A Comprehensive Review on the Heavy Metal Toxicity and Sequestration in Plants
Abstract
:1. Introduction
2. HM Toxicity Induces Morphological, Anatomical, and Physiological Changes in Plants
3. HM Toxicity Negatively Influence the Photosynthesis
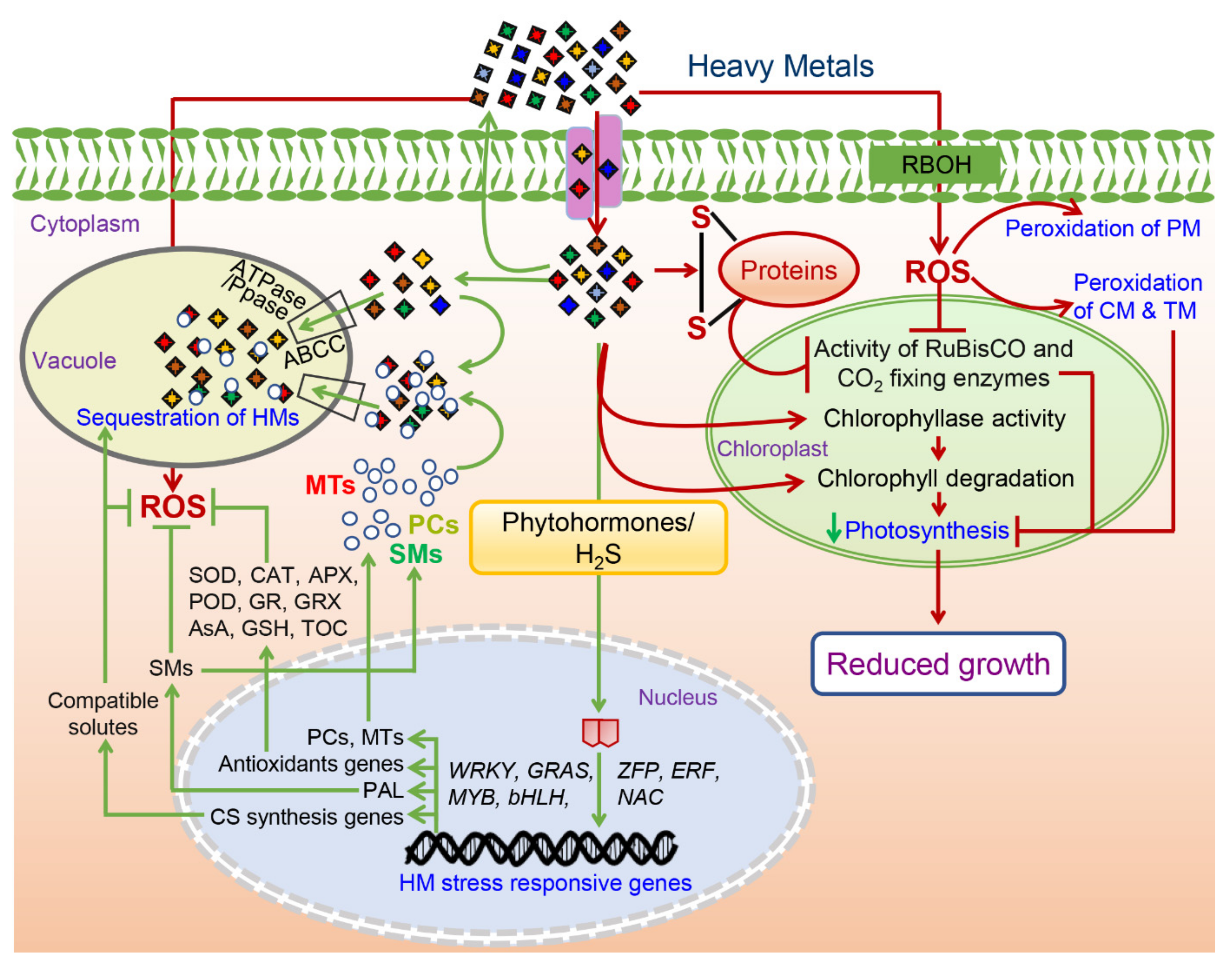
4. Antioxidant Enzymes Alleviate the HM Toxicity Induced Oxidative Stress
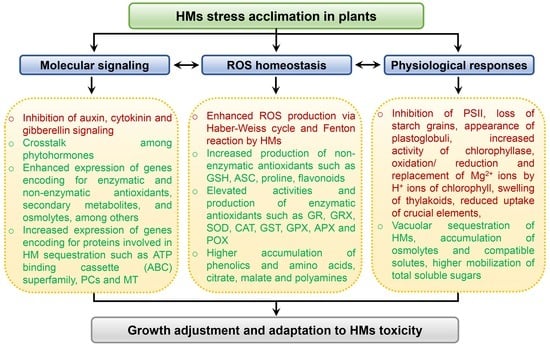
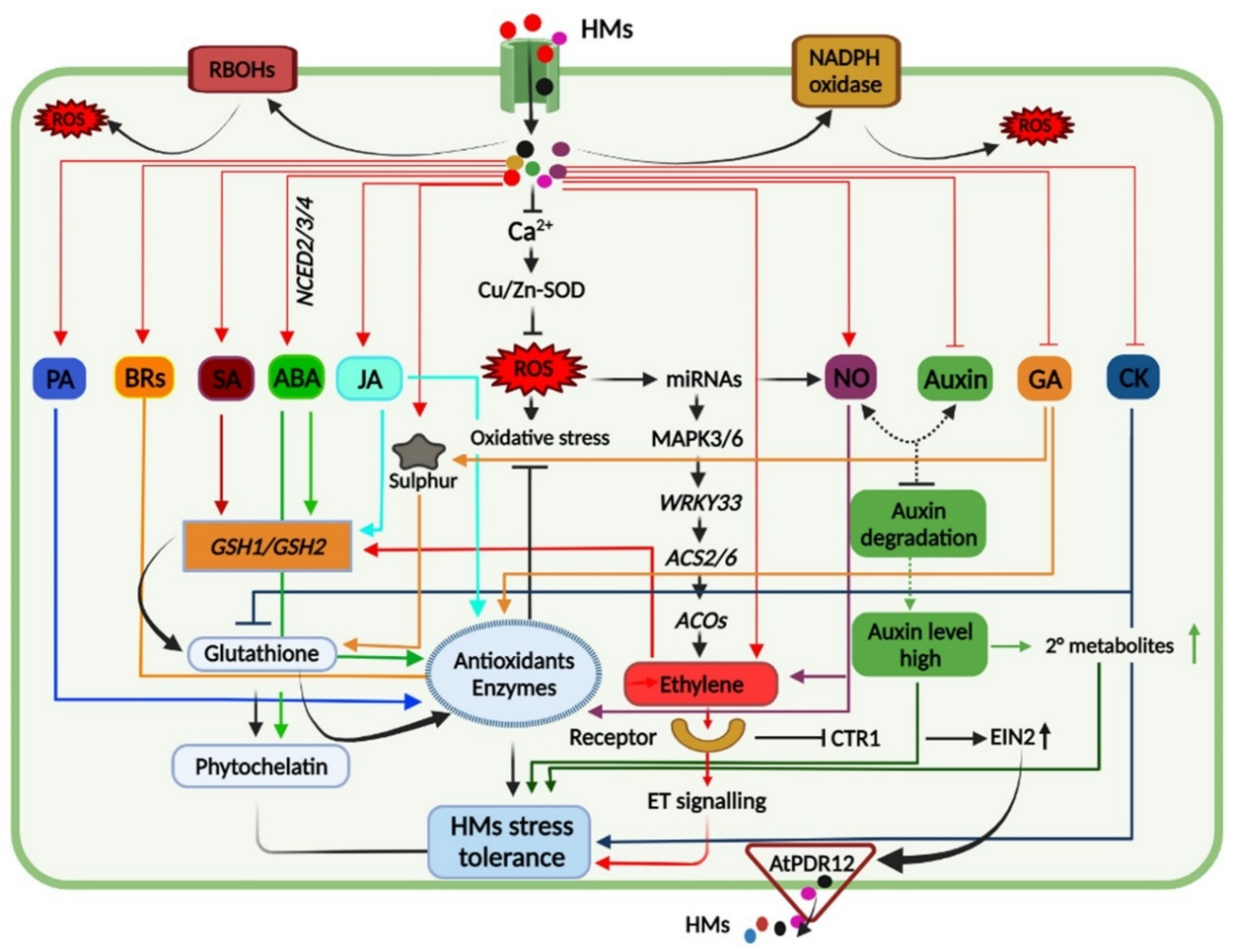
5. Tolerance to HM Toxicity Is Mediated by a Complex Signaling Network
6. Sequestration and Compartmentalization: Plants’ Way to Alleviate the HM Toxicity
7. Plants Retaliate against HM Toxicity by Elevating the Levels of Compatible Solutes
8. HM-Stress Tolerance Is Mediated by the Phytohormones Signaling
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riyazuddin, R.; Nisha, N.; Singh, K.; Verma, R.; Gupta, R. Involvement of dehydrin proteins in mitigating the negative effects of drought stress in plants. Plant Cell Rep. 2021, 1–15. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Boyd, R.S.; Rajakaruna, N. Heavy Metal Tolerance; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Tiwari, S.; Lata, C. Heavy metal stress, signaling, and tolerance due to plant-associated microbes: An overview. Front. Plant Sci. 2018, 9, 452. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Li, Y.; Zhang, C.; Liu, H.; Liu, J.; Zheng, W.; Kang, X.; Leng, X.; Zhao, K.; Gu, Y. Culturable heavy metal-resistant and plant growth promoting bacteria in V-Ti magnetite mine tailing soil from Panzhihua, China. PLoS ONE 2014, 9, e106618. [Google Scholar] [CrossRef]
- Gill, M. Heavy metal stress in plants: A review. Int. J. Adv. Res. 2014, 2, 1043–1055. [Google Scholar]
- Ghori, N.-H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy metal stress and responses in plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Seth, C.S.; Chaturvedi, P.K.; Misra, V. Toxic effect of arsenate and cadmium alone and in combination on giant duckweed (Spirodela polyrrhiza L.) in response to its accumulation. Environ. Toxicol. An. Int. J. 2007, 22, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Seth, C.S.; Misra, V.; Chauhan, L.K.S. Accumulation, detoxification, and genotoxicity of heavy metals in indian mustard (Brassica juncea L.). Int. J. Phytoremediation 2012, 14, 1–13. [Google Scholar] [CrossRef]
- Ozturk, M.; Yucel, E.; Gucel, S.; Sakçali, S.; Aksoy, A. 28 Plants as Biomonitors of Trace Elements Pollution in Soil; Academia: San Francisco, CA, USA, 2008. [Google Scholar]
- Öztürk, M.; Ashraf, M.; Aksoy, A.; Ahmad, M.S.A.; Hakeem, K.R. Plants, Pollutants and Remediation; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 9401771944. [Google Scholar]
- Hasan, M.; Cheng, Y.; Kanwar, M.K.; Chu, X.-Y.; Ahammed, G.J.; Qi, Z.-Y. Responses of plant proteins to heavy metal stress—a review. Front. Plant Sci. 2017, 8, 1492. [Google Scholar] [CrossRef] [Green Version]
- Adrees, M.; Ali, S.; Rizwan, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Zia-ur-Rehman, M.; Irshad, M.K.; Bharwana, S.A. The effect of excess copper on growth and physiology of important food crops: A review. Environ. Sci. Pollut. Res. 2015, 22, 8148–8162. [Google Scholar] [CrossRef]
- Tomulescu, I.M.; Radoviciu, E.M.; Merca, V.V.; Tuduce, A.D. Effect of copper, zinc and lead and their combinations on the germination capacity of two cereals. Acta Agrar. Debreceniensis 2004, 15, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Cokkizgin, A.; Cokkizgin, H. Effects of lead (PbCl2) stress on germination of lentil (Lens culinaris Medic.) lines. Afr. J. Biotechnol. 2010, 9, 8608–8612. [Google Scholar]
- Islam, E.; Yang, X.; Li, T.; Liu, D.; Jin, X.; Meng, F. Effect of Pb toxicity on root morphology, physiology and ultrastructure in the two ecotypes of Elsholtzia argyi. J. Hazard. Mater. 2007, 147, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Sengar, R.S.; Gautam, M.; Sengar, K.; Chaudhary, R.; Garg, S. Physiological and metabolic effect of mercury accumulation in higher plants system. Toxicol. Environ. Chem. 2010, 92, 1265–1281. [Google Scholar] [CrossRef]
- Ashraf, R.; Ali, T.A. Effect of heavy metals on soil microbial community and mung beans seed germination. Pakistan J. Bot. 2007, 39, 629. [Google Scholar]
- Sedzik, M.; Smolik, B.; Malkiewicz, M.K. Effect of lead on germination and some morphological and physiological parameters of 10-day-old seedlings of various plant species. Environ. Prot. Nat. Resour. 2015, 26, 22–27. [Google Scholar]
- Zhang, Y.; Deng, B.; Li, Z. Inhibition of NADPH oxidase increases defense enzyme activities and improves maize seed germination under Pb stress. Ecotoxicol. Environ. Saf. 2018, 158, 187–192. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Farooq, M.; Hussain, S.; Maqsood, M.; Hussain, M.; Ishfaq, M.; Ahmad, M.; Anjum, M.Z. Lead toxicity in plants: Impacts and remediation. J. Environ. Manag. 2019, 250, 109557. [Google Scholar] [CrossRef]
- Neelima, P.; Reddy, K.J. Differential effect of cadmium and mercury on growth and metabolism of Solanum melongena L. seedlings. J. Environ. Biol. 2003, 24, 453–460. [Google Scholar]
- Cui, L.; Feng, X.; Lin, C.; Wang, X.; Meng, B.; Wang, X.; Wang, H. Accumulation and translocation of 198Hg in four crop species. Environ. Toxicol. Chem. 2014, 33, 334–340. [Google Scholar] [CrossRef]
- Pasternak, T.; Rudas, V.; Potters, G.; Jansen, M.A.K. Morphogenic effects of abiotic stress: Reorientation of growth in Arabidopsis thaliana seedlings. Environ. Exp. Bot. 2005, 53, 299–314. [Google Scholar] [CrossRef]
- Hasnain, S.; Sabri, A.N. Growth stimulation of Triticum aestivum seedlings under Cr-stresses by non-rhizospheric pseudomonad strains. Environ. Pollut. 1997, 97, 265–273. [Google Scholar] [CrossRef]
- Sofo, A.; Bochicchio, R.; Amato, M.; Rendina, N.; Vitti, A.; Nuzzaci, M.; Altamura, M.M.; Falasca, G.; Della Rovere, F.; Scopa, A. Plant architecture, auxin homeostasis and phenol content in Arabidopsis thaliana grown in cadmium-and zinc-enriched media. J. Plant Physiol. 2017, 216, 174–180. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, F.; Yuan, J.; Zheng, Z.; Wong, M. Responses of Sesbania rostrata and S. cannabina to Pb, Zn, Cu and Cd toxi-cities. J. Environ. Sci. 2004, 16, 670–673. [Google Scholar]
- Schützendübel, A.; Schwanz, P.; Teichmann, T.; Gross, K.; Langenfeld-Heyser, R.; Godbold, D.L.; Polle, A. Cadmium-induced changes in antioxidative systems, hydrogen peroxide content, and differentiation in Scots pine roots. Plant Physiol. 2001, 127, 887–898. [Google Scholar] [CrossRef]
- Rucińska, R.; Sobkowiak, R.; Gwóźdź, E.A. Genotoxicity of lead in lupin root cells as evaluated by the comet assay. Cell Mol. Biol Lett 2004, 9, 519–528. [Google Scholar] [PubMed]
- Kopittke, P.M.; Asher, C.J.; Kopittke, R.A.; Menzies, N.W. Toxic effects of Pb2+ on growth of cowpea (Vigna unguiculata). Environ. Pollut. 2007, 150, 280–287. [Google Scholar] [CrossRef] [Green Version]
- Terzano, R.; Al Chami, Z.; Vekemans, B.; Janssens, K.; Miano, T.; Ruggiero, P. Zinc distribution and speciation within rocket plants (Eruca vesicaria L. Cavalieri) grown on a polluted soil amended with compost as determined by XRF microtomography and micro-XANES. J. Agric. Food Chem. 2008, 56, 3222–3231. [Google Scholar] [CrossRef]
- Rucińska-Sobkowiak, R. Water relations in plants subjected to heavy metal stresses. Acta Physiol. Plant. 2016, 38, 257. [Google Scholar] [CrossRef] [Green Version]
- Baster, P.; Robert, S.; Kleine-Vehn, J.; Vanneste, S.; Kania, U.; Grunewald, W.; De Rybel, B.; Beeckman, T.; Friml, J. SCFTIR1/AFB-auxin signalling regulates PIN vacuolar trafficking and auxin fluxes during root gravitropism. EMBO J. 2013, 32, 260–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Smet, S.; Cuypers, A.; Vangronsveld, J.; Remans, T. Gene networks involved in hormonal control of root development in Arabidopsis thaliana: A framework for studying its disturbance by metal stress. Int. J. Mol. Sci. 2015, 16, 19195–19224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sofo, A.; Vitti, A.; Nuzzaci, M.; Tataranni, G.; Scopa, A.; Vangronsveld, J.; Remans, T.; Falasca, G.; Altamura, M.M.; Degola, F. Correlation between hormonal homeostasis and morphogenic responses in Arabidopsis thaliana seedlings growing in a Cd/Cu/Zn multi-pollution context. Physiol. Plant. 2013, 149, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Huang, X. Inhibition of root meristem growth by cadmium involves nitric oxide-mediated repression of auxin accumulation and signalling in Arabidopsis. Plant Cell Environ. 2016, 39, 120–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Zhu, C.; Gan, L.; Ng, D.; Xia, K. GA 3 enhances root responsiveness to exogenous IAA by modulating auxin transport and signalling in Arabidopsis. Plant Cell Rep. 2015, 34, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Pető, A.; Lehotai, N.; Lozano-Juste, J.; León, J.; Tari, I.; Erdei, L.; Kolbert, Z. Involvement of nitric oxide and auxin in signal transduction of copper-induced morphological responses in Arabidopsis seedlings. Ann. Bot. 2011, 108, 449–457. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Wang, J.; Lv, J. Identifying quantitative sources and spatial distributions of potentially toxic elements in soils by using three receptor models and sequential indicator simulation. Chemosphere 2020, 242, 125266. [Google Scholar] [CrossRef] [PubMed]
- Rout, G.R.; Samantaray, S.; Das, P. Differential chromium tolerance among eight mungbean cultivars grown in nutrient culture. J. Plant Nutr. 1997, 20, 473–483. [Google Scholar] [CrossRef]
- Shanker, A.K.; Cervantes, C.; Loza-Tavera, H.; Avudainayagam, S. Chromium toxicity in plants. Environ. Int. 2005, 31, 739–753. [Google Scholar] [CrossRef]
- Anderson, A.J.; Meyer, D.R.; Mayer, F.K. Heavy metal toxicities: Levels of nickel, cobalt and chromium in the soil and plants associated with visual symptoms and variation in growth of an oat crop. Aust. J. Agric. Res. 1973, 24, 557–571. [Google Scholar] [CrossRef]
- Gorsuch, J.W.; Ritter, M.; Anderson, E.R. Comparative toxicities of six heavy metals using root elongation and shoot growth in three plant species. In Environmental Toxicology and Risk Assessment: Third Volume; ASTM International: West Conshohocken, PA, USA, 1995. [Google Scholar]
- Sharma, D.C.; Sharma, C.P. Chromium uptake and its effects on growth and biological yield of wheat. Cereal Res. Commun. 1993, 21, 317–322. [Google Scholar]
- Shu, X.; Yin, L.; Zhang, Q.; Wang, W. Effect of Pb toxicity on leaf growth, antioxidant enzyme activities, and photosynthesis in cuttings and seedlings of Jatropha curcas L. Environ. Sci. Pollut. Res. 2012, 19, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Pál, M.; Horváth, E.; Janda, T.; Páldi, E.; Szalai, G. Physiological changes and defense mechanisms induced by cadmium stress in maize. J. Plant Nutr. Soil Sci. 2006, 169, 239–246. [Google Scholar] [CrossRef]
- Hayat, S.; Khalique, G.; Irfan, M.; Wani, A.S.; Tripathi, B.N.; Ahmad, A. Physiological changes induced by chromium stress in plants: An overview. Protoplasma 2012, 249, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Xia, X.; Lan, X.; Liu, M.; Zhao, L.; Zhang, P.; Wu, Y. Influence of arsenic stress on physiological, biochemical, and morphological characteristics in seedlings of two cultivars of maize (Zea mays L.). Water Air, Soil Pollut. 2017, 228, 55. [Google Scholar] [CrossRef]
- He, J.; Ren, Y.; Chen, X.; Chen, H. Protective roles of nitric oxide on seed germination and seedling growth of rice (Oryza sativa L.) under cadmium stress. Ecotoxicol. Environ. Saf. 2014, 108, 114–119. [Google Scholar] [CrossRef]
- Kieffer, P.; Planchon, S.; Oufir, M.; Ziebel, J.; Dommes, J.; Hoffmann, L.; Hausman, J.-F.; Renaut, J. Combining proteomics and metabolite analyses to unravel cadmium stress-response in poplar leaves. J. Proteome Res. 2009, 8, 400–417. [Google Scholar] [CrossRef]
- Gabbrielli, P.; Pandolfini, T.; Vergnano, O.; Palandri, M.R. Comparison of two serpentine species with different nickel tolerance strategies. Plant Soil 1990, 122, 271–277. [Google Scholar] [CrossRef]
- Visviki, I.; Rachlin, J.W. The toxic action and interactions of copper and cadmium to the marine alga Dunaliella minuta, in both acute and chronic exposure. Arch. Environ. Contam. Toxicol. 1991, 20, 271–275. [Google Scholar] [CrossRef]
- Patra, M.; Bhowmik, N.; Bandopadhyay, B.; Sharma, A. Comparison of mercury, lead and arsenic with respect to genotoxic effects on plant systems and the development of genetic tolerance. Environ. Exp. Bot. 2004, 52, 199–223. [Google Scholar] [CrossRef]
- Yadav, G.; Srivastava, P.K.; Singh, V.P.; Prasad, S.M. Light intensity alters the extent of arsenic toxicity in Helianthus annuus L. seedlings. Biol. Trace Elem. Res. 2014, 158, 410–421. [Google Scholar] [CrossRef]
- Nair, P.M.G.; Chung, I.M. Study on the correlation between copper oxide nanoparticles induced growth suppression and enhanced lignification in Indian mustard (Brassica juncea L.). Ecotoxicol. Environ. Saf. 2015, 113, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.P.R.; de Vasconcelos, L.L.; da Conceição de Souza Braga, P.; Rossini, F.P.; Conde, L.T.; de Almeida Rodrigues, L.C.; Falqueto, A.R.; Gontijo, A.B.P.L. Morphophysiological responses, bioaccumulation and tolerance of Alternanthera tenella Colla (Amaranthaceae) to excess copper under in vitro conditions. Plant Cell Tissue Organ Cult. 2020, 143, 303–318. [Google Scholar] [CrossRef]
- Alsokari, S.S.; Aldesuquy, H.S. Synergistic effect of polyamines and waste water on leaf turgidity, heavy metals accumulation in relation to grain yield. J. Appl. Sci. Res. 2011, 7, 376–384. [Google Scholar]
- Tripathi, A.K.; Tripathi, S. Changes in some physiological and biochemical characters in Albizia lebbek as bio-indicators of heavy metal toxicity. J. Environ. Biol. 1999, 20, 93–98. [Google Scholar]
- Zaheer, I.E.; Ali, S.; Rizwan, M.; Abbas, Z.; Bukhari, S.A.H.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Zinc-lysine prevents chromium-induced morphological, photosynthetic, and oxidative alterations in spinach irrigated with tannery wastewater. Environ. Sci. Pollut. Res. 2019, 26, 28951–28961. [Google Scholar] [CrossRef]
- Chatterjee, J.; Chatterjee, C. Phytotoxicity of cobalt, chromium and copper in cauliflower. Environ. Pollut. 2000, 109, 69–74. [Google Scholar] [CrossRef]
- Karunyal, S.; Renuga, G.; Kailash, P. Effects of tannery effluent on seed germination, leaf area, biomass and mineral content of some plants. Bioresour. Technol. 1994, 47, 215–218. [Google Scholar] [CrossRef]
- Buendía-González, L.; Orozco-Villafuerte, J.; Cruz-Sosa, F.; Barrera-Díaz, C.E.; Vernon-Carter, E.J. Prosopis laevigata a potential chromium (VI) and cadmium (II) hyperaccumulator desert plant. Bioresour. Technol. 2010, 101, 5862–5867. [Google Scholar] [CrossRef]
- Radha, J.; Srivastava, S.; Madan, V.K. Influence of chromium on growth and cell division of sugarcane. Indian J. Plant Physiol. 2000, 5, 228–231. [Google Scholar]
- Sharma, J.; Shrivastava, S. Physiological and morphological responses of Phaseolus vulgaris caused by mercury stress under lab conditions. Recent Adv. Biol Med. 2014, 1, 136. [Google Scholar] [CrossRef]
- Sagardoy, R.; Vázquez, S.; Florez-Sarasa, I.D.; Albacete, A.; Ribas-Carbó, M.; Flexas, J.; Abadía, J.; Morales, F. Stomatal and mesophyll conductances to CO2 are the main limitations to photosynthesis in sugar beet (Beta vulgaris) plants grown with excess zinc. New Phytol. 2010, 187, 145–158. [Google Scholar] [CrossRef]
- Kasim, W.A. Changes induced by copper and cadmium stress in the anatomy and grain yield of Sorghum bicolor (L.) Moench. Int. J. Agric. Biol 2006, 8, 123–128. [Google Scholar]
- Kastori, R.; Petrović, M.; Petrović, N. Effect of excess lead, cadmium, copper, and zinc on water relations in sunflower. J. Plant Nutr. 1992, 15, 2427–2439. [Google Scholar] [CrossRef]
- Gupta, P.; Bhatnagar, A.K. Spatial distribution of arsenic in different leaf tissues and its effect on structure and development of stomata and trichomes in mung bean, Vigna radiata (L.) Wilczek. Environ. Exp. Bot. 2015, 109, 12–22. [Google Scholar] [CrossRef]
- Souza, J.F.; Dolder, H.; Cortelazzo, A.L. Effect of excess cadmium and zinc ions on roots and shoots of maize seedlings. J. Plant Nutr. 2005, 28, 1923–1931. [Google Scholar] [CrossRef]
- Gomes, M.P.; de Sá e Melo Marques, T.C.L.L.; de Oliveira Gonçalves Nogueira, M.; de Castro, E.M.; Soares, Â.M. Ecophysiological and anatomical changes due to uptake and accumulation of heavy metal in Brachiaria decumbens. Sci. Agric. 2011, 68, 566–573. [Google Scholar] [CrossRef] [Green Version]
- Malar, S.; Vikram, S.S.; Favas, P.J.C.; Perumal, V. Lead heavy metal toxicity induced changes on growth and antioxidative enzymes level in water hyacinths [Eichhornia crassipes (Mart.)]. Bot. Stud. 2016, 55, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paunov, M.; Koleva, L.; Vassilev, A.; Vangronsveld, J.; Goltsev, V. Effects of different metals on photosynthesis: Cadmium and zinc affect chlorophyll fluorescence in durum wheat. Int. J. Mol. Sci. 2018, 19, 787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latif, U.; Farid, M.; Rizwan, M.; Ishaq, H.K.; Farid, S.; Ali, S.; El-Sheikh, M.A.; Alyemeni, M.N.; Wijaya, L. Physiological and Biochemical Response of Alternanthera bettzickiana (Regel) G. Nicholson under Acetic Acid Assisted Phytoextraction of Lead. Plants 2020, 9, 1084. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Chang, X.; Yang, Y.; Song, Z. Foliar graphene oxide treatment increases photosynthetic capacity and reduces oxidative stress in cadmium-stressed lettuce. Plant Physiol. Biochem. 2020, 154, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Yotsova, E.; Dobrikova, A.; Stefanov, M.; Misheva, S.; Bardáčová, M.; Matušíková, I.; Žideková, L.; Blehová, A.; Apostolova, E. Effects of cadmium on two wheat cultivars depending on different nitrogen supply. Plant Physiol. Biochem. 2020, 155, 789–799. [Google Scholar] [CrossRef]
- Mazur, R.; Sadowska, M.; Kowalewska, Ł.; Abratowska, A.; Kalaji, H.M.; Mostowska, A.; Garstka, M.; Krasnodębska-Ostręga, B. Overlapping toxic effect of long term thallium exposure on white mustard (Sinapis alba L.) photosynthetic activity. BMC Plant Biol. 2016, 16, 191. [Google Scholar] [CrossRef] [Green Version]
- Appenroth, K.-J.; Keresztes, A.; Sárvári, É.; Jaglarz, A.; Fischer, W. Multiple effects of chromate on Spirodela polyrhiza: Electron microscopy and biochemical investigations. Plant Biol. 2003, 5, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Hegedüs, A.; Erdei, S.; Horváth, G. Comparative studies of H2O2 detoxifying enzymes in green and greening barley seedlings under cadmium stress. Plant Sci. 2001, 160, 1085–1093. [Google Scholar] [CrossRef]
- Gill, S.S.; Anjum, N.A.; Ahmad, I.; Thangavel, P.; Sridevi, G.; Pacheco, M.; Duarte, A.C.; Umar, S.; Khan, N.A.; Pereira, M.E. Metal hyperaccumulation and tolerance in Alyssum, Arabidopsis and Thlaspi: An overview. Plant Fam. Brassicaceae 2012, 21, 99–137. [Google Scholar]
- Moradi, L.; Ehsanzadeh, P. Effects of Cd on photosynthesis and growth of safflower (Carthamus tinctorius L.) genotypes. Photosynthetica 2015, 53, 506–518. [Google Scholar] [CrossRef]
- Sela, M.; Garty, J.; TEL-OR, E. The accumulation and the effect of heavy metals on the water fern Azolla filiculoides. New Phytol. 1989, 112, 7–12. [Google Scholar] [CrossRef]
- Bertrand, M.; Poirier, I. Photosynthetic organisms and excess of metals. Photosynthetica 2005, 43, 345–353. [Google Scholar] [CrossRef]
- Singh, H.P.; Mahajan, P.; Kaur, S.; Batish, D.R.; Kohli, R.K. Chromium toxicity and tolerance in plants. Environ. Chem. Lett. 2013, 11, 229–254. [Google Scholar] [CrossRef]
- Shahzad, B.; Tanveer, M.; Rehman, A.; Cheema, S.A.; Fahad, S.; Rehman, S.; Sharma, A. Nickel; whether toxic or essential for plants and environment-A review. Plant Physiol. Biochem. 2018, 132, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K. Photosynthetic response of plants under different abiotic stresses: A review. J. Plant Growth Regul. 2019, 1–23. [Google Scholar] [CrossRef]
- Juarez, A.B.; Barsanti, L.; Passarelli, V.; Evangelista, V.; Vesentini, N.; Conforti, V.; Gualtieri, P. In vivo microspectroscopy monitoring of chromium effects on the photosynthetic and photoreceptive apparatus of Eudorina unicocca and Chlorella kessleri. J. Environ. Monit. 2008, 10, 1313–1318. [Google Scholar] [CrossRef]
- Wang, X.; Ma, R.; Cui, D.; Cao, Q.; Shan, Z.; Jiao, Z. Physio-biochemical and molecular mechanism underlying the enhanced heavy metal tolerance in highland barley seedlings pre-treated with low-dose gamma irradiation. Sci. Rep. 2017, 7, 14233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, W.; Chen, X.; Song, G.; Wang, Q.; Chang, C.C. Effects of copper and cadmium on heavy metal polluted waterbody restoration by duckweed (Lemna minor). Plant Physiol. Biochem. 2007, 45, 62–69. [Google Scholar] [CrossRef]
- Garg, P.; Chandra, P.; Devi, S. Chromium (VI) induced morphological changes in Limnanthemum cristatum Griseb.: A possible bioindicator. Phytomorphology 1994, 44, 201–206. [Google Scholar]
- Piotrowska, A.; Bajguz, A.; Godlewska-Żyłkiewicz, B.; Czerpak, R.; Kamińska, M. Jasmonic acid as modulator of lead toxicity in aquatic plant Wolffia arrhiza (Lemnaceae). Environ. Exp. Bot. 2009, 66, 507–513. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef] [Green Version]
- Ehsan, S.; Ali, S.; Noureen, S.; Mahmood, K.; Farid, M.; Ishaque, W.; Shakoor, M.B.; Rizwan, M. Citric acid assisted phytoremediation of cadmium by Brassica napus L. Ecotoxicol. Environ. Saf. 2014, 106, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Afshan, S.; Ali, S.; Bharwana, S.A.; Rizwan, M.; Farid, M.; Abbas, F.; Ibrahim, M.; Mehmood, M.A.; Abbasi, G.H. Citric acid enhances the phytoextraction of chromium, plant growth, and photosynthesis by alleviating the oxidative damages in Brassica napus L. Environ. Sci. Pollut. Res. 2015, 22, 11679–11689. [Google Scholar] [CrossRef]
- Rizwan, M.; Meunier, J.-D.; Davidian, J.-C.; Pokrovsky, O.S.; Bovet, N.; Keller, C. Silicon alleviates Cd stress of wheat seedlings (Triticum turgidum L. cv. Claudio) grown in hydroponics. Environ. Sci. Pollut. Res. 2016, 23, 1414–1427. [Google Scholar] [CrossRef]
- Keller, C.; Rizwan, M.; Davidian, J.-C.; Pokrovsky, O.S.; Bovet, N.; Chaurand, P.; Meunier, J.-D. Effect of silicon on wheat seedlings (Triticum turgidum L.) grown in hydroponics and exposed to 0 to 30 µM Cu. Planta 2015, 241, 847–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuriakose, S.V.; Prasad, M.N. V Cadmium stress affects seed germination and seedling growth in Sorghum bicolor (L.) Moench by changing the activities of hydrolyzing enzymes. Plant Growth Regul. 2008, 54, 143–156. [Google Scholar] [CrossRef]
- Soudek, P.; Petrová, Š.; Vaňková, R.; Song, J.; Vaněk, T. Accumulation of heavy metals using Sorghum sp. Chemosphere 2014, 104, 15–24. [Google Scholar] [CrossRef]
- Qin, W.; Bazeille, N.; Henry, E.; Zhang, B.; Deprez, E.; Xi, X.-G. Mechanistic insight into cadmium-induced inactivation of the Bloom protein. Sci. Rep. 2016, 6, 26225. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.; Wang, X.; Shi, C.; Guo, J.; Ma, P.; Ren, X.; Wei, T.; Liu, H.; Li, J. Hydrogen sulfide decreases Cd translocation from root to shoot through increasing Cd accumulation in cell wall and decreasing Cd2+ influx in Isatis indigotica. Plant Physiol. Biochem. 2020, 155, 605–612. [Google Scholar] [CrossRef]
- Ekmekçi, Y.; Tanyolaç, D.; Ayhan, B. A crop tolerating oxidative stress induced by excess lead: Maize. Acta Physiol. Plant. 2009, 31, 319–330. [Google Scholar] [CrossRef]
- Souri, Z.; Cardoso, A.A.; da-Silva, C.J.; de Oliveira, L.M.; Dari, B.; Sihi, D.; Karimi, N. Heavy metals and photosynthesis: Recent developments. In Photosynthesis, Productivity, and Environmental Stress; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 107–134. [Google Scholar]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.-J. The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci. 2009, 14, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Piyatida, P.; da Silva, J.A.T.; Fujita, M. Molecular mechanism of heavy metal toxicity and tolerance in plants: Central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot. 2012, 2012, 872875. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Stress-triggered redox signalling: What’s in pROSpect? Plant Cell Environ. 2016, 39, 951–964. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J.; Bagchi, D. Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 1995, 18, 321–336. [Google Scholar] [CrossRef] [Green Version]
- SONG, W.; MENDOZA-CÓZATL, D.G.; Lee, Y.; Schroeder, J.I.; AHN, S.; LEE, H.; Wicker, T.; Martinoia, E. Phytochelatin–metal (loid) transport into vacuoles shows different substrate preferences in barley and A rabidopsis. Plant Cell Environ. 2014, 37, 1192–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahid, M.; Pourrut, B.; Dumat, C.; Nadeem, M.; Aslam, M.; Pinelli, E. Heavy-metal-induced reactive oxygen species: Phytotoxicity and physicochemical changes in plants. Rev. Environ. Contam. Toxicol. 2014, 232, 1–44. [Google Scholar] [PubMed]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.I.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef]
- Mittler, R.; Zilinskas, B.A. Molecular cloning and characterization of a gene encoding pea cytosolic ascorbate peroxidase. J. Biol. Chem. 1992, 267, 21802–21807. [Google Scholar] [CrossRef]
- Jiménez, A.; Hernández, J.A.; Pastori, G.; del Rıo, L.A.; Sevilla, F. Role of the ascorbate-glutathione cycle of mitochondria and peroxisomes in the senescence of pea leaves. Plant Physiol. 1998, 118, 1327–1335. [Google Scholar] [CrossRef] [Green Version]
- Panda, S.K.; Matsumoto, H. Changes in antioxidant gene expression and induction of oxidative stress in pea (Pisum sativum L.) under Al stress. Biometals 2010, 23, 753–762. [Google Scholar] [CrossRef]
- Pandey, V.; Dixit, V.; Shyam, R. Antioxidative responses in relation to growth of mustard (Brassica juncea cv. Pusa Jaikisan) plants exposed to hexavalent chromium. Chemosphere 2005, 61, 40–47. [Google Scholar] [CrossRef]
- Adhikari, A.; Adhikari, S.; Ghosh, S.; Azahar, I.; Shaw, A.K.; Roy, D.; Roy, S.; Saha, S.; Hossain, Z. Imbalance of redox homeostasis and antioxidant defense status in maize under chromium (VI) stress. Environ. Exp. Bot. 2020, 169, 103873. [Google Scholar] [CrossRef]
- Dixit, V.; Pandey, V.; Shyam, R. Differential antioxidative responses to cadmium in roots and leaves of pea (Pisum sativum L. cv. Azad). J. Exp. Bot. 2001, 52, 1101–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yannarelli, G.G.; Fernández-Alvarez, A.J.; Santa-Cruz, D.M.; Tomaro, M.L. Glutathione reductase activity and isoforms in leaves and roots of wheat plants subjected to cadmium stress. Phytochemistry 2007, 68, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Sobrino-Plata, J.; Ortega-Villasante, C.; Flores-Cáceres, M.L.; Escobar, C.; Del Campo, F.F.; Hernández, L.E. Differential alterations of antioxidant defenses as bioindicators of mercury and cadmium toxicity in alfalfa. Chemosphere 2009, 77, 946–954. [Google Scholar] [CrossRef]
- Wang, X.; Wei, Z.; Liu, D.; Zhao, G. Effects of NaCl and silicon on activities of antioxidative enzymes in roots, shoots and leaves of alfalfa. Afr. J. Biotechnol. 2011, 10, 545–549. [Google Scholar]
- Sobrino-Plata, J.; Herrero, J.; Carrasco-Gil, S.; Pérez-Sanz, A.; Lobo, C.; Escobar, C.; Millán, R.; Hernández, L.E. Specific stress responses to cadmium, arsenic and mercury appear in the metallophyte Silene vulgaris when grown hydroponically. RSC Adv. 2013, 3, 4736–4744. [Google Scholar] [CrossRef]
- Sobrino-Plata, J.; Carrasco-Gil, S.; Abadía, J.; Escobar, C.; Álvarez-Fernández, A.; Hernández, L.E. The role of glutathione in mercury tolerance resembles its function under cadmium stress in Arabidopsis. Metallomics 2014, 6, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Rouhier, N.; San Koh, C.; Gelhaye, E.; Corbier, C.; Favier, F.; Didierjean, C.; Jacquot, J.-P. Redox based anti-oxidant systems in plants: Biochemical and structural analyses. Biochim. Biophys. Acta (BBA)-General Subj. 2008, 1780, 1249–1260. [Google Scholar] [CrossRef]
- El-Kereamy, A.; Bi, Y.-M.; Mahmood, K.; Ranathunge, K.; Yaish, M.W.; Nambara, E.; Rothstein, S.J. Overexpression of the CC-type glutaredoxin, OsGRX6 affects hormone and nitrogen status in rice plants. Front. Plant Sci. 2015, 6, 934. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Dubey, A.K.; Kumar, V.; Ansari, M.A.; Narayan, S.; Kumar, S.; Pandey, V.; Shirke, P.A.; Pande, V.; Sanyal, I. Over-expression of chickpea glutaredoxin (CaGrx) provides tolerance to heavy metals by reducing metal accumulation and improved physiological and antioxidant defence system. Ecotoxicol. Environ. Saf. 2020, 192, 110252. [Google Scholar] [CrossRef]
- Verma, P.K.; Verma, S.; Pande, V.; Mallick, S.; Deo Tripathi, R.; Dhankher, O.P.; Chakrabarty, D. Overexpression of rice glutaredoxin OsGrx_C7 and OsGrx_C2. 1 reduces intracellular arsenic accumulation and increases tolerance in Arabidopsis thaliana. Front. Plant Sci. 2016, 7, 740. [Google Scholar]
- Chowardhara, B.; Borgohain, P.; Saha, B.; Awasthi, J.P.; Panda, S.K. Differential oxidative stress responses in Brassica juncea (L.) Czern and Coss cultivars induced by cadmium at germination and early seedling stage. Acta Physiol. Plant. 2020, 42, 105. [Google Scholar] [CrossRef]
- Tyagi, S.; Singh, K.; Upadhyay, S.K. Molecular characterization revealed the role of catalases under abiotic and arsenic stress in bread wheat (Triticum aestivum L.). J. Hazard. Mater. 2021, 403, 123585. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Zhao, L.; Li, J.; Huang, S.; Tang, H.; Chang, L.; Dai, Z.; Chen, A.; Li, D.; Li, Z. Physiological responses and tolerance of flax (Linum usitatissimum L.) to lead stress. Acta Physiol. Plant. 2020, 42, 113. [Google Scholar] [CrossRef]
- Jia, H.; Wang, X.; Dou, Y.; Liu, D.; Si, W.; Fang, H.; Zhao, C.; Chen, S.; Xi, J.; Li, J. Hydrogen sulfide-cysteine cycle system enhances cadmium tolerance through alleviating cadmium-induced oxidative stress and ion toxicity in Arabidopsis roots. Sci. Rep. 2016, 6, 39702. [Google Scholar] [CrossRef]
- López-Martín, M.C.; Becana, M.; Romero, L.C.; Gotor, C. Knocking out cytosolic cysteine synthesis compromises the antioxidant capacity of the cytosol to maintain discrete concentrations of hydrogen peroxide in Arabidopsis. Plant Physiol. 2008, 147, 562–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, S.; Gao, W.; Chen, Q.; Ramalingam, S.; Chye, M. Overexpression of membrane-associated acyl-CoA-binding protein ACBP1 enhances lead tolerance in Arabidopsis. Plant J. 2008, 54, 141–151. [Google Scholar] [CrossRef]
- DU, Z.; CHEN, M.; CHEN, Q.; GU, J.; CHYE, M. Expression of A rabidopsis acyl-CoA-binding proteins AtACBP 1 and AtACBP 4 confers P b (II) accumulation in B rassica juncea roots. Plant Cell Environ. 2015, 38, 101–117. [Google Scholar] [CrossRef]
- Sunkar, R.; Kaplan, B.; Bouché, N.; Arazi, T.; Dolev, D.; Talke, I.N.; Maathuis, F.J.M.; Sanders, D.; Bouchez, D.; Fromm, H. Expression of a truncated tobacco NtCBP4 channel in transgenic plants and disruption of the homologous Arabidopsis CNGC1 gene confer Pb2+ tolerance. Plant J. 2000, 24, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, I.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. Time course analysis of gene regulation under cadmium stress in rice. Plant Soil 2009, 325, 97–108. [Google Scholar] [CrossRef] [Green Version]
- He, F.; Liu, Q.; Zheng, L.; Cui, Y.; Shen, Z.; Zheng, L. RNA-Seq analysis of rice roots reveals the involvement of post-transcriptional regulation in response to cadmium stress. Front. Plant Sci. 2015, 6, 1136. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Fang, H.; Pei, Y.; Jin, Z.; Zhang, L.; Liu, D. WRKY transcription factors down-regulate the expression of H 2 S-generating genes, LCD and DES in Arabidopsis thaliana. Sci. Bull. 2015, 60, 995–1001. [Google Scholar] [CrossRef] [Green Version]
- Rui, H.; Zhang, X.; Shinwari, K.I.; Zheng, L.; Shen, Z. Comparative transcriptomic analysis of two Vicia sativa L. varieties with contrasting responses to cadmium stress reveals the important role of metal transporters in cadmium tolerance. Plant Soil 2018, 423, 241–255. [Google Scholar] [CrossRef]
- Chen, P.; Chen, T.; Li, Z.; Jia, R.; Luo, D.; Tang, M.; Lu, H.; Hu, Y.; Yue, J.; Huang, Z. Transcriptome analysis revealed key genes and pathways related to cadmium-stress tolerance in Kenaf (Hibiscus cannabinus L.). Ind. Crops Prod. 2020, 158, 112970. [Google Scholar] [CrossRef]
- Weber, M.; Trampczynska, A.; Clemens, S. Comparative transcriptome analysis of toxic metal responses in Arabidopsis thaliana and the Cd2+−hypertolerant facultative metallophyte Arabidopsis halleri. Plant Cell Environ. 2006, 29, 950–963. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, S.; Guan, H.; Ma, L.; Chen, Z.; Gu, H.; Qu, L.-J. Transcriptional profiling of Arabidopsis seedlings in response to heavy metal lead (Pb). Environ. Exp. Bot. 2009, 67, 377–386. [Google Scholar] [CrossRef]
- Chen, J.; Yang, L.; Yan, X.; Liu, Y.; Wang, R.; Fan, T.; Ren, Y.; Tang, X.; Xiao, F.; Liu, Y. Zinc-finger transcription factor ZAT6 positively regulates cadmium tolerance through the glutathione-dependent pathway in Arabidopsis. Plant Physiol. 2016, 171, 707–719. [Google Scholar] [CrossRef]
- He, G.; Qin, L.; Tian, W.; Meng, L.; He, T.; Zhao, D. Heavy metal Transporters-Associated proteins in Solanum tuberosum: Genome-wide identification, comprehensive gene feature, evolution and expression analysis. Genes 2020, 11, 1269. [Google Scholar] [CrossRef]
- Clemens, S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 2006, 88, 1707–1719. [Google Scholar] [CrossRef] [PubMed]
- Arslan Topal, E.I.; Topal, M.; Öbek, E. Assessment of heavy metal accumulations and health risk potentials in tomatoes grown in the discharge area of a municipal wastewater treatment plant. Int. J. Environ. Health Res. 2020, 1–13. [Google Scholar] [CrossRef]
- Clemens, S. Molecular mechanisms of plant metal tolerance and homeostasis. Planta 2001, 212, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Cobbett, C.; Goldsbrough, P. Phytochelatins and metallothioneins: Roles in heavy metal detoxification and homeostasis. Annu. Rev. Plant Biol. 2002, 53, 159–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anjum, N.A.; Hasanuzzaman, M.; Hossain, M.A.; Thangavel, P.; Roychoudhury, A.; Gill, S.S.; Rodrigo, M.A.M.; Adam, V.; Fujita, M.; Kizek, R. Jacks of metal/metalloid chelation trade in plants—An overview. Front. Plant Sci. 2015, 6, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frey, B.; Keller, C.; Zierold, K. Distribution of Zn in functionally different leaf epidermal cells of the hyperaccumulator Thlaspi caerulescens. Plant Cell Environ. 2000, 23, 675–687. [Google Scholar] [CrossRef]
- Zhao, F.J.; Lombi, E.; Breedon, T. Zinc hyperaccumulation and cellular distribution in Arabidopsis halleri. Plant Cell Environ. 2000, 23, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Küpper, H.; Lombi, E.; Zhao, F.-J.; McGrath, S.P. Cellular compartmentation of cadmium and zinc in relation to other elements in the hyperaccumulator Arabidopsis halleri. Planta 2000, 212, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Hu, P.-J.; Qiu, R.-L.; Senthilkumar, P.; Jiang, D.; Chen, Z.-W.; Tang, Y.-T.; Liu, F.-J. Tolerance, accumulation and distribution of zinc and cadmium in hyperaccumulator Potentilla griffithii. Environ. Exp. Bot. 2009, 66, 317–325. [Google Scholar] [CrossRef]
- Brooks, R.R.; Reeves, R.D.; Morrison, R.S.; Malaisse, F. Hyperaccumulation of copper and cobalt—A review. Bull. la Société R. Bot. Belgique/Bulletin van K. Belgische Bot. Ver. 1980, 113, 166–172. [Google Scholar]
- Salt, D.E.; Prince, R.C.; Pickering, I.J.; Raskin, I. Mechanisms of cadmium mobility and accumulation in Indian mustard. Plant Physiol. 1995, 109, 1427–1433. [Google Scholar] [CrossRef] [Green Version]
- Chardonnens, A.N.; Ten Bookum, W.M.; Kuijper, L.D.J.; Verkleij, J.A.C.; Ernst, W.H.O. Distribution of cadmium in leaves of cadmium tolerant and sensitive ecotypes of Silene vulgaris. Physiol. Plant. 1998, 104, 75–80. [Google Scholar] [CrossRef]
- Masood, A.; Iqbal, N.; Khan, N.A. Role of ethylene in alleviation of cadmium-induced photosynthetic capacity inhibition by sulphur in mustard. Plant Cell Environ. 2012, 35, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Carrier, P.; Baryla, A.; Havaux, M. Cadmium distribution and microlocalization in oilseed rape (Brassica napus) after long-term growth on cadmium-contaminated soil. Planta 2003, 216, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Thangavel, P.; Long, S.; Minocha, R. Changes in phytochelatins and their biosynthetic intermediates in red spruce (Picea rubens Sarg.) cell suspension cultures under cadmium and zinc stress. Plant Cell. Tissue Organ Cult. 2007, 88, 201–216. [Google Scholar] [CrossRef]
- Dago, À.; González, I.; Ariño, C.; Díaz-Cruz, J.M.; Esteban, M. Chemometrics applied to the analysis of induced phytochelatins in Hordeum vulgare plants stressed with various toxic non-essential metals and metalloids. Talanta 2014, 118, 201–209. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Z.; Zhu, C. Heteroexpression of the wheat phytochelatin synthase gene (TaPCS1) in rice enhances cadmium sensitivity. Acta Biochim. Biophys. Sin. 2012, 44, 886–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhigang, A.; Cuijie, L.; Yuangang, Z.; Yejie, D.; Wachter, A.; Gromes, R.; Rausch, T. Expression of BjMT2, a metallothionein 2 from Brassica juncea, increases copper and cadmium tolerance in Escherichia coli and Arabidopsis thaliana, but inhibits root elongation in Arabidopsis thaliana seedlings. J. Exp. Bot. 2006, 57, 3575–3582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, G.; Kushwaha, H.R.; Panjabi-Sabharwal, V.; Kumari, S.; Joshi, R.; Karan, R.; Mittal, S.; Pareek, S.L.S.; Pareek, A. Clustered metallothionein genes are co-regulated in rice and ectopic expression of OsMT1e-P confers multiple abiotic stress tolerance in tobacco via ROS scavenging. BMC Plant Biol. 2012, 12, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015, 2015, 756120. [Google Scholar] [CrossRef]
- Zhou, B.; Yao, W.; Wang, S.; Wang, X.; Jiang, T. The metallothionein gene, TaMT3, from Tamarix androssowii confers Cd2+ tolerance in tobacco. Int. J. Mol. Sci. 2014, 15, 10398–10409. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Qi, Y.; Yuan, Y.; Wang, G.; Cui, J.; Chen, Y.; Zhang, H.; Shen, Z. Overexpression of Elsholtzia haichowensis metallothionein 1 (EhMT1) in tobacco plants enhances copper tolerance and accumulation in root cytoplasm and decreases hydrogen peroxide production. J. Hazard. Mater. 2012, 233, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Emamverdian, A.; Ding, Y.; Xie, Y.; Sangari, S. Silicon mechanisms to ameliorate heavy metal stress in plants. Biomed. Res. Int. 2018, 2018, 8492898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics, and ionomics. Front. Plant Sci. 2016, 6, 1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, G.; Cai, Q.; Liu, C.; Wu, L. Silicon alleviates cadmium toxicity in peanut plants in relation to cadmium distribution and stimulation of antioxidative enzymes. Plant Growth Regul. 2010, 61, 45–52. [Google Scholar] [CrossRef]
- Song, A.; Li, Z.; Zhang, J.; Xue, G.; Fan, F.; Liang, Y. Silicon-enhanced resistance to cadmium toxicity in Brassica chinensis L. is attributed to Si-suppressed cadmium uptake and transport and Si-enhanced antioxidant defense capacity. J. Hazard. Mater. 2009, 172, 74–83. [Google Scholar] [CrossRef]
- Feng, J.; Shi, Q.; Wang, X.; Wei, M.; Yang, F.; Xu, H. Silicon supplementation ameliorated the inhibition of photosynthesis and nitrate metabolism by cadmium (Cd) toxicity in Cucumis sativus L. Sci. Hortic. 2010, 123, 521–530. [Google Scholar] [CrossRef]
- Farooq, M.A.; Ali, S.; Hameed, A.; Ishaque, W.; Mahmood, K.; Iqbal, Z. Alleviation of cadmium toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes; suppressed cadmium uptake and oxidative stress in cotton. Ecotoxicol. Environ. Saf. 2013, 96, 242–249. [Google Scholar] [CrossRef]
- Su, N.; Ling, F.; Xing, A.; Zhao, H.; Zhu, Y.; Wang, Y.; Deng, X.; Wang, C.; Xu, X.; Hu, Z. Lignin synthesis mediated by CCoAOMT enzymes is required for the tolerance against excess Cu in Oryza sativa. Environ. Exp. Bot. 2020, 175, 104059. [Google Scholar] [CrossRef]
- Kupper, H.; Mijovilovich, A.; Meyer-Klaucke, W.; Kroneck, P.M.H. Tissue-and age-dependent differences in the complexation of cadmium and zinc in the cadmium/zinc hyperaccumulator Thlaspi caerulescens (Ganges ecotype) revealed by X-ray absorption spectroscopy. Plant Physiol. 2004, 134, 748–757. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, A.A.A.; Dardiry, M.H.O.; Samad, A.; Abdelrady, E. Exposure to Lead (Pb) Induced Changes in the Metabolite Content, Antioxidant Activity and Growth of Jatropha curcas (L.). Trop. Plant Biol. 2020, 13, 150–161. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, Y.; Ge, Q.; Li, Y.; Sun, J.; Zhang, Y.; Liu, X. Comparative physiological responses of Solanum nigrum and Solanum torvum to cadmium stress. New Phytol. 2012, 196, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Lwalaba, J.L.W.; Zvobgo, G.; Mwamba, T.M.; Louis, L.T.; Fu, L.; Kirika, B.A.; Tshibangu, A.K.; Adil, M.F.; Sehar, S.; Mukobo, R.P. High accumulation of phenolics and amino acids confers tolerance to the combined stress of cobalt and copper in barley (Hordeum vulagare). Plant Physiol. Biochem. 2020, 155, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, N.; Hermans, C.; Schat, H. Molecular mechanisms of metal hyperaccumulation in plants. New Phytol. 2009, 181, 759–776. [Google Scholar] [CrossRef] [PubMed]
- Solanki, R.; Dhankhar, R. Biochemical changes and adaptive strategies of plants under heavy metal stress. Biologia 2011, 66, 195–204. [Google Scholar] [CrossRef]
- Song, W.-Y.; Yamaki, T.; Yamaji, N.; Ko, D.; Jung, K.-H.; Fujii-Kashino, M.; An, G.; Martinoia, E.; Lee, Y.; Ma, J.F. A rice ABC transporter, OsABCC1, reduces arsenic accumulation in the grain. Proc. Natl. Acad. Sci. USA 2014, 111, 15699–15704. [Google Scholar] [CrossRef] [Green Version]
- Rea, P.A. MRP subfamily ABC transporters from plants and yeast. J. Exp. Bot. 1999, 50, 895–913. [Google Scholar] [CrossRef]
- Martinoia, E.; Klein, M.; Geisler, M.; Bovet, L.; Forestier, C.; Kolukisaoglu, U.; MuÈller-RoÈber, B.; Schulz, B. Multifunctionality of plant ABC transporters–more than just detoxifiers. Planta 2002, 214, 345–355. [Google Scholar] [CrossRef]
- Huang, D.; Huo, J.; Liao, W. Hydrogen sulfide: Roles in plant abiotic stress response and crosstalk with other signals. Plant Sci. 2020, 302, 110733. [Google Scholar] [CrossRef]
- Bhati, K.K.; Sharma, S.; Aggarwal, S.; Kaur, M.; Shukla, V.; Kaur, J.; Mantri, S.; Pandey, A.K. Genome-wide identification and expression characterization of ABCC-MRP transporters in hexaploid wheat. Front. Plant Sci. 2015, 6, 488. [Google Scholar] [CrossRef]
- Zhang, X.D.; Zhao, K.X.; Yang, Z.M. Identification of genomic ATP binding cassette (ABC) transporter genes and Cd-responsive ABCs in Brassica napus. Gene 2018, 664, 139–151. [Google Scholar] [CrossRef]
- Gaillard, S.; Jacquet, H.; Vavasseur, A.; Leonhardt, N.; Forestier, C. AtMRP6/AtABCC6, an ATP-binding cassette transporter gene expressed during early steps of seedling development and up-regulated by cadmium in Arabidopsis thaliana. BMC Plant Biol. 2008, 8, 22. [Google Scholar] [CrossRef] [Green Version]
- Brunetti, P.; Zanella, L.; De Paolis, A.; Di Litta, D.; Cecchetti, V.; Falasca, G.; Barbieri, M.; Altamura, M.M.; Costantino, P.; Cardarelli, M. Cadmium-inducible expression of the ABC-type transporter AtABCC3 increases phytochelatin-mediated cadmium tolerance in Arabidopsis. J. Exp. Bot. 2015, 66, 3815–3829. [Google Scholar] [CrossRef] [Green Version]
- Shi, M.; Wang, S.; Zhang, Y.; Wang, S.; Zhao, J.; Feng, H.; Sun, P.; Fang, C.; Xie, X. Genome-wide characterization and expression analysis of ATP-binding cassette (ABC) transporters in strawberry reveal the role of FvABCC11 in cadmium tolerance. Sci. Hortic. 2020, 271, 109464. [Google Scholar] [CrossRef]
- Park, J.; Song, W.; Ko, D.; Eom, Y.; Hansen, T.H.; Schiller, M.; Lee, T.G.; Martinoia, E.; Lee, Y. The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. Plant J. 2012, 69, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Bovet, L.; Maeshima, M.; Martinoia, E.; Lee, Y. The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance. Plant J. 2007, 50, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Yang, J.; Wan, Y.; Xiang, S.; Guan, M.; Du, H.; Tang, Z.; Lu, K.; Li, J.; Qu, C. A Genome-Wide Survey of MATE Transporters in Brassicaceae and Unveiling Their Expression Profiles under Abiotic Stress in Rapeseed. Plants 2020, 9, 1072. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Sun, J.; Chen, Y.; Shi, L.; Yang, L.; Wang, Y. The molecular mechanism underlying cadmium resistance in NHX1 transgenic Lemna turonifera was studied by comparative transcriptome analysis. Plant Cell Tissue Organ Cult. 2020, 143, 189–200. [Google Scholar] [CrossRef]
- Zhang, X.D.; Meng, J.G.; Zhao, K.X.; Chen, X.; Yang, Z.M. Annotation and characterization of Cd-responsive metal transporter genes in rapeseed (Brassica napus). Biometals 2018, 31, 107–121. [Google Scholar] [CrossRef]
- Komal, T.; Mustafa, M.; Ali, Z.; Kazi, A.G. Heavy metal uptake and transport in plants. In Heavy Metal Contamination of Soils; Springer: Berlin/Heidelberg, Germany, 2015; pp. 181–194. [Google Scholar]
- Vert, G.; Grotz, N.; Dédaldéchamp, F.; Gaymard, F.; Guerinot, M.L.; Briat, J.-F.; Curie, C. IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 2002, 14, 1223–1233. [Google Scholar] [CrossRef] [Green Version]
- Connolly, E.L.; Fett, J.P.; Guerinot, M. Lou Expression of the IRT1 metal transporter is controlled by metals at the levels of transcript and protein accumulation. Plant Cell 2002, 14, 1347–1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milner, M.J.; Seamon, J.; Craft, E.; Kochian, L.V. Transport properties of members of the ZIP family in plants and their role in Zn and Mn homeostasis. J. Exp. Bot. 2013, 64, 369–381. [Google Scholar] [CrossRef] [Green Version]
- González-Guerrero, M.; Escudero, V.; Saéz, Á.; Tejada-Jiménez, M. Transition metal transport in plants and associated endosymbionts: Arbuscular mycorrhizal fungi and rhizobia. Front. Plant Sci. 2016, 7, 1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Zaal, B.J.; Neuteboom, L.W.; Pinas, J.E.; Chardonnens, A.N.; Schat, H.; Verkleij, J.A.C.; Hooykaas, P.J.J. Overexpression of a novel Arabidopsis gene related to putative zinc-transporter genes from animals can lead to enhanced zinc resistance and accumulation. Plant Physiol. 1999, 119, 1047–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assunção, A.G.L.; Martins, P.D.C.; De Folter, S.; Vooijs, R.; Schat, H.; Aarts, M.G.M. Elevated expression of metal transporter genes in three accessions of the metal hyperaccumulator Thlaspi caerulescens. Plant Cell Environ. 2001, 24, 217–226. [Google Scholar] [CrossRef]
- Delhaize, E.; Kataoka, T.; Hebb, D.M.; White, R.G.; Ryan, P.R. Genes encoding proteins of the cation diffusion facilitator family that confer manganese tolerance. Plant Cell 2003, 15, 1131–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verret, F.; Gravot, A.; Auroy, P.; Leonhardt, N.; David, P.; Nussaume, L.; Vavasseur, A.; Richaud, P. Overexpression of AtHMA4 enhances root-to-shoot translocation of zinc and cadmium and plant metal tolerance. FEBS Lett. 2004, 576, 306–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohli, S.K.; Handa, N.; Bali, S.; Arora, S.; Sharma, A.; Kaur, R.; Bhardwaj, R. Modulation of antioxidative defense expression and osmolyte content by co-application of 24-epibrassinolide and salicylic acid in Pb exposed Indian mustard plants. Ecotoxicol. Environ. Saf. 2018, 147, 382–393. [Google Scholar] [CrossRef]
- Bali, S.; Jamwal, V.L.; Kaur, P.; Kohli, S.K.; Ohri, P.; Gandhi, S.G.; Bhardwaj, R.; Al-Huqail, A.A.; Siddiqui, M.H.; Ahmad, P. Role of P-type ATPase metal transporters and plant immunity induced by jasmonic acid against Lead (Pb) toxicity in tomato. Ecotoxicol. Environ. Saf. 2019, 174, 283–294. [Google Scholar] [CrossRef]
- Jalmi, S.K.; Bhagat, P.K.; Verma, D.; Noryang, S.; Tayyeba, S.; Singh, K.; Sharma, D.; Sinha, A.K. Traversing the links between heavy metal stress and plant signaling. Front. Plant Sci. 2018, 9, 12. [Google Scholar] [CrossRef]
- Chaturvedi, R.; Varun, M.; Paul, M.S. Phytoremediation: Uptake and role of metal transporters in some members of Brassicaceae. In Phytoremediation; Springer: Berlin/Heidelberg, Germany, 2016; pp. 453–468. [Google Scholar]
- Ricachenevsky, F.K.; Menguer, P.K.; Sperotto, R.A.; Williams, L.E.; Fett, J.P. Roles of plant metal tolerance proteins (MTP) in metal storage and potential use in biofortification strategies. Front. Plant Sci. 2013, 4, 144. [Google Scholar] [CrossRef] [Green Version]
- Jozefczak, M.; Remans, T.; Vangronsveld, J.; Cuypers, A. Glutathione is a key player in metal-induced oxidative stress defenses. Int. J. Mol. Sci. 2012, 13, 3145–3175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rui, H.; Chen, C.; Zhang, X.; Shen, Z.; Zhang, F. Cd-induced oxidative stress and lignification in the roots of two Vicia sativa L. varieties with different Cd tolerances. J. Hazard. Mater. 2016, 301, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Shahzad, B.; Ashraf, U.; Fahad, S.; Hassan, W.; Jan, S.; Khan, I.; Saleem, M.F. Osmoregulation and antioxidant production in maize under combined cadmium and arsenic stress. Environ. Sci. Pollut. Res. 2016, 23, 11864–11875. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.H.; Murata, N. Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr. Opin. Plant Biol. 2002, 5, 250–257. [Google Scholar] [CrossRef]
- Riyazuddin, R.; Verma, R.; Singh, K.; Nisha, N.; Keisham, M.; Bhati, K.K.; Kim, S.T.; Gupta, R. Ethylene: A master regulator of salinity stress tolerance in plants. Biomolecules 2020, 10, 959. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shahzad, B.; Kumar, V.; Kohli, S.K.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Bhardwaj, R.; Zheng, B. Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules 2019, 9, 285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline mechanisms of stress survival. Antioxid. Redox Signal. 2013, 19, 998–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Yang, H. Exogenous polyamines alleviate the lipid peroxidation induced by cadmium chloride stress in Malus hupehensis Rehd. Sci. Hortic. 2008, 116, 442–447. [Google Scholar] [CrossRef]
- Hsu, Y.T.; Kao, C.H. Cadmium-induced oxidative damage in rice leaves is reduced by polyamines. Plant Soil 2007, 291, 27–37. [Google Scholar] [CrossRef]
- Rady, M.M.; Hemida, K.A. Modulation of cadmium toxicity and enhancing cadmium-tolerance in wheat seedlings by exogenous application of polyamines. Ecotoxicol. Environ. Saf. 2015, 119, 178–185. [Google Scholar] [CrossRef]
- Rady, M.M.; El-Yazal, M.A.S.; Taie, H.A.A.; Ahmed, S.M.A. Response of wheat growth and productivity to exogenous polyamines under lead stress. J. Crop Sci. Biotechnol. 2016, 19, 363–371. [Google Scholar] [CrossRef]
- Choudhary, S.P.; Kanwar, M.; Bhardwaj, R.; Yu, J.-Q.; Tran, L.-S.P. Chromium stress mitigation by polyamine-brassinosteroid application involves phytohormonal and physiological strategies in Raphanus sativus L. PLoS ONE 2012, 7, e33210. [Google Scholar] [CrossRef] [Green Version]
- Podlešáková, K.; Ugena, L.; Spíchal, L.; Doležal, K.; De Diego, N. Phytohormones and polyamines regulate plant stress responses by altering GABA pathway. N. Biotechnol. 2019, 48, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Groppa, M.D.; Tomaro, M.L.; Benavides, M.P. Polyamines and heavy metal stress: The antioxidant behavior of spermine in cadmium-and copper-treated wheat leaves. Biometals 2007, 20, 185–195. [Google Scholar] [CrossRef]
- Wang, X.; Shi, G.; Xu, Q.; Hu, J. Exogenous polyamines enhance copper tolerance of Nymphoides peltatum. J. Plant Physiol. 2007, 164, 1062–1070. [Google Scholar] [CrossRef]
- Tang, C.F.; Zhang, R.Q.; Wen, S.Z.; Li, C.F.; Guo, X.F.; Liu, Y.G. Effects of exogenous spermidine on subcellular distribution and chemical forms of cadmium in Typha latifolia L. under cadmium stress. Water Sci. Technol. 2009, 59, 1487–1493. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.P.; Oral, H.V.; Bhardwaj, R.; Yu, J.-Q.; Tran, L.-S.P. Interaction of brassinosteroids and polyamines enhances copper stress tolerance in Raphanus sativus. J. Exp. Bot. 2012, 63, 5659–5675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, X.; Liu, Y.; Huang, D.; Zeng, G.; Liu, S.; Tang, H.; Zhou, L.; Hu, X.; Zhou, Y.; Tan, X. Effects of exogenous calcium and spermidine on cadmium stress moderation and metal accumulation in Boehmeria nivea (L.) Gaudich. Environ. Sci. Pollut. Res. 2016, 23, 8699–8708. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Rahman, A.; Alam, M.; Mahmud, J.-A.; Suzuki, T.; Fujita, M. Polyamines confer salt tolerance in mung bean (Vigna radiata L.) by reducing sodium uptake, improving nutrient homeostasis, antioxidant defense, and methylglyoxal detoxification systems. Front. Plant Sci. 2016, 7, 1104. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, R.; Hu, X.; Song, J.; Li, B.; Ou, D.; Hu, X.; Zhao, Y. Exogenous spermidine elevating cadmium tolerance in Salix matsudana involves cadmium detoxification and antioxidant defense. Int. J. Phytoremediation 2019, 21, 305–315. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Aziz, U.; Alsahli, A.; Alyemeni, M.N.; Ahmad, P. Combined kinetin and spermidine treatments ameliorate growth and photosynthetic inhibition in vigna angularis by up-regulating antioxidant and nitrogen metabolism under cadmium stress. Biomolecules 2020, 10, 147. [Google Scholar] [CrossRef] [Green Version]
- Naz, R.; Sarfraz, A.; Anwar, Z.; Yasmin, H.; Nosheen, A.; Keyani, R.; Roberts, T.H. Combined ability of salicylic acid and spermidine to mitigate the individual and interactive effects of drought and chromium stress in maize (Zea mays L.). Plant Physiol. Biochem. 2021, 159, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Singh, H.P.; Khan, M.I.R.; Masood, A.; Per, T.S.; Negi, A.; Batish, D.R.; Khan, N.A.; Duarte, A.C.; Pereira, E. Too much is bad—An appraisal of phytotoxicity of elevated plant-beneficial heavy metal ions. Environ. Sci. Pollut. Res. 2015, 22, 3361–3382. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.-T.; Qi, Y.-P.; Jiang, H.-X.; Chen, L.-S. Roles of organic acid anion secretion in aluminium tolerance of higher plants. Biomed. Res. Int. 2013, 2013, 173682. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.F.; Zheng, C.; Hu, Y.T.; Jiang, T.A.O.; Liu, Y.U.; Dong, N.Y.; Yang, J.L.; Zheng, S.J. Cadmium-induced oxalate secretion from root apex is associated with cadmium exclusion and resistance in Lycopersicon esulentum. Plant Cell Environ. 2011, 34, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-Y.; Jung, J.-Y.; Song, W.-Y.; Suh, H.-S.; Lee, Y. Identification of rice varieties with high tolerance or sensitivity to lead and characterization of the mechanism of tolerance. Plant Physiol. 2000, 124, 1019–1026. [Google Scholar] [CrossRef] [Green Version]
- Nriagu, J.O. Zinc in the Environment. Part II: Health Effects; John Wiley Sons: New York, NY, USA, 1980; p. 10016. [Google Scholar]
- Tolrà, R.P.; Poschenrieder, C.; Barceló, J. Zinc hyperaccumulation in Thlaspi caerulescens. II. Influence on organic acids. J. Plant Nutr. 1996, 19, 1541–1550. [Google Scholar] [CrossRef]
- Sun, R.; Zhou, Q.; Jin, C. Cadmium accumulation in relation to organic acids in leaves of Solanum nigrum L. as a newly found cadmium hyperaccumulator. Plant Soil 2006, 285, 125–134. [Google Scholar] [CrossRef]
- Krämer, U.; Cotter-Howells, J.D.; Charnock, J.M.; Baker, A.J.M.; Smith, J.A.C. Free histidine as a metal chelator in plants that accumulate nickel. Nature 1996, 379, 635–638. [Google Scholar] [CrossRef]
- Salt, D.E.; Prince, R.C.; Baker, A.J.M.; Raskin, I.; Pickering, I.J. Zinc ligands in the metal hyperaccumulator Thlaspi caerulescens as determined using X-ray absorption spectroscopy. Environ. Sci. Technol. 1999, 33, 713–717. [Google Scholar] [CrossRef]
- Wycisk, K.; Kim, E.J.; Schroeder, J.I.; Krämer, U. Enhancing the first enzymatic step in the histidine biosynthesis pathway increases the free histidine pool and nickel tolerance in Arabidopsis thaliana. FEBS Lett. 2004, 578, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.-L.; Zhou, Q.-X.; Sun, F.-H.; Jin, C.-X. Antioxidative defense and proline/phytochelatin accumulation in a newly discovered Cd-hyperaccumulator, Solanum nigrum L. Environ. Exp. Bot. 2007, 60, 468–476. [Google Scholar] [CrossRef]
- Zoufan, P.; Jalali, R.; Hassibi, P.; Neisi, E.; Rastegarzadeh, S. Evaluation of antioxidant bioindicators and growth responses in Malva parviflora L. exposed to cadmium. Physiol. Mol. Biol. Plants 2018, 24, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Dinakar, N.; Nagajyothi, P.C.; Suresh, S.; Damodharam, T.; Suresh, C. Cadmium induced changes on proline, antioxidant enzymes, nitrate and nitrite reductases in Arachis hypogaea L. J. Environ. Biol. 2009, 30, 289–294. [Google Scholar]
- Nikolic, N.; Kojic, D.; Pilipovic, A.; Pajevic, S.; Krstic, B.; Borisev, M.; Orlovic, S. Responses of hybrid poplar to cadmium stress: Photosynthetic characteristics, cadmium and proline accumulation, and antioxidant enzyme activity. Acta Biol. Cracoviensia Ser. Bot. 2008, 50, 95–103. [Google Scholar]
- Yılmaz, D.D.; Parlak, K.U. Changes in proline accumulation and antioxidative enzyme activities in Groenlandia densa under cadmium stress. Ecol. Indic. 2011, 11, 417–423. [Google Scholar] [CrossRef]
- Mishra, S.; Dubey, R.S. Inhibition of ribonuclease and protease activities in arsenic exposed rice seedlings: Role of proline as enzyme protectant. J. Plant Physiol. 2006, 163, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Hoque, M.A.; Okuma, E.; Jannat, R.; Banu, M.N.A.; Jahan, M.S.; Nakamura, Y.; Murata, Y. Proline and glycinebetaine confer cadmium tolerance on tobacco bright yellow-2 cells by increasing ascorbate-glutathione cycle enzyme activities. Biosci. Biotechnol. Biochem. 2009, 909031637. [Google Scholar] [CrossRef] [Green Version]
- De Carvalho, K.; de Campos, M.K.F.; Domingues, D.S.; Pereira, L.F.P.; Vieira, L.G.E. The accumulation of endogenous proline induces changes in gene expression of several antioxidant enzymes in leaves of transgenic Swingle citrumelo. Mol. Biol. Rep. 2013, 40, 3269–3279. [Google Scholar] [CrossRef]
- Jabeen, N.; Abbas, Z.; Iqbal, M.; Rizwan, M.; Jabbar, A.; Farid, M.; Ali, S.; Ibrahim, M.; Abbas, F. Glycinebetaine mediates chromium tolerance in mung bean through lowering of Cr uptake and improved antioxidant system. Arch. Agron. Soil Sci. 2016, 62, 648–662. [Google Scholar] [CrossRef]
- Bhatti, K.H.; Anwar, S.; Nawaz, K.; Hussain, K.; Siddiqi, E.H.; Sharif, R.U.; Talat, A.; Khalid, A. Effect of exogenous application of glycinebetaine on wheat (Triticum aestivum L.) under heavy metal stress. Middle East J. Sci. Res. 2013, 14, 130–137. [Google Scholar]
- Stoyanova, S.; Geuns, J.; Hideg, E.; Van den Ende, W. The food additives inulin and stevioside counteract oxidative stress. Int. J. Food Sci. Nutr. 2011, 62, 207–214. [Google Scholar] [CrossRef]
- Rahoui, S.; Chaoui, A.; Ben, C.; Rickauer, M.; Gentzbittel, L.; El Ferjani, E. Effect of cadmium pollution on mobilization of embryo reserves in seedlings of six contrasted Medicago truncatula lines. Phytochemistry 2015, 111, 98–106. [Google Scholar] [CrossRef]
- Dhir, B.; Nasim, S.A.; Samantary, S.; Srivastava, S. Assessment of osmolyte accumulation in heavy metal exposed Salvinia natans. Int. J. Bot. 2012, 8, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Adrees, M.; Ali, S.; Iqbal, M.; Bharwana, S.A.; Siddiqi, Z.; Farid, M.; Ali, Q.; Saeed, R.; Rizwan, M. Mannitol alleviates chromium toxicity in wheat plants in relation to growth, yield, stimulation of anti-oxidative enzymes, oxidative stress and Cr uptake in sand and soil media. Ecotoxicol. Environ. Saf. 2015, 122, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Habiba, U.; Ali, S.; Rizwan, M.; Ibrahim, M.; Hussain, A.; Shahid, M.R.; Alamri, S.A.; Alyemeni, M.N.; Ahmad, P. Alleviative role of exogenously applied mannitol in maize cultivars differing in chromium stress tolerance. Environ. Sci. Pollut. Res. 2019, 26, 5111–5121. [Google Scholar] [CrossRef]
- Demecsová, L.; Zelinová, V.; Liptáková, Ľ.; Tamás, L. Mild cadmium stress induces auxin synthesis and accumulation, while severe cadmium stress causes its rapid depletion in barley root tip. Environ. Exp. Bot. 2020, 175, 104038. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, A.; Bhardwaj, R. Plant steroidal hormone epibrassinolide regulate–Heavy metal stress tolerance in Oryza sativa L. by modulating antioxidant defense expression. Environ. Exp. Bot. 2016, 122, 1–9. [Google Scholar] [CrossRef]
- Mazorra, L.M.; Nunez, M.; Hechavarria, M.; Coll, F.; Sánchez-Blanco, M.J. Influence of brassinosteroids on antioxidant enzymes activity in tomato under different temperatures. Biol. Plant. 2002, 45, 593–596. [Google Scholar] [CrossRef]
- Özdemir, F.; Bor, M.; Demiral, T.; Türkan, İ. Effects of 24-epibrassinolide on seed germination, seedling growth, lipid peroxidation, proline content and antioxidative system of rice (Oryza sativa L.) under salinity stress. Plant Growth Regul. 2004, 42, 203–211. [Google Scholar] [CrossRef]
- Bajguz, A.; Hayat, S. Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol. Biochem. 2009, 47, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, Z.; Si, J.; Di, C.; Han, J.; An, L. Brassinosteroids alleviate chilling-induced oxidative damage by enhancing antioxidant defense system in suspension cultured cells of Chorispora bungeana. Plant Growth Regul. 2009, 59, 207–214. [Google Scholar] [CrossRef]
- Alam, M.M.; Hayat, S.; Ali, B.; Ahmad, A. Effect of 28-homobrassinolide on nickel induced changes in Brassica juncea. Photosynthetica 2007, 45, 139. [Google Scholar] [CrossRef]
- Anuradha, S.; Rao, S.S.R. The effect of brassinosteroids on radish (Raphanus sativus L.) seedlings growing under cadmium stress. Plant Soil Environ. 2007, 53, 465. [Google Scholar] [CrossRef] [Green Version]
- Kagale, S.; Divi, U.K.; Krochko, J.E.; Keller, W.A.; Krishna, P. Brassinosteroid confers tolerance in Arabidopsis thaliana and Brassica napus to a range of abiotic stresses. Planta 2007, 225, 353–364. [Google Scholar] [CrossRef]
- Sharma, P.; Bhardwaj, R. Effects of 24-epibrassinolide on growth and metal uptake in Brassica juncea L. under copper metal stress. Acta Physiol. Plant. 2007, 29, 259–263. [Google Scholar] [CrossRef]
- Hu, Z.; Fu, Q.; Zheng, J.; Zhang, A.; Wang, H. Transcriptomic and metabolomic analyses reveal that melatonin promotes melon root development under copper stress by inhibiting jasmonic acid biosynthesis. Hortic. Res. 2020, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Chopra, P.; Chhillar, H.; Ahanger, M.A.; Hussain, S.J.; Maheshwari, C. Regulatory hubs and strategies for improving heavy metal tolerance in plants: Chemical messengers, omics and genetic engineering. Plant Physiol. Biochem. 2021, 164, 260–278. [Google Scholar] [CrossRef] [PubMed]

| Plant | Gene(s) | Metal(s) | Reported Phenotypes | References |
|---|---|---|---|---|
| Lemna turonifera | AtNHX1 | Cadmium | vacuolar sequestration of metabolites and improved tolerance | Yao et al., 2020 |
| Triticum aestivum L. | TaCATs | Arsenic | Stress tolerance | Tyagi et al., 2020 |
| Oryza sativa | CCoAOMT | Copper | Lignin production and enhanced tolerance | Su et al., 2020 |
| Oryza sativa | cadA and bmtA | Cadmium | Cd accumulation and Cd-nanoparticles (CdNPs) biosynthesis and improved tolerance by decreasing oxidative stress | Shi et al., 2020 |
| Hordeum vulagare | HvPAL, HvMDH andHvCSY | Copper and Cobalt | Accumulation of phenolics and amino acids and increased tolerance | Lwalaba et al., 2020 |
| Jatropha curcas | JcMT2a andJcPAL | Lead | Accumulation of antioxidants, e.g., flavonoids and phenolics and metal detoxification | Mohamed et al., 2020 |
| Tobacco | EhMT1 | Copper | Decreased hydrogen peroxide (H2O2) formation and increased tolerance | Xia et al., 2012 |
| Tobacco | TaMT3 | Cadmium | Increased superoxide dismutase (SOD) activity and conferred tolerance | Zhou et al., 2014 |
| Tobacco | OSMT1e-p | Copper and Zinc | ROS scavenging and enhanced tolerance | Kumar et al., 2012 |
| Arabidopsis thaliana | BjMT2 | Copper and Cadmium | Inhibits root elongation but increased tolerance | Zhigang et al., 2006 |
| Hibiscus cannabinus L. | WRKY, GRAS, MYB, bHLH, ZFP, ERF, and NAC | Cadmium | Enhanced tolerance via molecular mechanism | Chen et al., 2020 |
| Tobacco | NtCBP4 | Lead | Increased tolerance | Sunkar et al., 2000 |
| Arabidopsis thaliana | ACBP1 | Lead | Higher gene expression and enhanced tolerance | Xiao et al., 2008; Du et al., 2015 |
| Linum usitatissimum L. | LuACBP1 and LuACBP2 | Lead | Transcript level was higher in transgenic and improved tolerance | Pan et al., 2020 |
| Oryza sativa | OsSTAR1 and OsSTAR2 | Aluminium | Decreased aluminium level in cell wall and enhanced tolerance | Huang et al., 2020 |
| Fragaria vesca | FvABCC11 | Cadmium | Increased tolerance via ATP binding cassette (ABC) transporters | Shi et al., 2020 |
| Arabidopsis thaliana | AtABCC3 and AtABCC6 | Cadmium | Phytochelatin mediated tolerance during seedling development | Brunetti et al., 2015; Gaillard et al., 2008 |
| Oryza sativa | OsABCC1 | Arsenic | Increased tolerance via vacuolar sequestration | Song et al., 2014 |
| Arabidopsis thaliana | AtABCC1 and AtABCC2 | Cadmium and Mercury | Enhanced tolerance via vacuolar sequestration | Park et al., 2012 |
| Brassica napus | BnaABCC3 and BnaABCC4 | Cadmium | Enhanced stress tolerance | Zhang et al., 2018 |
| Triticum aestivum | TaABCC | Cadmium | Distinct molecular expression and increased tolerance | Bhati et al., 2015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riyazuddin, R.; Nisha, N.; Ejaz, B.; Khan, M.I.R.; Kumar, M.; Ramteke, P.W.; Gupta, R. A Comprehensive Review on the Heavy Metal Toxicity and Sequestration in Plants. Biomolecules 2022, 12, 43. https://doi.org/10.3390/biom12010043
Riyazuddin R, Nisha N, Ejaz B, Khan MIR, Kumar M, Ramteke PW, Gupta R. A Comprehensive Review on the Heavy Metal Toxicity and Sequestration in Plants. Biomolecules. 2022; 12(1):43. https://doi.org/10.3390/biom12010043
Chicago/Turabian StyleRiyazuddin, Riyazuddin, Nisha Nisha, Bushra Ejaz, M. Iqbal R. Khan, Manu Kumar, Pramod W. Ramteke, and Ravi Gupta. 2022. "A Comprehensive Review on the Heavy Metal Toxicity and Sequestration in Plants" Biomolecules 12, no. 1: 43. https://doi.org/10.3390/biom12010043
APA StyleRiyazuddin, R., Nisha, N., Ejaz, B., Khan, M. I. R., Kumar, M., Ramteke, P. W., & Gupta, R. (2022). A Comprehensive Review on the Heavy Metal Toxicity and Sequestration in Plants. Biomolecules, 12(1), 43. https://doi.org/10.3390/biom12010043