Morphological, Gene, and Hormonal Changes in Gonads and In-Creased Micrococcal Nuclease Accessibility of Sperm Chromatin Induced by Mercury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Mussels Sampling and Exposure to HgCl2
2.3. Gonad Sampling and PL Proteins Extraction
2.4. RNA Extraction, cDNA Synthesis and RT-qPCR
2.5. Sperm Nuclei Preparation and MNase Assay
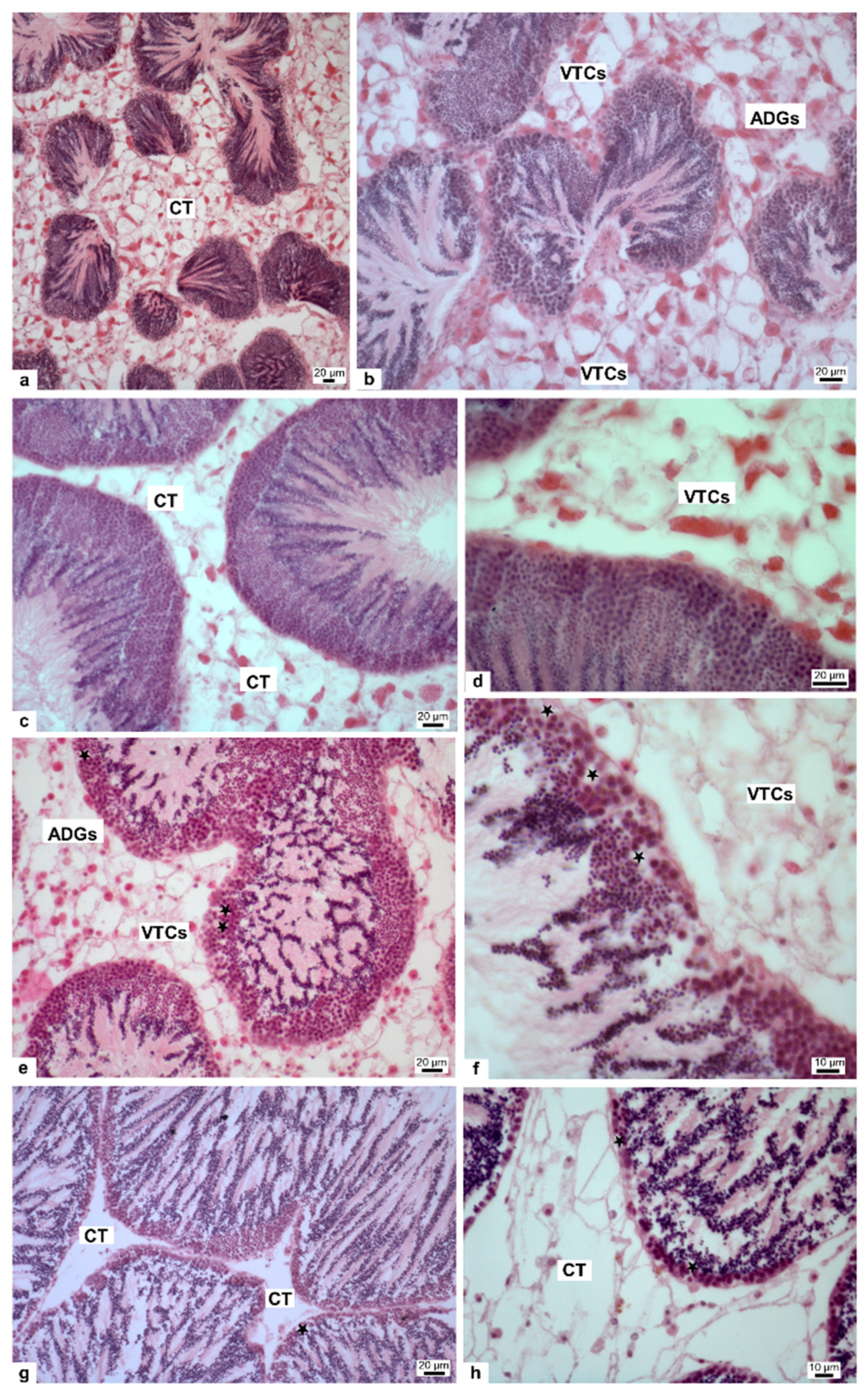
2.6. Morphological Analyses of Gonad
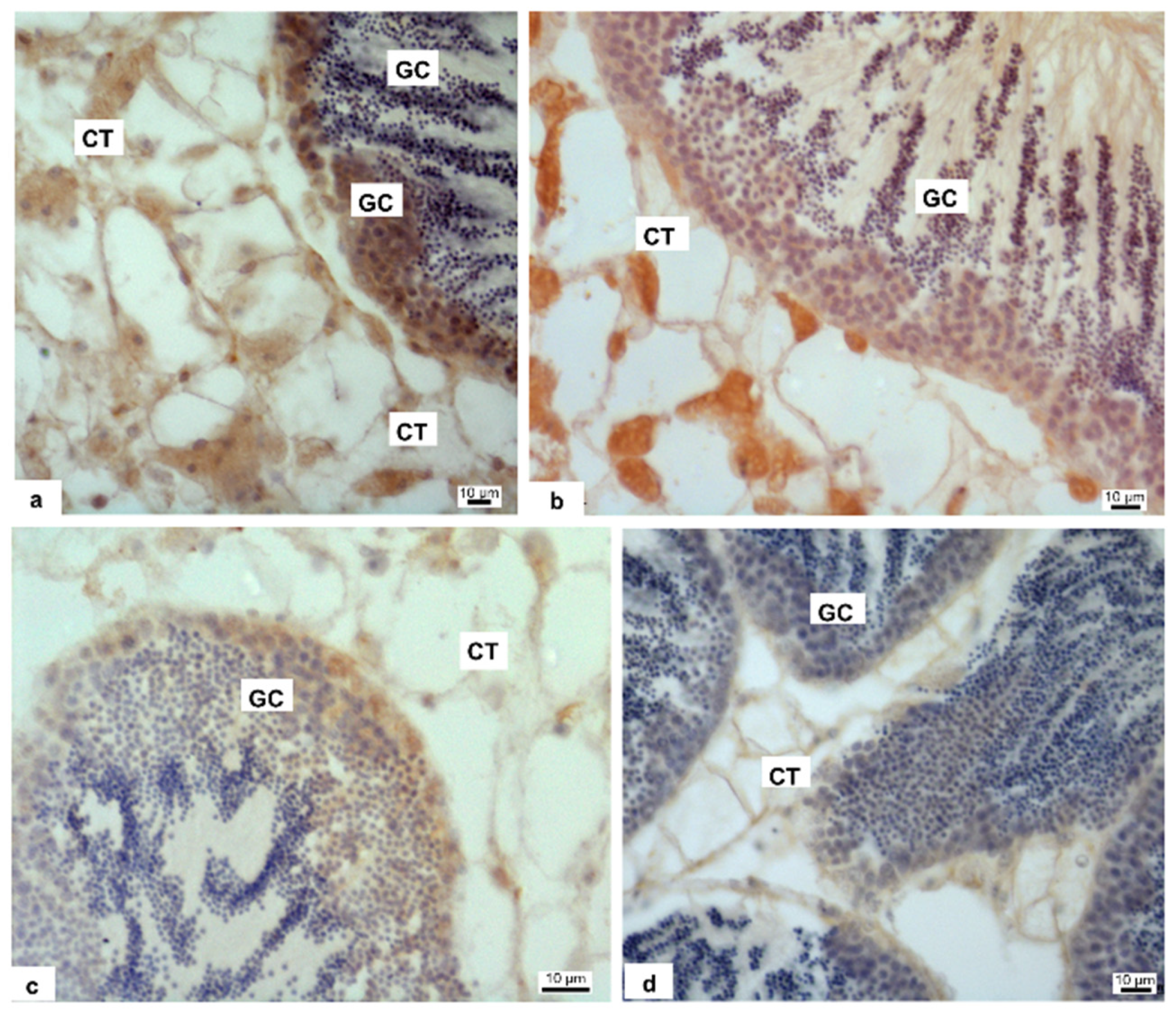
2.7. Immunohistochemistry Analyses
2.8. Statistical Analysis
3. Results
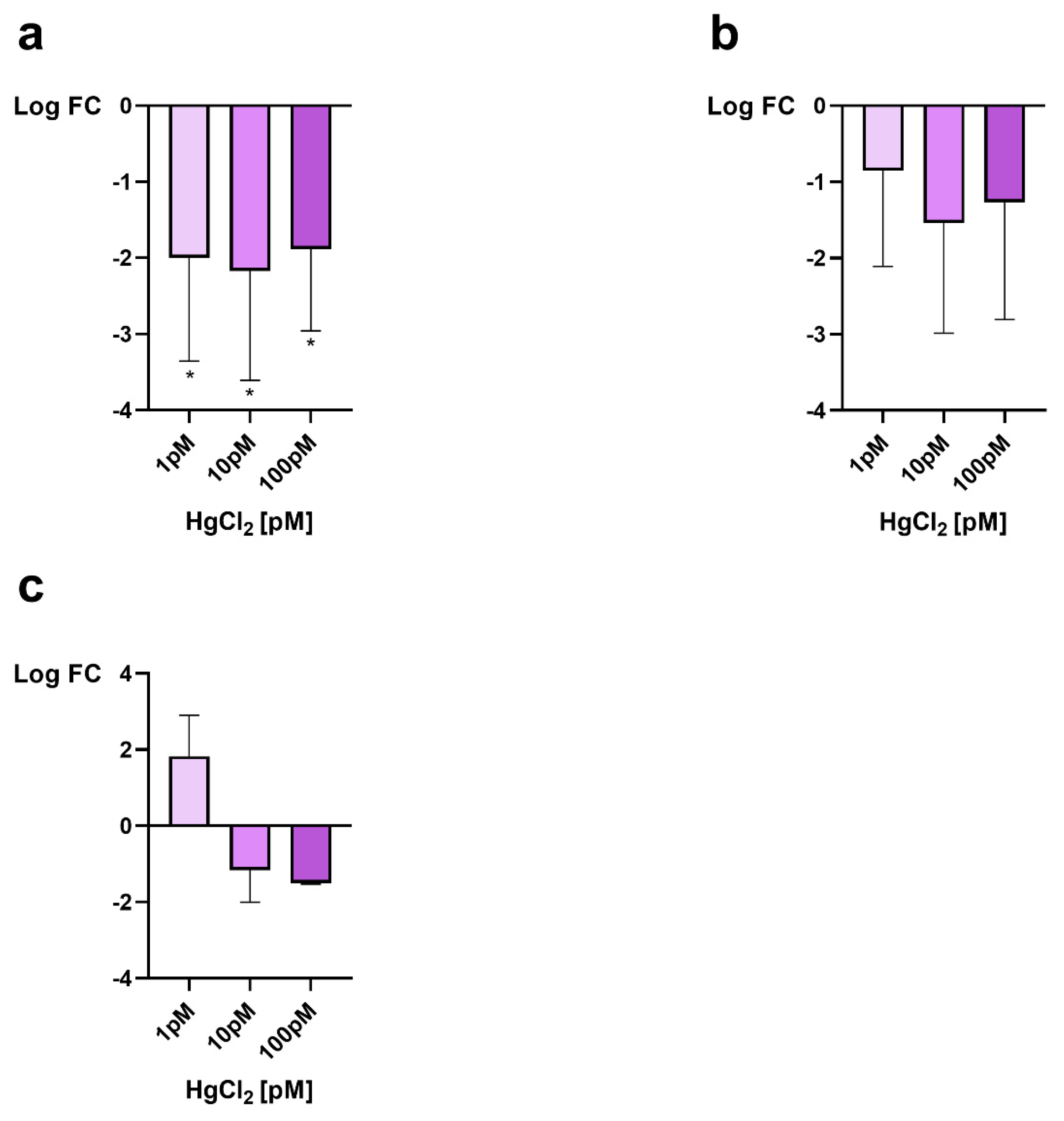
3.1. Gonadal mt10, πgst and hsp70 Expression
3.2. Morphological Analisys of Gonad
3.3. Immunohistochemistry
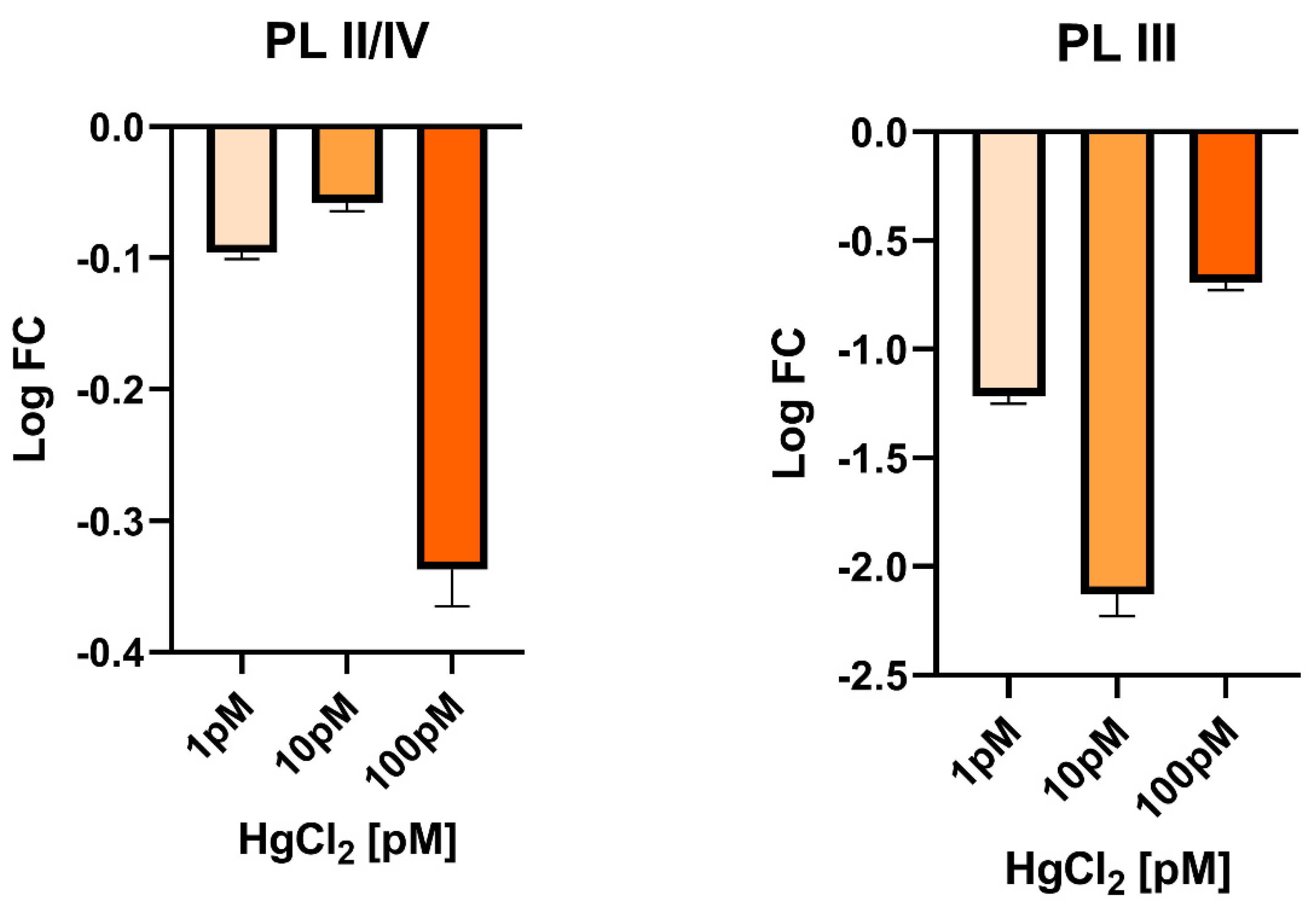
3.4. Gonadal PL-Proteins Genes Expression
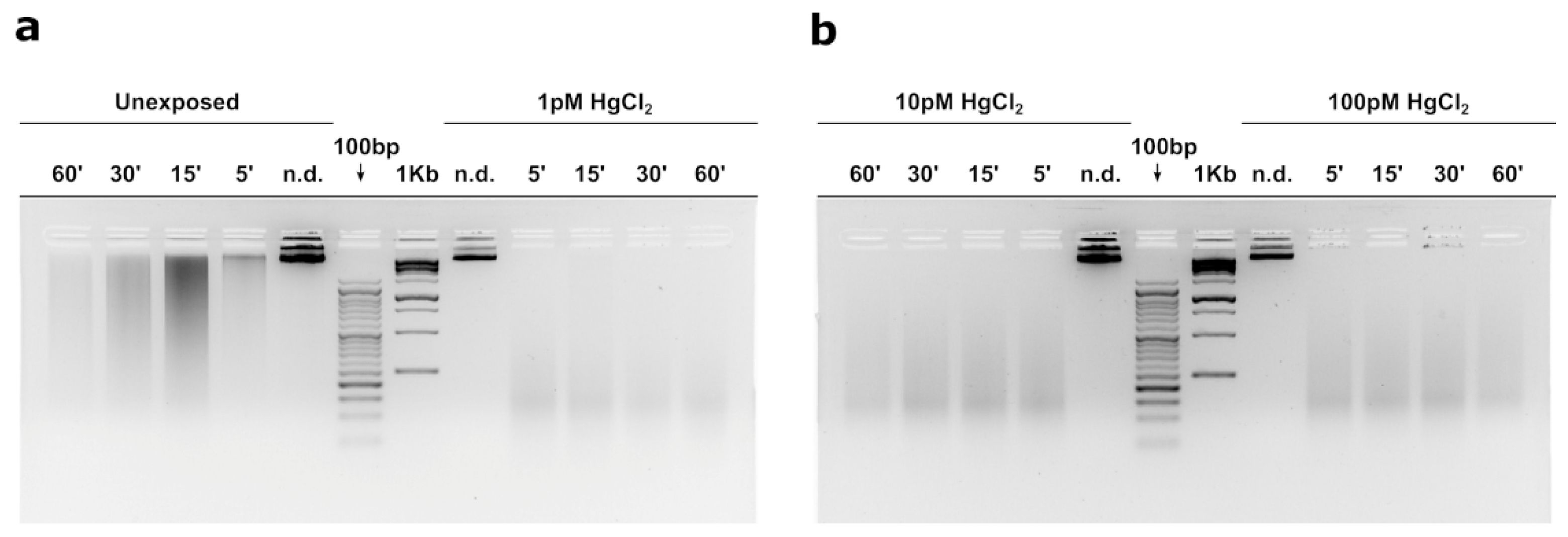
3.5. MNase Digestion Pattern of M. Galloprovincialis’sperm Nuclei
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CT | Connective tissue |
| VTC | Vesicular connective tissue cells |
| ADGs | Adipogranular cells |
| 17β-HSD | 17β-Hydroxysteroid dehydrogenases |
| 3β-HSD | β-Hydroxysteroid dehydrogenase/Δ5−4 isomerase |
| PL- proteins | Protamine-Like |
| H&E | Hematoxylin and Eosin |
| ASD | Androstenedione |
| T | Testosterone |
| DHEA | Dehydroepiandrosterone |
| n.d. MNase | Undigest Micrococcal nuclease |
References
- Outridge, P.M.; Mason, R.P.; Wang, F.; Guerrero, S.; Heimbürger-Boavida, L.E. Updated Global and Oceanic Mercury Budgets for the United Nations Global Mercury Assessment 2018. Environ. Sci. Technol. 2018, 52, 11466–11477. [Google Scholar] [CrossRef] [PubMed]
- Tortora, F.; Notariale, R.; Maresca, V.; Good, K.V.; Sorbo, S.; Basile, A.; Piscopo, M.; Manna, C. Phenol-Rich Feijoa Sellowiana (Pineapple guava) Extracts Protect Human Red Blood Cells from Mercury-Induced Cellular Toxicity. Antioxidants 2019, 8, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piscopo, M.; Notariale, R.; Tortora, F.; Lettieri, G.; Palumbo, G.; Manna, C. Novel Insights into Mercury Effects on Hemoglobin and Membrane Proteins in Human Erythrocytes. Molecules 2020, 25, 3278. [Google Scholar] [CrossRef]
- Officioso, A.; Alzoubi, K.; Lang, F.; Manna, C. Hydroxytyrosol Inhibits Phosphatidylserine Exposure and Suicidal Death Induced by Mercury in Human Erythrocytes: Possible Involvement of the Glutathione Pathway. Food Chem. Toxicol. 2016, 89, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Fathallah, S.; Medhioub, M.N.; Medhioub, A.; Kraiem, M.M. Toxicity of Hg, Cu and Zn on Early Developmental Stages of the European Clam (Ruditapes decussatus) with Potential Application in Marine Water Quality Assessment. Environ. Monit. Assess. 2010, 171, 661–669. [Google Scholar] [CrossRef]
- Depledge, M.H.; Aagaard, A.; Györkös, P. Assessment of Trace Metal Toxicity Using Molecular, Physiological and Behavioural Biomarkers. Mar. Pollut. Bull. 1995, 31, 19–27. [Google Scholar] [CrossRef]
- Bresler, V.; Bissinger, V.; Abelson, A.; Dizer, H.; Sturm, A.; Kratke, R.; Fishelson, L.; Hansen, P.-D. Marine Molluscs and Fish as Biomarkers of Pollution Stress in Littoral Regions of the Red Sea, Mediterranean Sea and North Sea. Helgol. Mar. Res. 1999, 53, 219–243. [Google Scholar] [CrossRef]
- Viarengo, A.; Canesi, L. Mussels as Biological Indicators of Pollution. Aquaculture 1991, 94, 225–243. [Google Scholar] [CrossRef]
- Piscopo, M. Seasonal Dependence of Cadmium Molecular Effects on Mytilus galloprovincialis (Lamarck, 1819) Protamine-like Protein Properties. Mol. Reprod. Dev. 2019, 86, 1418–1429. [Google Scholar] [CrossRef]
- Lettieri, G.; Notariale, R.; Carusone, N.; Giarra, A.; Trifuoggi, M.; Manna, C.; Piscopo, M. New Insights into Alterations in PL Proteins Affecting Their Binding to DNA after Exposure of Mytilus galloprovincialis to Mercury-A Possible Risk to Sperm Chromatin Structure? Int. J. Mol. Sci. 2021, 22, 5893. [Google Scholar] [CrossRef]
- Lettieri, G.; Notariale, R.; Ambrosino, A.; Di Bonito, A.; Giarra, A.; Trifuoggi, M.; Manna, C.; Piscopo, M. Spermatozoa Transcriptional Response and Alterations in PL Proteins Properties after Exposure of Mytilus galloprovincialis to Mercury. Int. J. Mol. Sci. 2021, 22, 1618. [Google Scholar] [CrossRef]
- Lettieri, G.; Mollo, V.; Ambrosino, A.; Caccavale, F.; Troisi, J.; Febbraio, F.; Piscopo, M. Molecular Effects of Copper on the Reproductive System of Mytilus galloprovincialis. Mol. Reprod. Dev. 2019, 86, 1357–1368. [Google Scholar] [CrossRef]
- Piscopo, M.; Notariale, R.; Rabbito, D.; Ausió, J.; Olanrewaju, O.S.; Guerriero, G. Mytilus galloprovincialis (Lamarck, 1819) Spermatozoa: Hsp70 Expression and Protamine-like Protein Property Studies. Environ. Sci. Pollut. Res. Int. 2018, 25, 12957–12966. [Google Scholar] [CrossRef]
- Piscopo, M.; Trifuoggi, M.; Notariale, R.; Labar, S.; Troisi, J.; Giarra, A.; Rabbito, D.; Puoti, R.; de Benedictis, D.; Brundo, M.V.; et al. Protamine-like Proteins’ Analysis as an Emerging Biotechnique for Cadmium Impact Assessment on Male Mollusk Mytilus galloprovincialis (Lamarck 1819). Acta Biochim. Pol. 2018, 65, 259–267. [Google Scholar] [CrossRef]
- Vassalli, Q.A.; Caccavale, F.; Avagnano, S.; Murolo, A.; Guerriero, G.; Fucci, L.; Ausió, J.; Piscopo, M. New Insights into Protamine-like Component Organization in Mytilus galloprovincialis’ Sperm Chromatin. DNA Cell Biol. 2015, 34, 162–169. [Google Scholar] [CrossRef]
- Briant, N.; Chouvelon, T.; Martinez, L.; Brach-Papa, C.; Chiffoleau, J.; Savoye, N.; Sonke, J.; Knoery, J. Spatial and Temporal Distribution of Mercury and Methylmercury in Bivalves from the French Coastline. Mar. Pollut. Bull. 2017, 114, 1096–1102. [Google Scholar] [CrossRef] [Green Version]
- Cinnirella, S.; Bruno, D.E.; Pirrone, N.; Horvat, M.; Živković, I.; Evers, D.C.; Johnson, S.; Sunderland, E.M. Mercury Concentrations in Biota in the Mediterranean Sea, a Compilation of 40 Years of Surveys. Sci. Data 2019, 6, 205. [Google Scholar] [CrossRef] [Green Version]
- Piscopo, M.; Ricciardiello, M.; Palumbo, G.; Troisi, J. Selectivity of Metal Bioaccumulation and Its Relationship with Glutathione S-Transferase Levels in Gonadal and Gill Tissues of Mytilus galloprovincialis Exposed to Ni (II), Cu (II) and Cd (II). Rend. Fis. Acc. Lincei 2016, 27, 737–748. [Google Scholar] [CrossRef] [Green Version]
- Lettieri, G.; Maione, M.; Ranauda, M.A.; Mele, E.; Piscopo, M. Molecular Effects on Spermatozoa of Mytilus galloprovincialis Exposed to Hyposaline Conditions. Mol. Reprod. Dev. 2019, 86, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Olivares, C.; Ruiz, S. Nucleosomal Organization of Chromatin in Sperm Nuclei of the Bivalve Mollusc Aulacomya Ater. Mol. Cell Biochem. 1991, 101, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Prisco, M.; Agnese, M.; De Marino, A.; Andreuccetti, P.; Rosati, L. Spermatogenic Cycle and Steroidogenic Control of Spermatogenesis in Mytilus galloprovincialis Collected in the Bay of Naples. Anat. Rec. 2017, 300, 1881–1894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- al-Hashimi, A.H.; al-Zorba, M.A. Mercury in Some Commercial Fish from Kuwait: A Pilot Study. Sci. Total Environ. 1991, 106, 71–82. [Google Scholar] [CrossRef]
- Ganguli, P.M.; Conaway, C.H.; Swarzenski, P.W.; Izbicki, J.A.; Flegal, A.R. Mercury Speciation and Transport via Submarine Groundwater Discharge at a Southern California Coastal Lagoon System. Environ. Sci. Technol. 2012, 46, 1480–1488. [Google Scholar] [CrossRef] [PubMed]
- Thain, J.E. Effects of Mercury on the Prosobranch Mollusc Crepidula fornicata: Acute Lethal Toxicity and Effects on Growth and Reproduction of Chronic Exposure. Mar. Environ. Res. 1984, 12, 285–309. [Google Scholar] [CrossRef]
- Stankovic, S.; Jovic, M. Health Risks of Heavy Metals in the Mediterranean Mussels as Seafood. Environ. Chem. Lett. 2012, 10, 119–130. [Google Scholar] [CrossRef]
- Almeida, Â.; Esteves, V.I.; Soares, A.M.V.M.; Freitas, R. Effects of Carbamazepine in Bivalves: A Review. In Reviews of Environmental Contamination and Toxicology Volume 254; de Voogt, P., Ed.; Reviews of Environmental Contamination and Toxicology; Springer International Publishing: Cham, Switzerland, 2021; pp. 163–181. ISBN 978-3-030-68530-0. [Google Scholar]
- Rodrigues, J.; Albino, S.; Silva, S.; Cravo, A.; Cardoso, V.V.; Benoliel, M.J.; Almeida, C.M.M. Development of a Multiresidue Method for the Determination of 24 Pharmaceuticals in Clams by QuEChERS and Liquid Chromatography-Triple Quadrupole Tandem Mass Spectrometry. Food Anal. Methods 2019, 12, 838–851. [Google Scholar] [CrossRef]
- Ambrósio, A.F.; Soares-da-Silva, P.; Carvalho, C.M.; Carvalho, A.P. Mechanisms of Action of Carbamazepine and Its Derivatives, Oxcarbazepine, BIA 2-093, and BIA 2-024. Neurochem. Res. 2002, 27, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Lusher, A.L.; Rotchell, J.M.; Deudero, S.; Turra, A.; Bråte, I.L.N.; Sun, C.; Shahadat Hossain, M.; Li, Q.; Kolandhasamy, P.; et al. Using Mussel as a Global Bioindicator of Coastal Microplastic Pollution. Environ. Pollut. 2019, 244, 522–533. [Google Scholar] [CrossRef]
- Zaynab, M.; Al-Yahyai, R.; Ameen, A.; Sharif, Y.; Ali, L.; Fatima, M.; Khan, K.A.; Li, S. Health and Environmental Effects of Heavy Metals. J. King Saud Univ. Sci. 2022, 34, 101653. [Google Scholar] [CrossRef]
- Khayat, A.; Dencker, L. Organ and Cellular Distribution of Inhaled Metallic Mercury in the Rat and Marmoset Monkey (Callithrix jacchus): Influence of Ethyl Alcohol Pretreatment. Acta Pharmacol. Toxicol. 1984, 55, 145–152. [Google Scholar] [CrossRef]
- Viganò, L.; Casatta, N.; Farkas, A.; Mascolo, G.; Roscioli, C.; Stefani, F.; Vitelli, M.; Olivo, F.; Clerici, L.; Robles, P.; et al. Embryo/Larval Toxicity and Transcriptional Effects in Zebrafish (Danio rerio) Exposed to Endocrine Active Riverbed Sediments. Environ. Sci. Pollut. Res. Int. 2020, 27, 10729–10747. [Google Scholar] [CrossRef]
- Köhler, K.; Riisgård, H.U. Formation of Metallothioneins in Relation to Accumulation of Cadmium in the Common Mussel Mytilus Edulis. Mar. Biol. 1982, 66, 53–58. [Google Scholar] [CrossRef]
- Ceratto, N.; Dondero, F.; van de Loo, J.W.; Burlando, B.; Viarengo, A. Cloning and Sequencing of a Novel Metallothionein Gene in Mytilus galloprovincialis Lam. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2002, 131, 217–222. [Google Scholar] [CrossRef]
- Banni, M.; Dondero, F.; Jebali, J.; Guerbej, H.; Boussetta, H.; Viarengo, A. Assessment of Heavy Metal Contamination Using Real-Time PCR Analysis of Mussel Metallothionein Mt10 and Mt20 Expression: A Validation along the Tunisian Coast. Biomarkers 2007, 12, 369–383. [Google Scholar] [CrossRef]
- Dondero, F.; Piacentini, L.; Banni, M.; Rebelo, M.; Burlando, B.; Viarengo, A. Quantitative PCR Analysis of Two Molluscan Metallothionein Genes Unveils Differential Expression and Regulation. Gene 2005, 345, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-X.; Rainbow, P.S. Influence of Metal Exposure History on Trace Metal Uptake and Accumulation by Marine Invertebrates. Ecotoxicol. Environ. Saf. 2005, 61, 145–159. [Google Scholar] [CrossRef]
- Meng, J.; Wang, W.; Shi, R.; Song, K.; Li, L.; Que, H.; Zhang, G. Identification of SNPs Involved in Zn and Cu Accumulation in the Pacific Oyster (Crassostrea gigas) by Genome-Wide Association Analysis. Ecotoxicol. Environ. Saf. 2020, 192, 110208. [Google Scholar] [CrossRef] [PubMed]
- Menezo, Y.; Russo, G.; Tosti, E.; El Mouatassim, S.; Benkhalifa, M. Expression Profile of Genes Coding for DNA Repair in Human Oocytes Using Pangenomic Microarrays, with a Special Focus on ROS Linked Decays. J. Assist. Reprod. Genet. 2007, 24, 513–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aitken, A. Protein Consensus Sequence Motifs. Mol. Biotechnol. 1999, 12, 13. [Google Scholar] [CrossRef]
- Nover, L.; Scharf, K.D. Heat Stress Proteins and Transcription Factors. Cell Mol. Life Sci. 1997, 53, 80–103. [Google Scholar] [CrossRef] [PubMed]
- Feder, M.E.; Hofmann, G.E. Heat-Shock Proteins, Molecular Chaperones, and the Stress Response: Evolutionary and Ecological Physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franzellitti, S.; Fabbri, E. Differential HSP70 Gene Expression in the Mediterranean Mussel Exposed to Various Stressors. Biochem. Biophys. Res. Commun. 2005, 336, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Koagouw, W.; Ciocan, C. Effects of Short-Term Exposure of Paracetamol in the Gonads of Blue Mussels Mytilus Edulis. Environ. Sci. Pollut. Res. Int. 2020, 27, 30933–30944. [Google Scholar] [CrossRef]
- Albakova, Z.; Armeev, G.A.; Kanevskiy, L.M.; Kovalenko, E.I.; Sapozhnikov, A.M. HSP70 Multi-Functionality in Cancer. Cells 2020, 9, 587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Han, C.; Huang, H.; Xin, Y.; Xu, Y.; Luo, L.; Yin, Z. Heat Shock Protein 70 Together with Its Co-Chaperone CHIP Inhibits TNF-Alpha Induced Apoptosis by Promoting Proteasomal Degradation of Apoptosis Signal-Regulating Kinase1. Apoptosis 2010, 15, 822–833. [Google Scholar] [CrossRef]
- Guo, F.; Sigua, C.; Bali, P.; George, P.; Fiskus, W.; Scuto, A.; Annavarapu, S.; Mouttaki, A.; Sondarva, G.; Wei, S.; et al. Mechanistic Role of Heat Shock Protein 70 in Bcr-Abl–Mediated Resistance to Apoptosis in Human Acute Leukemia Cells. Blood 2005, 105, 1246–1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoarau, P.; Damiens, G.; Roméo, M.; Gnassia-Barelli, M.; Bebianno, M.J. Cloning and Expression of a GST-Pi Gene in Mytilus Galloprovincialis. Attempt to Use the GST-Pi Transcript as a Biomarker of Pollution. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2006, 143, 196–203. [Google Scholar] [CrossRef]
- Maretta, M.; Marettová, E.; Skrobánek, P.; Ledec, M. Effect of Mercury on the Seminiferous Epithelium of the Fowl Testis. Acta Vet. Hung. 1995, 43, 153–161. [Google Scholar]
- Dondero, F.; Piacentini, L.; Marsano, F.; Rebelo, M.; Vergani, L.; Venier, P.; Viarengo, A. Gene Transcription Profiling in Pollutant Exposed Mussels (Mytilus spp.) Using a New Low-Density Oligonucleotide Microarray. Gene 2006, 376, 24–36. [Google Scholar] [CrossRef]
- Vergílio, C.S.; Moreira, R.V.; Carvalho, C.E.V.; Melo, E.J.T. Effects of in Vitro Exposure to Mercury on Male Gonads and Sperm Structure of the Tropical Fish Tuvira Gymnotus carapo (L.). J. Fish Dis. 2014, 37, 543–551. [Google Scholar] [CrossRef]
- Nagar, R.N.; Bhattacharya, L. Effect of Mercuric Chloride on Testicular Activities in Mice, Musculus Albinus. J. Environ. Biol. 2001, 22, 15–18. [Google Scholar] [PubMed]
- Boujbiha, M.A.; Hamden, K.; Guermazi, F.; Bouslama, A.; Omezzine, A.; Kammoun, A.; El Feki, A. Testicular Toxicity in Mercuric Chloride Treated Rats: Association with Oxidative Stress. Reprod. Toxicol. 2009, 28, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Janer, G.; Porte, C. Sex Steroids and Potential Mechanisms of Non-Genomic Endocrine Disruption in Invertebrates. Ecotoxicology 2007, 16, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, D.; Loi, B.; Porte, C. Biosynthesis and Metabolism of Steroids in Molluscs. J. Steroid. Biochem. Mol. Biol. 2011, 127, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Lehoux, J.G.; Sandor, T. The Occurrence of Steroids and Steroid Metabolizing Enzyme Systems in Invertebrates. A Review. Steroids 1970, 16, 141–171. [Google Scholar] [CrossRef]
- Lafont, R. Reverse Endocrinology, or “Hormones” Seeking Functions. Insect Biochem. 1991, 21, 697–721. [Google Scholar] [CrossRef]
- Lafont, R.; Mathieu, M. Steroids in Aquatic Invertebrates. Ecotoxicology 2007, 16, 109–130. [Google Scholar] [CrossRef] [PubMed]
- Crump, K.L.; Trudeau, V.L. Mercury-Induced Reproductive Impairment in Fish. Environ. Toxicol. Chem. 2009, 28, 895–907. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Ekstrand, B.; Zamaratskaia, G. Regulation of 3β-Hydroxysteroid Dehydrogenase/Δ5-Δ4 Isomerase: A Review. Int. J. Mol. Sci. 2013, 14, 17926–17942. [Google Scholar] [CrossRef] [Green Version]
- Mindnich, R.; Möller, G.; Adamski, J. The Role of 17 Beta-Hydroxysteroid Dehydrogenases. Mol. Cell Endocrinol. 2004, 218, 7–20. [Google Scholar] [CrossRef] [PubMed]
- De Guglielmo, V.; Puoti, R.; Notariale, R.; Maresca, V.; Ausió, J.; Troisi, J.; Verrillo, M.; Basile, A.; Febbraio, F.; Piscopo, M. Alterations in the Properties of Sperm Protamine-like II Protein after Exposure of Mytilus galloprovincialis (Lamarck 1819) to Sub-Toxic Doses of Cadmium. Ecotoxicol. Environ. Saf. 2019, 169, 600–606. [Google Scholar] [CrossRef]
- Hedayati, A.; Hosseini, A.R. Endocrine Disruptions Induced by Artificial Induction of Mercury Chloride on Sea Bream. Comp. Clin. Pathol. 2013, 22, 679–684. [Google Scholar] [CrossRef]
- Alonso, A.; Suárez, P.; Ruiz, Y.; Dobal, V.; San Juan, F. Gonadal Histopathological Disorders in Mytilus Galloprovincialis Male Exposed to Tars Used in Mussel Farms. Front. Mar. Sci. 2019, 6. [Google Scholar] [CrossRef] [Green Version]
- Freeman, H.C.; Sangalang, G.B. A Study of the Effects of Methyl Mercury, Cadmium, Arsenic, Selenium, and a PCB, (Aroclor 1254) on Adrenal and Testicular Steroidogeneses in Vitro, by the Gray Seal Halichoerus grypus. Arch. Environ. Contam. Toxicol. 1977, 5, 369–383. [Google Scholar] [CrossRef] [PubMed]
- McNeil, S.I.; Bhatnagar, M.K. Ultrastructure of the Testis of Pekin Ducks Fed Methyl Mercury Chloride: Seminiferous Epithelium. Am. J. Vet. Res. 1985, 46, 2019–2025. [Google Scholar] [PubMed]
- Burton, G.V.; Meikle, A.W. Acute and Chronic Methyl Mercury Poisoning Impairs Rat Adrenal and Testicular Function. J. Toxicol. Environ. Health 1980, 6, 597–606. [Google Scholar] [CrossRef]
- Kirubagaran, R.; Joy, K.P. Inhibition of Testicular 3 Beta-Hydroxy-Delta 5-Steroid Dehydrogenase (3 Beta-HSD) Activity in Catfish Clarias batrachus (L.) by Mercurials. Indian J. Exp. Biol. 1988, 26, 907–908. [Google Scholar] [PubMed]
- Ng, T.B.; Liu, W.K. Toxic Effect of Heavy Metals on Cells Isolated from the Rat Adrenal and Testis. In Vitro Cell Dev. Biol. 1990, 26, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, G.; D’Agostino, G.; Mele, E.; Cardito, C.; Esposito, R.; Cimmino, A.; Giarra, A.; Trifuoggi, M.; Raimondo, S.; Notari, T.; et al. Discovery of the Involvement in DNA Oxidative Damage of Human Sperm Nuclear Basic Proteins of Healthy Young Men Living in Polluted Areas. Int. J. Mol. Sci. 2020, 21, 4198. [Google Scholar] [CrossRef]
- Lettieri, G.; Marra, F.; Moriello, C.; Prisco, M.; Notari, T.; Trifuoggi, M.; Giarra, A.; Bosco, L.; Montano, L.; Piscopo, M. Molecular Alterations in Spermatozoa of a Family Case Living in the Land of Fires. A First Look at Possible Transgenerational Effects of Pollutants. Int. J. Mol. Sci. 2020, 21, 6710. [Google Scholar] [CrossRef] [PubMed]
- Baby, J.; Raj, J.; Biby, E.; Sankarganesh, P.; Jeevitha, M.; Ajisha, S.; Rajan, S. Toxic Effect of Heavy Metals on Aquatic Environment. Int. J. Biol. Chem. Sci. 2011, 4. [Google Scholar] [CrossRef] [Green Version]
| Gene | F-Primer | F-Primer Length | R-Primer | R-Primer Length | Accession Number |
|---|---|---|---|---|---|
| Gapdh | CTGCACCACCAACTGCTT | 18 | TTCTGGGTGGCAGTGATG | 18 | SY171038758-018/019 |
| Hsp70 | CGCGATGCCAAACTAGACAA | 20 | TCACCTGACAAAATGGCTGC | 20 | AY861684 |
| Mt10 | GCCTGCACCTTGTAACTGTAT | 21 | CTGTACACCCTGCTTCACAC | 20 | AY566248 |
| Gst | AGTTAGAGGCCGAGCTGAA | 19 | TGGAAACCGTCATCATCTG | 19 | SY140930374-050/051 |
| Pl III | CACCCAACAAGAAGGATGCC | 20 | CCTTGCCCTTTTCTTTCCCC | 20 | SY140930274 |
| Pl II/IV | AAGCCCAAGTAGACGTTCCA | 20 | TCCGAGGTGTGATGTGTTGA | 20 | SY140930274 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lettieri, G.; Carusone, N.; Notariale, R.; Prisco, M.; Ambrosino, A.; Perrella, S.; Manna, C.; Piscopo, M. Morphological, Gene, and Hormonal Changes in Gonads and In-Creased Micrococcal Nuclease Accessibility of Sperm Chromatin Induced by Mercury. Biomolecules 2022, 12, 87. https://doi.org/10.3390/biom12010087
Lettieri G, Carusone N, Notariale R, Prisco M, Ambrosino A, Perrella S, Manna C, Piscopo M. Morphological, Gene, and Hormonal Changes in Gonads and In-Creased Micrococcal Nuclease Accessibility of Sperm Chromatin Induced by Mercury. Biomolecules. 2022; 12(1):87. https://doi.org/10.3390/biom12010087
Chicago/Turabian StyleLettieri, Gennaro, Nadia Carusone, Rosaria Notariale, Marina Prisco, Alessia Ambrosino, Shana Perrella, Caterina Manna, and Marina Piscopo. 2022. "Morphological, Gene, and Hormonal Changes in Gonads and In-Creased Micrococcal Nuclease Accessibility of Sperm Chromatin Induced by Mercury" Biomolecules 12, no. 1: 87. https://doi.org/10.3390/biom12010087
APA StyleLettieri, G., Carusone, N., Notariale, R., Prisco, M., Ambrosino, A., Perrella, S., Manna, C., & Piscopo, M. (2022). Morphological, Gene, and Hormonal Changes in Gonads and In-Creased Micrococcal Nuclease Accessibility of Sperm Chromatin Induced by Mercury. Biomolecules, 12(1), 87. https://doi.org/10.3390/biom12010087








