Towards Mapping of the Human Brain N-Glycome with Standardized Graphitic Carbon Chromatography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Biosynthesis of Isotope-Encoded Standard Glycan Structures
2.3. Mass Spectrometric Analysis
2.4. Binary Gradient Mixed-Mode Chromatography of Fluorescent Derivatives
3. Results
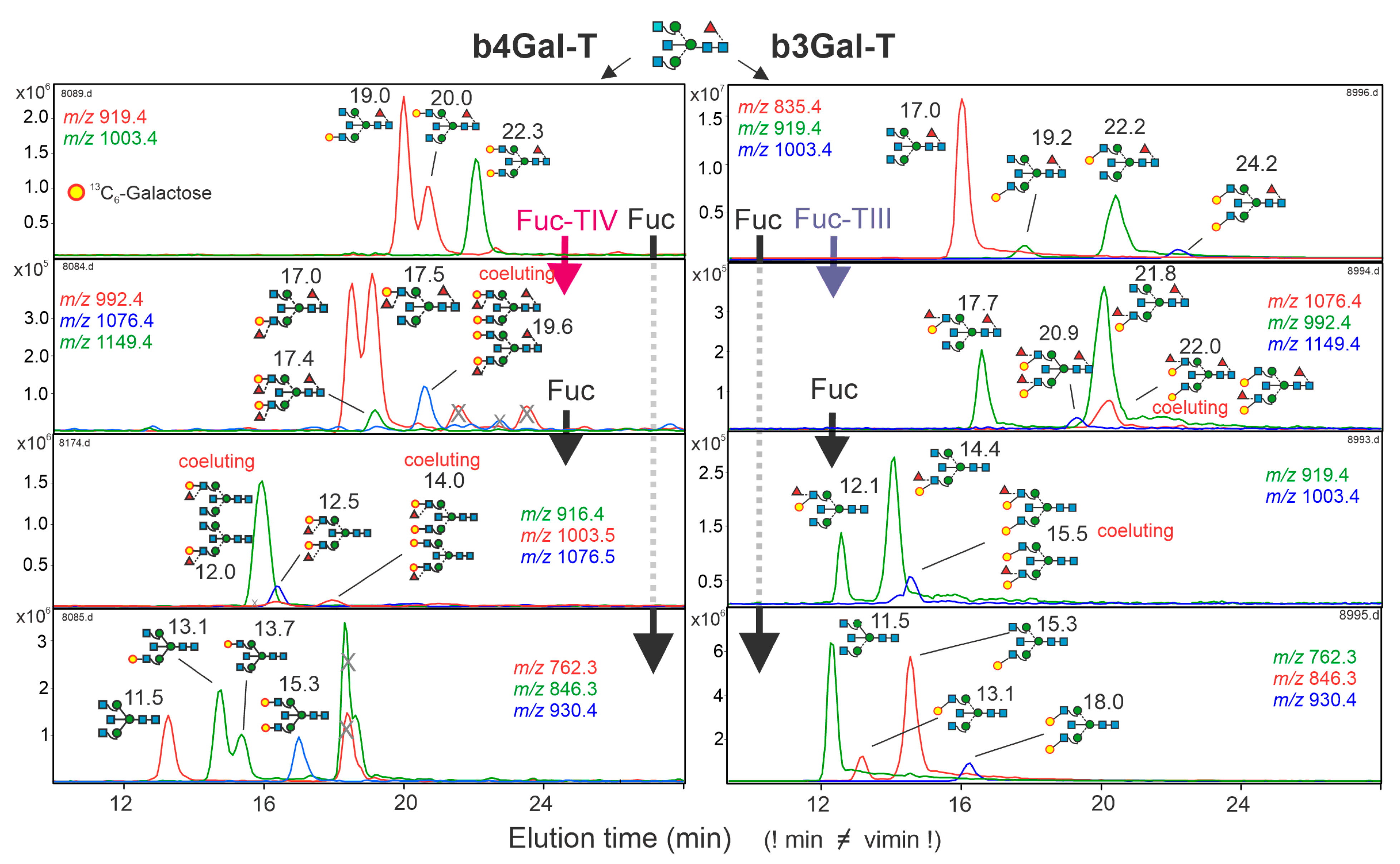
3.1. Biosynthesis of Glycan Standards
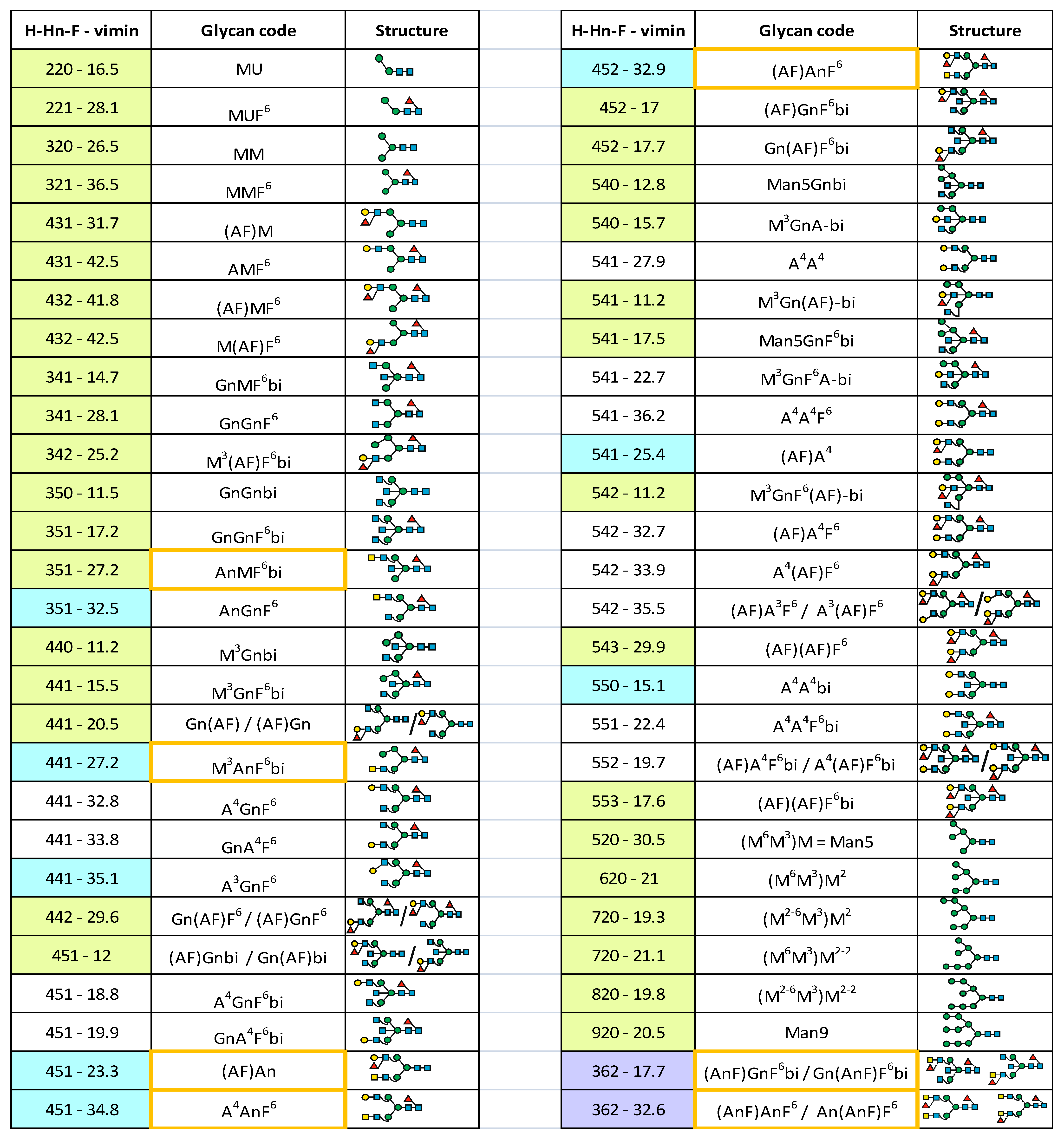
3.2. The Virtual Minute Retention Time Library
3.3. Analysis of Brain N-Glycans—The Concept
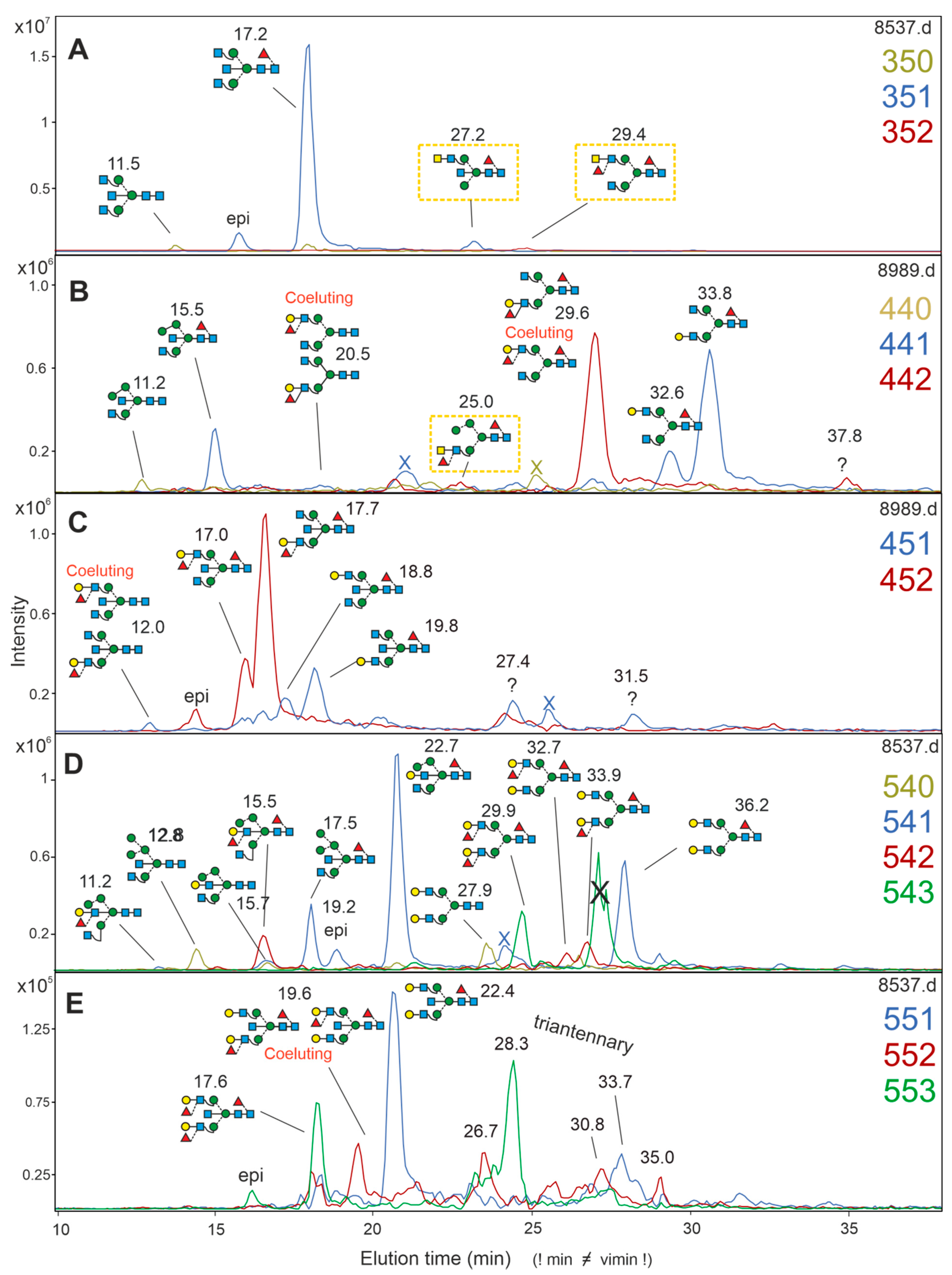
3.4. Analysis of Brain N-Glycans—Neutral Structures
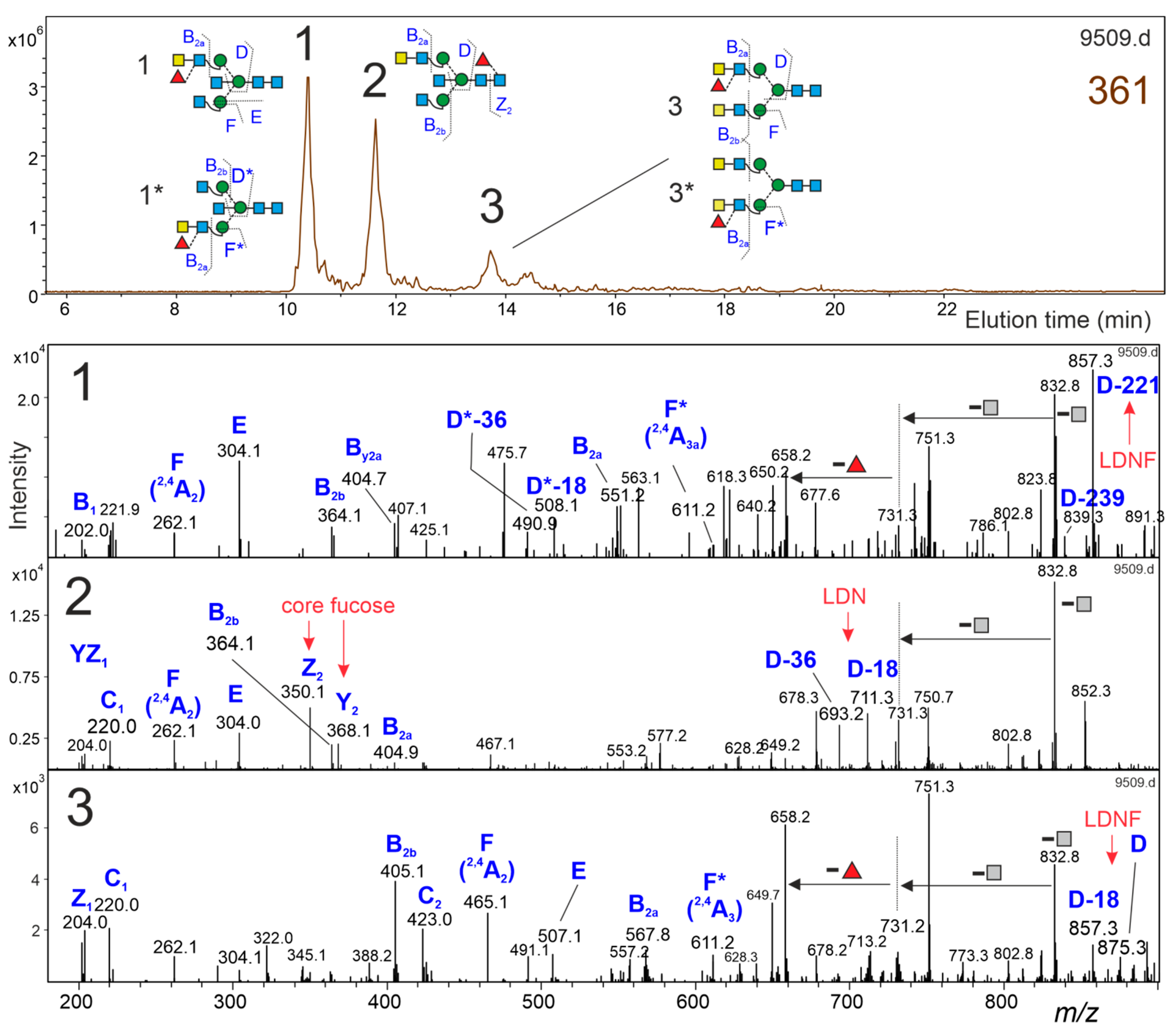
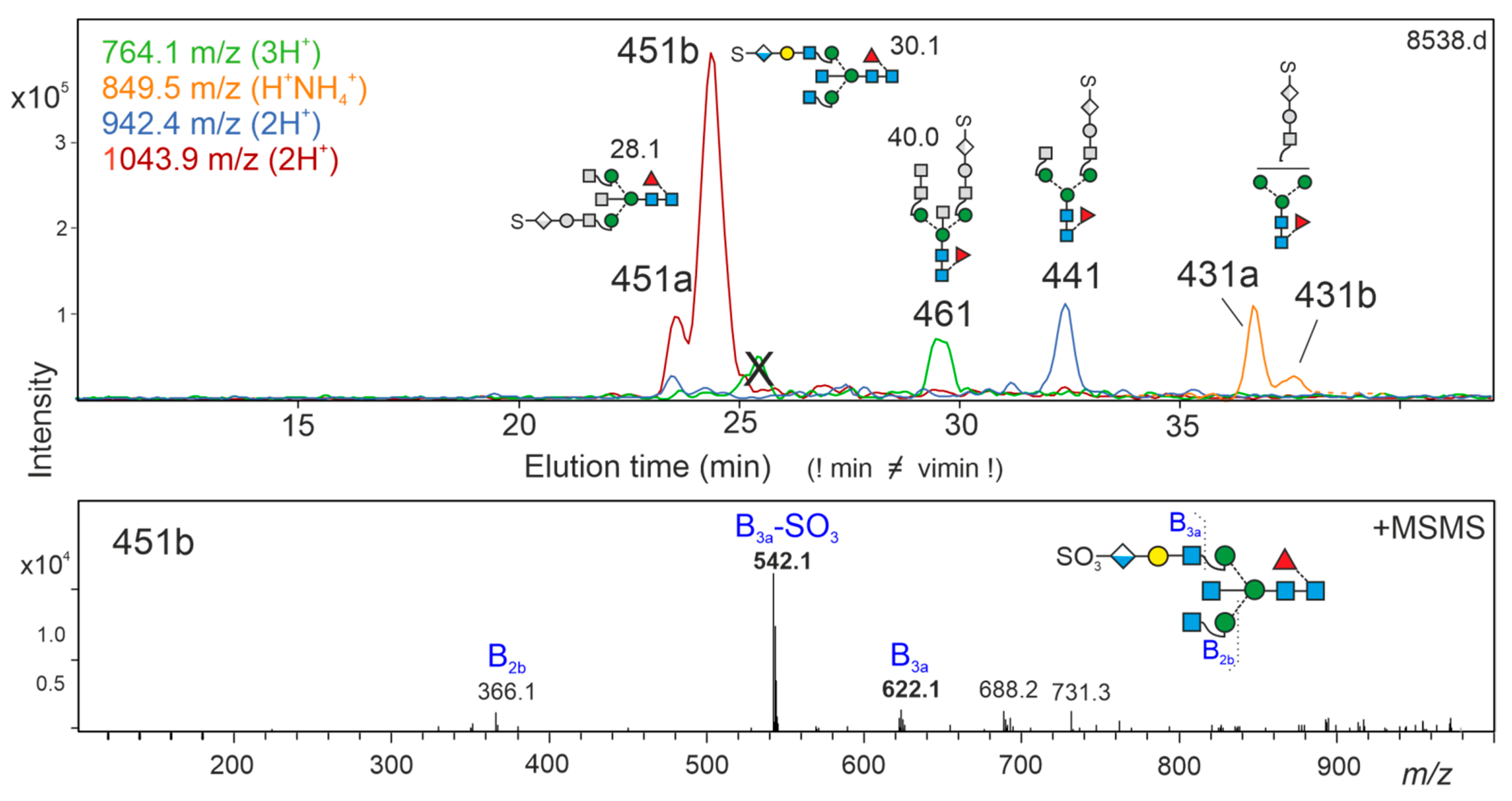
3.5. Brain N-Glycans with an LDNF Determinant
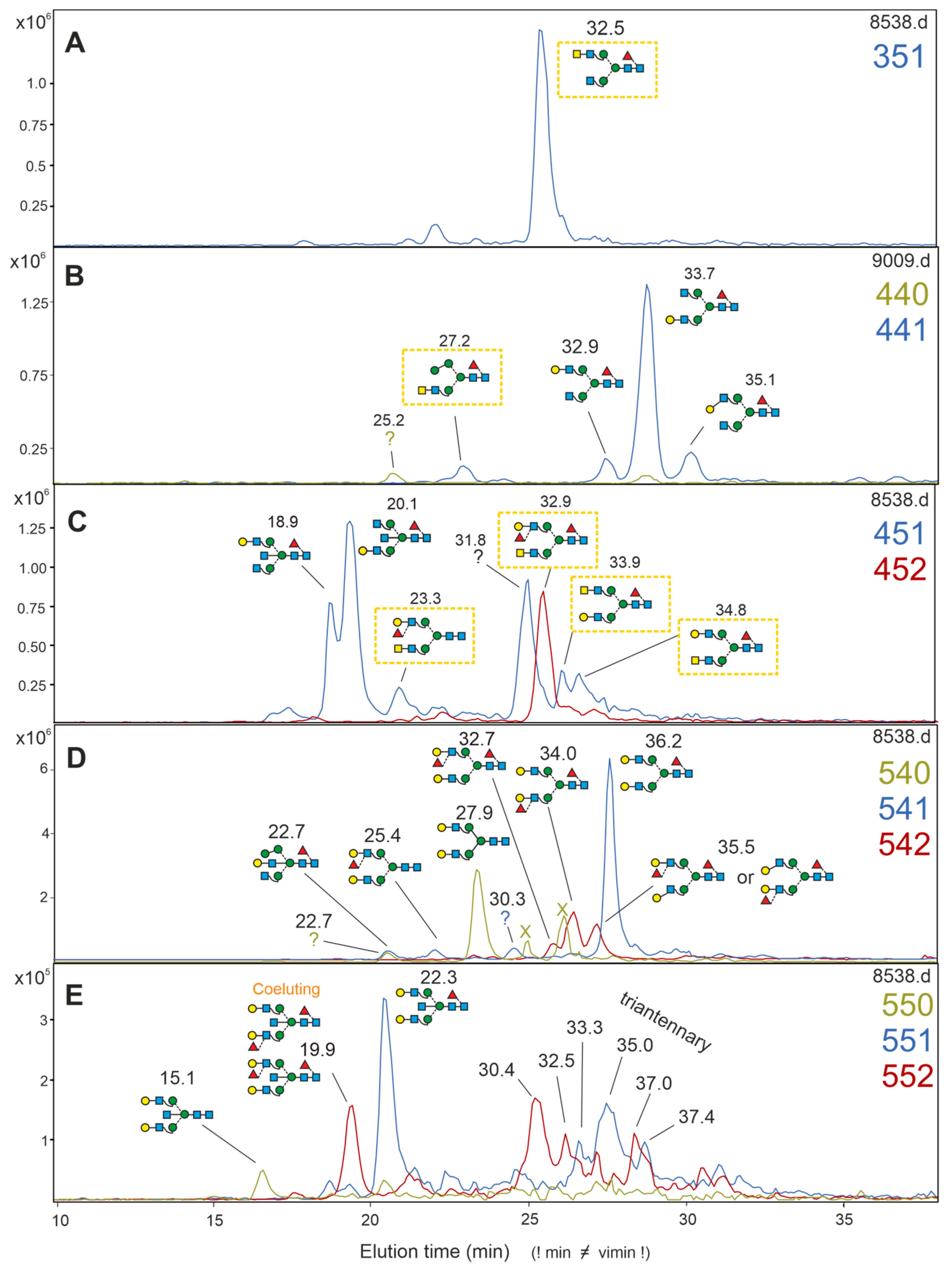
3.6. Analysis of Brain N-Glycans—De-Sialylated Structures
3.7. HNK-1 Structures
3.8. Occurrence of β1,3-Linked Galactose
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A4A4 and similar | N-glycan structure (see Figure 6) |
| b4Gal-T | β1,4-galactosyltransferase |
| CID | Collision-induced dissociation |
| ESI-MS | Electrospray ioniztion |
| Fuc-T | Fucosyltransferase |
| Gal | Galactose |
| GDP | Guanosine diphosphate |
| Glc | Glucose |
| GlcA | Glucuronic acid |
| GlcNAc | N-acetylglucosamine |
| Gn(AF)F6 and similar | N-glycan structure (see Figure 6) |
| LeX | Lewis X |
| M3GnF6bi and similar | N-glycan structure (see Figure 6) |
| MS | Mass spectrometry |
| PGC | Porous graphitic carbon |
References
- Paprocka, J.; Jezela-Stanek, A.; Tylki-Szymanska, A.; Grunewald, S. Congenital Disorders of Glycosylation from a Neurological Perspective. Brain Sci. 2021, 11, 88. [Google Scholar] [CrossRef]
- Gaunitz, S.; Tjernberg, L.O.; Schedin-Weiss, S. The N-glycan profile in cortex and hippocampus is altered in Alzheimer disease. J. Neurochem. 2020, 159, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Schedin-Weiss, S.; Gaunitz, S.; Sui, P.; Chen, Q.; Haslam, S.M.; Blennow, K.; Winblad, B.; Dell, A.; Tjernberg, L.O. Glycan biomarkers for Alzheimer disease correlate with T-tau and P-tau in cerebrospinal fluid in subjective cognitive impairment. FEBS J. 2020, 287, 3221–3234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, P.; Xie, J.; Sang, S.; Zhang, L.; Liu, M.; Yang, L.; Xu, Y.; Yan, G.; Yao, J.; Gao, X.; et al. Multilayered N-Glycoproteome Profiling Reveals Highly Heterogeneous and Dysregulated Protein N-Glycosylation Related to Alzheimer’s Disease. Anal. Chem. 2020, 92, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Boll, I.; Jensen, P.; Schwammle, V.; Larsen, M.R. Depolarization-dependent Induction of Site-specific Changes in Sialylation on N-linked Glycoproteins in Rat Nerve Terminals. Mol. Cell. Proteom. 2020, 19, 1418–1435. [Google Scholar] [CrossRef]
- Matthies, I.; Abrahams, J.L.; Jensen, P.; Oliveira, T.; Kolarich, D.; Larsen, M.R. N-Glycosylation in isolated rat nerve terminals. Mol. Omics 2021, 17, 517–532. [Google Scholar] [CrossRef] [PubMed]
- Barboza, M.; Solakyildirim, K.; Knotts, T.A.; Luke, J.; Gareau, M.G.; Raybould, H.E.; Lebrilla, C.B. Region-Specific Cell Membrane N-Glycome of Functional Mouse Brain Areas Revealed by nanoLC-MS Analysis. Mol. Cell. Proteom. 2021, 20, 100130. [Google Scholar] [CrossRef]
- Lee, J.; Ha, S.; Kim, M.; Kim, S.W.; Yun, J.; Ozcan, S.; Hwang, H.; Ji, I.J.; Yin, D.; Webster, M.J.; et al. Spatial and temporal diversity of glycome expression in mammalian brain. Proc. Natl. Acad. Sci. USA 2020, 117, 28743–28753. [Google Scholar] [CrossRef]
- Chen, Y.J.; Wing, D.R.; Guile, G.R.; Dwek, R.A.; Harvey, D.J.; Zamze, S. Neutral N-glycans in adult rat brain tissue--complete characterisation reveals fucosylated hybrid and complex structures. Eur. J. Biochem. 1998, 251, 691–703. [Google Scholar] [CrossRef]
- Horstkorte, R.; Schachner, M.; Magyar, J.P.; Vorherr, T.; Schmitz, B. The fourth immunoglobulin-like domain of NCAM contains a carbohydrate recognition domain for oligomannosidic glycans implicated in association with L1 and neurite outgrowth. J. Cell Biol. 1993, 121, 1409–1421. [Google Scholar] [CrossRef] [Green Version]
- Zamze, S.; Harvey, D.J.; Chen, Y.J.; Guile, G.R.; Dwek, R.A.; Wing, D.R. Sialylated N-glycans in adult rat brain tissue--a widespread distribution of disialylated antennae in complex and hybrid structures. Eur. J. Biochem. 1998, 258, 243–270. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Mishra, S.K.; Tokoro, Y.; Sato, K.; Nakajima, K.; Yamaguchi, Y.; Taniguchi, N.; Kizuka, Y. Bisecting GlcNAc Is a General Suppressor of Terminal Modification of N-glycan. Mol. Cell. Proteom. 2019, 18, 2044–2057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taniguchi, N.; Ohkawa, Y.; Maeda, K.; Harada, Y.; Nagae, M.; Kizuka, Y.; Ihara, H.; Ikeda, Y. True significance of N-acetylglucosaminyltransferases GnT-III, V and alpha1,6 fucosyltransferase in epithelial-mesenchymal transition and cancer. Mol. Asp. Med. 2021, 79, 100905. [Google Scholar] [CrossRef]
- Kizuka, Y.; Nakano, M.; Miura, Y.; Taniguchi, N. Epigenetic regulation of neural N-glycomics. Proteomics 2016, 16, 2854–2863. [Google Scholar] [CrossRef] [PubMed]
- Kizuka, Y.; Taniguchi, N. Neural functions of bisecting GlcNAc. Glycoconj. J. 2018, 35, 345–351. [Google Scholar] [CrossRef]
- Ohkawa, Y.; Kizuka, Y.; Takata, M.; Nakano, M.; Ito, E.; Mishra, S.K.; Akatsuka, H.; Harada, Y.; Taniguchi, N. Peptide Sequence Mapping around Bisecting GlcNAc-Bearing N-Glycans in Mouse Brain. Int. J. Mol. Sci. 2021, 22, 8579. [Google Scholar] [CrossRef]
- Lieberoth, A.; Splittstoesser, F.; Katagihallimath, N.; Jakovcevski, I.; Loers, G.; Ranscht, B.; Karagogeos, D.; Schachner, M.; Kleene, R. Lewis(x) and alpha2,3-sialyl glycans and their receptors TAG-1, Contactin, and L1 mediate CD24-dependent neurite outgrowth. J. Neurosci. 2009, 29, 6677–6690. [Google Scholar] [CrossRef] [Green Version]
- Torii, T.; Yoshimura, T.; Narumi, M.; Hitoshi, S.; Takaki, Y.; Tsuji, S.; Ikenaka, K. Determination of major sialylated N-glycans and identification of branched sialylated N-glycans that dynamically change their content during development in the mouse cerebral cortex. Glycoconj. J. 2014, 31, 671–683. [Google Scholar] [CrossRef] [Green Version]
- Sytnyk, V.; Leshchyns’ka, I.; Schachner, M. Neural glycomics: The sweet side of nervous system functions. Cell. Mol. Life Sci. 2021, 78, 93–116. [Google Scholar] [CrossRef]
- de Freitas Junior, J.C.; Morgado-Diaz, J.A. The role of N-glycans in colorectal cancer progression: Potential biomarkers and therapeutic applications. Oncotarget 2016, 7, 19395–19413. [Google Scholar] [CrossRef] [Green Version]
- Holst, S.; Wilding, J.L.; Koprowska, K.; Rombouts, Y.; Wuhrer, M. N-Glycomic and Transcriptomic Changes Associated with CDX1 mRNA Expression in Colorectal Cancer Cell Lines. Cells 2019, 8, 273. [Google Scholar] [CrossRef] [Green Version]
- Attrill, H.; Takazawa, H.; Witt, S.; Kelm, S.; Isecke, R.; Brossmer, R.; Ando, T.; Ishida, H.; Kiso, M.; Crocker, P.R.; et al. The structure of siglec-7 in complex with sialosides: Leads for rational structure-based inhibitor design. Biochem. J. 2006, 397, 271–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey, D.J.; Mattu, T.S.; Wormald, M.R.; Royle, L.; Dwek, R.A.; Rudd, P.M. “Internal residue loss”: Rearrangements occurring during the fragmentation of carbohydrates derivatized at the reducing terminus. Anal. Chem. 2002, 74, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Lettow, M.; Mucha, E.; Manz, C.; Thomas, D.A.; Marianski, M.; Meijer, G.; von Helden, G.; Pagel, K. The role of the mobile proton in fucose migration. Anal. Bioanal. Chem. 2019, 411, 4637–4645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wuhrer, M.; Koeleman, C.A.; Hokke, C.H.; Deelder, A.M. Mass spectrometry of proton adducts of fucosylated N-glycans: Fucose transfer between antennae gives rise to misleading fragments. Rapid Commun. Mass Spectrom. 2006, 20, 1747–1754. [Google Scholar] [CrossRef]
- Shen, J.; Jia, L.; Dang, L.; Su, Y.; Zhang, J.; Xu, Y.; Zhu, B.; Chen, Z.; Wu, J.; Lan, R.; et al. StrucGP: De novo structural sequencing of site-specific N-glycan on glycoproteins using a modularization strategy. Nat. Methods 2021, 18, 921–929. [Google Scholar] [CrossRef]
- Samal, J.; Saldova, R.; Rudd, P.M.; Pandit, A.; O’Flaherty, R. Region-Specific Characterization of N-Glycans in the Striatum and Substantia Nigra of an Adult Rodent Brain. Anal. Chem. 2020, 92, 12842–12851. [Google Scholar] [CrossRef]
- Helm, J.; Grunwald-Gruber, C.; Thader, A.; Urteil, J.; Fuehrer, J.; Stenitzer, D.; Maresch, D.; Neumann, L.; Pabst, M.; Altmann, F. Bisecting Lewis X in hybrid-type N-glycans of human brain revealed by deep structural glycomics. Anal. Chem. 2021, 93, 15175–15182. [Google Scholar] [CrossRef]
- Seo, Y.; Oh, M.J.; Park, J.Y.; Ko, J.K.; Kim, J.Y.; An, H.J. Comprehensive Characterization of Biotherapeutics by Selective Capturing of Highly Acidic Glycans Using Stepwise PGC-SPE and LC/MS/MS. Anal. Chem. 2019, 91, 6064–6071. [Google Scholar] [CrossRef]
- Grass, J.; Pabst, M.; Kolarich, D.; Poltl, G.; Leonard, R.; Brecker, L.; Altmann, F. Discovery and structural characterization of fucosylated oligomannosidic N-glycans in mushrooms. J. Biol. Chem. 2011, 286, 5977–5984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stadlmann, J.; Pabst, M.; Kolarich, D.; Kunert, R.; Altmann, F. Analysis of immunoglobulin glycosylation by LC-ESI-MS of glycopeptides and oligosaccharides. Proteomics 2008, 8, 2858–2871. [Google Scholar] [CrossRef] [PubMed]
- Zeleny, R.; Altmann, F.; Praznik, W. A capillary electrophoretic study on the specificity of beta-galactosidases from Aspergillus oryzae, Escherichia coli, Streptococcus pneumoniae, and Canavalia ensiformis (jack bean). Anal. Biochem. 1997, 246, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.; Schoberer, J.; Jin, C.; Glossl, J.; Mach, L.; Steinkellner, H. Molecular cloning and characterization of Arabidopsis thaliana Golgi alpha-mannosidase II, a key enzyme in the formation of complex N-glycans in plants. Plant. J. 2006, 45, 789–803. [Google Scholar] [CrossRef]
- Grunwald-Gruber, C.; Thader, A.; Maresch, D.; Dalik, T.; Altmann, F. Determination of true ratios of different N-glycan structures in electrospray ionization mass spectrometry. Anal. Bioanal. Chem. 2017, 409, 2519–2530. [Google Scholar] [CrossRef] [Green Version]
- Townsend, R.R.; Lipniunas, P.H.; Bigge, C.; Ventom, A.; Parekh, R. Multimode high-performance liquid chromatography of fluorescently labeled oligosaccharides from glycoproteins. Anal. Biochem. 1996, 239, 200–207. [Google Scholar] [CrossRef]
- Tosh, N.; Quadrelli, S.; Galloway, G.; Mountford, C. Two New Fucose-alpha (1-2)-Glycans Assigned in the Healthy Human Brain Taking the Number to Seven. Sci Rep. 2019, 9, 18806. [Google Scholar] [CrossRef]
- Smalla, K.H.; Angenstein, F.; Richter, K.; Gundelfinger, E.D.; Staak, S. Identification of fucose alpha(1-2) galactose epitope-containing glycoproteins from rat hippocampus. Neuroreport 1998, 9, 813–817. [Google Scholar] [CrossRef]
- Harvey, D.J. Negative Ion Mass Spectrometry for the Analysis of N-Linked Glycans. Mass Spectrom. Rev. 2020, 39, 586–679. [Google Scholar] [CrossRef]
- Gallego, R.G.; Blanco, J.L.; Thijssen-van Zuylen, C.W.; Gotfredsen, C.H.; Voshol, H.; Duus, J.O.; Schachner, M.; Vliegenthart, J.F. Epitope diversity of N-glycans from bovine peripheral myelin glycoprotein P0 revealed by mass spectrometry and nano probe magic angle spinning 1H NMR spectroscopy. J. Biol. Chem. 2001, 276, 30834–30844. [Google Scholar] [CrossRef] [Green Version]
- Albach, C.; Klein, R.A.; Schmitz, B. Do Rodent and Human Brains Have Different N-Glycosylation Patterns? Biol. Chem. 2001, 382, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.R. Structure, mechanism and inhibition of Golgi alpha-mannosidase II. Curr. Opin. Struct. Biol. 2012, 22, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Harvey, D.J.; Crispin, M.; Scanlan, C.; Singer, B.B.; Lucka, L.; Chang, V.T.; Radcliffe, C.M.; Thobhani, S.; Yuen, C.T.; Rudd, P.M. Differentiation between isomeric triantennary N-linked glycans by negative ion tandem mass spectrometry and confirmation of glycans containing galactose attached to the bisecting (beta1-4-GlcNAc) residue in N-glycans from IgG. Rapid Commun. Mass Spectrom. 2008, 22, 1047–1052. [Google Scholar] [CrossRef]
- Okamoto, Y.; Omichi, K.; Yamanaka, S.; Ikenaka, K.; Hase, S. Conversion of brain-specific complex type sugar chains by N-acetyl-beta-D-hexosaminidase B. J. Biochem. 1999, 125, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Pabst, M.; Grass, J.; Toegel, S.; Liebminger, E.; Strasser, R.; Altmann, F. Isomeric analysis of oligomannosidic N-glycans and their dolichol-linked precursors. Glycobiology 2012, 22, 389–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, S.B.; Chao, Y.B.; van Halbeek, H. Novel Asn-linked oligosaccharides terminating in GalNAc beta (1-->4)[Fuc alpha (1-->3)]GlcNAc beta (1-->.) are present in recombinant human protein C expressed in human kidney 293 cells. Glycobiology 1993, 3, 597–608. [Google Scholar] [CrossRef]
- Coddeville, B.; Strecker, G.; Wieruszeski, J.M.; Vliegenthart, J.F.; van Halbeek, H.; Peter-Katalinic, J.; Egge, H.; Spik, G. Heterogeneity of bovine lactotransferrin glycans. Characterization of alpha-D-Galp-(1-->3)-beta-D-Gal- and alpha-NeuAc-(2-->6)-beta-D-GalpNAc-(1-->4)- beta-D-GlcNAc-substituted N-linked glycans. Carbohydr. Res. 1992, 236, 145–164. [Google Scholar] [CrossRef] [Green Version]
- Chan, A.L.; Morris, H.R.; Panico, M.; Etienne, A.T.; Rogers, M.E.; Gaffney, P.; Creighton-Kempsford, L.; Dell, A. A novel sialylated N-acetylgalactosamine-containing oligosaccharide is the major complex-type structure present in Bowes melanoma tissue plasminogen activator. Glycobiology 1991, 1, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Nimtz, M.; Grabenhorst, E.; Conradt, H.S.; Sanz, L.; Calvete, J.J. Structural characterization of the oligosaccharide chains of native and crystallized boar seminal plasma spermadhesin PSP-I and PSP-II glycoforms. Eur. J. Biochem. 1999, 265, 703–718. [Google Scholar] [CrossRef] [Green Version]
- Voshol, H.; van Zuylen, C.W.; Orberger, G.; Vliegenthart, J.F.; Schachner, M. Structure of the HNK-1 carbohydrate epitope on bovine peripheral myelin glycoprotein P0. J. Biol. Chem. 1996, 271, 22957–22960. [Google Scholar] [CrossRef] [Green Version]
- She, Y.M.; Tam, R.Y.; Li, X.; Rosu-Myles, M.; Sauve, S. Resolving Isomeric Structures of Native Glycans by Nanoflow Porous Graphitized Carbon Chromatography-Mass Spectrometry. Anal. Chem. 2020, 92, 14038–14046. [Google Scholar] [CrossRef]
- Nagae, M.; Kanagawa, M.; Morita-Matsumoto, K.; Hanashima, S.; Kizuka, Y.; Taniguchi, N.; Yamaguchi, Y. Atomic visualization of a flipped-back conformation of bisected glycans bound to specific lectins. Sci. Rep. 2016, 6, 22973. [Google Scholar] [CrossRef] [Green Version]
- Akasaka-Manya, K.; Manya, H.; Sakurai, Y.; Wojczyk, B.S.; Kozutsumi, Y.; Saito, Y.; Taniguchi, N.; Murayama, S.; Spitalnik, S.L.; Endo, T. Protective effect of N-glycan bisecting GlcNAc residues on beta-amyloid production in Alzheimer’s disease. Glycobiology 2010, 20, 99–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bleckmann, C.; Geyer, H.; Reinhold, V.; Lieberoth, A.; Schachner, M.; Kleene, R.; Geyer, R. Glycomic analysis of N-linked carbohydrate epitopes from CD24 of mouse brain. J. Proteome Res. 2009, 8, 567–582. [Google Scholar] [CrossRef] [PubMed]
- Kalovidouris, S.A.; Gama, C.I.; Lee, L.W.; Hsieh-Wilson, L.C. A role for fucose alpha(1-2) galactose carbohydrates in neuronal growth. J. Am. Chem. Soc. 2005, 127, 1340–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawade, H.; Morise, J.; Mishra, S.K.; Tsujioka, S.; Oka, S.; Kizuka, Y. Tissue-Specific Regulation of HNK-1 Biosynthesis by Bisecting GlcNAc. Molecules 2021, 26, 5176. [Google Scholar] [CrossRef]
- Takegawa, Y.; Deguchi, K.; Nakagawa, H.; Nishimura, S. Structural analysis of an N-glycan with “beta1-4 bisecting branch” from human serum IgG by negative-ion MSn spectral matching and exoglycosidase digestion. Anal. Chem. 2005, 77, 6062–6068. [Google Scholar] [CrossRef]
- Mondal, N.; Dykstra, B.; Lee, J.; Ashline, D.J.; Reinhold, V.N.; Rossi, D.J.; Sackstein, R. Distinct human alpha(1,3)-fucosyltransferases drive Lewis-X/sialyl Lewis-X assembly in human cells. J. Biol. Chem. 2018, 293, 7300–7314. [Google Scholar] [CrossRef] [Green Version]
- Nishihara, S.; Iwasaki, H.; Nakajima, K.; Togayachi, A.; Ikehara, Y.; Kudo, T.; Kushi, Y.; Furuya, A.; Shitara, K.; Narimatsu, H. Alpha1,3-fucosyltransferase IX (Fut9) determines Lewis X expression in brain. Glycobiology 2003, 13, 445–455. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, N.; Shinomi, M.; Hirano, K.; Ui-Tei, K.; Nishihara, S. LacdiNAc (GalNAcbeta1-4GlcNAc) contributes to self-renewal of mouse embryonic stem cells by regulating leukemia inhibitory factor/STAT3 signaling. Stem Cells 2011, 29, 641–650. [Google Scholar] [CrossRef]
- Machado, E.; Kandzia, S.; Carilho, R.; Altevogt, P.; Conradt, H.S.; Costa, J. N-Glycosylation of total cellular glycoproteins from the human ovarian carcinoma SKOV3 cell line and of recombinantly expressed human erythropoietin. Glycobiology 2011, 21, 376–386. [Google Scholar] [CrossRef] [Green Version]
- Gotoh, M.; Sato, T.; Kiyohara, K.; Kameyama, A.; Kikuchi, N.; Kwon, Y.D.; Ishizuka, Y.; Iwai, T.; Nakanishi, H.; Narimatsu, H. Molecular cloning and characterization of beta1,4-N-acetylgalactosaminyltransferases IV synthesizing N,N’-diacetyllactosediamine. FEBS Lett. 2004, 562, 134–140. [Google Scholar] [CrossRef] [Green Version]
- Srivatsan, J.; Smith, D.F.; Cummings, R.D. The human blood fluke Schistosoma mansoni synthesizes glycoproteins containing the Lewis X antigen. J. Biol. Chem. 1992, 267, 20196–20203. [Google Scholar] [CrossRef]
- van Remoortere, A.; Hokke, C.H.; van Dam, G.J.; van Die, I.; Deelder, A.M.; van den Eijnden, D.H. Various stages of schistosoma express Lewis(x), LacdiNAc, GalNAcbeta1-4 (Fucalpha1-3)GlcNAc and GalNAcbeta1-4(Fucalpha1-2Fucalpha1-3)GlcNAc carbohydrate epitopes: Detection with monoclonal antibodies that are characterized by enzymatically synthesized neoglycoproteins. Glycobiology 2000, 10, 601–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, I.B.; Paschinger, K. Sweet secrets of a therapeutic worm: Mass-spectrometric N-glycomic analysis of Trichuris suis. Anal. Bioanal. Chem. 2016, 408, 461–471. [Google Scholar] [CrossRef] [Green Version]
- Van Die, I.; Cummings, R.D. Glycan gimmickry by parasitic helminths: A strategy for modulating the host immune response? Glycobiology 2010, 20, 2–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergwerff, A.A.; Thomas-Oates, J.E.; van Oostrum, J.; Kamerling, J.P.; Vliegenthart, J.F. Human urokinase contains GalNAc beta (1-4)[Fuc alpha (1-3)]GlcNAc beta (1-2) as a novel terminal element in N-linked carbohydrate chains. FEBS Lett. 1992, 314, 389–394. [Google Scholar] [CrossRef] [Green Version]
- Andre, M.; Morelle, W.; Planchon, S.; Milhiet, P.E.; Rubinstein, E.; Mollicone, R.; Chamot-Rooke, J.; Le Naour, F. Glycosylation status of the membrane protein CD9P-1. Proteomics 2007, 7, 3880–3895. [Google Scholar] [CrossRef]
- Kubelka, V.; Altmann, F.; Staudacher, E.; Tretter, V.; Marz, L.; Hard, K.; Kamerling, J.P.; Vliegenthart, J.F. Primary structures of the N-linked carbohydrate chains from honeybee venom phospholipase A2. Eur. J. Biochem. 1993, 213, 1193–1204. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helm, J.; Hirtler, L.; Altmann, F. Towards Mapping of the Human Brain N-Glycome with Standardized Graphitic Carbon Chromatography. Biomolecules 2022, 12, 85. https://doi.org/10.3390/biom12010085
Helm J, Hirtler L, Altmann F. Towards Mapping of the Human Brain N-Glycome with Standardized Graphitic Carbon Chromatography. Biomolecules. 2022; 12(1):85. https://doi.org/10.3390/biom12010085
Chicago/Turabian StyleHelm, Johannes, Lena Hirtler, and Friedrich Altmann. 2022. "Towards Mapping of the Human Brain N-Glycome with Standardized Graphitic Carbon Chromatography" Biomolecules 12, no. 1: 85. https://doi.org/10.3390/biom12010085
APA StyleHelm, J., Hirtler, L., & Altmann, F. (2022). Towards Mapping of the Human Brain N-Glycome with Standardized Graphitic Carbon Chromatography. Biomolecules, 12(1), 85. https://doi.org/10.3390/biom12010085






