Abstract
Oxidative stress and mitochondrial dysfunction are associated with the pathogenesis of several human diseases. The excessive generation of reactive oxygen species (ROS) and/or lack of adequate antioxidant defenses causes DNA mutations in mitochondria, damages the mitochondrial respiratory chain, and alters membrane permeability and mitochondrial defense mechanisms. All these alterations are linked to the development of numerous diseases. Curcumin, an active ingredient of turmeric plant rhizomes, exhibits numerous biological activities (i.e., antioxidant, anti-inflammatory, anticancer, and antimicrobial). In recent years, many researchers have shown evidence that curcumin has the ability to reduce the oxidative stress- and mitochondrial dysfunction-associated diseases. In this review, we discuss curcumin’s antioxidant mechanism and significance in oxidative stress reduction and suppression of mitochondrial dysfunction in mammals. We also discuss the research gaps and give our opinion on how curcumin research in mammals should proceed moving forward.
1. Introduction
Mitochondria are membrane-bound organelles involved in oxidative phosphorylation, which is solely responsible for synthesizing phospholipids, heme, and adenosine-5′-triphosphate (ATP). It has a considerable role in calcium proportion, induction of apoptosis, and cell senescence [1]. Mitochondria possess their own genetic material and are introspective of bacterial origin. The nuclear genome encodes a few respiratory proteins of mitochondria and some mitochondrial tRNA encoded by mitochondrial genes [2]. The biogenesis of mitochondria requires both nuclear and mitochondrial genomes to express respiratory chain proteins [3]. Defects in mitochondrial expression may lead to numerous dysfunctions including diabetes mellitus, leigh syndrome, and Leber’s hereditary optic neuropathy [4,5]. Mitochondrial dysfunction is defined by the deficit of its efficacy in minimizing high-energy molecules, namely ATP required for metabolism in the body. It is related to aging and is also important in many chronic diseases [6]. Mitochondrial dysfunction is often maternally inherited by offspring from mothers. It may also arise due to inadequate mitochondrial numbers in a cell or even mutations in the mitochondrial DNA. The symptom is chronic fatigue, and the disorder cannot be cured completely but can be sustained with supportive treatments [7].
ATP synthesis occurs in mitochondria as it is the site for oxidative metabolism. Glucose, a main energy source for cellular metabolism, which is absorbed from the food consumed by the mitochondria to produce energy moieties known as ATP. Glucose is converted to ATP through the following cycles: glycolysis, Kreb’s cycle, and oxidative phosphorylation. In the glycolysis pathway, glucose is converted into pyruvate, generating two ATP molecules which are a very low energy source. Simultaneously, pyruvate is transformed to acetyl coenzyme A (acetyl Co-A), which on entering Kreb’s cycle generates 36–38 ATP molecules, enabling the oxidation of NADH and FADH2. These two molecules are utilized by the mitochondrial respiratory chain, protein complexes catalyze ADP phosphorylation to ATP by generating a high proton gradient across the inner mitochondrial membrane. These ATP molecules are further utilized by the other cells of our body to perform various metabolic activities [8,9].
Oxidative stress, a disproportion between the generation and over-accumulation of reactive oxygen species (ROS) and antioxidants [10]. ROS are free oxidative radicals and no radical derivatives of oxygen present in the body that can readily combine with other biomolecules resulting in the formation of toxic substances. These toxic substances formed from ROS can eventually lead to cell death. ROS includes peroxides, superoxides, hydroxyl radicals, ozone, and nascent oxygen molecules [11]. Under favorable and regulated conditions, these ROS act as signaling molecules in the cell organelles. When produced in higher amounts, it becomes a toxicant, damaging most of the biomolecules because of its ability to oxidize nucleic acids, lipids, and proteins [12]. ROS generally forms highly stable molecules, but after being oxidized with the free compounds available in the body, it becomes a toxic substance in the organelle [13].
In eukaryotes, mitochondria are a rich source of free radicals [14]. Oxidative damage induced by free radicals can potentially damage the mitochondrial DNA (mtDNA), which affects its function within the cells and contributes to redox signaling for the rest of the cell organelle [15]. ROS is generated in the mitochondrial respiratory chain with the help of enzymes and matrix proteins of the TCA cycle, and the first report of mitochondrial ROS was in 1966 [16]. To mitigate and regulate oxidative stress caused in the body, mitochondrial ROS scavenging systems come into the act, where hydrogen peroxide arises from superoxide radical dismutation with superoxide dismutase are detoxified with catalysts such as glutathione peroxidase breaking down hydrogen peroxide into water [17]. Mitochondria possesses sodium dismutase (SOD), MnSOD, which explains mitochondrial superoxide production [18]. Deregulated ROS and oxidative levels in mitochondria lead to various pathogenesis in the human body, causing mitochondrial dysfunction. Mitochondrial ROS pool has a major role in disease pathophysiology and therapeutic purpose [19]. The dysfunction in mitochondria is characterized by higher oxidative stress, nitric oxide (NO) synthesis, and declined ATP production/oxygen consumption [20]. Antioxidants scavenge ROS by donating their electron to prevalent ROS and neutralizing it. This scavenging activity of antioxidants decreases or delays the capacity of damage to macromolecules [21]. Antioxidants such as glutathione and uric acid are found during the body’s normal metabolism. At the same time, some others are found in our diet. Other lighter antioxidants, namely vitamin E, C, and β-carotene, must be supplied through diet [22,23,24].
In the view of increasing disease conditions in humans, many medicinal and dietary plants grabbed the attraction of researchers as therapeutic agents. One such plant compound is curcumin. It is a polyphenol compound present in rhizomes of turmeric plants (Curcuma spp.). Curcumin is beneficial as it has antioxidant, anti-inflammatory, antimicrobial, antimutagenic, and anticancer activities [25,26,27,28]. Another important mechanism is the antioxidant action of curcumin against oxidative stress [29] by increasing the effect of superoxide dismutase, glutathione and catalase, which reduces mitochondrial oxidative stress [30]. The three redox sites of curcumin can undergo oxidation and hydrogen abstraction, resulting in the formation of phenoxy radicals and stabilization across the keto-enol structure. The administration of curcumin can inactivate stress-sensitive kinases by scavenging free radicals, which could significantly prevent cell damage [31]. Consumption of natural antioxidants such as curcumin will be useful to control oxidative stress caused in our body. It combats various forms of free radicals, namely ROS and reactive nitrogen species (RNS); it modifies glutathione, SOD, and catalase activity to neutralize ROS/RNS and inhibits certain enzymes generated by ROS, such as cyclooxygenase/lipoxygenase and xanthine hydrogenase/oxidase [32,33]. It is an effective scavenger of peroxy radical as it is a lipophilic compound similar to vitamin E, and is considered a chain-breaking antioxidant [34]. Curcumin increases the ROS levels in cancer cells, which leads to apoptosis using caspase enzymes and Cytochrome C release from mitochondria. It has a potent role in regulating cancer cell proliferation and apoptosis activation in different types of cancer [35]. Curcumin can act as an antioxidant that can potentially neutralize free radical ROS in mitochondria and other cellular parts, proving a dynamic approach to controlling mitochondrial oxidative stress. In this review, we discuss curcumin’s antioxidant mechanism and the positive effect rendered by curcumin on oxidative stress-mediated mitochondrial dysfunction in mammals. In addition, we evaluate the study gaps and offer our thoughts on future curcumin research in mammals
2. Antioxidant Mechanism of Curcumin
Curcumin possesses numerous pharmacological activities (antiangiogenic, antioxidant, antiviral, anti-inflammatory, antileukemic, immunostimulant, decarboxylase inhibitor, COX-2 inhibitor, metal chelator); therefore, it has been utilized experimentally and therapeutically in humans and animals. Of these, the antioxidant is remarkable, and the existing research outcome showed that it is an effective antioxidant that reduces the negative effects of oxidative stress [36,37]. Curcumin has the ability to prevent oxidative degradation of lipids, hemoglobin, and DNA by potentially chelating heavy metals or controlling the activity of many enzymes [38]. Curcumin’s antioxidant mechanism is summarized in Figure 1. Regarding curcumin’s antioxidant properties, research revealed that its two phenolic sites enable it to scavenge a number of free radicals directly. It has been shown to be effective against ROS and RNS production in the microenvironment. Additionally, curcumin lowers low-density lipoprotein (LDL) and prevents DNA and protein damage.
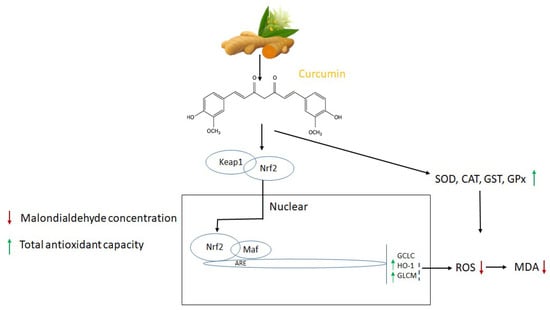
Figure 1.
Molecular targets and antioxidant mechanism of curcumin. The activities of superoxide dismutases (SOD), catalase (CAT), reactive oxygen species (ROS), malondialdehyde (MDA), glutathione S-transferases (GST), and glutathione peroxidase (GPx) adapted from [39,40,44,45].
On an enzyme level, curcumin reduces the production of ROS by the enzymes (i.e., lipoxygenase/cyclooxygenase and xanthine dehydrogenase/oxidase). It increases the enzyme activity SOD and POD, which are known as the first line of defense against oxygen-free radicals [39,40]. The topical application of curcumin is well-known to obstruct TPA-induced H2O2 production in the epidermis [41]. In a study, curcumin alleviated the decrease in cardiac antioxidant enzymes (SOD and CAT) and glutathione levels (glutathione-S-transferase) in diabetic rats [42]. After inducing Al3+ metal ion into Drosophila melanogaster, Oyetayo et al. (2020) [43] found that antioxidants, including catalase, glutathione-S-transferase, and glutathione, decreased whereas H2O2 and NO, which are related to free radical precursors were increased. Notably, oxidative damage induced by the Al3+ ion was reduced by curcumin in a concentration-based dosage.
3. The Physiological and Molecular role of Curcumin in Reducing Oxidative Stress and Preventing Mitochondrial Dysfunction
Elevated ROS levels and oxidative stress are associated with mitochondrial dysfunction, affecting various cellular activities. Curcumin has phenolic and β-diketone functional groups, which helps it to be an antioxidant and free radical scavenger. It enhances the activities of SOD, CAT, and GPX. Curcumin’s ability to penetrate mitochondria protects against oxidative damage and prevents mitochondrial dysfunction. Curcumin’s significance in reducing mitochondrial dysfunction in various organs depicted in Figure 2.
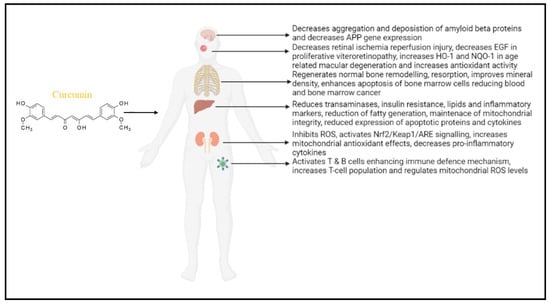
Figure 2.
Role of curcumin in reducing mitochondrial dysfunction in various organs: in the brain—Alzheimer’s disease; eye—retinal infection; skeletal system; liver function; kidney disease, and lymphocyte regulation. Curcumin mainly regulates ROS levels and maintains the antioxidant system for proper regulation of mitochondrial function. (APP—amyloid precursor protein; EGF—epidermal growth factor; HO-1—Heme oxygenase 1; NQO1—NAD(P)H quinone oxidoreductase 1; Nrf2—nuclear factor erythroid 2-related factor 2; Keap1—Kelch-like ECH-associated protein 1; ARE—antioxidant response element.).
3.1. The Effect of Curcumin in Neurodegenerative Diseases
Neurodegenerative disease (ND) is characterized by losing an accessible population of anatomical and physiological related neurons due to other metabolic or toxic disorders. It is classified based on clinical features, anatomic distribution, and molecular abnormality [46,47]. Despite various symptoms and pathology in neurological disorders, recent evidence shows that mitochondria damage plays a considerable role in the progression of neurodegenerative disease [48]. In ND patients, combining quercetin and curcumin enhanced neuro and mitochondrial-protective effects against the side effects of oxaliplatin. It declines lipid peroxidation levels, protein carbonyl content, and simultaneous oxidative stress in mitochondria. It also increases electron transport chain complex enzymes and alters enzymatic and non-enzymatic antioxidants [49]. Curcumin can protect the central nervous system in NDs. It prevents dysfunction in mitochondria and suppresses neuronal death by targeting various pathways, including ROS, intrinsic/extrinsic apoptosis pathway, inflammatory mediators, and microglial cells. It also reduces the loss of neurons and neurotoxic compounds. Curcumin also protects the central nervous system (CNS) against ischemia-induced mitochondrial dysfunction and the onset of NDs [50].
Alzheimer’s disease (AD) is the most common ND characterized by oxidative damage in mitochondria leading to degeneration. These damages enhance oxidative stress causing mitochondrial dysfunction in AD [51]. Studies revealed that higher levels of oxidative damage to biomolecules were observed in AD patient’s brains. β-amyloid is an essential protein in AD which misfolds and forms aggregates due to oxidative stress. The β-amyloid protein in mitochondria interacts with alcohol dehydrogenase inhibiting cytochrome c oxidase [52,53,54]. In human brain tissues and transgenic mice from AD, it was observed that there are direct interactions between ROS and amyloid plaques [55]. The β-amyloid protein impairs mitochondrial dynamics, declines mitochondrial biogenesis, synaptic activity, and improves mitochondrial function. Curcumin enhances mitochondrial fusion activity, biogenesis, and synaptic proteins. It also increases cell function and viability in SHSY5Y cells [56].
3.2. The Effect of Curcumin in Liver Function (Alcoholic Fatty Liver and Obesity)
Fatty liver is the condition in which over-accumulation of fats occurs in hepatocytes [57]. It is the earliest change observed in the pathology of non-alcoholic fatty liver disease (NAFLD) and alcoholic fatty liver disease (AFLD) [58]. NAFLD may be due to the insulin resistance linked with metabolic risk factors [59]. AFLD is due to excessive consumption of alcohol [49]. Both cases lead to steatohepatitis followed by fibrosis, cirrhosis, hepatocellular carcinoma (HCC), and simultaneously death [60]. In patients with NAFLD, insulin resistance is likely to occur along with mitochondrial dysfunction, which plays a significant role in the progression of NAFLD to non-alcoholic steatohepatitis (NASH) [61,62]. High free fatty acids (HFFAs) induced mitochondrial impairment and oxidative stress in primary hepatocytes. Treatment with curcumin inhibited ROS production, ATP depletion, and lipoapoptosis, contributing to the cell’s survival and regaining the membrane potential of mitochondria. Curcumin also increases the copy number of mitochondrial DNA (mtDNA) along with higher levels of transcription factors, namely peroxisome proliferator-activated receptor-γ coactivator 1α (PGC1α), nuclear respiratory factor 1 (NRF1), and mitochondrial transcription factor A (Tfam) regulating mitochondrial biogenesis [63,64]. Curcumin reverses the role of HFFA-induced enhancement of PGC-1α levels, thereby upregulating mitochondrial biogenesis in fatty liver patients [65]. In an experiment with high-fat diet (HFD)-induced obese mice (OM), mitochondria from the liver were isolated, showing high oxidative stress. On treatment with curcumin, it was observed that there was increased oxygen consumption and decreased lipid and protein oxidation levels in isolated mitochondria compared with untreated obese mice [66,67]. Curcumin reduces body weight, decreasing body fat in HFD-induced OM mice [68].
3.3. The Effect of Curcumin in Renal Function
The kidney filters out waste products and withholds other proteins and other components. In the case of damage, these products will drain into urine from the blood. The filters were slowly shut down and lost their ability to filter [69]. Chronic kidney disease (CKD) and chronic renal failure (CRF) are the disease condition that prevails with the abnormality of kidney failure for more than three months period [70]. CKD occurs when a person is diagnosed with anemia, hypertension, breathing shortness, kidney function alterations, itches, cramps, damage to the glomerular capillary tubes, cognitive changes, and occurrence of peripheral odema due to accumulation of sodium [71]. CKD patients are more prone to muscle atrophy with the decline in physical exercise, which contributes to hazardous situations and decreases the quality of life. However, no preventive measures or treatment for muscle atrophy have been devised so far [72,73]. Oxidative stress-mediated mitochondrial dysfunction contributes widely to muscle atrophy. Mitochondria has a considerable role in generating energy as ATP for metabolism in muscle and ROS production [74]. Curcumin, a promising candidate, prevents CKD-induced muscle atrophy by improving mitochondrial dysfunction, biogenesis, and oxidative metabolism of mitochondria. Thereby, it decreases the level of oxidative stress in CKD patients with muscle atrophy [75,76]. Furthermore, pre-treatment with curcumin will protect the renal function by the early decline in changes of CKD-induced mitochondrial biogenesis, oxidative damage, and dynamics [77]. Patients with CRF are inevitably prone to cardiovascular diseases linked with higher oxidative stress associated with mitochondrial dysfunction. Renal failure was observed after nephrectomy in Wistar rats, followed by alteration in cardiac functions. Treatment with curcumin ameliorates the cardiac problem linked with the decline in ROS production, a decrease in oxidative stress markers, and enhanced antioxidant activities [78].
Various documentation alleviates the role of curcumin protective activities against several disease models mediating the mechanism of protecting mitochondrial function and maintaining its integrity [79]. A study using curcumin enhanced the antioxidant enzyme activity levels and the oxidative stress decline. It prevented the capacity of respiration in mitochondrial isolation in nephrectomy, followed by heart failure induced by cardiac reperfusion rats [79,80]. Gentamicin (GM)-induced renal injury is closely linked with mitochondrial dysfunction in proximal convoluted tubules [81]. An experiment conducted in both in vitro cell culture of tubular cells and in vivo studies in rat kidneys exposed to GM brings about the disruption of mitochondrial membrane potential, decline in production of ATP, release of cytochrome c oxidase, apoptosis, and decrease in antioxidant status [82,83]. Treatment with curcumin rendered a positive effect against GM-induced renal injury by protecting antioxidants and modifying the inflammatory response by nuclear factor—κB [84,85]. In 5/6 nephrectomized rats (a rat model with one kidney removed totally and the other 2/3 of the other kidney removed a week later), curcumin reduced the expression of NFκB signaling, and increased NRF2 translocation, enhanced antioxidant enzymes, and decreased inflammation [86,87].
3.4. Effect of Curcumin in Eyes (Retina)
Curcumin has been shown to have negative effects on RPE cells at concentrations indicated as effective in the treatment of tumor cells and reducing the death of retinal neurons (∼10 µm). It is recommended that the function of retina must be closely monitored while taking curcumin as a concurrent therapy for cancer or in the treatment of visual problems. Increased oxidative stress has a role in the etiology of a number of binding retinal disorders (i.e., diabetic retinopathy, retinitis pigmentosa, and age-related macular degeneration) [88]. Antioxidants may reduce the likelihood of developing age-related macular degeneration [89]. Curcumin has been proposed to have potential benefits in reducing the progress of diabetic retinopathy, age-related macular degeneration, and retinitis pigmentosa based on results acquired in animal models of retinopathies and cultured retinal cells [90]. In the retina of hyperglycemic rats, dietary curcumin reduced oxidative alterations and suppressed the increased activity of interleukin-1β, tumor necrosis factor (TNF)-α, and vascular endothelial growth factor (VEGF) [91]. In a rat model of light-induced retinal degeneration, dietary curcumin also prevented the activation of inflammatory genes and protected retinal cells from oxidative damage and simultaneous leading to cell death [92]. Curcumin reduced staurosporine-induced retinal ganglion cell and murine amacrine cell death, decreased neuronal apoptosis, and microvessel degeneration in an experimental ischemia-reperfusion retinal injury model in rats with retinitis pigmentosa. It has also been found that curcumin causes human retinal endothelial cells to undergo apoptosis [93].
It is unclear how curcumin affects retinal pigment epithelial (RPE) cells. RPE cells defend the outer retina from photo-oxidative stress during the digestion of shed photoreceptor outer segments transporting oxidized lipids, avoiding retinal edema and neovascularization [94]. Age-related macular degeneration’s pathogenesis heavily depends on RPE cell malfunction and degeneration [95]. The wet form is recognized by choroidal neovascularization and subretinal edema brought on by outer retinal hypoxia, whereas the dry form is distinguished by the presence of lipofuscin inside the RPE and drusen under the RPE, both of which include photoreceptor-derived oxidized lipids. VEGF is the main hypoxia-induced angiogenic agent that triggers the development of retinal neovascularization and edema. RPE cells are a significant source of VEGF in the retina. Basic fibroblast growth factor (BFGF), a growth factor in addition to VEGF, regulates retinal neovascular diseases such as diabetic retinopathy and the most common kind of age-dependent macular retinopathy. In hyperactive retinopathies and choroidal neovascularization, HGF-induced cell scattering is a necessary step for RPE cell migration and proliferation. Recent research has shown that curcumin reduces RPE cell viability by inducing caspase activation [96]. Researchers evaluated the toxicity of curcumin in human RPE cells as well as curcumin’s effects on the production and release of angiogenic factors from the cells.
The retina is a component in the CNS that comprises the posterior region of the optic globe, and is directly in touch with the vitreous fluid. It is made up of several cell types, of which two kinds of photoreceptors are described in detail: the rods, which are intense at the retina’s periphery, function in scotopic bright conditions (< 0.1 lux, night vision) and are especially susceptible to darkness; while the cones are intense in the macula lutea, and are more sensitive to fine shape and light, being able to distinguish colors and working in photopic light conditions (> 10 lux). The retina also has bipolar cells, amacrine cells, horizontal cells, Muller cells, and retinal pigment epithelial cells in addition to photoreceptors (RPEC). The RPE is a retinal layer that is responsible for numerous important tasks, including the digesting of damaged photoreceptor outer segments (POS) and maintenance of retinal structure. Munia et al. studied the effects of resveratrol, lutein, and curcumin on human retinal epithelial cells, finding that pre-treatment with these nutraceuticals shielded these cells from demise following oxidative stress. The retina is a continual oxidative stress target. It is identified as being mitochondrial-rich and with capillaries that are constantly affected by photons of light. This explains why the majority of retinal disease developments include an oxidative stress equilibrium with high amount of ROS and low levels of antioxidant scavengers.
Furthermore, it is important to note that the retina has a high amount of polyunsaturated fatty acids (PUFA) and, as previously stated, a higher oxygen and glucose intake in contrast to other tissues; these properties make the retina very susceptible to oxidative stress. The RGCS and photoreceptors, in particular, are vulnerable to oxidative stress damage. ROS imbalance is well known to be involved in several retinal diseases, including uveitis, age-related macular degeneration (AMD), diabetic retinopathy (DR), central serous chorioretinopathy (CSC), macular edema (ME), and from uncommon etiologies, retinal ischemia-reperfusion injury (RIRI), retinal and choroidal tumors, proliferative vitreoretinopathy (PVR), hereditary tapeto-retinal degenerations, and retinal and choroidal tumors.
Curcumin substantially decreased retinal vascular leakage in a diabetic retinopathy (DR) animal model. (1) Curcumin is an antioxidant that inhibits free radical formation [97]. (2) Curcumin boosts the mRNA expression of antioxidant enzymes such as SOD and catalase by lowering oxidative DNA damage and controlling nitrosative DNA damage [98]. (3) Curcumin activates a mitochondrial pathway by controlling the respiratory activity of mitochondrial complexes I, II, III, and V while simultaneously activating Nrf2 [99]. (4) Curcumin, a dual inhibitor of dual inhibitor of arachidonic acid, can increase antioxidant capacity in the retina of diabetic rats as well as hypoglycemic and preventive anti-inflammatory activity by lowering levels of proinflammatory cytokines such as IL-1, tumor necrosis factor-alpha, VEGF and 5-hydroxyeicosatetraenoic acid [100]. (5) Curcumin inhibits the migration of retinal human endothelial cells and functions as an antiangiogenic drug by reducing stromal cell-derived factor 1 alpha.
3.5. Effect of Curcumin on the Skeletal System
Mitochondrial dysfunction can cause damage to the osteoblasts, the cells that help the bone formation and mineral absorption, ultimately leading to bone diseases [34]. Mitochondrial oxidative stress can cause an imbalance between osteoblast and osteoclast’s functions within the skeletal bones [101]. Thus, this imbalance will lead to bone-related disorders such as osteoporosis, osteoarthritis, and the demineralization of bones. Consumption of curcumin can improve the proliferation and differentiation of these cells resulting in normal bone remodeling, resorption, and improving the mineral density of the skeletal system [102]. However, some experiments demonstrate that curcumin administered at appropriate dosages can regulate the reactive oxygen species levels in normal proportions within a cell, beyond which it could have some negative impacts and inhibiting effects on the cell organelles [103]. Apart from oxidative stress and mitochondrial dysfunction, curcumin is also known to cause apoptosis of bone marrow cells, reducing the risk of blood and bone marrow cancer.
3.6. Effect of Curcumin on the Lymphatic System
It is known that curcumin can activate T and B cells of the lymphatic system providing an efficient immune defense mechanism to the body with the help of using natural compounds [104]. Curcumin can increase the T cell population over the tumor site of the affected individuals or organisms in the circulating lymphatic system, proving that the compound can be used to fight against malignant cancers known so far [105]. It inhibits the tube formation in rat lymphatic endothelial cells, thereby exhibiting anti-lymphangiogenic effects [106]. It can regulate the mitochondrial ROS level of lymphocytes, thus aiding in the lymphatic system’s normal metabolism [107].
3.7. Effect of Curcumin on Psychiatric Disorders
Depression is a chronic psychological condition that reduces one’s quality of life and increases one’s risk to death. It is a complex disorder with multifactorial etiologies that includes genetic and environmental influences [108]. Curcumin has been widely used as an antidepressant in modulating neurotransmitters (increases in noradrenaline, dopamine, serotonin and decreases in monoamine oxidase enzymes), improving mitochondrial protection, decreasing levels of oxidative markers and nitric oxide, increasing antioxidant enzyme activity, restoring HPA axis dysfunction and enhancing auto-immuno inflammatory action [109]. Table 1 and Table 2 illustrate the cause of mitochondrial dysfunction and the role of curcumin against mitochondrial dysfunction in various organs, respectively.

Table 1.
The molecular causes of mitochondrial dysfunction in various organs in the body.

Table 2.
The role of curcumin in alleviating mitochondrial dysfunction in different organs.
4. Conclusions and Future Perspectives
In comparison to other plant-derived compounds, curcumin has garnered considerable attention for its therapeutic value over the years. In this review, according to the content mentioned, numerous research has demonstrated curcumin’s potent ability to reduce oxidative stress and prevent mitochondrial dysfunction. However, they are limited to in vitro and in vivo studies, and no detailed data for clinical trials about the long-term effects and precise mechanisms of curcumin on oxidative stress in humans were available. As a result, we know little about the potential risks of curcumin and its modified formulations in human. Therefore, a greater number of clinical studies must be conducted to comprehend the possible advantages of curcumin and its modified formulations and related risks in humans. Curcumin’s mechanism of action is complex and linked to multiple signaling pathways. Its targeting mechanisms are not well understood. Therefore, the precise molecular targets and regulatory mechanisms of curcumin require further investigation. It is hoped that further studies on curcumin will provide novel insights to curcumin’s role in reducing oxidative stress and preventing mitochondrial dysfunction.
Author Contributions
Conceptualization: M.S. and A.K.; writing—original draft preparation: M.S., L.C.P.D. and A.K.; writing—review and editing: A.K., M.S., S.K. and T.M.; funding acquisition: A.K. and T.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Creative Challenge Program (2020R1|1A1A01060923) to Adhimoolam Karthikeyan (A.K.) and the Basic Science Research Program (2019R1A6A1A11052070 and 2022R1A2B5B02001711) to Taesun Min (T.M.) through the National Research Foundation of Korea (NRF), Ministry of Science and ICT.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Duchen, M.R. Mitochondria and Calcium: From Cell Signalling to Cell Death. J. Physiol. 2000, 529, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Andersson, S.G.E.; Zomorodipour, A.; Andersson, J.O.; Sicheritz-Pontén, T.; Alsmark, U.C.M.; Podowski, R.M.; Näslund, A.K.; Eriksson, A.-S.; Winkler, H.H.; Kurland, C.G. The Genome Sequence of Rickettsia Prowazekii and the Origin of Mitochondria. Nature 1998, 396, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.; Bankier, A.T.; Barrell, B.G.; de Bruijn, M.H.L.; Coulson, A.R.; Drouin, J.; Eperon, I.C.; Nierlich, D.P.; Roe, B.A.; Sanger, F.; et al. Sequence and Organization of the Human Mitochondrial Genome. Nature 1981, 290, 457–465. [Google Scholar] [CrossRef]
- Santorelli, F.M.; Shanske, S.; Macaya, A.; DeVivo, D.C.; DiMauro, S. The Mutation at Nt 8993 of Mitochondrial DNA Is a Common Cause of Leigh’s Syndrome. Ann. Neurol. 1993, 34, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.P.; Köhler, M.; Graff, C.; Oldfors, A.; Magnuson, M.A.; Berggren, P.-O.; Larsson, N.-G. Impaired Insulin Secretion and β-Cell Loss in Tissue-Specific Knockout Mice with Mitochondrial Diabetes. Nat. Genet. 2000, 26, 336–340. [Google Scholar] [CrossRef]
- Swerdlow, R.H. Brain Aging, Alzheimer’s Disease, and Mitochondria. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2011, 1812, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- Twig, G.; Shirihai, O.S. The Interplay Between Mitochondrial Dynamics and Mitophagy. Antioxid. Redox Signal. 2011, 14, 1939–1951. [Google Scholar] [CrossRef]
- Bonora, M.; Patergnani, S.; Rimessi, A.; de Marchi, E.; Suski, J.M.; Bononi, A.; Giorgi, C.; Marchi, S.; Missiroli, S.; Poletti, F.; et al. ATP Synthesis and Storage. Purinergic Signal. 2012, 8, 343–357. [Google Scholar] [CrossRef]
- Tarasov, A.I.; Griffiths, E.J.; Rutter, G.A. Regulation of ATP Production by Mitochondrial Ca2+. Cell Calcium 2012, 52, 28–35. [Google Scholar] [CrossRef]
- Ferrer, M.; Sureda, A.; Mestre, A.; Tur, J.; Pons, A. The Double Edge of Reactive Oxygen Species as Damaging and Signaling Molecules in HL60 Cell Culture. Cell. Physiol. Biochem. 2010, 25, 241–252. [Google Scholar] [CrossRef]
- Sato, H.; Shibata, M.; Shimizu, T.; Shibata, S.; Toriumi, H.; Ebine, T.; Kuroi, T.; Iwashita, T.; Funakubo, M.; Kayama, Y.; et al. Differential Cellular Localization of Antioxidant Enzymes in the Trigeminal Ganglion. Neuroscience 2013, 248, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Yepes, J.; Zavala-Flores, L.; Anandhan, A.; Wang, F.; Skotak, M.; Chandra, N.; Li, M.; Pappa, A.; Martinez-Fong, D.; del Razo, L.M.; et al. Antioxidant Gene Therapy against Neuronal Cell Death. Pharmacol. Ther. 2014, 142, 206–230. [Google Scholar] [CrossRef]
- Wu, J.Q.; Kosten, T.R.; Zhang, X.Y. Free Radicals, Antioxidant Defense Systems, and Schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 46, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Andreyev, A.Y.; Kushnareva, Y.E.; Starkov, A.A. Mitochondrial Metabolism of Reactive Oxygen Species. Biochemistry 2005, 70, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, Oxidants, and Aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.K. Antimycin-Insensitive Oxidation of Succinate and Reduced Nicotinamide-Adenine Dinucleotide in Electron-Transport Particles I. PH Dependency and Hydrogen Peroxide Formation. Biochim. Biophys. Acta (BBA) Enzymol. Biol. Oxid. 1966, 122, 157–166. [Google Scholar] [CrossRef]
- Loschen, G.; Azzi, A.; Richter, C.; Flohé, L. Superoxide Radicals as Precursors of Mitochondrial Hydrogen Peroxide. FEBS Lett. 1974, 42, 68–72. [Google Scholar] [CrossRef]
- Weisiger, R.A.; Fridovich, I. Superoxide Dismutase. J. Biol. Chem. 1973, 248, 3582–3592. [Google Scholar] [CrossRef]
- Anglevo, P.R.; Abramov, A.Y. Role of mitochondrial ROS in the brain: From physiology to neurodegeneration. FEBS Lett. 2018, 592, 692–702. [Google Scholar] [CrossRef]
- Martínez-Morúa, A.; Soto-Urquieta, M.G.; Franco-Robles, E.; Zúñiga-Trujillo, I.; Campos-Cervantes, A.; Pérez-Vázquez, V.; Ramírez-Emiliano, J. Curcumin Decreases Oxidative Stress in Mitochondria Isolated from Liver and Kidneys of High-Fat Diet-Induced Obese Mice. J. Asian Nat. Prod. Res. 2013, 15, 905–915. [Google Scholar] [CrossRef]
- Halliwell, B. How to Characterize an Antioxidant: An Update. Biochem. Soc. Symp. 1995, 61, 73–101. [Google Scholar] [CrossRef] [PubMed]
- Levine, M. Criteria and Recommendations for Vitamin C Intake. JAMA 1999, 281, 1415. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Noguchi, N.; Niki, E. Comparative Study on Dynamics of Antioxidative Action of α-Tocopheryl Hydroquinone, Ubiquinol, and α-Tocopherol against Lipid Peroxidation. Free Radic. Biol. Med. 1999, 27, 334–346. [Google Scholar] [CrossRef]
- Priyadarsini, K. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef]
- Vera-Ramirez, L.; Pérez-Lopez, P.; Varela-Lopez, A.; Ramirez-Tortosa, M.; Battino, M.; Quiles, J.L. Curcumin and Liver Disease. BioFactors 2013, 39, 88–100. [Google Scholar] [CrossRef]
- Reddy, R.C.; Vatsala, P.G.; Keshamouni, V.G.; Padmanaban, G.; Rangarajan, P.N. Curcumin for Malaria Therapy. Biochem. Biophys. Res. Commun. 2005, 326, 472–474. [Google Scholar] [CrossRef]
- Lestari, M.L.A.D.; Indrayanto, G. Curcumin. Profiles Drug Subst. Excip. Relat. Methodol. 2014, 39, 113–204. [Google Scholar]
- Sahebkar, A.; Serban, M.-C.; Ursoniu, S.; Banach, M. Effect of Curcuminoids on Oxidative Stress: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Funct. Foods 2015, 18, 898–909. [Google Scholar] [CrossRef]
- Banach, M.; Serban, C.; Aronow, W.S.; Rysz, J.; Dragan, S.; Lerma, E.V.; Apetrii, M.; Covic, A. Lipid, Blood Pressure and Kidney Update 2013. Int. Urol. Nephrol. 2014, 46, 947–961. [Google Scholar] [CrossRef]
- Marchiani, A.; Rozzo, C.; Fadda, A.; Delogu, G.; Ruzza, P. Curcumin and Curcumin-like Molecules: From Spice to Drugs. Curr. Med. Chem. 2013, 21, 204–222. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Springer: Boston, MA, USA, 2007; Volume 595, pp. 105–125. [Google Scholar] [CrossRef]
- Lin, Y.G.; Kunnumakkara, A.B.; Nair, A.; Merritt, W.M.; Han, L.Y.; Armaiz-Pena, G.N.; Kamat, A.A.; Spannuth, W.A.; Gershenson, D.M.; Lutgendorf, S.K.; et al. Curcumin Inhibits Tumor Growth and Angiogenesis in Ovarian Carcinoma by Targeting the Nuclear Factor-ΚB Pathway. Clin. Cancer Res. 2007, 13, 3423–3430. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I.; Maity, D.K.; Naik, G.H.; Kumar, M.S.; Unnikrishnan, M.K.; Satav, J.G.; Mohan, H. Role of Phenolic O-H and Methylene Hydrogen on the Free Radical Reactions and Antioxidant Activity of Curcumin. Free Radic. Biol. Med. 2003, 35, 475–484. [Google Scholar] [CrossRef]
- Bimonte, S.; Barbieri, A.; Palma, G.; Rea, D.; Luciano, A.; D’Aiuto, M.; Arra, C.; Izzo, F. Dissecting the Role of Curcumin in Tumour Growth and Angiogenesis in Mouse Model of Human Breast Cancer. BioMed Res. Int. 2015, 2015, 878134. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, A.; Senthil, N.; Min, T. Nanocurcumin: A promising candidate for therapeutic applications. Front. Pharmacol. 2020, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, A.; Young, K.N.; Moniruzzaman, M.; Beyene, A.M.; Do, K.; Kalaiselvi, S.; Min, T. Curcumin and its modified formulations on inflammatory bowel disease (IBD): The story so far and future outlook. Pharmaceutics 2021, 13, 484. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, K.; Druzga, A.; Katarzyna, J.; Skonieczna-Zydecka, K. Antioxidant potential of curcumin-A meta-analysis of randomized clinical trials. Antioxidants 2020, 9, 1092. [Google Scholar] [CrossRef]
- Shen, L.; Ji, H.F. The pharmacology of curcumin: Is it the degradation products? Trends Mol. Med. 2012, 18, 138–144. [Google Scholar] [CrossRef]
- Del Peado-Audelo, M.L.; Caballero-Floran, I.H.; Meza-Toledo, J.A.; Mendoza-Munoz, N.; Gonzalez-Torres, M.; Floran, B.; Cortes, H.; Levya-Gomez, G. Formulations of curcumin nanoparticles for brain diseases. Biomolecules 2019, 9, 56. [Google Scholar] [CrossRef]
- Huang, M.T. Antioxidant and antitumorigenic properties of curcumin. In Food Factors for Cancer Revention; Ohigashi, H., Osawa, T., Terao, J., Watanabe, S., Yoshikawa, T., Eds.; Springer: Tokyo, Japan, 1997; pp. 249–252. [Google Scholar] [CrossRef]
- Abo-Salem, O.M.; Harisa, G.I.; Ali, T.M.; El-Sayed, E.S.M.; Abou-Elnour, F.M. Curcumin ameliorates streptozotocin-induced heart injury in rats. J. Biochem. Mol. Toxicol. 2014, 28, 263–270. [Google Scholar] [CrossRef]
- Oyetayo, B.O.; Abolaji, A.O.; Fasae, K.D.; Aderibigbe, A. Ameliorative role of diets fortified with curcumin in a Drosophila melanogaster model of aluminium chloride-induced neurotoxicity. J. Funct. Foods 2020, 71, 104035. [Google Scholar] [CrossRef]
- Lin, X.; Bai, D.; Wei, Z.; Zhang, Y.; Huang, Y.; Deng, H.; Huang, X. Curcumin attenuates oxidative stress in RAW264.7 cells by increasing the activity of antioxidant enzymes and activating the Nrf2-Keap1 pathway. PLoS ONE 2019, 14, e0216711. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Calina, D.; Docea, A.O.; Koirala, N.; Aryal, S.; Lombardo, D.; Pasqua, L.; Taheri, Y.; Marina Salgado Castillo, C.; Martorell, M.; et al. Curcumin’s Nanomedicine Formulations for Therapeutic Application in Neurological Diseases. J. Clin. Med. 2020, 9, 430. [Google Scholar] [CrossRef]
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef] [PubMed]
- Wojda, U.; Salinska, E.; Kuznicki, J. Calcium Ions in Neuronal Degeneration. IUBMB Life 2008, 60, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Kasote, D.M.; Hegde, M.V.; Katyare, S.S. Mitochondrial Dysfunction in Psychiatric and Neurological Diseases: Cause(s), Consequence(s), and Implications of Antioxidant Therapy. BioFactors 2013, 39, 392–406. [Google Scholar] [CrossRef] [PubMed]
- Waseem, M.; Parvez, S. Neuroprotective Activities of Curcumin and Quercetin with Potential Relevance to Mitochondrial Dysfunction Induced by Oxaliplatin. Protoplasma 2016, 253, 417–430. [Google Scholar] [CrossRef]
- Gemmell, E.; Bosomworth, H.; Allan, L.; Hall, R.; Khundakar, A.; Oakley, A.E.; Deramecourt, V.; Polvikoski, T.M.; O’Brien, J.T.; Kalaria, R.N. Hippocampal Neuronal Atrophy and Cognitive Function in Delayed Poststroke and Aging-Related Dementias. Stroke 2012, 43, 808–814. [Google Scholar] [CrossRef]
- Ferreiro, E.; Baldeiras, I.; Ferreira, I.L.; Costa, R.O.; Rego, A.C.; Pereira, C.F.; Oliveira, C.R. Mitochondrial- and Endoplasmic Reticulum-Associated Oxidative Stress in Alzheimer’s Disease: From Pathogenesis to Biomarkers. Int. J. Cell Biol. 2012, 2012, 735206. [Google Scholar] [CrossRef]
- Giasson, B.I.; Ischiropoulos, H.; Lee, V.M.-Y.; Trojanowski, J.Q. The Relationship between Oxidative/Nitrative Stress and Pathological Inclusions in Alzheimer’s and Parkinson’s Diseases1,2 11Guest Editors: Mark A. Smith and George Perry 22This Article Is Part of a Series of Reviews on “Causes and Consequences of Oxidative Stress in Alzheimer’s Disease.” The Full List of Papers May Be Found on the Homepage of the Journal. Free Radic. Biol. Med. 2002, 32, 1264–1275. [Google Scholar] [CrossRef]
- Andersen, J.K. Oxidative Stress in Neurodegeneration: Cause or Consequence? Nat. Med. 2004, 10, S18–S25. [Google Scholar] [CrossRef] [PubMed]
- Lustbader, J.W.; Cirilli, M.; Lin, C.; Xu, H.W.; Takuma, K.; Wang, N.; Caspersen, C.; Chen, X.; Pollak, S.; Chaney, M.; et al. ABAD Directly Links Aß to Mitochondrial Toxicity in Alzheimer’s Disease. Science 2004, 304, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Dumont, M.; Ho, D.J.; Calingasan, N.Y.; Xu, H.; Gibson, G.; Beal, M.F. Mitochondrial Dihydrolipoyl Succinyltransferase Deficiency Accelerates Amyloid Pathology and Memory Deficit in a Transgenic Mouse Model of Amyloid Deposition. Free Radic. Biol. Med. 2009, 47, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H.; Manczak, M.; Yin, X.; Grady, M.C.; Mitchell, A.; Kandimalla, R.; Kuruva, C.S. Protective Effects of a Natural Product, Curcumin, against Amyloid β Induced Mitochondrial and Synaptic Toxicities in Alzheimer’s Disease. J. Investig. Med. 2016, 64, 1220–1234. [Google Scholar] [CrossRef] [PubMed]
- Lakshman, R.; Shah, R.; Reyes-Gordillo, K.; Varatharajalu, R. Synergy between NAFLD and AFLD and Potential Biomarkers. Clin. Res. Hepatol. Gastroenterol. 2015, 39, S29–S34. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.M.; deVera, M.E.; Fontes, P.; Shaikh, O.; Ahmad, J. Outcome After Liver Transplantation for NASH Cirrhosis. Am. J. Transplant. 2009, 9, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.P.; Pitts, A.; Younossi, Z.M. Increased Overall Mortality and Liver-Related Mortality in Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2008, 49, 608–612. [Google Scholar] [CrossRef]
- Lefkowitch, J.H. Morphology of Alcoholic Liver Disease. Clin. Liver Dis. 2005, 9, 37–53. [Google Scholar] [CrossRef]
- Siegel, A.B.; Zhu, A.X. Metabolic Syndrome and Hepatocellular Carcinoma. Cancer 2009, 115, 5651–5661. [Google Scholar] [CrossRef]
- Rao, M.S.; Reddy, J.K. PPAR? In the Pathogenesis of Fatty Liver Disease. Hepatology 2004, 40, 783–786. [Google Scholar] [CrossRef]
- Feldstein, A.E.; Canbay, A.; Guicciardi, M.E.; Higuchi, H.; Bronk, S.F.; Gores, G.J. Diet Associated Hepatic Steatosis Sensitizes to Fas Mediated Liver Injury in Mice. J. Hepatol. 2003, 39, 978–983. [Google Scholar] [CrossRef]
- Rafiee, P.; Binion, D.G.; Wellner, M.; Behmaram, B.; Floer, M.; Mitton, E.; Nie, L.; Zhang, Z.; Otterson, M.F. Modulatory Effect of Curcumin on Survival of Irradiated Human Intestinal Microvascular Endothelial Cells: Role of Akt/MTOR and NF-ΚB. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G865–G877. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, M.A.; Rushworth, S.A. Curcumin: Potential for Hepatic Fibrosis Therapy? Br. J. Pharmacol. 2008, 153, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.-J.; Chang, H.-H.; Tsai, T.-H.; Lee, T.-Y. Curcumin Ameliorates Mitochondrial Dysfunction Associated with Inhibition of Gluconeogenesis in Free Fatty Acid-Mediated Hepatic Lipoapoptosis. Int. J. Mol. Med. 2012, 30, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; West, N.E.J.; Pillai, R.; Taggart, D.P.; Channon, K.M. Nitric Oxide Modulates Superoxide Release and Peroxynitrite Formation in Human Blood Vessels. Hypertension 2002, 39, 1088–1094. [Google Scholar] [CrossRef]
- Saavedra-Molina, A.; Ramírez-Emiliano, J.; Clemente-Guerrero, M.; Pérez-Vázquez, V.; Aguilera-Aguirre, L.; González-Hernández, J.C. Mitochondrial Nitric Oxide Inhibits ATP Synthesis Effect of Free Calcium in Rat Heart. Amino Acids 2003, 24, 95–102. [Google Scholar] [CrossRef]
- Weisberg, S.P.; Leibel, R.; Tortoriello, D.V. Dietary Curcumin Significantly Improves Obesity-Associated Inflammation and Diabetes in Mouse Models of Diabesity. Endocrinology 2008, 149, 3549–3558. [Google Scholar] [CrossRef]
- Razmaria, A.A. Chronic Kidney Disease. JAMA 2016, 315, 2248. [Google Scholar] [CrossRef]
- Chen, T.K.; Knicely, D.H.; Grams, M.E. Chronic Kidney Disease Diagnosis and Management. JAMA 2019, 322, 1294. [Google Scholar] [CrossRef]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Ábrigo, J.; Elorza, A.A.; Riedel, C.A.; Vilos, C.; Simon, F.; Cabrera, D.; Estrada, L.; Cabello-Verrugio, C. Role of Oxidative Stress as Key Regulator of Muscle Wasting during Cachexia. Oxidative Med. Cell. Longev. 2018, 2018, 2063179. [Google Scholar] [CrossRef] [PubMed]
- Moorthi, R.N.; Avin, K.G. Clinical Relevance of Sarcopenia in Chronic Kidney Disease. Curr. Opin. Nephrol. Hypertens. 2017, 26, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Leitner, L.M.; Wilson, R.J.; Yan, Z.; Gödecke, A. Reactive Oxygen Species/Nitric Oxide Mediated Inter-Organ Communication in Skeletal Muscle Wasting Diseases. Antioxid. Redox Signal. 2017, 26, 700–717. [Google Scholar] [CrossRef]
- Wu, H.; Kanatous, S.B.; Thurmond, F.A.; Gallardo, T.; Isotani, E.; Bassel-Duby, R.; Williams, R.S. Regulation of Mitochondrial Biogenesis in Skeletal Muscle by CaMK. Science 2002, 296, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, J.; Liu, X.; Zheng, P.; Song, G.; Yi, T.; Li, S. RETRACTED ARTICLE: A Chinese Herbal Formula, Jian-Pi-Yi-Shen Decoction, Improves Muscle Atrophy via Regulating Mitochondrial Quality Control Process in 5/6 Nephrectomised Rats. Sci. Rep. 2017, 7, 9253. [Google Scholar] [CrossRef]
- Aparicio-Trejo, O.E.; Tapia, E.; Molina-Jijón, E.; Medina-Campos, O.N.; Macías-Ruvalcaba, N.A.; León-Contreras, J.C.; Hernández-Pando, R.; García-Arroyo, F.E.; Cristóbal, M.; Sánchez-Lozada, L.G.; et al. Curcumin Prevents Mitochondrial Dynamics Disturbances in Early 5/6 Nephrectomy: Relation to Oxidative Stress and Mitochondrial Bioenergetics. BioFactors 2017, 43, 293–310. [Google Scholar] [CrossRef]
- Correa, F.; Buelna-Chontal, M.; Hernández-Reséndiz, S.; García-Niño, W.R.; Roldán, F.J.; Soto, V.; Silva-Palacios, A.; Amador, A.; Pedraza-Chaverrí, J.; Tapia, E.; et al. Curcumin Maintains Cardiac and Mitochondrial Function in Chronic Kidney Disease. Free Radic. Biol. Med. 2013, 61, 119–129. [Google Scholar] [CrossRef]
- Trujillo, J.; Granados-Castro, L.F.; Zazueta, C.; Andérica-Romero, A.C.; Chirino, Y.I.; Pedraza-Chaverrí, J. Mitochondria as a Target in the Therapeutic Properties of Curcumin. Arch. Pharm. 2014, 347, 873–884. [Google Scholar] [CrossRef]
- González-Salazar, A.; Molina-Jijón, E.; Correa, F.; Zarco-Márquez, G.; Calderón-Oliver, M.; Tapia, E.; Zazueta, C.; Pedraza-Chaverri, J. Curcumin Protects from Cardiac Reperfusion Damage by Attenuation of Oxidant Stress and Mitochondrial Dysfunction. Cardiovasc. Toxicol. 2011, 11, 357–364. [Google Scholar] [CrossRef]
- Quiros, Y.; Vicente-Vicente, L.; Morales, A.I.; Lopez-Novoa, J.M.; Lopez-Hernandez, F.J. An Integrative Overview on the Mechanisms Underlying the Renal Tubular Cytotoxicity of Gentamicin. Toxicol. Sci. 2011, 119, 245–256. [Google Scholar] [CrossRef]
- Chen, J.; Wong, H.S.; Leung, H.Y.; Leong, P.K.; Chan, W.M.; Chen, N.; Ko, K.M. An Ursolic Acid-Enriched Extract of Cynomorium Songaricum Protects against Carbon Tetrachloride Hepatotoxicity and Gentamicin Nephrotoxicity in Rats Possibly through a Mitochondrial Pathway: A Comparison with Ursolic Acid. J. Funct. Foods 2014, 7, 330–341. [Google Scholar] [CrossRef]
- Negrette-Guzmán, M.; Huerta-Yepez, S.; Medina-Campos, O.N.; Zatarain-Barrón, Z.L.; Hernández-Pando, R.; Torres, I.; Tapia, E.; Pedraza-Chaverri, J. Sulforaphane Attenuates Gentamicin-Induced Nephrotoxicity: Role of Mitochondrial Protection. Evid. Based Complement. Altern. Med. 2013, 2013, 135314. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, R.; Beulaja, M.; Thiagarajan, R.; Priyadarsini, A.; Saravanan, R.; Arumugam, M. Ameliorative Effects of Curcumin against Renal Injuries Mediated by Inducible Nitric Oxide Synthase and Nuclear Factor Kappa B during Gentamicin-Induced Toxicity in Wistar Rats. Eur. J. Pharmacol. 2011, 670, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.H.; Al-Wabel, N.; Mahmoud, O.; Mousa, H.M.; Hashad, M. Curcumin Has a Palliative Action on Gentamicin-Induced Nephrotoxicity in Rats. Fundam. Clin. Pharmacol. 2005, 19, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.S.; Massey, H.D.; Krieg, R.; Fazelbhoy, Z.A.; Ghosh, S.; Sica, D.A.; Fakhry, I.; Gehr, T.W.B. Curcumin Ameliorates Renal Failure in 5/6 Nephrectomized Rats: Role of Inflammation. Am. J. Physiol. Ren. Physiol. 2009, 296, F1146–F1157. [Google Scholar] [CrossRef]
- Tapia, E.; Soto, V.; Ortiz-Vega, K.M.; Zarco-Márquez, G.; Molina-Jijón, E.; Cristóbal-García, M.; Santamaría, J.; García-Niño, W.R.; Correa, F.; Zazueta, C.; et al. Curcumin Induces Nrf2 Nuclear Translocation and Prevents Glomerular Hypertension, Hyperfiltration, Oxidant Stress, and the Decrease in Antioxidant Enzymes in 5/6 Nephrectomized Rats. Oxidative Med. Cell. Longev. 2012, 2012, 269039. [Google Scholar] [CrossRef]
- Gao, X.; Deeb, D.; Jiang, H.; Liu, Y.B.; Dulchavsky, S.A.; Gautam, S.C. Curcumin differentially sensitizes malignant glioma cells to TRAIL/Apo2L-mediated apoptosis through activation of procaspases and release of cytochrome c from mitochondria. J. Exp. Ther. Oncol. 2005, 5, 39–48. [Google Scholar]
- Mares-Perlman, J.A.; Klein, R.; Klein, B.E.; Greger, J.L.; Brady, W.E.; Palta, M.; Ritter, L.L. Association of zinc and antioxidant nutrients with age-related maculopathy. Arch. Ophthalmol. 1996, 114, 991–997. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Kanwar, M. Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr. Metab. 2007, 4, 8. [Google Scholar] [CrossRef]
- Vasireddy, V.; Chavali, V.R.; Joseph, V.T.; Kadam, R.; Lin, J.H.; Jamison, J.A.; Ayyagari, R. Rescue of photoreceptor degeneration by curcumin in transgenic rats with P23H rhodopsin mutation. PLoS ONE 2011, 6, e21193. [Google Scholar] [CrossRef]
- Mandal, M.N.A.; Patlolla, J.M.; Zheng, L.; Agbaga, M.P.; Tran, J.T.A.; Wicker, L.; Anderson, R.E. Curcumin protects retinal cells from light-and oxidant stress-induced cell death. Free Radic. Biol. Med. 2009, 46, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Premanand, C.; Rema, M.; Sameer, M.Z.; Sujatha, M.; Balasubramanyam, M. Effect of curcumin on proliferation of human retinal endothelial cells under in vitro conditions. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2179–2184. [Google Scholar] [CrossRef] [PubMed]
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef] [PubMed]
- Pinlaor, S.; Yongvanit, P.; Prakobwong, S.; Kaewsamut, B.; Khoontawad, J.; Pinlaor, P.; Hiraku, Y. Curcumin reduces oxidative and nitrative DNA damage through balancing of oxidant–antioxidant status in hamsters infected with Opisthorchis viverrini. Mol. Nutr. Food Res. 2009, 53, 1316–1328. [Google Scholar] [CrossRef] [PubMed]
- Alex, A.F.; Spitznas, M.; Tittel, A.P.; Kurts, C.; Eter, N. Inhibitory effect of epigallocatechin gallate (EGCG), resveratrol, and curcumin on proliferation of human retinal pigment epithelial cells in vitro. Curr. Eye Res. 2010, 35, 1021–1033. [Google Scholar] [CrossRef]
- Roth, F.; Bindewald, A.; Holz, F.G. Keypathophysiologic pathways in age-related macular disease. Graefe’s Arch. Clin. Exp. Ophthalmol. 2004, 242, 710–716. [Google Scholar] [CrossRef]
- Molina-Jijón, E.; Tapia, E.; Zazueta, C.; El Hafidi, M.; Zatarain-Barrón, Z.L.; Hernández-Pando, R.; Pedraza-Chaverri, J. Curcumin prevents Cr (VI)-induced renal oxidant damage by a mitochondrial pathway. Free Radic. Biol. Med. 2011, 51, 1543–1557. [Google Scholar] [CrossRef]
- Flynn, D.L.; Rafferty, M.F.; Boctor, A.M. Inhibition of 5-hydroxy-eicosatetraenoic acid (5-HETE) formation in intact human neutrophils by naturally-occurring diarylheptanoids: Inhibitory activities of curcuminoids and yakuchinones. Prostaglandins Leukot. Med. 1986, 22, 357–360. [Google Scholar] [CrossRef]
- Terruzzi, I.; Montesano, A.; Senesi, P.; Villa, I.; Ferraretto, A.; Bottani, M.; Rubinacci, A. L-Carnitine reduces oxidative stress and promotes cells differentiation and bone matrix proteins expression in human osteoblast-like cells. BioMed Res. Int. 2019, 2019, 5678548. [Google Scholar] [CrossRef]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209. [Google Scholar] [CrossRef]
- Peddada, K.V.; Peddada, K.V.; Shukla, S.K.; Mishra, A.; Verma, V. Role of curcumin in common musculoskeletal disorders: A review of current laboratory, translational, and clinical data. Orthop. Surg. 2015, 7, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Rohanizadeh, R.; Deng, Y.; Verron, E. Therapeutic actions of curcumin in bone disorders. BoneKEy Rep. 2016, 5, 793. [Google Scholar] [CrossRef] [PubMed]
- Jagetia, G.C.; Aggarwal, B.B. “Spicing up” of the immune system by curcumin. J. Clin. Immunol. 2007, 27, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Da, W.; Zhang, J.; Zhang, R.; Zhu, J. Curcumin inhibits the lymphangiogenesis of gastric cancer cells by inhibition of HMGB1/VEGF-D signaling. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419861600. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Md Sakib Hossain, D.; Mohanty, S.; Sankar Sen, G.; Chattopadhyay, S.; Banerjee, S.; Chakraborty, J.; Das, K.; Sarkar, D.; Das, T.; et al. Curcumin reverses T cell-mediated adaptive immune dysfunctions in tumor-bearing hosts. Cell Mol. Immunol. 2010, 7, 306–315. [Google Scholar] [CrossRef]
- Sivertsen, H.; Bjorkorf, G.H.; Engedal, K.; Selbaek, G.; Helvik, A.S. Depression and Quality of Life in Older Persons: A Review. Dement. Geriatr. Cogn. Disord. 2015, 40, 311–339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, L.; Zhang, J. Curcumin in Antidepressant treatments: An overview of potential mechanisms, preclinical/clinical trials and ongoing challenges. Basic Clin. Pharmacol. Toxicol. 2020, 127, 243–253. [Google Scholar] [CrossRef]
- Norat, P.; Soldozy, S.; Sokolowski, J.D.; Gorick, C.M.; Kumar, J.S.; Chae, Y.; Yağmurlu, K.; Prada, F.; Walker, M.; Levitt, M.R.; et al. Mitochondrial Dysfunction in Neurological Disorders: Exploring Mitochondrial Transplantation. NPJ Regen. Med. 2020, 5, 22. [Google Scholar] [CrossRef]
- Mansouri, A.; Gattolliat, C.-H.; Asselah, T. Mitochondrial Dysfunction and Signaling in Chronic Liver Diseases. Gastroenterology 2018, 155, 629–647. [Google Scholar] [CrossRef]
- Cloonan, S.M.; Kim, K.; Esteves, P.; Trian, T.; Barnes, P.J. Mitochondrial Dysfunction in Lung Ageing and Disease. Eur. Respir. Rev. 2020, 29, 200165. [Google Scholar] [CrossRef]
- Schrier, S.A.; Falk, M.J. Mitochondrial Disorders and the Eye. Curr. Opin. Ophthalmol. 2011, 22, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Chanséaume, E.; Morio, B. Potential Mechanisms of Muscle Mitochondrial Dysfunction in Aging and Obesity and Cellular Consequences. Int. J. Mol. Sci. 2009, 10, 306–324. [Google Scholar] [CrossRef] [PubMed]
- Gouspillou, G.; Hepple, R.T. Editorial: Mitochondria in Skeletal Muscle Health, Aging and Diseases. Front. Physiol. 2016, 7, 446. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.A.; Volpi, S.; Sims, K.B.; Walter, J.E.; Traggiai, E. Powering the Immune System: Mitochondria in Immune Function and Deficiency. J. Immunol. Res. 2014, 2014, 164309. [Google Scholar] [CrossRef]
- Wang, D.; Yang, Y.; Zou, X.; Zheng, Z.; Zhang, J. Curcumin Ameliorates CKD-Induced Mitochondrial Dysfunction and Oxidative Stress through Inhibiting GSK-3β Activity. J. Nutr. Biochem. 2020, 83, 108404. [Google Scholar] [CrossRef]
- Daverey, A.; Agrawal, S.K. Curcumin Alleviates Oxidative Stress and Mitochondrial Dysfunction in Astrocytes. Neuroscience 2016, 333, 92–103. [Google Scholar] [CrossRef]
- Soto-Urquieta, M.G.; López-Briones, S.; Pérez-Vázquez, V.; Saavedra-Molina, A.; González-Hernández, G.A.; Ramírez-Emiliano, J. Curcumin Restores Mitochondrial Functions and Decreases Lipid Peroxidation in Liver and Kidneys of Diabetic Db/Db Mice. Biol. Res. 2014, 47, 74. [Google Scholar] [CrossRef]
- Sabet, N.S.; Atashbar, S.; Khanlou, E.M.; Kahrizi, F.; Salimi, A. Curcumin Attenuates Bevacizumab-Induced Toxicity via Suppressing Oxidative Stress and Preventing Mitochondrial Dysfunction in Heart Mitochondria. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 1447–1457. [Google Scholar] [CrossRef]
- Li, L.; Liu, S.; Zhou, Y.; Zhao, M.; Wang, Y.; Wang, C.; Lou, P.; Huang, R.; Ma, L.; Lu, Y.; et al. Indispensable Role of Mitochondria in Maintaining the Therapeutic Potential of Curcumin in Acute Kidney Injury. J. Cell. Mol. Med. 2021, 25, 9863–9877. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).