IL-10 and IL-17 as Progression Markers of Syphilis in People Living with HIV: A Systematic Review
Abstract
1. Introduction
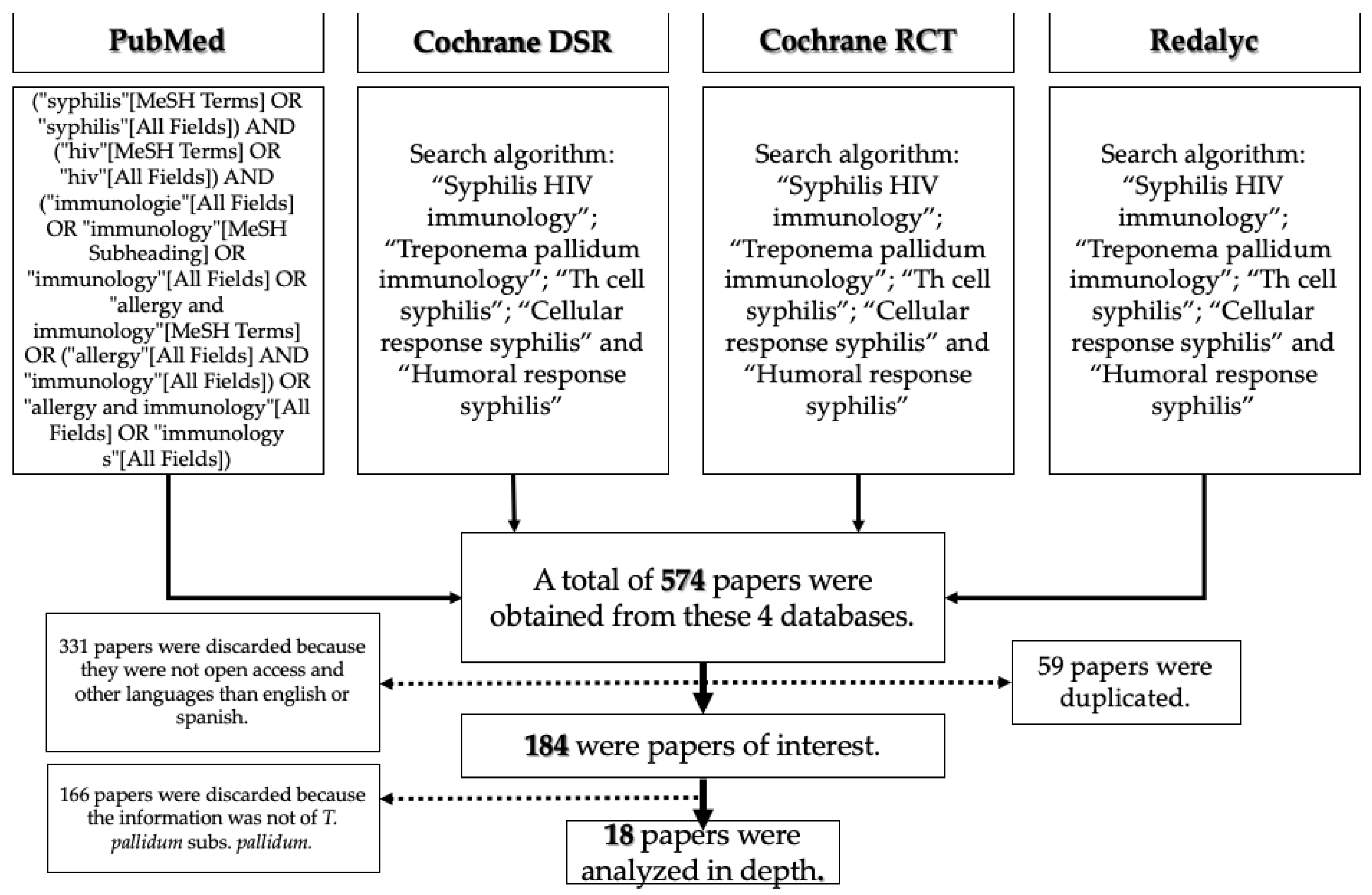
2. Materials and Methods
- (“treponema pallidum” [MeSH Terms] OR (“treponema” [All Fields] AND “pallidum” [All Fields]) OR “treponema pallidum” [All Fields]) AND (“immunologie” [All Fields] OR “immunology” [MeSH Subheading] OR “immunology” [All Fields] OR “allergy and immunology” [MeSH Terms] OR (“allergy” [All Fields] AND “immunology” [All Fields]) OR “allergy and immunology” [All Fields] OR “immunology” [All Fields])
- (“syphilis” [MeSH Terms] OR “syphilis” [All Fields]) AND (“hiv” [MeSH Terms] OR “hiv” [All Fields]) AND (“immunologie” [All Fields] OR “immunology” [MeSH Subheading] OR “immunology” [All Fields] OR “allergy and immunology” [MeSH Terms] OR (“allergy” [All Fields] AND “immunology” [All Fields]) OR “allergy and immunology” [All Fields] OR “immunology s” [All Fields])
- (“cells” [MeSH Terms] OR “cells” [All Fields] OR “cellular” [All Fields]) AND (“response” [All Fields] OR “responses” [All Fields] OR “responsive” [All Fields] OR “responsiveness” [All Fields] OR “responsivenesses” [All Fields] OR “responsives” [All Fields] OR “responsivities” [All Fields] OR “responsivity” [All Fields]) AND (“syphilis” [MeSH Terms] OR “syphilis” [All Fields])
- (“humoral” [All Fields] OR “humorally” [All Fields] OR “humoral” [All Fields]) AND (“response” [All Fields] OR “responses” [All Fields] OR “responsive” [All Fields] OR “responsiveness” [All Fields] OR “responsivenesses” [All Fields] OR “responsives” [All Fields] OR “responsivities” [All Fields] OR “responsivity” [All Fields]) AND (“syphilis” [MeSH Terms] OR “syphilis” [All Fields])
3. Results
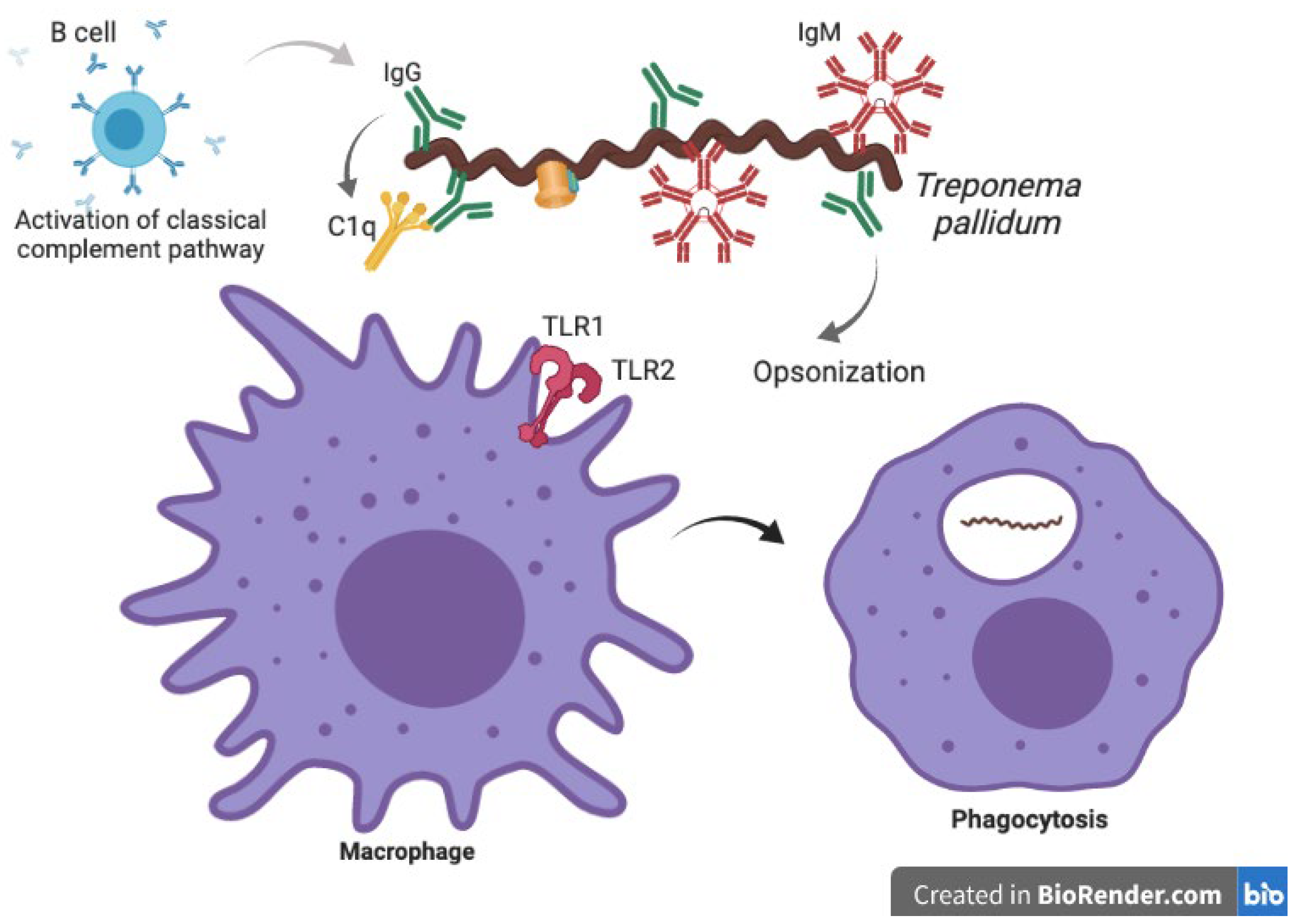
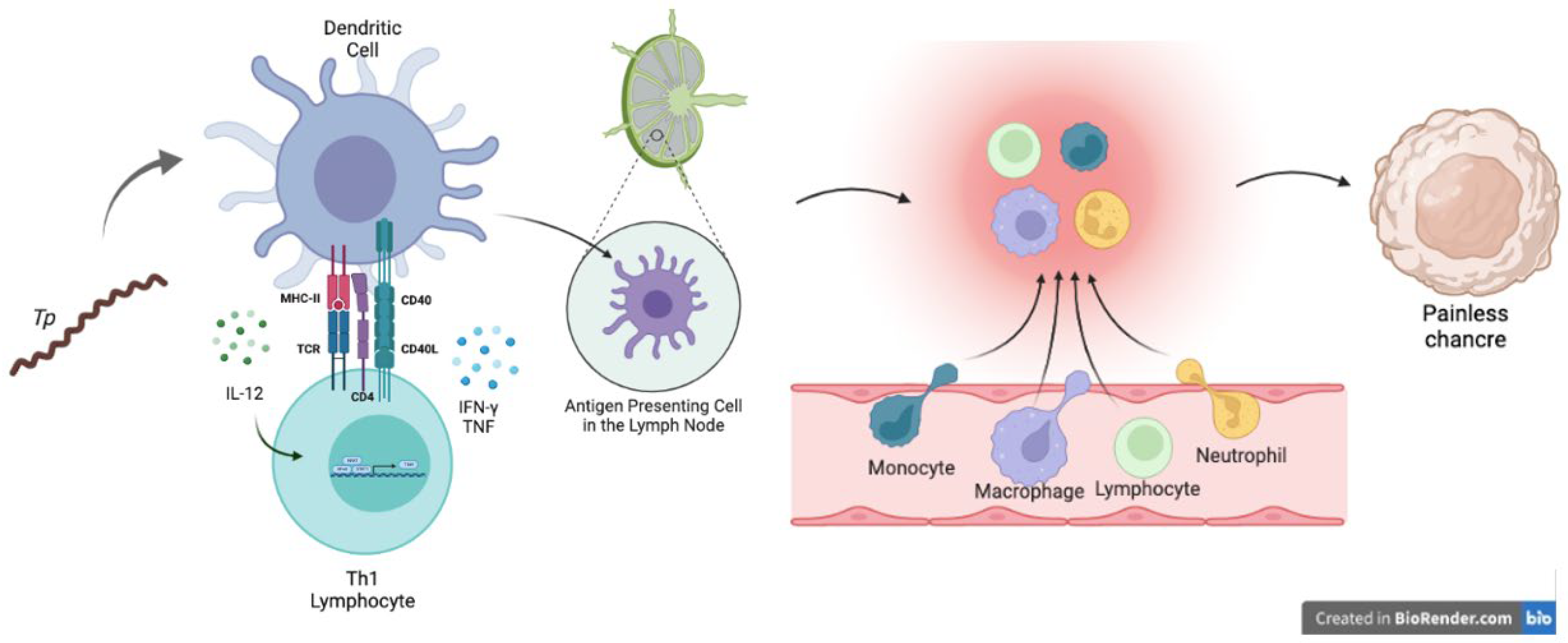
3.1. Innate Immune Response against Treponema pallidum
3.2. Adaptive Immune Response against Syphilis
3.3. Co-Infection: HIV/Syphilis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. New Study Hihglights Unacceptably High Global Prevalence of Syphilis among Men Who Have Sex with Men. 2021. Available online: https://www.who.int/news/item/09-07-2021-new-study-highlights-unacceptably-high-global-prevalence-of-syphilis-among-men-who-have-sex-with-men (accessed on 10 September 2022).
- Hook, E.W. Syphilis. Lancet 2017, 389, 1550–1557. [Google Scholar] [CrossRef]
- Peeling, R.W.; Mabey, D.; Kamb, M.L.; Chen, X.S.; Radolf, J.D.; Benzaken, A.S. Syphilis. Nat. Rev. Dis. Primer 2017, 3, 17073. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, O.M.; Vandenbroucke, J.P.; Cevallos, M.; Renehan, A.G.; Altman, D.G.; Egger, M. COSMOS-E: Guidance on conducting systematic reviews and meta-analyses of observational studies of etiology. PLoS Med. 2019, 16, e1002742. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.R.; Ramirez, L.G.; Zuluaga, A.V.; Pillay, A.; Abreu, C.; Valencia, C.A.; La Vake, C.; Cervantes, J.L.; Dunham-Ems, S.; Cartun, R.; et al. Immune evasion and recognition of the syphilis spirochete in blood and skin of secondary syphilis patients: Two immunologically distinct compartments. PLoS Negl. Trop. Dis. 2012, 6, e1717. [Google Scholar] [CrossRef]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; Van Der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef]
- Bekkering, S.; Domínguez-Andrés, J.; Joosten, L.A.B.; Riksen, N.P.; Netea, M.G. Trained Immunity: Reprogramming Innate Immunity in Health and Disease. Annu. Rev. Immunol. 2021, 39, 667–693. [Google Scholar] [CrossRef]
- Bekkering, S.; Domínguez-Andrés, J.; Joosten, L.A.; Riksen, N.P.; Netea, M.G. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [Google Scholar]
- Babolin, C.; Amedei, A.; Ozolins, D.; Zilevica, A.; D’Elios, M.M.; de Bernard, M. TpF1 from Treponema pallidum activates inflammasome and promotes the development of regulatory T cells. J. Immunol. 2011, 187, 1377–1384. [Google Scholar] [CrossRef]
- Salazar, J.C.; Cruz, A.R.; Pope, C.D.; Valderrama, L.; Trujillo, R.; Saravia, N.G.; Radolf, J.D. Treponema pallidum Elicits Innate and Adaptive Cellular Immune Responses in Skin and Blood during Secondary Syphilis: A Flow-Cytometric Analysis. J. Infect. Dis. 2007, 195, 879–887. [Google Scholar] [CrossRef]
- Roberts, C.P.; Klausner, J.D. Global challenges in human immunodeficiency virus and syphilis coinfection among men who have sex with men. Expert. Rev. Anti Infect. Ther. 2016, 14, 1037–1046. [Google Scholar] [CrossRef]
- van Voorhis, W.C.; Barrett, L.K.; Nasio, J.M.; Plummer, F.A.; Lukehart, S.A. Lesions of primary and secondary syphilis contain activated cytolytic T cells. Infect. Immun. 1996, 64, 1048–1050. [Google Scholar] [CrossRef]
- Leader, B.T.; Godornes, C.; VanVoorhis, W.C.; Lukehart, S.A. CD4+ lymphocytes and gamma interferon predominate in local immune responses in early experimental syphilis. Infect. Immun. 2007, 75, 3021–3026. [Google Scholar] [CrossRef]
- Wang, J.; Lu, S.; Zheng, K.; He, Z.; Li, W.; Liu, J.; Guo, N.; Xie, Y.; Chen, D.; Xu, M.; et al. Treponema pallidum delays the apoptosis of human polymorphonuclear neutrophils through the intrinsic and extrinsic pathways. Mol. Immunol. 2022, 147, 157–169. [Google Scholar] [CrossRef]
- Carlson, J.A.; Dabiri, G.; Cribier, B.; Sell, S. The immunopathobiology of syphilis: The manifestations and course of syphilis are determined by the level of delayed-type hypersensitivity. Am. J. Dermatopathol. 2011, 33, 433–460. [Google Scholar] [CrossRef]
- Bedoya, S.K.; Lam, B.; Lau, K.; Larkin, J. Th17 Cells in Immunity and Autoimmunity. Clin. Dev. Immunol. 2013, 2013, 986789. [Google Scholar] [CrossRef]
- Yasuda, K.; Takeuchi, Y.; Hirota, K. The pathogenicity of Th17 cells in autoimmune diseases. Semin. Immunopathol. 2019, 41, 283–297. [Google Scholar] [CrossRef]
- Zhao, J.; Ma, J.; Zhang, X.; Li, Q.; Yang, X. Equilibrium of Treg/Th17 cells of peripheral blood in syphilitic patients with sero-resistance. Exp. Ther. Med. 2016, 11, 2300–2304. [Google Scholar] [CrossRef]
- Wicher, K.; Wicher, V. Autoimmunity in syphilis. Immunol. Ser. 1990, 52, 101–124. [Google Scholar]
- Fornaciari, G. Renaissance mummies in Italy. Med. Secoli. 1999, 11, 85–105. [Google Scholar]
- Fornaciari, G.; Castagna, M.; Tognetti, A.; Tornaboni, D.; Bruno, J. Syphilis in a renaissance italian mummy. Lancet 1989, 334, 614. [Google Scholar] [CrossRef]
- Knudsen, A.; Benfield, T.; Kofoed, K. Cytokine expression during syphilis infection in HIV-1-infected individuals. Sex Transm. Dis. 2009, 36, 300–304. [Google Scholar] [CrossRef]
- Kotsafti, O.; Paparizos, V.; Kourkounti, S.; Chatziioannou, A.; Nicolaidou, E.; Kapsimali, V.; Antoniou, C. Early syphilis affects markers of HIV infection. Int. J. STD AIDS 2016, 27, 739–745. [Google Scholar] [CrossRef]
- Li, Z.; Lu, X.; Hu, Z.; Luo, Z.; Jiang, W.; Wu, H.; Gao, Y.; Yan, J.; Zhang, Q.; Song, A.; et al. Syphilis Infection Differentially Regulates the Phenotype and Function of γδ T Cells in HIV-1-Infected Patients Depends on the HIV-1 Disease Stage. Front. Immunol. 2017, 8, 991. [Google Scholar] [CrossRef]
- Guo, N.; Liu, L.; Yang, X.; Song, T.; Li, G.; Li, L.; Jiang, T.; Gao, Y.; Zhang, T.; Su, B.; et al. Immunological Changes in Monocyte Subsets and Their Association with Foxp3+ Regulatory T Cells in HIV-1-Infected Individuals with Syphilis: A Brief Research Report. Front. Immunol. 2019, 10, 714. [Google Scholar] [CrossRef]
- Guo, N.; Chen, Y.; Su, B.; Yang, X.; Zhang, Q.; Song, T.; Wu, H.; Liu, C.; Liu, L.; Zhang, T. Alterations of CCR2 and CX3CR1 on Three Monocyte Subsets During HIV-1/Treponema pallidum Coinfection. Front. Med. 2020, 7, 272. [Google Scholar] [CrossRef]
- Kenyon, C.; Osbak, K.K.; Crucitti, T.; Kestens, L. The immunological response to syphilis differs by HIV status; a prospective observational cohort study. BMC Infect. Dis. 2017, 17, 111. [Google Scholar] [CrossRef]
- Yan, J.; Luo, M.L.; Han, J.; Yan, D.; Zhang, B.; Zhang, Z.; Shi, J.; Zhu, M.; Yu, J.; Liu, S.; et al. Comparing Noninvasive Predictors of Neurosyphilis Among Syphilis Patients with and without HIV Co-Infection Based on the Real-World Diagnostic Criteria: A Single-Center, Retrospective Cohort Study in China. AIDS Res. Hum. Retrovir. 2021, 38, 406–414. [Google Scholar] [CrossRef]
- Díaz Betancourt, M.L.; Klínger Hernández, J.C.; Niño Castaño, V.E. Profound CD4+ T lymphocytopenia in human immunodeficiency virus negative individuals, improved with anti-human herpes virus treatment. Colomb. Medica Cali Colomb. 2012, 43, 305–311. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, L.; Gao, Z.; Guan, Z.; Lu, H.; Shi, M.; Gao, Y.; Xu, H.; Yang, X.F.; Zhou, P. Increased interleukin-17 in peripheral blood and cerebrospinal fluid of neurosyphilis patients. PLoS Negl. Trop. Dis. 2014, 8, e3004. [Google Scholar] [CrossRef]
- González-Domenech, C.M.; Antequera Martín-Portugués, I.; Clavijo-Frutos, E.; Márquez-Solero, M.; Santos-González, J.; Palacios-Muñoz, R. Sífilis e infección por el virus de la inmunodeficiencia humana: Una endemia en hombres que tienen sexo con hombres. Enferm. Infecc. Microbiol. Clínica 2015, 33, 32–36. [Google Scholar] [CrossRef]
- Das, A.; Harly, C.; Ding, Y.; Bhandoola, A. ILC Differentiation from Progenitors in the Bone Marrow. In Innate Lymphoid Cells; Advances in Experimental Medicine and Biology Book Series; Sun, X.H., Ed.; Springer Nature: Singapore, 2022; Volume 1365, pp. 7–24. Available online: https://link.springer.com/10.1007/978-981-16-8387-9_2 (accessed on 21 June 2022).
- Liu, W.N.; Wu, K.X.; Wang, X.T.; Lin, L.R.; Tong, M.L.; Liu, L.L. LncRNA-ENST00000421645 promotes T cells to secrete IFN-γ by sponging PCM1 in neurosyphilis. Epigenomics 2021, 13, 1187–1203. [Google Scholar] [CrossRef] [PubMed]
- He, W.Q.; Wang, H.L.; Zhong, D.Q.; Lin, L.Y.; Qiu, X.S.; Yang, R.D. Treponemal antibody in CSF and cellular immunity in peripheral blood of syphilitic patients with persisting positive rapid plasma regain. Int. J. Clin. Exp. Pathol. 2015, 8, 5775–5780. [Google Scholar] [PubMed]
- Yu, Q.; Cheng, Y.; Wang, Y.; Wang, C.; Lu, H.; Guan, Z.; Huang, J.; Gong, W.; Shi, M.; Ni, L.; et al. Aberrant Humoral Immune Responses in Neurosyphilis: CXCL13/CXCR5 Play a Pivotal Role for B-Cell Recruitment to the Cerebrospinal Fluid. J. Infect. Dis. 2017, 216, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.L.; Chung, K.Y.; Kang, J.M.; Lee, T.H.; Lee, M.G. The effects of Treponema pallidum on human dendritic cells. Yonsei Med. J. 2004, 45, 515–522. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Pliego, A.; Vergara-Ortega, D.N.; Herrera-Ortíz, A.; Toledano-Jaimes, C.; Esquivel-Guadarrama, F.R.; Sánchez-Alemán, M.Á. IL-10 and IL-17 as Progression Markers of Syphilis in People Living with HIV: A Systematic Review. Biomolecules 2022, 12, 1472. https://doi.org/10.3390/biom12101472
Hernández-Pliego A, Vergara-Ortega DN, Herrera-Ortíz A, Toledano-Jaimes C, Esquivel-Guadarrama FR, Sánchez-Alemán MÁ. IL-10 and IL-17 as Progression Markers of Syphilis in People Living with HIV: A Systematic Review. Biomolecules. 2022; 12(10):1472. https://doi.org/10.3390/biom12101472
Chicago/Turabian StyleHernández-Pliego, Adriana, Dayana Nicté Vergara-Ortega, Antonia Herrera-Ortíz, Cairo Toledano-Jaimes, Fernando R. Esquivel-Guadarrama, and Miguel Ángel Sánchez-Alemán. 2022. "IL-10 and IL-17 as Progression Markers of Syphilis in People Living with HIV: A Systematic Review" Biomolecules 12, no. 10: 1472. https://doi.org/10.3390/biom12101472
APA StyleHernández-Pliego, A., Vergara-Ortega, D. N., Herrera-Ortíz, A., Toledano-Jaimes, C., Esquivel-Guadarrama, F. R., & Sánchez-Alemán, M. Á. (2022). IL-10 and IL-17 as Progression Markers of Syphilis in People Living with HIV: A Systematic Review. Biomolecules, 12(10), 1472. https://doi.org/10.3390/biom12101472







