Integrated Quantitative Targeted Lipidomics and Proteomics Reveal Unique Fingerprints of Multiple Metabolic Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Study Population and Samples
2.3. Ethics Approval and Consent to Participate
2.4. Sample Preparation and Targeted Lipidomics Analysis
2.5. Categorization of Lipid Species by FA Carbon Chain Length and Saturation
2.6. Sample Preparation and Targeted Proteomics Analysis
2.7. Data Processing and Statistical Analysis
3. Results
3.1. Comparative Assessment of Lipidomic Profiles across Different Metabolic Categories
3.2. Comparative Assessment of Proteins
3.3. Lipid–Protein Correlation Analysis
3.4. Lipid–Protein Network Analysis by Sample Groups
3.5. Pathway Enrichment Analysis Based on Protein Data
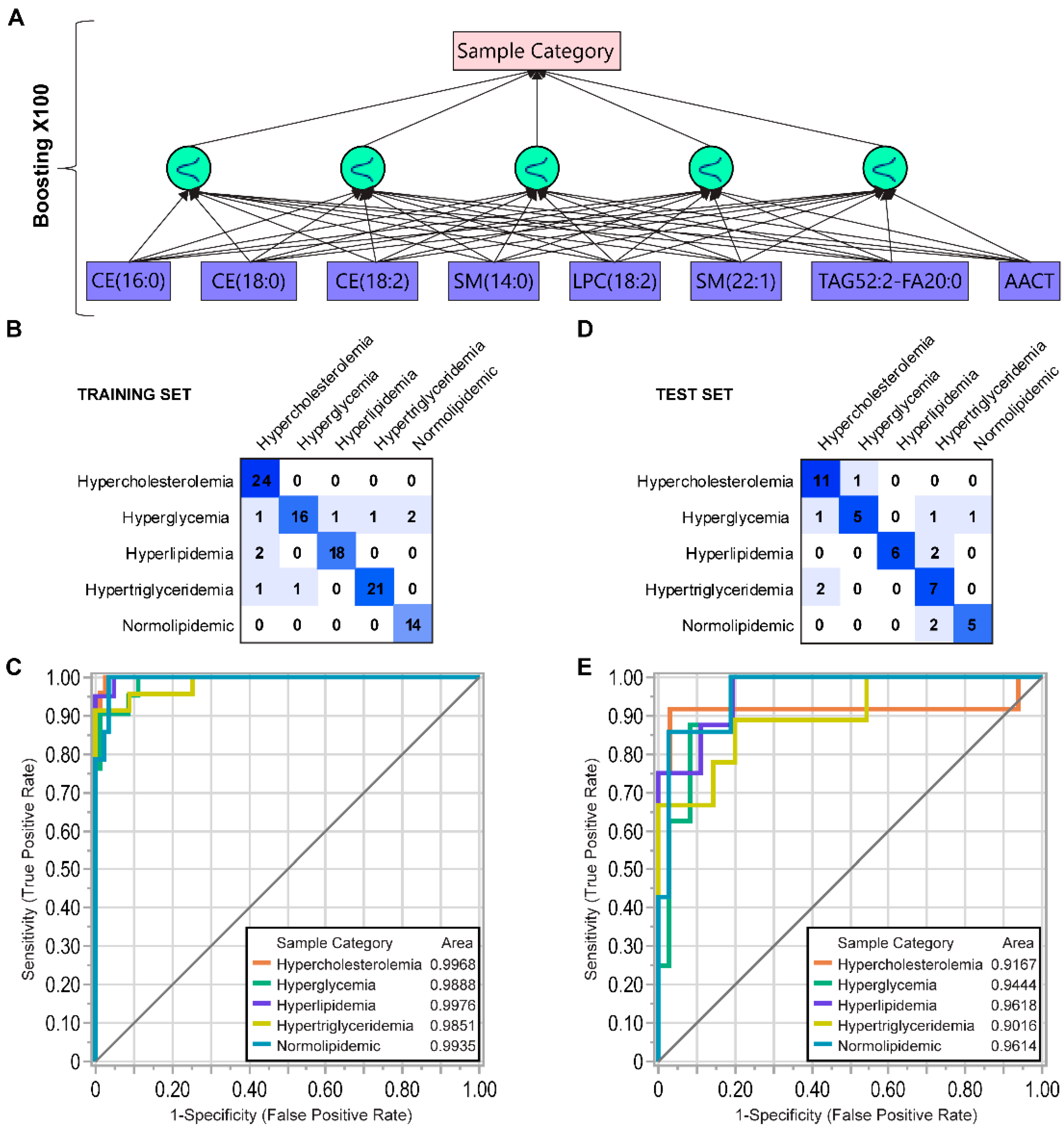
3.6. Data-Driven Parameter Screening and Artificial Neural Network Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Athyros, V.G.; Doumas, M.; Imprialos, K.P.; Stavropoulos, K.; Georgianou, E.; Katsimardou, A.; Karagiannis, A. Diabetes and lipid metabolism. Hormones 2018, 17, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Cisa-Wieczorek, S.; Hernandez-Alvarez, M.I. Deregulation of Lipid Homeostasis: A Fa(c)t in the Development of Metabolic Diseases. Cells 2020, 9, 2605. [Google Scholar] [CrossRef] [PubMed]
- Stegemann, C.; Pechlaner, R.; Willeit, P.; Langley, S.R.; Mangino, M.; Mayr, U.; Menni, C.; Moayyeri, A.; Santer, P.; Rungger, G.; et al. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based bruneck study. Circulation 2014, 129, 1821–1831. [Google Scholar] [CrossRef] [PubMed]
- Vega, G.L.; Wang, J.; Grundy, S.M. Prevalence and significance of risk enhancing biomarkers in the United States population at intermediate risk for atherosclerotic disease. J. Clin. Lipidol. 2022, 16, 66–74. [Google Scholar] [CrossRef]
- Jacobson, T.A.; Ito, M.K.; Maki, K.C.; Orringer, C.E.; Bays, H.E.; Jones, P.H.; McKenney, J.M.; Grundy, S.M.; Gill, E.A.; Wild, R.A.; et al. National Lipid Association recommendations for patient-centered management of dyslipidemia: Part 1—Executive summary. J. Clin. Lipidol. 2014, 8, 473–488. [Google Scholar] [CrossRef]
- Sachdeva, A.; Cannon, C.P.; Deedwania, P.C.; LaBresh, K.A.; Smith Jr, S.C.; Dai, D.; Hernandez, A.; Fonarow, G.C. Lipid levels in patients hospitalized with coronary artery disease: An analysis of 136,905 hospitalizations in Get With The Guidelines. Am. Heart J. 2009, 157, 111–117.e2. [Google Scholar] [CrossRef]
- Yanai, H.; Yoshida, H. Secondary dyslipidemia: Its treatments and association with atherosclerosis. Glob. Health Med. 2021, 3, 15–23. [Google Scholar] [CrossRef]
- Schaefer, E.J.; Geller, A.S.; Endress, G. The biochemical and genetic diagnosis of lipid disorders. Curr. Opin. Lipidol. 2019, 30, 56–62. [Google Scholar] [CrossRef]
- Taghizadeh, E.; Esfehani, R.J.; Sahebkar, A.; Parizadeh, S.M.; Rostami, D.; Mirinezhad, M.; Poursheikhani, A.; Mobarhan, M.G.; Pasdar, A. Familial combined hyperlipidemia: An overview of the underlying molecular mechanisms and therapeutic strategies. IUBMB Life 2019, 71, 1221–1229. [Google Scholar] [CrossRef]
- Chou, R.; Dana, T.; Blazina, I.; Daeges, M.; Bougatsos, C.; Jeanne, T.L. Screening for Dyslipidemia in Younger Adults: A Systematic Review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2016, 165, 560–564. [Google Scholar] [CrossRef]
- Kwiterovich Jr, P.O. Diagnosis and management of familial dyslipoproteinemias. Curr. Cardiol. Rep. 2013, 15, 371. [Google Scholar] [CrossRef]
- Zannis, V.I.; Fotakis, P.; Koukos, G.; Kardassis, D.; Ehnholm, C.; Jauhiainen, M.; Chroni, A. Hdl biogenesis, remodeling, and catabolism. Handb. Exp. Pharmacol. 2015, 224, 53–111. [Google Scholar] [PubMed]
- Ramasamy, I. Recent advances in physiological lipoprotein metabolism. Clin. Chem. Lab. Med. 2014, 52, 1695–1727. [Google Scholar] [PubMed]
- Alaupovic, P. The concept of apolipoprotein-defined lipoprotein families and its clinical significance. Curr. Atheroscler. Rep. 2003, 5, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Segrest, J.P. Computational studies of plasma lipoprotein lipids. Biochim. Biophys. Acta Biomembr. 2016, 1858, 2401–2420. [Google Scholar] [CrossRef] [PubMed]
- McLeod, R.S.; Yao, Z. Assembly and Secretion of Triglyceride-Rich Lipoproteins. In Biochemistry of Lipids, Lipoproteins and Membranes, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 459–488. [Google Scholar]
- Melchior, J.T.; Street, S.E.; Vaisar, T.; Hart, R.; Jerome, J.; Kuklenyik, Z.; Clouet-Foraison, N.; Thornock, C.; Bedi, S.; Shah, A.S.; et al. Apolipoprotein A-I modulates HDL particle size in the absence of apolipoprotein A-II. J. Lipid Res. 2021, 62, 100099. [Google Scholar] [CrossRef] [PubMed]
- Manchekar, M.; Richardson, P.E.; Forte, T.M.; Datta, G.; Segrest, J.P.; Dashti, N. Apolipoprotein b-containing lipoprotein particle assembly—Lipid capacity of the nascent lipoprotein particle. J. Biol. Chem. 2004, 279, 39757–39766. [Google Scholar] [CrossRef]
- Siekmeier, R.; Scharnagl, H.; Kostner, G.M.; Grammer, T.; Stojakovic, T.; März, W. Lipoprotein(a)—Structure, epidemiology and function. LaboratoriumsMedizin 2007, 31, 109–124. [Google Scholar] [CrossRef]
- Gao, X.; Yuan, S.; Jayaraman, S.; Gursky, O. Role of apolipoprotein A-II in the structure and remodeling of human high-density lipoprotein (HDL): Protein conformational ensemble on HDL. Biochemistry 2012, 51, 4633–4641. [Google Scholar] [CrossRef]
- Walker, R.G.; Deng, X.; Melchior, J.T.; Morris, J.; Tso, P.; Jones, M.K.; Segrest, J.P.; Thompson, T.B.; Davidson, W.S. The structure of human apolipoprotein A-IV as revealed by stable isotope-assisted cross-linking, molecular dynamics, and small angle X-ray scattering. J. Biol. Chem. 2014, 289, 5596–5608. [Google Scholar] [CrossRef]
- Zhu, L.; Luu, T.; Emfinger, C.H.; Parks, B.A.; Shi, J.; Trefts, E.; Zeng, F.; Kuklenyik, Z.; Harris, R.C.; Wasserman, D.H.; et al. CETP inhibition improves hdl function but leads to fatty liver and insulin resistance in CETP-expressing transgenic mice on a high-fat diet. Diabetes 2018, 67, 2494–2506. [Google Scholar] [CrossRef] [PubMed]
- Meyers, N.L.; Larsson, M.; Olivecrona, G.; Small, D.M. A pressure-dependent model for the regulation of lipoprotein lipase by apolipoprotein C-II. J. Biol. Chem. 2015, 290, 18029–18044. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Mahley, R.W. Apolipoprotein E: Structure and function in lipid metabolism, neurobiology, and Alzheimer′s diseases. Neurobiol. Dis. 2014, 72, 3–12. [Google Scholar] [CrossRef]
- Darabi, M.; Guillas-Baudouin, I.; Le Goff, W.; Chapman, M.J.; Kontush, A. Therapeutic applications of reconstituted HDL: When structure meets function. Pharmacol. Ther. 2016, 157, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Chièze, L.; Bolanos-Garcia, V.M.; Le Caër, G.; Renault, A.; Vié, V.; Beaufils, S. Difference in lipid packing sensitivity of exchangeable apolipoproteins apoA-I and apoA-II: An important determinant for their distinctive role in lipid metabolism. Biochim. Biophys. Acta Biomembr. 2012, 1818, 2732–2741. [Google Scholar] [CrossRef] [PubMed]
- Lecompte, M.F.; Bras, A.C.; Dousset, N.; Portas, I.; Salvayre, R.; Ayrault-Jarrier, M. Binding steps of apolipoprotein A-I with phospholipid monolayers: Adsorption and penetration. Biochemistry 1998, 37, 16165–16171. [Google Scholar] [CrossRef]
- Meyers, N.L.; Wang, L.; Gursky, O.; Small, D.M. Changes in helical content or net charge of apolipoprotein C-I alter its affinity for lipid/water interfaces. J. Lipid Res. 2013, 54, 1927–1938. [Google Scholar] [CrossRef]
- McKeone, B.J.; Massey, J.B.; Knapp, R.D.; Pownall, H.J. Apolipoproteins C-I, C-II, and C-III: Kinetics of association with model membranes and intermembrane transfer. Biochemistry 1988, 27, 4500–4505. [Google Scholar] [CrossRef]
- Vainio, P.; Virtanen, J.A.; Kinnunen, P.K.J.; Voyta, J.C.; Smith, L.C.; Gotto, A.M., Jr.; Sparrow, J.T.; Pattus, F.; Verger, R. Action of lipoprotein lipase on phospholipid monolayers. Activation by apolipoprotein C-II. Biochemistry 1983, 22, 2270–2275. [Google Scholar] [CrossRef]
- Niisuke, K.; Kuklenyik, Z.; Horvath, K.V.; Gardner, M.S.; Toth, C.A.; Asztalos, B.F. Composition-function analysis of HDL subpopulations: Influence of lipid composition on particle functionality. J. Lipid Res. 2020, 61, 306–315. [Google Scholar] [CrossRef]
- Kennedy, E.P. Sailing to Byzantium. Annu. Rev. Biochem. 1992, 61, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Holčapek, M.; Červená, B.; Cífková, E.; Lísa, M.; Chagovets, V.; Vostálová, J.; Bancířová, M.; Galuszka, J.; Hill, M. Lipidomic analysis of plasma, erythrocytes and lipoprotein fractions of cardiovascular disease patients using UHPLC/MS, MALDI-MS and multivariate data analysis. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 990, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Ubhi, B.K. Direct Infusion-Tandem Mass Spectrometry (DI-MS/MS) Analysis of Complex Lipids in Human Plasma and Serum Using the Lipidyzer™ Platform. In Clinical Metabolomics; Humana Press Inc.: Totowa, NJ, USA, 2018; Volume 1730, pp. 227–236. [Google Scholar]
- Postle, A.D.; Hunt, A.N. Dynamic lipidomics with stable isotope labelling. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 2716–2721. [Google Scholar] [CrossRef] [PubMed]
- Misra, B.B. New software tools, databases, and resources in metabolomics: Updates from 2020. Metabolomics 2021, 17, 49. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Niu, L.; Clerici, C.; Russo, R.; Byrd, M.; Setchell, K.D.R. Data analysis of MS-based clinical lipidomics studies with crossover design: A tutorial mini-review of statistical methods. Clin. Mass Spectrom. 2019, 13, 5–17. [Google Scholar] [CrossRef]
- Bowden, J.A.; Heckert, A.; Ulmer, C.Z.; Jones, C.M.; Koelmel, J.P.; Abdullah, L.; Ahonen, L.; Alnouti, Y.; Armando, A.M.; Asara, J.M.; et al. Harmonizing lipidomics: NIST interlaboratory comparison exercise for lipidomics using SRM 1950-Metabolites in frozen human plasma. J. Lipid Res. 2017, 58, 2275–2288. [Google Scholar] [CrossRef]
- Cajka, T.; Fiehn, O. Comprehensive analysis of lipids in biological systems by liquid chromatography-mass spectrometry. Trends Anal. Chem. 2014, 61, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, R.; Ripatti, S. Integrating lipidomics and genomics: Emerging tools to understand cardiovascular diseases. Cell. Mol. Life Sci. 2021, 78, 2565–2584. [Google Scholar] [CrossRef]
- Brown, M.F. Curvature forces in membrane lipid-protein interactions. Biochemistry 2012, 51, 9782–9795. [Google Scholar] [CrossRef]
- Brown, J.M.; Hazen, S.L. Seeking a unique lipid signature predicting cardiovascular disease risk. Circulation 2014, 129, 1799–1803. [Google Scholar] [CrossRef]
- Gardner, M.S.; McWilliams, L.G.; Jones, J.I.; Kuklenyik, Z.; Pirkle, J.L.; Barr, J.R. Simultaneous Quantification of Free Cholesterol, Cholesteryl Esters, and Triglycerides without Ester Hydrolysis by UHPLC Separation and In-Source Collision Induced Dissociation Coupled MS/MS. J. Am. Soc. Mass Spectrom. 2017, 28, 2319–2329. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Gardner, M.S.; Kuklenyik, Z.; Lehtikoski, A.; Carter, K.A.; McWilliams, L.G.; Kusovschi, J.; Bierbaum, K.; Jones, J.I.; Rees, J.; Reis, G.; et al. Development and application of a high throughput one-pot extraction protocol for quantitative LC-MS/MS analysis of phospholipids in serum and lipoprotein fractions in normolipidemic and dyslipidemic subjects. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1118–1119, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Bettcher, L.F.; Hsieh, W.-Y.; Hornburg, D.; Pearson, M.J.; Blomberg, N.; Giera, M.; Snyder, M.P.; Raftery, D.; Bensinger, S.J.; et al. A DMS Shotgun Lipidomics Workflow Application to Facilitate High-Throughput, Comprehensive Lipidomics. J. Am. Soc. Mass Spectrom. 2021, 32, 2655–2663. [Google Scholar] [CrossRef]
- Toth, C.A.; Kuklenyik, Z.; Jones, J.I.; Parks, B.A.; Gardner, M.S.; Schieltz, D.M.; Rees, J.C.; Andrews, M.L.; McWilliams, L.G.; Pirkle, J.L.; et al. On-column trypsin digestion coupled with LC-MS/MS for quantification of apolipoproteins. J. Proteom. 2017, 150, 258–267. [Google Scholar] [CrossRef]
- Smoot, M.E.; Ono, K.; Ruscheinski, J.; Wang, P.L.; Ideker, T. Cytoscape 2.8: New features for data integration and network visualization. Bioinformatics 2011, 27, 431–432. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, A.; Jupe, S.; Matthews, L.; Sidiropoulos, K.; Gillespie, M.; Garapati, P.; Haw, R.; Jassal, B.; Korninger, F.; May, B.; et al. The Reactome Pathway Knowledgebase. Nucl. Acids Res. 2018, 46, D649–D655. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Diaz, J.E.L.; Flamholz, Z.N.; Keenan, A.B.; Lachmann, A.; Wojciechowicz, M.L.; Cagan, R.L.; Ma’ayan, A. ModEnrichr: A suite of gene set enrichment analysis tools for model organisms. Nucl. Acids Res. 2019, 47, W183–W190. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.J.; Wu, T.Y.; Lin, C.L.; Su, C.Y.; Li, J.R.; Chung, Y.L.; Tien, N.; Lim, Y.P. Effects of Urate-Lowering Therapy on Risk of Hyperlipidemia in Gout by a Population-Based Cohort Study and on in Vitro Hepatic Lipogenesis-Related Gene Expression. Mediat. Inflamm. 2020, 2020, 8890300. [Google Scholar] [CrossRef]
- Ahmad, W.M.A.W.; Ahmed, F.; Noor, N.F.M.; Aleng, N.A.; Ghazali, F.M.M.; Alam, M.K. Prediction and Elucidation of Triglycerides Levels Using a Machine Learning and Linear Fuzzy Modelling Approach. BioMed Res. Int. 2022, 2022, 7511806. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Qin, S.; Wang, J.; Li, J.; Wang, H.; Li, H.; Chen, Z.; Li, C.; Wang, J.; Yuan, J. Develop and Evaluate a New and Effective Approach for Predicting Dyslipidemia in Steel Workers. Front. Bioeng. Biotechnol. 2020, 8, 839. [Google Scholar] [CrossRef] [PubMed]
- Krogh, A. What are artificial neural networks? Nat. Biotechnol. 2008, 26, 195–197. [Google Scholar] [CrossRef]
- Singh, S.A.; Andraski, A.B.; Pieper, B.; Goh, W.; Mendivil, C.O.; Sacks, F.M.; Aikawa, M. Multiple apolipoprotein kinetics measured in human HDL by high-resolution/accurate mass parallel reaction monitoring. J. Lipid Res. 2016, 57, 714–728. [Google Scholar] [CrossRef]
- Thongtang, N.; Diffenderfer, M.R.; Ooi, E.M.M.; Barrett, P.H.R.; Turner, S.M.; Le, N.A.; Brown, W.V.; Schaefer, E.J. Metabolism and proteomics of large and small dense LDL in combined hyperlipidemia: Effects of rosuvastatin. J. Lipid Res. 2017, 58, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Shahrokh, Z.; Nichols, A.V. Factors influencing interaction of human plasma low-density lipoproteins with discoidal complexes of apolipoprotein A-I and phosphatidylcholine. Biochim. Biophys. Acta Lipids Lipid Metab. 1986, 878, 152–158. [Google Scholar] [CrossRef]
- Jonas, A.; Kezdy, E.; Williams, M.I.; Rye, K.A. Lipid transfers between reconstituted high density lipoprotein complexes and low density lipoproteins: Effects of plasma protein factors. J. Lipid Res. 1988, 29, 1349–1357. [Google Scholar] [CrossRef]
- Hopkins, G.J.; Barter, P.J. Dissociation of the in vitro transfers of esterified cholesterol and triglyceride between human lipoproteins. Metabolism 1982, 31, 78–81. [Google Scholar] [CrossRef]
- Eisenberg, S. Effect of temperature and plasma on the exchange of apolipoproteins and phospholipids between rat plasma very low and high density lipoproteins. J. Lipid Res. 1978, 19, 229–236. [Google Scholar] [CrossRef]
- Fagone, P.; Jackowski, S. Membrane phospholipid synthesis and endoplasmic reticulum function. J. Lipid Res. 2009, 50, S311–S316. [Google Scholar] [CrossRef]
- Hamer, I.; Van Beersel, G.; Arnould, T.; Jadot, M. Lipids and lysosomes. Curr. Drug Metab. 2012, 13, 1371–1387. [Google Scholar] [CrossRef]
- Harkewicz, R.; Fahy, E.; Andreyev, A.; Dennis, E.A. Arachidonate-derived dihomoprostaglandin production observed in endotoxin-stimulated macrophage-like cells. J. Biol. Chem. 2007, 282, 2899–2910. [Google Scholar] [CrossRef] [PubMed]
- Bell, T.A., III; Wilson, M.D.; Kelley, K.; Sawyer, J.K.; Rudel, L.L. Monounsaturated fatty acyl-coenzyme A is predictive of atherosclerosis in human apoB-100 transgenic, LDLr-/- mice. J. Lipid Res. 2007, 48, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Bisgaier, C.L.; Siebenkas, M.V.; Brown, M.L.; Inazu, A.; Koizumi, J.; Mabuchi, H.; Tall, A.R. Familial Cholesteryl Ester Transfer Protein-Deficiency Is Associated with Triglyceride-Rich Low-Density Lipoproteins Containing Cholesteryl Esters of Probable Intracellular Origin. J. Lipid Res. 1991, 32, 21–33. [Google Scholar] [CrossRef]
- Diffenderfer, M.R.; Schaefer, E.J. The composition and metabolism of large and small LDL. Curr. Opin. Lipidol. 2014, 25, 221–226. [Google Scholar] [CrossRef]
- Brousseau, M.E.; Schaefer, E.J.; Stucchi, A.F.; Osada, J.; Vespa, D.B.; Ordovas, J.M.; Nicolosi, R.J. Diets enriched in unsaturated fatty acids enhance apolipoprotein A-I catabolism but do not affect either its production or hepatic mRNA abundance in cynomolgus monkeys. Atherosclerosis 1995, 115, 107–119. [Google Scholar] [CrossRef]
- Barber, M.N.; Risis, S.; Yang, C.; Meikle, P.J.; Staples, M.; Febbraio, M.A.; Bruce, C.R. Plasma lysophosphatidylcholine levels are reduced in obesity and type 2 diabetes. PLoS ONE 2012, 7, e41456. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Bhatnagar, S. Novel Lipidomic Biomarkers in Hyperlipidemia and Cardiovascular Diseases: An Integrative Biology Analysis. OMICS 2017, 21, 132–142. [Google Scholar] [CrossRef]
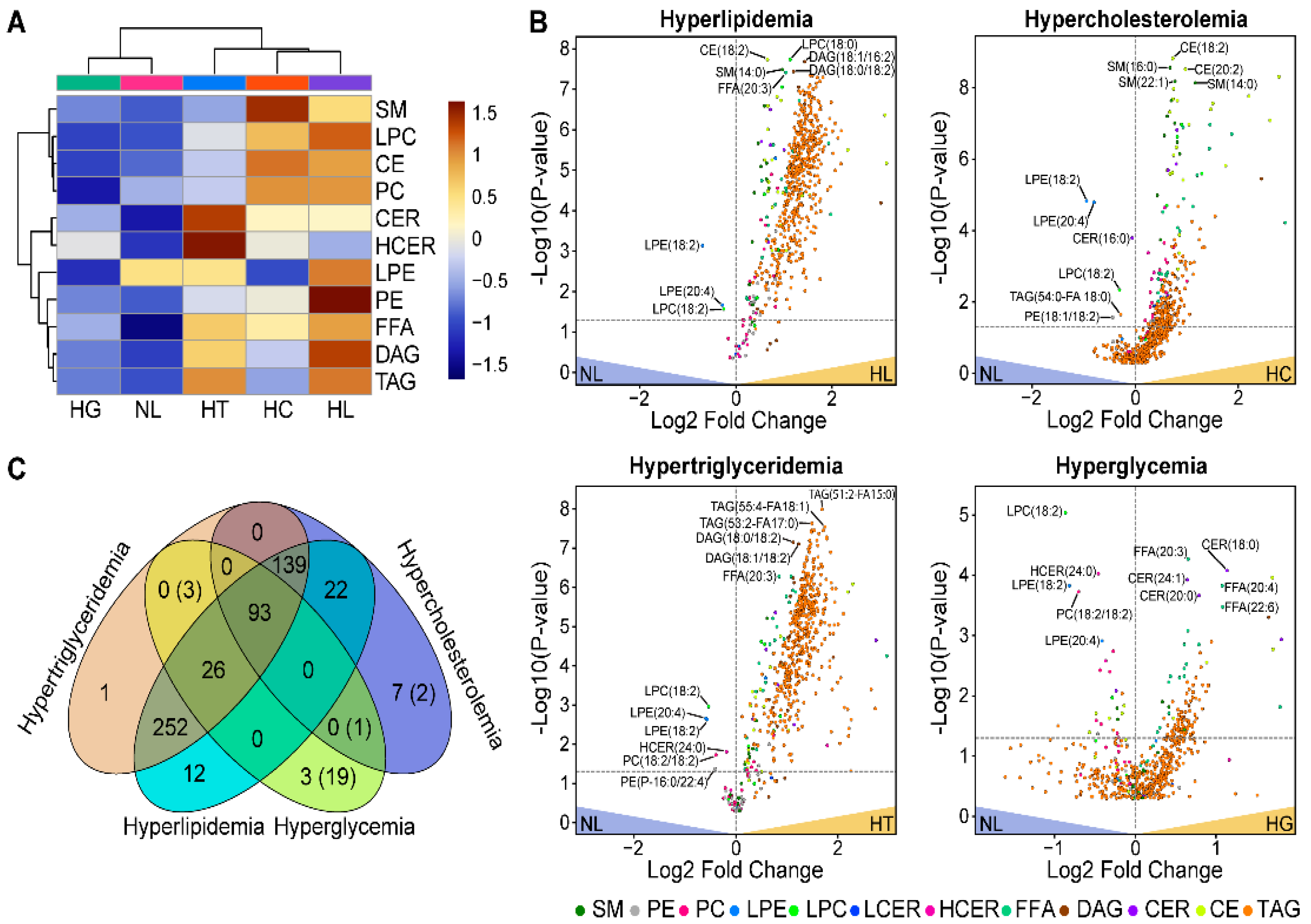
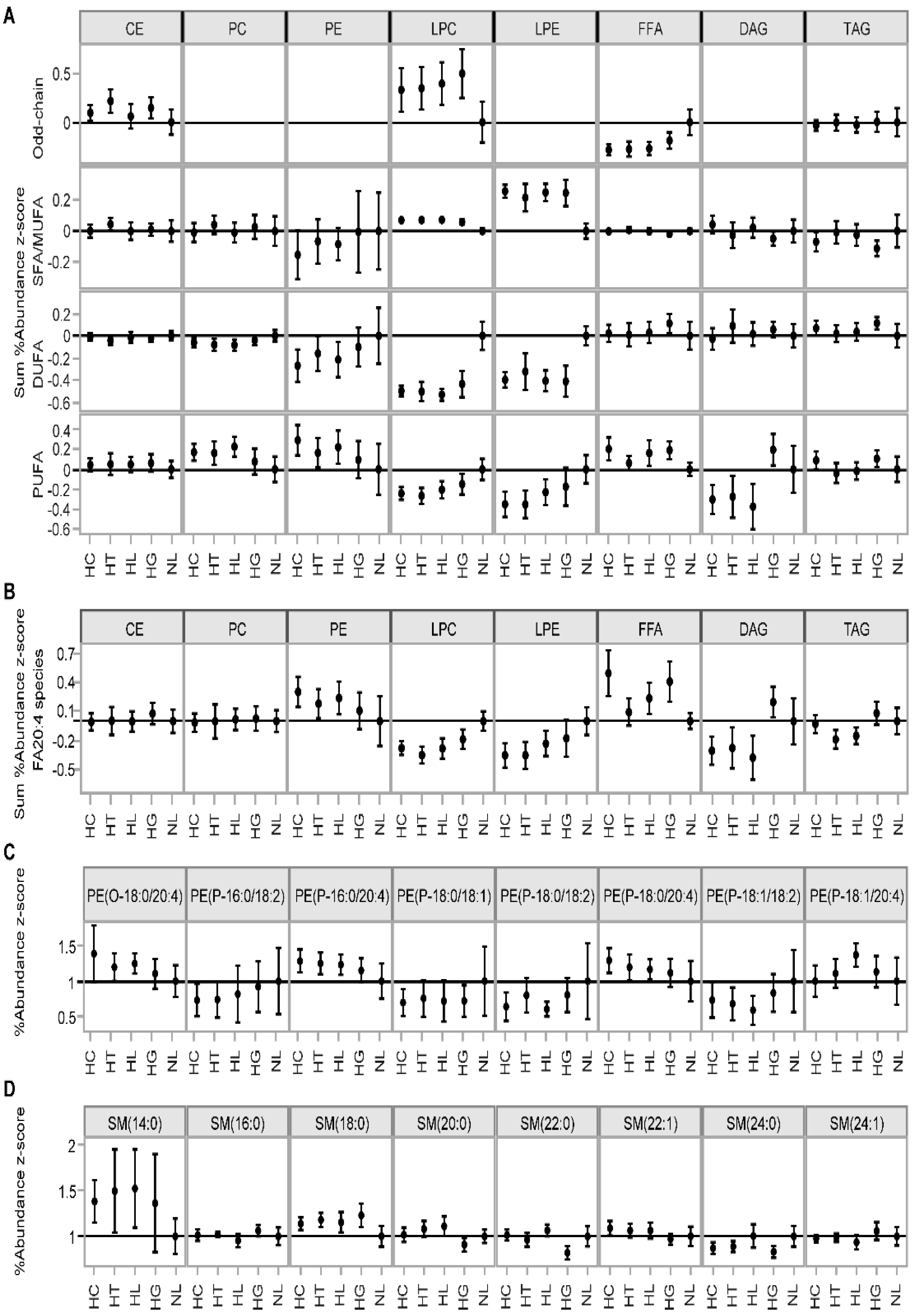
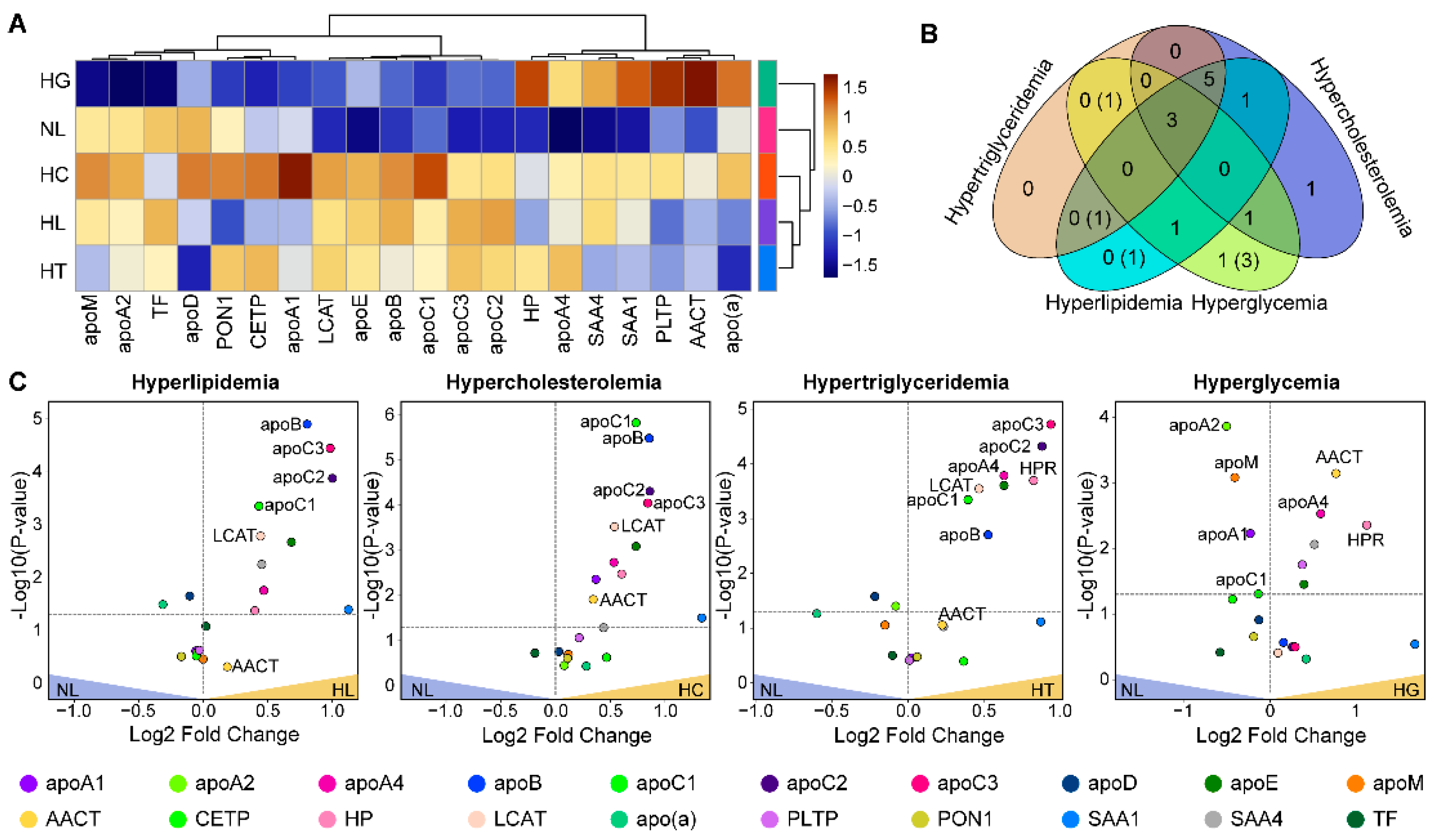
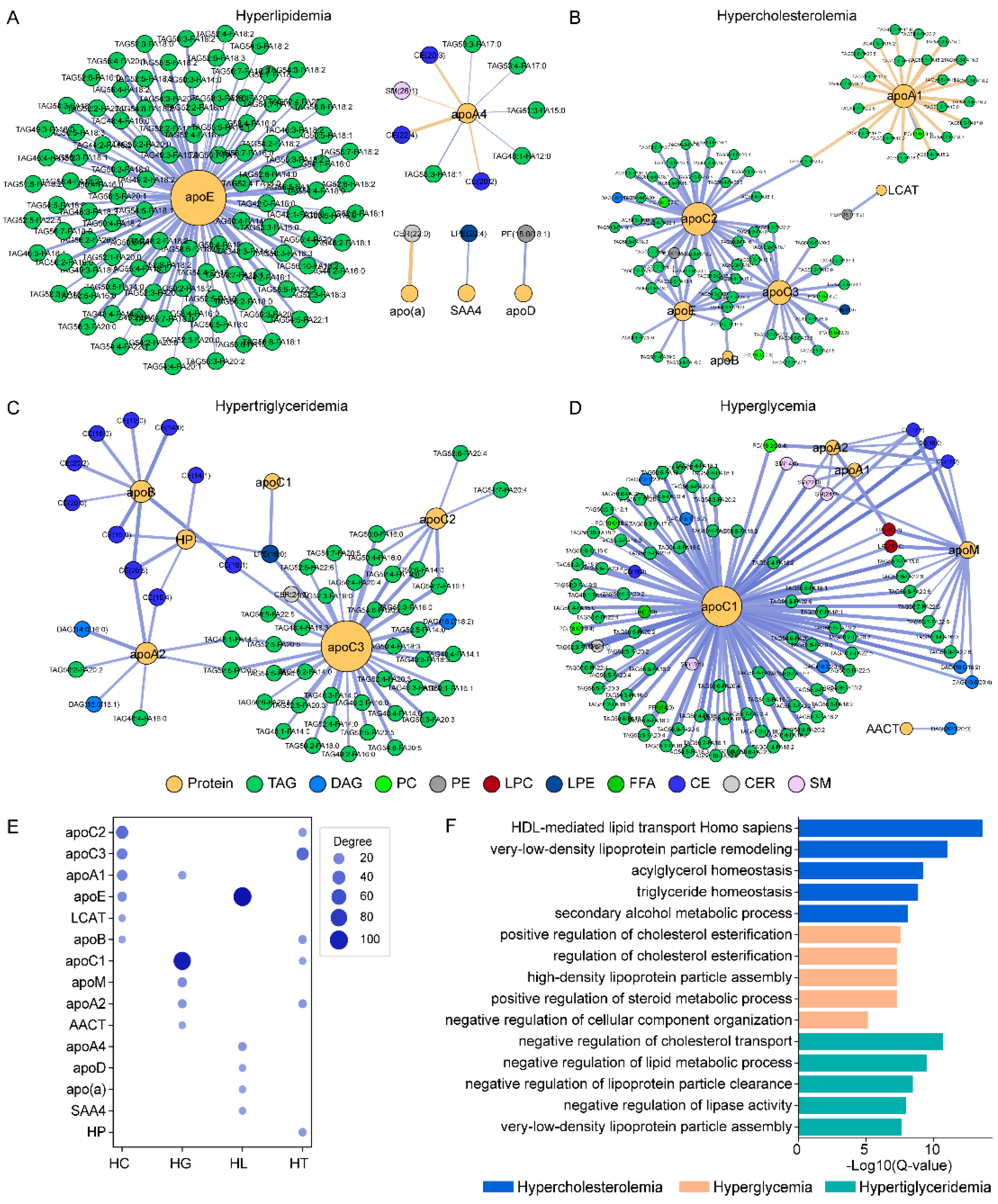
| Lipid Class | N | Mean Class Concentration (nmol/mL) | p-Value | FDR-Adjusted p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| HL (n = 28) | HC (n = 36) | HT (n = 32) | HG (n = 29) | NL (n = 21) | ||||
| CE | 22 | 6293.02 (3983.87/ 9655.85) | 6582.15 (2785.74/ 11914.27) | 4652.47 (2921.34/ 6470.10) | 3628.28 (1493.49/ 8586.73) | 3902.51 (2913.87/ 5032.15) | <0.0001 | <0.0001 |
| CER | 6 | 11.66 (6.31/ 26.26) | 11.74 (4.52/ 17.16) | 14.98 (5.58/ 101.21) | 9.97 (2.03/ 25.18) | 7.55 (4.53/ 15.77) | <0.0001 | <0.0001 |
| DAG | 17 | 112.60 (47.03/ 166.63) | 66.47 (16.74/ 175.28) | 91.61 (31.44/ 144.29) | 53.65 (10.23/ 127.48) | 43.65 (14.24/ 111.77) | <0.0001 | <0.0001 |
| FFA | 23 | 1631.17 (579.04/ 3571.34) | 1422.70 (477.73/ 3878.03) | 1541.25 (538.41/ 2572.79) | 1146.13 (435.13/ 2406.97) | 775.00 (361.17/ 1506.86) | <0.0001 | <0.0001 |
| HCER | 4 | 4.59 (2.42/ 11.78) | 5.04 (1.72/ 10.58) | 6.58 (2.05/ 53.38) | 4.96 (1.60/ 14.58) | 3.93 (2.80/ 7.66) | 0.0402 | 0.0442 |
| LPC | 9 | 1155.03 (588.88/ 2091.95) | 1043.70 (354.47/ 2142.20) | 834.88 (44.22/ 1328.24) | 611.82 (253.96/ 1220.26 | 637.43 (471.16/ 870.45) | <0.0001 | <0.0001 |
| LPE | 4 | 6.01 (3.77/ 10.20) | 4.84 (1.51/ 8.64) | 5.65 (2.53/ 9.59) | 4.74 (1.77/ 11.71) | 5.67 (3.00/ 11.70) | 0.0482 | 0.0482 |
| PC | 22 | 1935.99 (1411.48/ 3429.96) | 1943.40 (789.95/ 2986.54) | 1613.68 (107.25/ 3080.28) | 1333.21 (625.37/ 2998.97) | 1565.61 (1136.61/ 2293.60) | <0.0001 | <0.0001 |
| PE | 20 | 101.81 (24.89/ 162.07) | 79.43 (19.79/ 129.12) | 76.98 (3.95/ 126.43) | 69.64 (19.03/ 149.40) | 66.83 (21.74/ 161.89) | 0.0013 | 0.0016 |
| SM | 12 | 643.17 (449.83/ 961.88) | 750.77 (289.47/ 1240.61) | 516.03 (47.06/ 766.77) | 498.27 (289.84/ 909.32) | 473.00 (331.12/ 659.44) | <0.0001 | <0.0001 |
| TAG | 435 | 3225.76 (1931.14/ 7599.63) | 1651.70 (343.76/ 5488.81) | 3121.76 (1308.20/ 7254.68) | 1464.9 (297.58/ 4951.11) | 1272.10 (415.40/ 4257.05) | <0.0001 | <0.0001 |
| Protein | Mean Class Concentration (nmol/L) | p-Value | FDR-Adjusted p-Value | ||||
|---|---|---|---|---|---|---|---|
| HL (n = 28) | HC (n = 36) | HT (n = 32) | HG (n = 29) | NL (n = 21) | |||
| apoA1 | 42083.17 (12188.1/ 66169.25) | 56483.08 (25218.02/ 95088.75) | 44540.11 (32114.84/ 70397.61) | 37341.74 (21008.5/ 51743.20) | 43776.14 (27504.08/ 75445.27) | <0.0001 | <0.0001 |
| AACT | 31373.50 (7716.27/ 113819.02) | 34964.14 (5340.99/ 72572.32) | 32233.06 (18656.83/ 54612.17) | 46986.16 (9633.38/ 101862.96) | 27568.85 (21209.76/ 40478.80) | 0.0022 | 0.0039 |
| apoA2 | 37812.64 (5204.16/ 68486.21) | 41216.74 (5033.16/ 58668.71) | 36878.18 (22066.13/ 73083.55) | 27421.17 (7478.85/ 50263.66) | 39002.86 (28271.1/ 51640.55) | <0.0001 | <0.0001 |
| apoA4 | 1975.83 (174.47/ 5096.85) | 2061.60 (177.50/ 5947.96) | 2212.38 (653.78/ 4666.87) | 2146.20 (1095.56/ 4497.41) | 1425.91 (855.8/ 2412.68) | 0.0066 | 0.0110 |
| apoB | 2135.44 (438.21/ 4035.01) | 2207.65 (741.96/ 3748.06) | 1760.61 (894.51/ 3366.66) | 1360.11 (682.29/ 3038.84) | 1220.63 (801.20/ 2503.91) | <0.0001 | <0.0001 |
| apoC1 | 11107.72 (2495.45/ 17306.72) | 13685.27 (5109.03/ 43096.16) | 10828.05 (5094.76/ 16227.18) | 7486.51 (2593.07/ 17171.94) | 8227.14 (5379.98/ 13462.97) | <0.0001 | <0.0001 |
| apoC2 | 6016.09 (1563.75/ 11102.30) | 5443.62 (1401.34/ 11685.60) | 5535.84 (1740.988/ 9993.20) | 3590.02 (369.23/ 11300.71) | 2998.99 (1162.95/ 7131.60) | <0.0001 | <0.0001 |
| apoC3 | 15971.44 (1169.71/ 27413.37) | 14438.28 (1479.65/ 27848.45) | 15485.42 (6080.41/ 30489.17) | 9869.37 (1282.52/ 23024.64) | 8056.58 (4673.48/ 18165.67) | <0.0001 | <0.0001 |
| apoD | 2159.77 (314.833/ 11649.68) | 2370.71 (908.66/ 8286.79) | 1995.23 (1164.22/ 3638.30) | 2121.42 (1256.00/ 3785.67) | 2322.35 (1405.64/ 3247.54) | 0.2224 | 0.2694 |
| apoE | 1849.06 (428.23/ 4051.86) | 1912.59 (56.99/ 4304.84) | 1784.22 (1034.24/ 3063.06) | 1512. 58 (703.88/ 3917.80) | 1149.86 (658.55/ 2423.09) | 0.0014 | 0.0028 |
| apoM | 910.14 (249.59/ 2211.54) | 986.90 (240.97/ 1623.97) | 819.74 (449.36/ 1377.66) | 684.93 (340.00/ 1295.73) | 910.80 (534.75/ 1446.49) | 0.0007 | 0.0016 |
| CETP | 32.15 (3.86/ 114.79) | 46.06 (10.53/ 202.26) | 43.04 (9.40/ 216.23) | 24.63 (4.68/ 73.84) | 33.36 (14.47/ 74.90) | 0.2490 | 0.2767 |
| HP | 22627.85 (2830.18/ 49552.20) | 26019.40 (2791.24/ 73051.78) | 30434.21 (5063.66/ 65007.75) | 37418.85 (5029.59/ 96991.16) | 17136.29 (2724.09/ 39682.59) | 0.0011 | 0.0025 |
| LCAT | 104.62 (38.38/ 169.55) | 111.42 (35.77/ 216.33) | 106.36 (25.29/ 176.85) | 81.85 (25.05/ 154.67) | 76.81 (57.80/ 123.69) | <0.0001 | 0.0003 |
| apo(a) | 66.42 (13.84/ 468.77) | 100.22 (13.67/ 380.86) | 54.40 (9.95/ 230.45) | 110.40 (22.10/ 505.71) | 82.53 (23.09/ 205.41) | 0.0610 | 0.0813 |
| PLTP | 74.76 (38.26/ 134.08) | 88.63 (19.33/ 150.89) | 76.80 (27.86/ 126.74) | 98.94 (61.72/ 195.06) | 76.33 (57.17/ 113.82) | 0.0226 | 0.0347 |
| PON1 | 1462.74 (255.70/ 2981.05) | 1774.69 (268.72/ 3472.60 | 1715.17 (579.82/ 3216.10) | 1442.07 (329.14/ 3697.67) | 1644.84 (747.88/ 3996.51) | 0.2681 | 0.2823 |
| SAA1 | 1032.14 (142.51/ 12268.88) | 1190.87 (203.90/ 9550.01) | 866.73 (115.45/ 4341.76) | 1520.89 (62.15/ 14491.09) | 472.2 8(82.01/ 2027.73) | 0.2290 | 0.2694 |
| SAA4 | 2347.91 (351.09/ 4767.17) | 2320.39 (765.17/ 7827.47) | 2015.95 (980.35/ 4298.63) | 2452.47 (561.43/ 5812.54) | 1713.41 (1124.85/ 3026.67) | 0.0583 | 0.0813 |
| TF | 12208.70 (3204.43/ 75876.58) | 10557.5 8(3015.28/ 53645.74) | 11204.71 (3628.40/ 64739.72) | 8036.81 (2535.74/ 14123.04) | 1230.71 (3922.83/ 105927.36) | 0.6225 | 0.6225 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, A.A.; Rees, J.C.; Parks, B.A.; Andrews, M.; Gardner, M.; Grigorutsa, E.; Kuklenyik, Z.; Pirkle, J.L.; Barr, J.R. Integrated Quantitative Targeted Lipidomics and Proteomics Reveal Unique Fingerprints of Multiple Metabolic Conditions. Biomolecules 2022, 12, 1439. https://doi.org/10.3390/biom12101439
Ivanova AA, Rees JC, Parks BA, Andrews M, Gardner M, Grigorutsa E, Kuklenyik Z, Pirkle JL, Barr JR. Integrated Quantitative Targeted Lipidomics and Proteomics Reveal Unique Fingerprints of Multiple Metabolic Conditions. Biomolecules. 2022; 12(10):1439. https://doi.org/10.3390/biom12101439
Chicago/Turabian StyleIvanova, Anna A., Jon C. Rees, Bryan A. Parks, Michael Andrews, Michael Gardner, Eunice Grigorutsa, Zsuzsanna Kuklenyik, James L. Pirkle, and John R. Barr. 2022. "Integrated Quantitative Targeted Lipidomics and Proteomics Reveal Unique Fingerprints of Multiple Metabolic Conditions" Biomolecules 12, no. 10: 1439. https://doi.org/10.3390/biom12101439
APA StyleIvanova, A. A., Rees, J. C., Parks, B. A., Andrews, M., Gardner, M., Grigorutsa, E., Kuklenyik, Z., Pirkle, J. L., & Barr, J. R. (2022). Integrated Quantitative Targeted Lipidomics and Proteomics Reveal Unique Fingerprints of Multiple Metabolic Conditions. Biomolecules, 12(10), 1439. https://doi.org/10.3390/biom12101439







