim, K.; Lee, J.H.; Yu, H.J.; Mun, J.H. Comparative chloroplast genome analysis of Artemisia (Asteraceae) in East Asia: Insights into evolutionary divergence and phylogenomic implications. BMC Genom. 2020, 21, 415. [Google Scholar] [CrossRef]
- Huang, H.; Tong, Y.; Zhang, Q.J.; Gao, L.Z. Genome size variation among and within Camellia species by using flow cytometric analysis. PLoS ONE. 2013, 8, e64981. [Google Scholar] [CrossRef]
- Wei, C.; Yang, H.; Wang, S.; Zhao, J.; Liu, C.; Gao, L.; Xia, E.; Lu, Y.; Tai, Y.; She, G.; et al. Draft genome sequence of Camellia sinensis var. sinensis provides insights into the evolution of the tea genome and tea quality. Proc. Natl. Acad. Sci. USA 2018, 115, E4151–E4158. [Google Scholar] [CrossRef]
- Wei, S.; Lu, Y.; Ye, Q.; Tang, S. Population Genetic Structure and Phylogeography of Camellia flavida (Theaceae) Based on Chloroplast and Nuclear DNA Sequences. Front. Plant Sci. 2017; 8, 718. [Google Scholar]
- Tong, Y.; Wu, C.Y.; Gao, L.Z. Characterization of chloroplast microsatellite loci from whole chloroplast genome of Camellia taliensis and their utilization for evaluating genetic diversity of Camellia reticulata (Theaceae). Biochem. Syst. Ecol. 2013, 50, 207–211. [Google Scholar] [CrossRef]
- Lu, X.; Chen, H.; Wei, S.; Bin, X.; Tang, S. Chloroplast and nuclear DNA analyses provide insight into the phylogeography and conservation genetics of Camellia nitidissima (Theaceae) in southern Guangxi, China. Tree Genet. Genomes 2020, 16, 8. [Google Scholar] [CrossRef]
- Ryu, Y.; Kim, I.R.; Su, M.H.; Jung, J.; Choi, H.K.; Kim, C. Phylogeographical study of Camellia japonica inferred from AFLP and chloroplast DNA haplotype analyses. J. Plant Biol. 2019, 62, 14–26. [Google Scholar] [CrossRef]
- Fang, W.; Yang, J.; Yang, S.; Li, D. Phylogeny of Camellia sects. Longipedicellata, Chrysantha and Longissima (Theaceae) based on sequence data of four chloroplast DNA loci. Acta Bot. Yunnanica. 2010, 32, 1–13. [Google Scholar]
- Chen, S.; Zhou, Y.; Chen, Y.; Jia, G. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Dong, W.; Li, E.; Liu, Y.; Xu, C.; Wang, Y.; Liu, K.; Cui, X.; Sun, J.; Suo, Z.; Zhang, Z. Phylogenomic approaches untangle early divergences and complex diversifications of the olive plant family. BMC Biol. 2022, 20, 92. [Google Scholar] [CrossRef]
- Boetzer, M.; Henkel, C.V.; Jansen, H.J.; Butler, D.; Pirovano, W. Scaffolding pre-assembled contigs using SSPACE. Bioinformatics 2011, 27, 578–579. [Google Scholar] [CrossRef] [PubMed]
- Vanhercke, T.; El Tahchy, A.; Shrestha, P.; Zhou, X.R.; Singh, S.P.; Petrie, J.R. Synergistic effect of WRI1 and DGAT1 coexpression on triacylglycerol biosynthesis in plants. FEBS Lett. 2013, 587, 364–369. [Google Scholar] [CrossRef]
- Boetzer, M.; Pirovano, W. Toward almost closed genomes with GapFiller. Genome Biol. 2012, 13, R56. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, D.; Chen, G.L.; Locascio, P.F.; Land, M.L.; Larimer, F.W.; Hauser, L.J. Prodigal: Prokaryotic gene recognition and translation initiation site identification. BMC Bioinform. 2010, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Eddy, S.R. A probabilistic model of local sequence alignment that simplifies statistical significance estimation. PLoS Comp. Biol. 2008, 4, 2046–2052. [Google Scholar] [CrossRef] [PubMed]
- Dean, L.; Bjorn, C. Aragorn, a program to detect tRNA genes and tmRNA genes in nucleotide sequences. Nucleic Acids Res. 2004, 32, 11–16. [Google Scholar]
- Lohse, M.; Drechsel, O.; Bock, R. Organellar Genome DRAW (OGDRAW): A tool for the easy generation of high-quality custom graphical maps of plastid and mitochondrial genomes. Curr. Genet. 2007, 52, 267–274. [Google Scholar] [CrossRef]
- Brendel, V.; Kurtz, S.; Walbot, V. Comparative genomics of Arabidopsis and maize: Prospects and limitations. Genome Biol. 2002, 3, reviews100. [Google Scholar] [CrossRef]
- Sebastian, B.; Thomas, T.; Thomas, M.; Uwe, S.; Martin, M. MISA-web: A web server for microsatellite prediction. Bioinformatics 2017, 33, 2583. [Google Scholar]
- Darling, A.E.; Mau, B.; Perna, N.T. Progressive Mauve: Multiple genome alignment with gene gain, loss and rearrangement. PLoS ONE 2010, 5, e11147. [Google Scholar] [CrossRef]
- Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772. [Google Scholar]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analyses of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Zhang, Z.; Zhu, J.; Yu, J. KaKs_Calculator 2.0: A toolkit incorporating gamma-series methods and sliding window strategies. Genom. Proteom. Bioinf. 2010, 8, 77–80. [Google Scholar] [CrossRef]
- Jian, Y.; Hong, L.; Goddard, M.E.; Visscher, P.M. GCTA: A tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 2011, 88, 76–82. [Google Scholar]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van de Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liang, L.; Huelsenbeck, S.J.P. MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Ki-Joong, K.; Hae-Lim, L. Complete chloroplast genome sequences from Korean Ginseng (Panax schinseng Nees) and comparative analysis of sequence evolution among 17 vascular plants. DNA Res. 2005, 11, 247–261. [Google Scholar]
- Xu, D.; Abe, J.; Gai, J.; Shimamoto, Y. Diversity of chloroplast DNA SSRs in wild and cultivated soybeans: Evidence for multiple origins of cultivated soybean. Theor. Appl. Genet. 2002, 105, 645–653. [Google Scholar] [CrossRef]
- Li, W.; Zhang, C.P.; Guo, X.; Liu, Q.H.; Wang, K.L. Complete chloroplast genome of Camellia japonica genome structures, comparative and phylogenetic analysis. PLoS ONE 2019, 14, 1–18. [Google Scholar] [CrossRef]
- Doyle, J.J.; Da, V.J.; Soreng, R.J.; Garvin, D.; Anderson, M.J. Chloroplast DNA inversions and the origin of the grass family (Poaceae). Proc. Natl. Acad. Sci. USA 1992, 89, 7722–7726. [Google Scholar] [CrossRef]
- Wu, F.H.; Chan, M.T.; Liao, D.C.; Hsu, C.T.; Lee, Y.W.; Daniell, H.; Duvall, M.R.; Lin, C.S. Complete chloroplast genome of Oncidium Gower Ramsey and evaluation of molecular markers for identification and breeding in Oncidiinae. BMC Plant Biol. 2010, 10, 68. [Google Scholar] [CrossRef]
- Chang, C.C.; Lin, H.C.; Lin, I.P.; Chow, T.Y.; Chen, H.H.; Chen, W.H.; Cheng, C.H.; Lin, C.Y.; Liu, S.M.; Chang, C.C.; et al. The chloroplast genome of Phalaenopsis aphrodite (Orchidaceae): Comparative analysis of evolutionary rate with that of grasses and its phylogenetic implications. Mol. Biol. Evol. 2006, 23, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Yoshihiro, M.; Yukiko, Y.; Yasunari, O.; Koichiro, T. Whole chloroplast genome comparison of rice, maize, and wheat: Implications for chloroplast gene diversification and phylogeny of cereals. Mol. Biol. Evol. 2002, 19, 2084–2091. [Google Scholar]
- Goremykin, V.V.; Barbara, H.; Hirsch-Ernst, K.I.; Hellwig, F.H. Analysis of Acorus calamus Chloroplast Genome and Its Phylogenetic Implications. Mol. Biol. Evol. 2005, 22, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
- Tangphatsornruang, S.; Sangsrakru, D.; Chanprasert, J.; Uthaipaisanwong, P.; Yoocha, T.; Jomchai, N.; Tragoonrung, S. The chloroplast genome sequence of mungbean (Vigna radiata) determined by high-throughput pyrosequencing: Structural organization and phylogenetic relationships. DNA Res. 2010, 17, 11–22. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Yang, Y.; Xie, X.; Lu, Y.; Yang, Z.; Jin, X.; Dong, W.; Suo, Z. Interspecific chloroplast genome sequence diversity and genomic resources in Diospyros. BMC Plant Biol. 2018, 18, 210. [Google Scholar] [CrossRef]
- Davis, J.I.; Soreng, R.J. Migration of endpoints of two genes relative to boundaries between regions of the plastid genome in the grass family (Poaceae). Am. J. Bot. 2010, 97, 874–892. [Google Scholar] [CrossRef]
- Wang, R.J.; Cheng, C.L.; Chang, C.C.; Wu, C.L.; Su, T.M.; Chaw, S.M. Dynamics and evolution of the inverted repeat-large single copy junctions in the chloroplast genomes of monocots. BMC Evol. Biol. 2008, 8, 36. [Google Scholar] [CrossRef]
- Xu, Q.; Xiong, G.; Li, P.; He, F.; Huang, Y.; Wang, K.; Li, Z.; Hua, J. Analysis of complete nucleotide sequences of 12 Gossypium chloroplast genomes: Origin and evolution of allotetraploids. PLoS ONE 2012, 7, e37128. [Google Scholar] [CrossRef]
- Guisinger, M.M.; Kuehl, J.V.; Boore, J.L.; Jansen, R.K. Extreme reconfiguration of plastid genomes in the angiosperm family Geraniaceae: Rearrangements, repeats, and codon usage. Mol. Biol. Evol. 2011, 28, 583–600. [Google Scholar] [CrossRef]
- Weng, M.L.; Blazier, J.C.; Madhumita, G.; Jansen, R.K. Reconstruction of the ancestral plastid genome in Geraniaceae reveals a correlation between genome rearrangements, repeats, and nucleotide substitution rates. Mol. Biol. Evol. 2014, 31, 645–659. [Google Scholar] [CrossRef]
- Xue, S.; Shi, T.; Luo, W.; Ni, X.; Iqbal, S.; Ni, Z.X.; Huang, X.; Yao, D.; Shen, Z.; Gao, Z. Comparative analysis of the complete chloroplast genome among Prunus mume, P. armeniaca, and P. salicina. Hortic. Res. 2019, 6, 89. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, T.; Ma, Q.; Liang, L.; Wang, G. Comparative genomics and phylogenetic analysis revealed the chloroplast genome variation and interspecific relationships of Corylus (Betulaceae) species. Front. Plant Sci. 2018, 9, 927. [Google Scholar] [CrossRef]
- Takayuki, A.; Takahiko, T.; Sakiko, T.; Hiroaki, S.; Koh-Ichi, K. Complete nucleotide sequence of the sugarcane (Saccharum officinarum) chloroplast genome: A comparative analysis of four monocot chloroplast genomes. DNA Res. 2004, 11, 93–99. [Google Scholar]
- Cavalier-Smith, T. Chloroplast evolution: Secondary symbiogenesis and multiple losses. Curr. Biol. 2002, 12, R62–R64. [Google Scholar] [CrossRef]
- Yamane, K.; Yano, K.; Kawahara, T. Pattern and rate of indel evolution inferred from whole chloroplast intergenic regions in sugarcane, maize and rice. DNA Res. 2006, 13, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Mccluskey, K.; Wiest, A.E.; Grigoriev, I.V.; Lipzen, A.; Martin, J.; Schackwitz, W.; Baker, S.E. Rediscovery by whole genome sequencing: Classical mutations and genome polymorphisms in Neurospora crassa. G3 Genes Genomes Genet. 2011, 1, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, Y.; Yang, H.; Bergelson, J.; Kreitman, M.; Dacheng, T. Variation in the ratio of nucleotide substitution and Indel rates across genomes in mammals and bacteria. Mol. Biol. Evol. 2009, 28, 1523–1531. [Google Scholar] [CrossRef]
- Smith, S.A.; Donoghue, M.J. Rates of molecular evolution are linked to life history in flowering plants. Science 2008, 322, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Liu, C.; Shi, L.; Liu, R.; Liang, D.; Li, H.; Cherny, S.S.; Chen, S.; Elvira, H. Utility of the trnH–psbA intergenic spacer region and its combinations as plant DNA barcodes: A Meta-Analysis. PLoS ONE 2012, 7, e48833. [Google Scholar] [CrossRef] [PubMed]
- Asaf, S.; Waqas, M.; Khan, A.L.; Khan, M.A.; Kang, S.M.; Imran, Q.M.; Shahzad, R.; Bilal, S.; Yun, B.W.; Lee, I.J. The complete chloroplast genome of wild rice (Oryza minuta) and its comparison to related species. Front. Plant Sci. 2017, 8, 1–15. [Google Scholar] [CrossRef]
- Song, W.C.; Chen, Z.M.; He, L.; Feng, Q.; Zhang, H.R.; Du, G.L.; Shi, C.; Wang, S. Comparative Chloroplast Genome Analysis of Wax Gourd (Benincasa hispida) with Three Benincaseae Species, Revealing. Genes 2022, 13, 461. [Google Scholar] [CrossRef]
- Song, W.C.; Ji, C.X.; Chen, Z.M.; Cai, H.H.; Wu, X.M.; Shi, C.; Wang, S. Comparative Analysis the Complete Chloroplast Genomes of Nine Musa Species: Genomic Features, Comparative Analysis, and Phylogenetic Implications. Front. Plant Sci. 2022, 13, 1–15. [Google Scholar] [CrossRef]
- Park, I.; Choi, B.; Weiss-Schneeweiss, H.; So, S.; Myeong, H.H.; Jang, T.S. Comparative Analyses of Complete Chloroplast Genomes and Karyotypes of Allotetraploid Iris koreana and Its Putative Diploid Parental Species (Iris Series Chinenses, Iridaceae). Int. J. Mol. Sci. 2022, 23, 10929. [Google Scholar] [CrossRef]
- Liu, G.; Ning, H.; Ayidaerhan, N.; Aisa, H.A. Evaluation of DNA barcode candidates for the discrimination of Artemisia, L. Mitochondrial. DNA A 2016, 28, 956–964. [Google Scholar] [CrossRef]
- Liston, A.; Wheeler, J.A. The phylogenetic position of the genus Astragalus (fabaceae): Evidence from the chloroplast genes rpo C1 and rpo C2. Biochem. Syst. Ecol. 1994, 22, 377–388. [Google Scholar] [CrossRef]
- Dong, W.; Xu, C.; Li, C.; Sun, J.; Zuo, Y.; Shi, S.; Cheng, T.; Guo, J.; Zhou, S. ycf1, the most promising plastid DNA barcode of land plants. Sci. Rep. 2015, 5, 8348. [Google Scholar] [CrossRef]
- Drew, B.T.; Sytsma, K.J. Phylogenetics, biogeography, and staminal evolution in the tribe Mentheae (Lamiaceae). Am. J. Bot. 2012, 99, 933–953. [Google Scholar] [CrossRef]
- Parks, M.; Liston, A.; Cronn, R. Newly developed primers for complete ycf1 amplification in pinus (pinaceae) chloroplasts with possible family-wide utility. Am. J. Bot. 2011, 98, e185–e188. [Google Scholar]
- Hernández-León, S.; Gernandt, D.S.; Rosa, J.; Rbolla, L.B. Phylogenetic relationships and species delimitation in Pinus section Trifoliae inferrred from plastid DNA. PLoS ONE 2013, 8, e70501. [Google Scholar] [CrossRef] [PubMed]
- Dastpak, A.; Osaloo, S.K.; Maassoumi, A.A.; Safar, K.N. Molecular phylogeny of Astragalus sect. Ammodendron (Fabaceae) inferred from chloroplast ycf 1 gene. Ann. Bot. Fenn. 2018, 55, 75–82. [Google Scholar]
- Yang, J.; Lucía, V.; Chen, X.; Li, H.; Hao, Z.; Liu, Z.; Zhao, G. Development of chloroplast and nuclear DNA markers for Chinese oaks (Quercus Subgenus Quercus) and assessment of their utility as DNA barcodes. Front. Plant Sci. 2017, 8, 816. [Google Scholar] [CrossRef]
- Vries, J.D.; Sousa, F.L.; Bölter, B.; Soll, J.; Gould, S.B. YCF1: A green TIC? Plant Cell 2015, 27, 1827–1833. [Google Scholar] [CrossRef]
- Thode, V.A.; Lohmann, L.G. Comparative chloroplast genomics at low taxonomic levels: A case study using Amphilophium (Bignonieae, Bignoniaceae). Front. Plant Sci. 2019, 10, 796. [Google Scholar] [CrossRef]
- Park, S.; Ruhlman, T.A.; Weng, M.L.; Hajrah, ..H.; Sabir, J.S.; Jansen, R.K. Contrasting patterns of nucleotide substitution rates provide insight into dynamic evolution of plastid and mitochondrial genomes of Geranium. Genome Biol. Evol. 2017, 9, 1766–1780. [Google Scholar] [CrossRef]
- De Vries, J.; Archibald, J.M.; Gould, S.B. The carboxy terminus of YCF1 contains a motif conserved throughout >500 myr of streptophyte evolution. Genome Biol. Evol. 2017, 9, 473–479. [Google Scholar] [CrossRef]
- Dong, W.; Liu, J.; Yu, J.; Wang, L.; Zhou, S. Highly variable chloroplast markers for evaluating plant phylogeny at low taxonomic levels and for DNA barcoding. PLoS ONE 2012, 7, e35071. [Google Scholar] [CrossRef]


| Taxon | Genus | Section | Collection Sites | Geographical Origin | Morphological characters |
|---|---|---|---|---|---|
| Camellia oleifera var. 40 | Camellia | Oleifera | DFFZP | Zhejiang province, China | Shrub or dungarunga; elliptical or ovate leaves; white flower, petals 5–7 |
| Camellia oleifera var. 53 | Camellia | Oleifera | DFFZP | Zhejiang province, China | Shrub; elliptical or ovate leaves; white flower, petals 5–7 |
| Camellia oleifera var. GR3 | Camellia | Oleifera | DFFZP | Guangxi zhuang autonomous region, China | Shrub; elliptical or ovate leaves; white flower, petals 5–7 |
| Camellia oleifera var. MY6 | Camellia | Oleifera | DFFZP | Fujian province, China | Shrub; elliptical or ovate leaves; white flower, petals 5–7 |
| Camellia meiocarpa | Camellia | Oleifera | DFFZP | Jiangxi province, China | Shrub; elliptical or ovate leaves; white flower, petals 5–7 |
| Camellia nanyongensis | Camellia | Oleifera | ICSG | Guangxi zhuang autonomous region, China | Shrub; lanceolate leaves; white flower, petals 6 |
| Camellia sasanqua | Camellia | Oleifera | RISF | Zhejiang province, China | Dungarunga; elliptical leaves; red flower, petals 6–7 |
| Camellia vietnamensis | Camellia | Oleifera | RISF | Guangdong province, China | Shrub or dungarunga; elliptical or ovate leaves; white flower, petals 5–7 |
| Camellia gauchowensis | Camellia | Oleifera | RISF | Guangdong province, China | Shrub or dungarunga; elliptical leaves; white flower, petals 7–8 |
| Camellia chekiangoleosa var. Baihua | Camellia | Camellia | DFFZP | Zhejiang province, China | Dungarunga; elliptical leaves; white flower, petals 7 |
| Camellia semiserrata | Camellia | Camellia | RISF | Guangxi zhuang autonomous region, China | Dungarunga; elliptical leaves; red flower, petals 6–7 |
| Camellia grijsii | Camellia | Paracamellia | RISF | Hunan province, China | Shrub or dungarunga; oblong leaves; white flower, petals 5–6 |
| Genome Features | C. oleifera var. 40 | C. oleifera var. 53 | C. oleifera var. GR3 | C. oleifera var. MY6 | C. meiocarpa | C. nanyongensis | C. sasanqua | C. vietnamensis | C. gauchowensis | C. chekiangoleosa var. Baihua | C. semiserrata | C. grijsii |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome size (bp) | 156,975 | 156,975 | 156,978 | 156,975 | 156,550 | 157,021 | 156,545 | 156,910 | 157,003 | 156,606 | 156,833 | 157,004 |
| LSC size (bp) | 86,659 | 86,659 | 86,606 | 86,659 | 86,264 | 86,619 | 86,257 | 86,560 | 86,656 | 86,257 | 86,462 | 86,657 |
| SSC size (bp) | 18,408 | 18,408 | 18,290 | 18,408 | 18,400 | 18,282 | 18,402 | 18,300 | 18,297 | 18,415 | 18,269 | 18,297 |
| IR size (bp) | 25,954 | 25,954 | 26,041 | 25,954 | 25,943 | 26,060 | 25,943 | 26,025 | 26,025 | 25,967 | 26,051 | 26,025 |
| Number of genes | 134 | 134 | 134 | 134 | 134 | 134 | 134 | 134 | 134 | 134 | 134 | 134 |
| Protein coding genes (unique) | 87 (80) | 87 (80) | 87 (80) | 87 (80) | 87 (80) | 87 (80) | 87 (80) | 87 (80) | 87 (80) | 87 (80) | 87 (80) | 87 (80) |
| tRNA genes (unique) | 37 (29) | 37 (29) | 37 (29) | 37 (29) | 37 (29) | 37 (29) | 37 (29) | 37 (29) | 37 (29) | 37 (29) | 37 (29) | 37 (29) |
| rRNA genes (unique) | 8 (4) | 8 (4) | 8 (4) | 8 (4) | 8 (4) | 8 (4) | 8 (4) | 8 (4) | 8 (4) | 8 (4) | 8 (4) | 8 (4) |
| Duplicated genes in IR | 34 | 34 | 34 | 34 | 34 | 34 | 34 | 34 | 34 | 34 | 34 | 34 |
| GC content (%) | 37.29 | 37.29 | 37.29 | 37.29 | 37.32 | 37.30 | 37.32 | 37.30 | 37.29 | 37.32 | 37.33 | 37.29 |
| GC content in LSC (%) | 35.29 | 35.29 | 35.30 | 35.29 | 35.33 | 35.32 | 35.33 | 35.31 | 35.29 | 35.34 | 35.34 | 35.29 |
| GC content in SSC (%) | 30.53 | 30.53 | 30.52 | 30.53 | 30.58 | 30.61 | 30.57 | 30.52 | 30.55 | 30.53 | 30.59 | 30.55 |
| GC content in IR (%) | 43.03 | 43.03 | 42.99 | 43.03 | 43.03 | 42.94 | 43.03 | 42.99 | 42.98 | 43.01 | 42.98 | 42.98 |
| Total reads | 25,000,000 | 25,000,000 | 25,000,000 | 25,000,000 | 25,000,000 | 25,000,000 | 25,000,000 | 25,000,000 | 25,000,000 | 25,000,000 | 25,000,000 | 25,000,000 |
| Assembled reads | 371,694 | 356,834 | 133,442 | 202,707 | 57,181 | 206,798 | 197,214 | 152,500 | 138,312 | 439,221 | 105,258 | 95,232 |
| Average coverage | 860 | 738 | 334 | 495 | 112 | 415 | 395 | 306 | 275 | 884 | 205 | 208 |
| Average insert size (bp) | 373 | 336 | 379 | 377 | 313 | 319 | 315 | 321 | 310 | 333 | 296 | 356 |
| Category | Group of Genes | Name of Genes |
|---|---|---|
| Photosynthesis | Subunits of photosystem I | psaA, psaB, psaC, psaI, psaJ |
| Subunits of photosystem II | psbA, psbB, psbC, psbD, psbE, psbF, psbH, psbI, psbJ, psbK, psbL, psbM, psbN, psbT, psbZ | |
| Subunits of NADH dehydrogenase | ndhA *, ndhB *(2), ndhC, ndhD, ndhE, ndhF, ndhG, ndhH, ndhI, ndhJ, ndhK | |
| Subunits of cytochrome b/f complex | petA, petB *, petD *, petG, petL, petN | |
| Subunits of ATP synthase | atpA, atpB, atpE, atpF *, atpH, atpI | |
| Large subunit of rubisco | rbcL | |
| Self-replication | Proteins of large ribosomal subunit | rpl14, rpl16 *, rpl2 *(2), rpl20, rpl22, rpl23(2), rpl32, rpl33, rpl36 |
| Proteins of small ribosomal subunit | #rps19, rps11, rps12 **(2), rps14, rps15, rps16 *, rps18, rps19, rps2, rps3, rps4, rps7(2), rps8 | |
| Subunits of RNA polymerase | rpoA, rpoB, rpoC1 *, rpoC2 | |
| Ribosomal RNAs | rrn16(2), rrn23(2), rrn4.5(2), rrn5(2) | |
| Transfer RNAs | trnA-UGC *(2), trnC-GCA, trnD-GUC, trnE-UUC, trnF-GAA, trnG-UCC, trnG-UCC *, trnH-GUG, trnI-CAU(2), trnI-GAU *(2), trnK-UUU *, trnL-CAA(2), trnL-UAA *, trnL-UAG, trnM-CAU, trnN-GUU(2), trnP-UGG, trnQ-UUG, trnR-ACG(2), trnR-UCU, trnS-GCU, trnS-GGA, trnS-UGA, trnT-GGU, trnT-UGU, trnV-GAC(2), trnV-UAC *, trnW-CCA, trnY-GUA, trnfM-CAU | |
| Other genes | Maturase | matK |
| Protease | clpP ** | |
| Envelope membrane protein | cemA | |
| Acetyl-CoA carboxylase | accD | |
| c-type cytochrome synthesis gene | ccsA | |
| Translation initiation factor | infA | |
| Unknown function | Conserved hypothetical chloroplast ORF | # ycf1, ycf1, ycf15(2), ycf2(2), ycf3 **, ycf4 |
| Gene | Location | Exon I (bp) | Intron I (bp) | Exon II (bp) | Intron II (bp) | Exon III (bp) |
|---|---|---|---|---|---|---|
| trnK-UUU | LSC | 37 | 2488–2502 | 35 | ||
| rps16 | LSC | 39 | 851–875 | 225 | ||
| trnG-UCC | LSC | 34 or 23 | 690 or 695 | 43 or 48 | ||
| atpF | LSC | 159 | 704 | 408 | ||
| rpoC1 | LSC | 435 | 732 | 1626 | ||
| ycf3 | LSC | 126 | 737 or 747 | 228 | 722–725 | 153 |
| trnL-UAA | LSC | 37 | 523 or 519 | 50 | ||
| trnV-UAC | LSC | 39 | 586 | 37 | ||
| rps12 | IRa | 346 | 538 | 26 | ||
| clpP | LSC | 69 | 539–542 | 291 | 797–805 | 285 |
| petB | LSC | 6 | 756–762 | 657 | ||
| petD | LSC | 9 | 696 | 525 | ||
| rpl16 | LSC | 9 | 1014–1025 | 402 | ||
| rpl2 | IRb | 393 | 667 | 435 | ||
| ndhB | IRb | 777 | 679 | 756 | ||
| trnI-GAU | IRb | 42 | 947 or 948 | 35 | ||
| trnA-UGC | IRb | 38 | 812 | 35 | ||
| ndhA | SSC | 552 | 1084–1110 | 540 |
| Species | SSR Loci (N) | Region | Location | Styles | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LSC | SSC | IR | Intron | Exon | IGS | P1 a Loci (N) | P2 b Loci (N) | P3 c Loci (N) | P4 d Loci (N) | P6 e Loci (N) | Pc f Loci (N) | ||
| C. oleifera var. 40 | 237 | 139 | 50 | 48 | 33 | 88 | 116 | 145 | 5 | 68 | 10 | 2 | 8 |
| C. oleifera var. 53 | 237 | 139 | 50 | 48 | 33 | 88 | 116 | 144 | 5 | 68 | 10 | 2 | 8 |
| C. oleifera var. GR3 | 238 | 139 | 51 | 48 | 33 | 89 | 116 | 147 | 4 | 68 | 10 | 2 | 7 |
| C. oleifera var. MY6 | 237 | 139 | 50 | 48 | 33 | 88 | 116 | 145 | 5 | 67 | 11 | 2 | 7 |
| C. meiocarpa | 232 | 137 | 49 | 46 | 34 | 87 | 111 | 142 | 4 | 69 | 10 | 0 | 7 |
| C. nanyongensis | 242 | 142 | 52 | 48 | 33 | 89 | 120 | 149 | 5 | 69 | 10 | 2 | 7 |
| C. sasanqua | 233 | 137 | 50 | 46 | 34 | 88 | 111 | 143 | 4 | 69 | 10 | 0 | 7 |
| C. vietnamensis | 239 | 142 | 51 | 46 | 33 | 90 | 116 | 150 | 4 | 68 | 10 | 0 | 7 |
| C. gauchowensis | 237 | 140 | 51 | 46 | 33 | 90 | 114 | 149 | 4 | 68 | 10 | 0 | 6 |
| C. chekiangoleosa var. Baihua | 235 | 137 | 50 | 48 | 33 | 88 | 114 | 143 | 4 | 67 | 10 | 2 | 9 |
| C. semiserrata | 236 | 138 | 50 | 48 | 32 | 90 | 114 | 147 | 4 | 66 | 10 | 2 | 7 |
| C. grijsii | 237 | 140 | 51 | 46 | 33 | 90 | 114 | 148 | 4 | 68 | 10 | 0 | 7 |
| C. oleifera var. 40 | C. oleifera var. 53 | C. oleifera var. GR3 | C. oleifera var. MY6 | C. meiocarpa | C. nanyongensis | C. sasanqua | C. vietnamensis | C. gauchowensis | C. grijsii | C. chekiangoleosa var. Baihua | C. semiserrata | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C. oleifera var. 40 | - | 0 | 62 | 0 | 48 | 78 | 42 | 77 | 73 | 74 | 50 | 89 |
| C. oleifera var. 53 | 0 | - | 62 | 0 | 48 | 78 | 42 | 77 | 73 | 74 | 50 | 89 |
| C. oleifera var. GR3 | 153(2.47) | 153(2.47) | - | 62 | 68 | 71 | 66 | 64 | 62 | 63 | 73 | 80 |
| C. oleifera var. MY6 | 0 | 0 | 153(2.47) | - | 48 | 78 | 42 | 77 | 73 | 74 | 50 | 89 |
| C. meiocarpa | 89(1.85) | 89(1.85) | 130(1.91) | 89(1.85) | - | 81 | 6 | 68 | 70 | 70 | 59 | 87 |
| C. nanyongensis | 203(2.60) | 203(2.60) | 175(2.46) | 203(2.60) | 178(2.20) | - | 79 | 93 | 87 | 87 | 84 | 69 |
| C. sasanqua | 93(2.21) | 93(2.21) | 128(1.94) | 93(2.21) | 6(1.00) | 176(2.23) | - | 68 | 67 | 68 | 53 | 83 |
| C. vietnamensis | 170(2.21) | 170(2.21) | 121(1.89) | 170(2.21) | 143(2.10) | 191(2.05) | 147(2.16) | - | 42 | 42 | 80 | 98 |
| C. gauchowensis | 171(2.34) | 171(2.34) | 122(1.97) | 171(2.34) | 147(2.10) | 198(2.28) | 151(2.25) | 62(1.48) | - | 2 | 77 | 94 |
| C. grijsii | 171(2.31) | 171(2.31) | 123(1.95) | 171(2.31) | 146(2.09) | 198(2.28) | 150(2.21) | 62(1.48) | 0 | - | 78 | 95 |
| C. chekiangoleosa var. Baihua | 75(1.5) | 75(1.5) | 140(1.92) | 75(1.5) | 72(1.22) | 190(2.26) | 76(1.43) | 157(1.96) | 158(2.05) | 158(2.03) | - | 91 |
| C. semiserrata | 194(2.18) | 194(2.18) | 173(2.16) | 194(2.18) | 173(1.99) | 162(2.35) | 177(2.13) | 170(1.73) | 173(1.84) | 173(1.82) | 183(2.01) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, P.; Yin, H.; Wang, K.; Gao, H.; Liu, L.; Yao, X. Comparative Genomic Analysis Uncovers the Chloroplast Genome Variation and Phylogenetic Relationships of Camellia Species. Biomolecules 2022, 12, 1474. https://doi.org/10.3390/biom12101474
Lin P, Yin H, Wang K, Gao H, Liu L, Yao X. Comparative Genomic Analysis Uncovers the Chloroplast Genome Variation and Phylogenetic Relationships of Camellia Species. Biomolecules. 2022; 12(10):1474. https://doi.org/10.3390/biom12101474
Chicago/Turabian StyleLin, Ping, Hengfu Yin, Kailiang Wang, Haidong Gao, Lei Liu, and Xiaohua Yao. 2022. "Comparative Genomic Analysis Uncovers the Chloroplast Genome Variation and Phylogenetic Relationships of Camellia Species" Biomolecules 12, no. 10: 1474. https://doi.org/10.3390/biom12101474
APA StyleLin, P., Yin, H., Wang, K., Gao, H., Liu, L., & Yao, X. (2022). Comparative Genomic Analysis Uncovers the Chloroplast Genome Variation and Phylogenetic Relationships of Camellia Species. Biomolecules, 12(10), 1474. https://doi.org/10.3390/biom12101474





