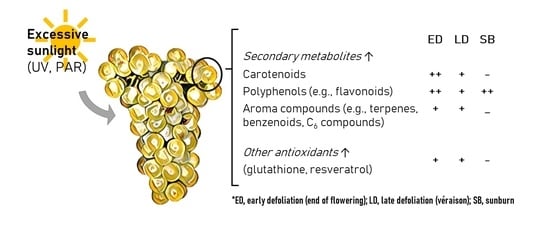
Secondary Metabolites Coordinately Protect Grapes from Excessive Light and Sunburn Damage during Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
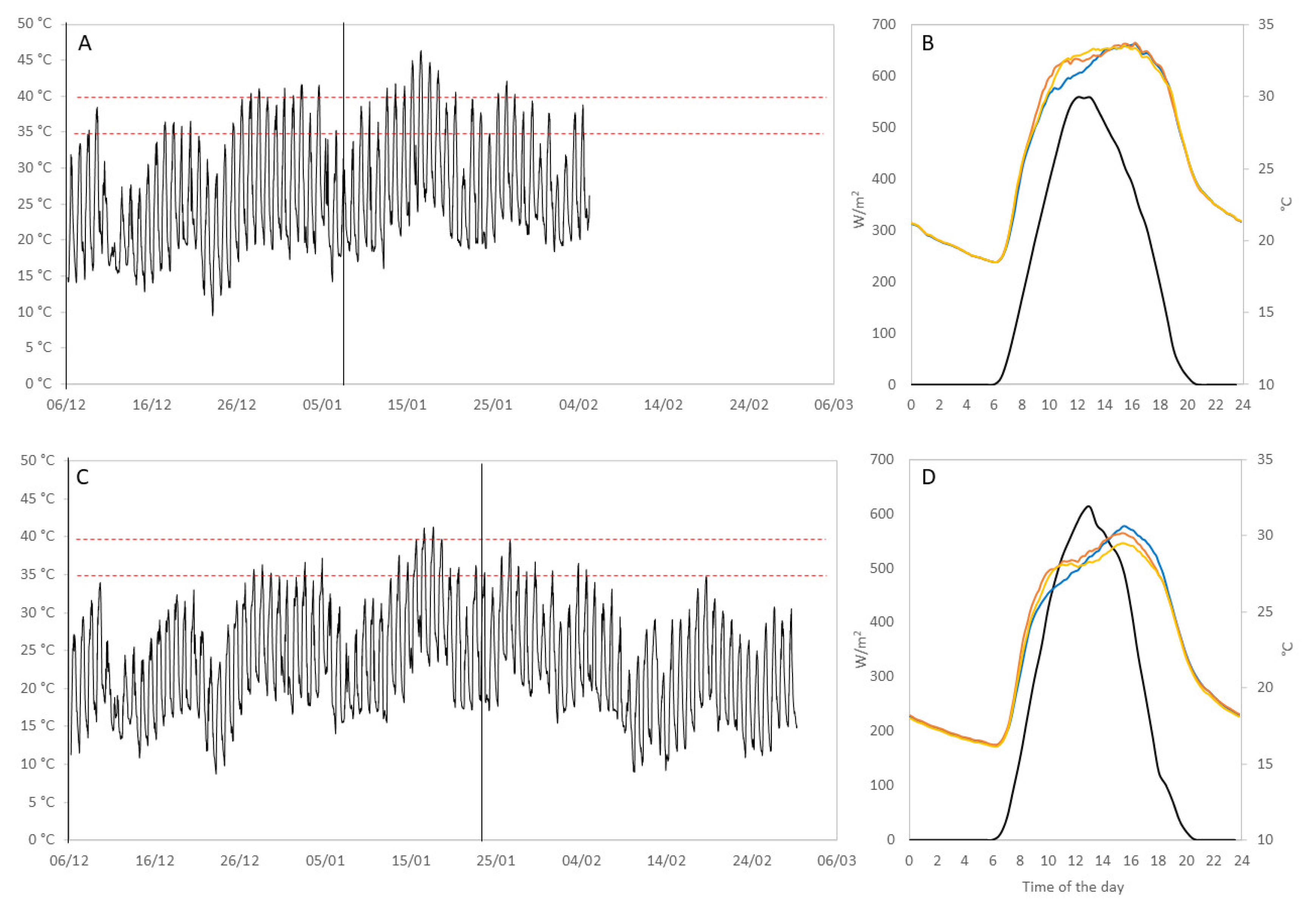
2.2. Climatic Monitoring and Vineyard Measurements
2.3. Sample Collection
2.4. Sunburn Damage Severity
2.5. Chemical Analyses
2.6. Statistical Analyses
3. Results
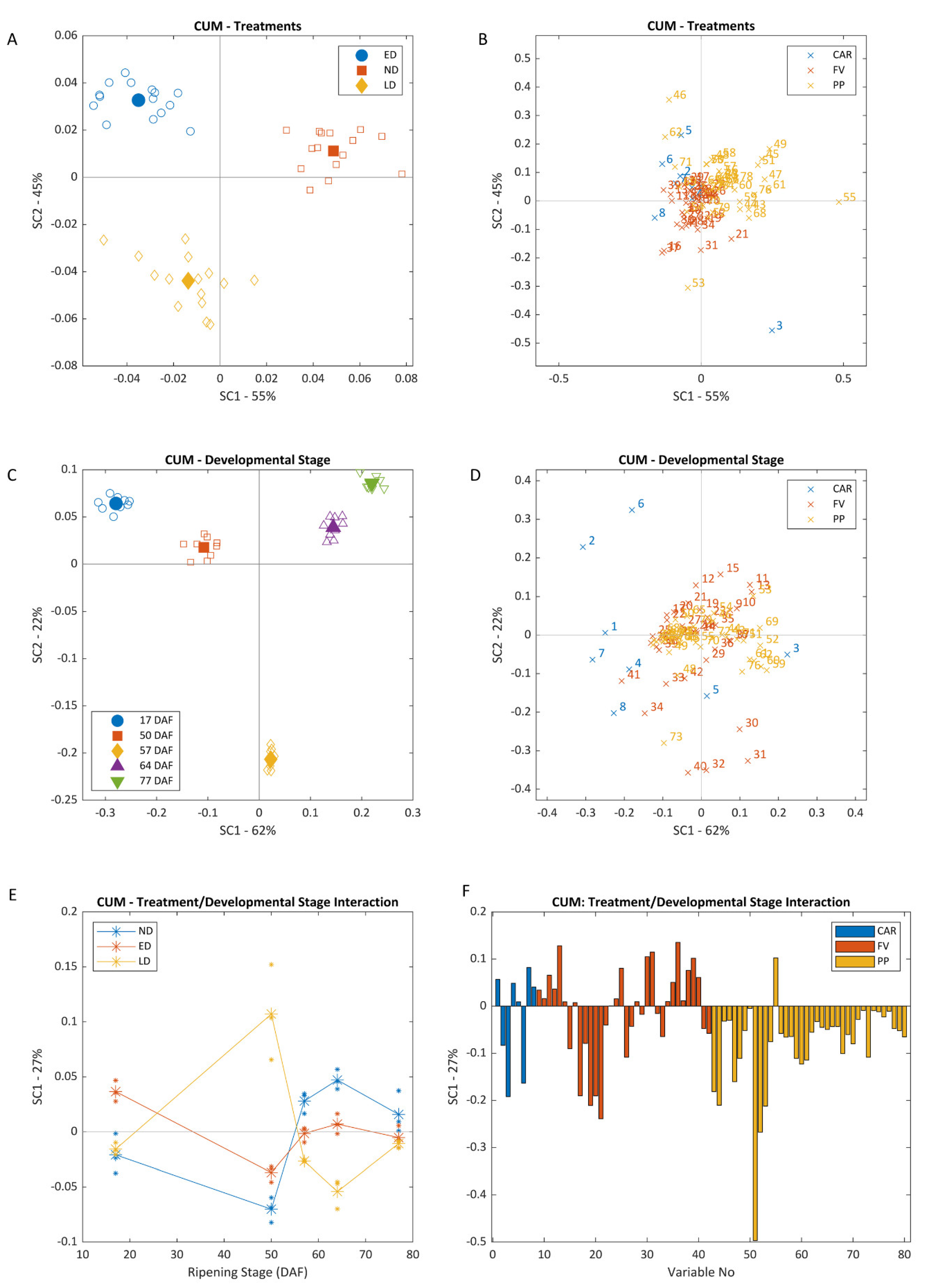
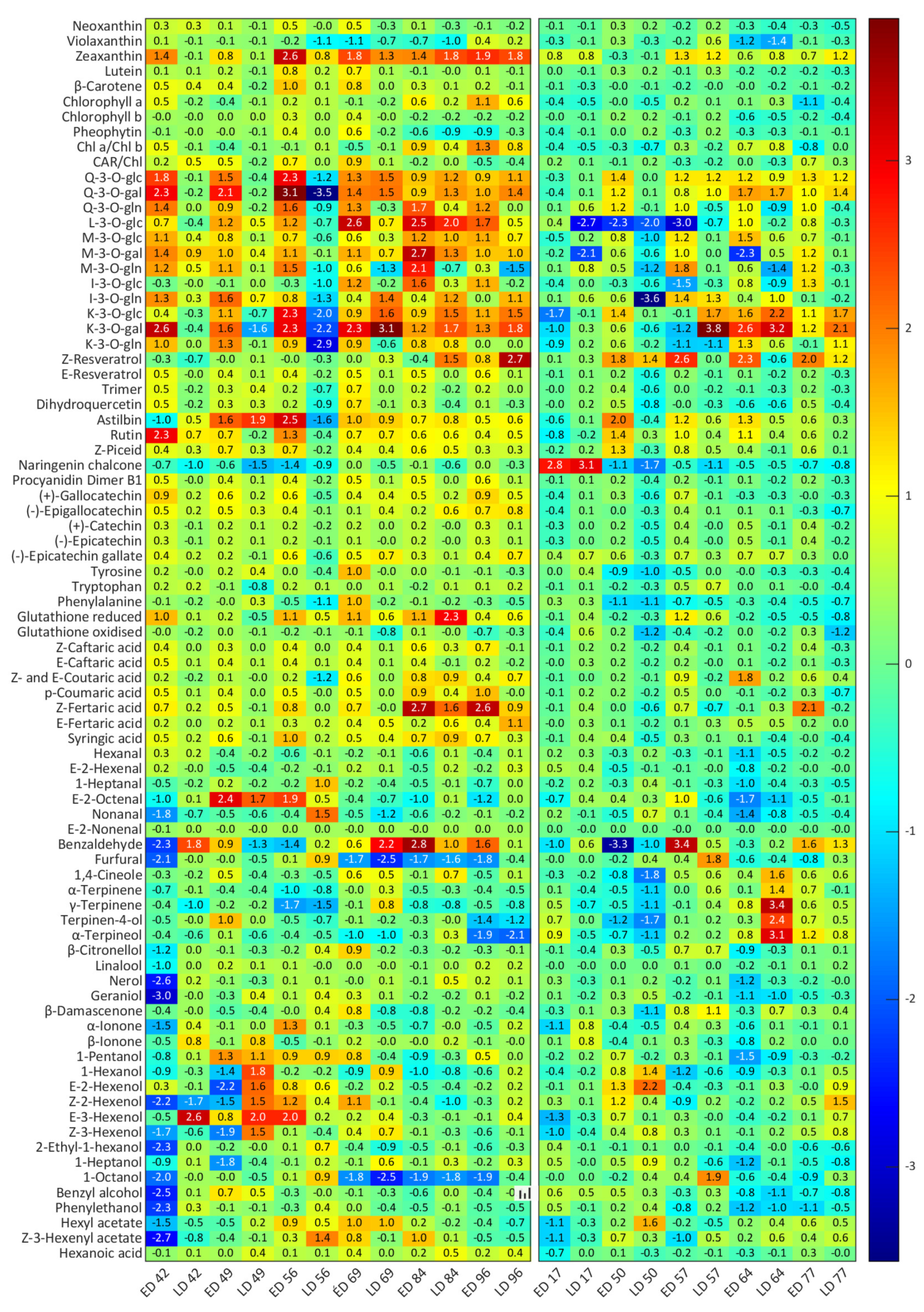
3.1. Effect of Different Light Exposure Timing on Composition
3.2. Effect of Extreme Temperatures on Secondary Metabolite Production
3.3. Level of Sunburn Damage
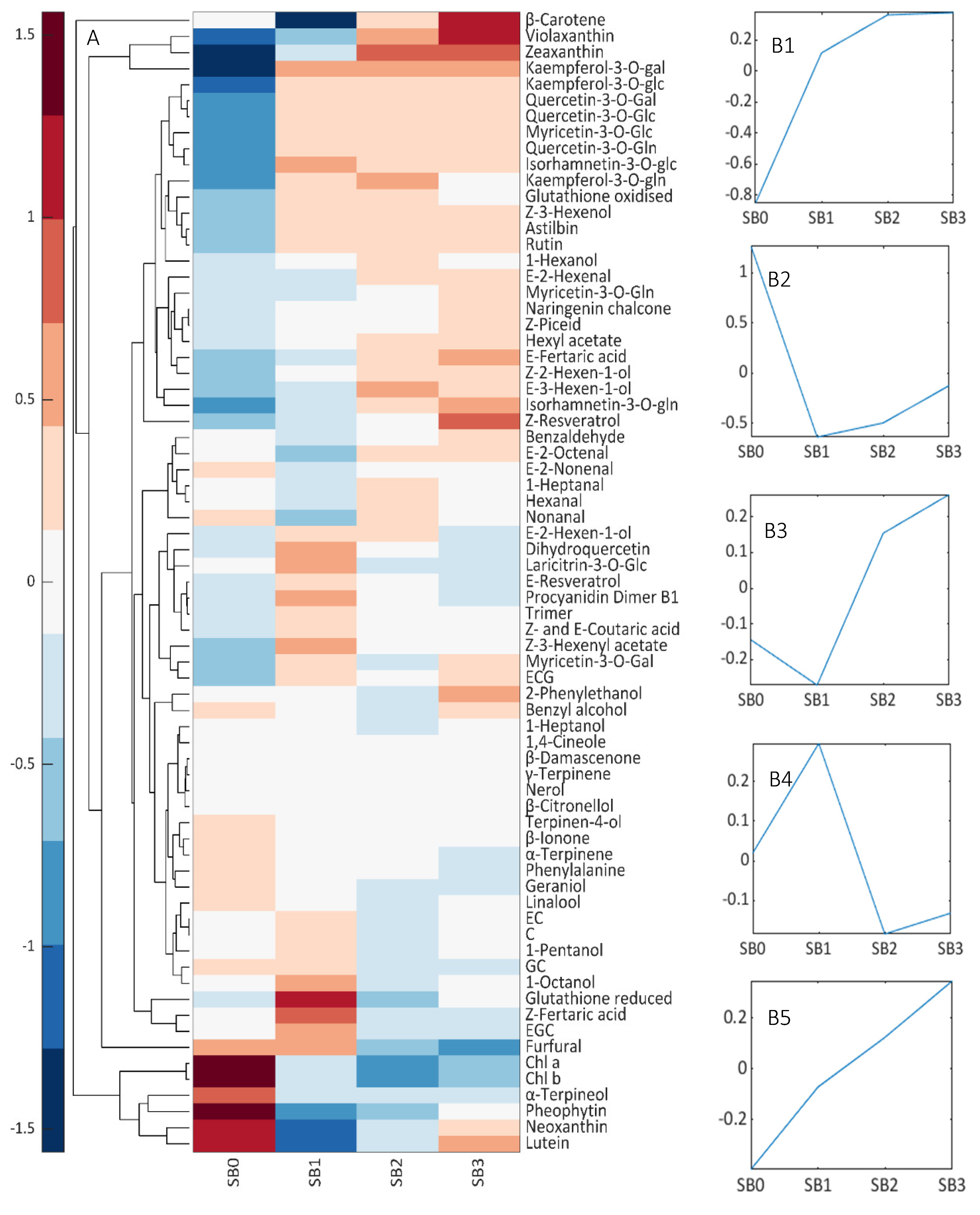
3.4. Composition of SB Levels
4. Discussion
4.1. Excessive Light Promotes Changes to Berry Composition
4.2. Heat Triggered FV Biosynthesis and the Degradation of CAR and PP Metabolites
4.3. Ripening Trumps All
4.4. Sunburn Damage
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zenoni, S.; Dal Santo, S.; Tornielli, G.B.; D’Incà, E.; Filippetti, I.; Pastore, C.; Allegro, G.; Silvestroni, O.; Lanari, V.; Pisciotta, A.; et al. Transcriptional Responses to Pre-Flowering Leaf Defoliation in Grapevine Berry from Different Growing Sites, Years, and Genotypes. Front. Plant Sci. 2017, 8, 630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gambetta, J.M.; Holzapfel, B.P.; Stoll, M.; Friedel, M. Sunburn in Grapes: A Review. Front. Plant Sci. 2021, 11, 604691. [Google Scholar] [CrossRef] [PubMed]
- Rustioni, L.; Milani, C.; Parisi, S.; Failla, O. Chlorophyll Role in Berry Sunburn Symptoms Studied in Different Grape (Vitis vinifera L.) Cultivars. Sci. Hortic. 2015, 185, 145–150. [Google Scholar] [CrossRef]
- Solovchenko, A.E. Photoprotection in Plants—Optical Screening Based Mechanisms; Springer Series in Biophysics; Springer: Moscow, Russia, 2010. [Google Scholar] [CrossRef] [Green Version]
- Du Plessis, K.; Young, P.R.; Eyéghé-Bickong, H.A.; Vivier, M.A. The Transcriptional Responses and Metabolic Consequences of Acclimation to Elevated Light Exposure in Grapevine Berries. Front. Plant Sci. 2017, 8, 1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munné-Bosch, S.; Vincent, C. Physiological Mechanisms Underlying Fruit Sunburn. Crit. Rev. Plant Sci. 2019, 38, 140–157. [Google Scholar] [CrossRef]
- Felicetti, D.A.; Schrader, L.E. Changes in Pigment Concentrations Associated with the Degree of Sunburn Browning of ‘Fuji’ Apple. J. Am. Soc. Hortic. Sci. 2008, 133, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Joubert, C.; Young, P.R.; Eyéghé-Bickong, H.A.; Vivier, M.A. Field-Grown Grapevine Berries Use Carotenoids and the Associated Xanthophyll Cycles to Acclimate to UV Exposure Differentially in High and Low Light (Shade) Conditions. Front. Plant Sci. 2016, 7, 786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolb, C.A.; Kopecký, J.; Riederer, M.; Pfündel, E.E. UV Screening by Phenolics in Berries of Grapevine (Vitis vinifera). Funct. Plant Biol. 2003, 30, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.; Xu, X.-Q.; Wang, Y.; Chen, W.-K.; Sun, R.-Z.; Cheng, G.; Liu, B.; Chen, W.; Duan, C.-Q.; Wang, J.; et al. Modulation of Volatile Compound Metabolome and Transcriptome in Grape Berries Exposed to Sunlight under Dry-Hot Climate. BMC Plant Biol. 2020, 20, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matus, J.T. Transcriptomic and Metabolomic Networks in the Grape Berry Illustrate That It Takes More than Flavonoids to Fight against Ultraviolet Radiation. Front. Plant Sci. 2016, 7, 1337. [Google Scholar] [CrossRef] [Green Version]
- Young, P.R.; Eyeghe-Bickong, H.A.; du Plessis, K.; Alexandersson, E.; Jacobson, D.A.; Coetzee, Z.; Deloire, A.; Vivier, M.A. Grapevine Plasticity in Response to an Altered Microclimate: Sauvignon Blanc Modulates Specific Metabolites in Response to Increased Berry Exposure. Plant Physiol. 2016, 170, 1235–1254. [Google Scholar] [CrossRef] [Green Version]
- Carbonell-Bejerano, P.; Diago, M.P.; Martínez-Abaigar, J.; Martínez-Zapater, J.M.; Tardáguila, J.; Núñez-Olivera, E. Solar Ultraviolet Radiation Is Necessary to Enhance Grapevine Fruit Ripening Transcriptional and Phenolic Responses. BMC Plant Biol. 2014, 14, 183. [Google Scholar] [CrossRef] [PubMed]
- Pastore, C.; Zenoni, S.; Fasoli, M.; Pezzotti, M.; Tornielli, G.B.; Filippetti, I. Selective Defoliation Affects Plant Growth, Fruit Transcriptional Ripening Program and Flavonoid Metabolism in Grapevine. BMC Plant Biol. 2013, 13, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz, P.; Munné-Bosch, S. Photo-Oxidative Stress during Leaf, Flower and Fruit Development. Plant Physiol. 2018, 176, 1004–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, P.; Li, X.P.; Niyogi, K.K. Non-Photochemical Quenching. A Response to Excess Light Energy. Plant Physiol. 2001, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hulands, S.; Greer, D.H.; Harper, J.D.I. The Interactive Effects of Temperature and Light Intensity on Vitis vinifera cv. “Semillon” Grapevines. II. Berry Ripening and Susceptibility to Sunburn at Harvest. Eur. J. Hortic. Sci. 2014, 79, 1–7. [Google Scholar]
- Rustioni, L.; Rocchi, L.; Guffanti, E.; Cola, G.; Failla, O. Characterization of Grape (Vitis vinifera L.) Berry Sunburn Symptoms by Reflectance. J. Agric. Food Chem. 2014, 62, 3043–3046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verdenal, T.; Zufferey, V.; Dienes-Nagy, A.; Belcher, S.; Lorenzini, F.; Rösti, J.; Koestel, C.; Gindro, K.; Spring, J.-L. Intensity and Timing of Defoliation on White Cultivar Chasselas under the Temperate Climate of Switzerland. Oeno One 2018, 52, 93–104. [Google Scholar] [CrossRef]
- Northcote, K.H. A Factual Key for the Recognition of Australian Soils, 4th ed.; Rellim Technical Publications: Glenside, SA, Australia, 1979; ISBN 9780959998900. [Google Scholar]
- Coombe, B.G. Growth Stages of the Grapevine: Adoption of a System for Identifying Grapevine Growth Stages. Aust. J. Grape Wine Res. 1995, 1, 104–110. [Google Scholar] [CrossRef]
- Gouot, J.C.; Smith, J.; Holzapfel, B.; Barril, C. Single and Cumulative Effects of Whole-Vine Heat Events on Shiraz Berry Composition. Oeno One 2019, 53, 171–187. [Google Scholar] [CrossRef]
- Gambetta, J.M.; Cozzolino, D.; Bastian, S.E.P.; Jeffery, D.W. Towards the Creation of a Wine Quality Prediction Index: Correlation of Chardonnay Juice and Wine Compositions from Different Regions and Quality Levels. Food Anal. Methods 2016, 9, 2842–2855. [Google Scholar] [CrossRef]
- Iland, P.; Bruer, N.; Edwards, G.; Weeks, S.; Wilkes, E. Chemical Analysis of Grapes and Wine: Techniques and Concepts; Patrick Iland Wine Promotions: Campbelltown, NSW, Australia, 2004; ISBN 9780958160513. [Google Scholar]
- Wehrens, R.; Carvalho, E.; Masuero, D.; de Juan, A.; Martens, S. High-Throughput Carotenoid Profiling Using Multivariate Curve Resolution. Anal. Bioanal. Chem. 2013, 405, 5075–5086. [Google Scholar] [CrossRef] [PubMed]
- Lashbrooke, J.G.; Young, P.R.; Strever, A.E.; Stander, C.; Vivier, M.A. The Development of a Method for the Extraction of Carotenoids and Chlorophylls from Grapevine Leaves and Berries for HPLC Profiling. Aust. J. Grape Wine Res. 2010, 16, 349–360. [Google Scholar] [CrossRef]
- Jansen, J.; Engel, J. ASCA: The implementation of design of experiments into multivariate modelling in chemometrics. In Comprehensive Analytical Chemistry; Jaumot, J., Bedia, C., Tauler, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 82, pp. 301–335. [Google Scholar]
- Hardie, W.J.; Aggenbach, S.J.; Jaudzems, V.G. The Plastids of the Grape Pericarp and Their Significance in Isoprenoid Synthesis. Aust. J. Grape Wine Res. 1996, 2, 144–154. [Google Scholar] [CrossRef]
- Brandt, M.; Scheidweiler, M.; Rauhut, D.; Patz, C.D.; Will, F.; Zorn, H.; Stoll, M. The Influence of Temperature and Solar Radiation on Phenols in Berry Skin and Maturity Parameters of Vitis vinifera L. cv. Riesling. Oeno One 2019, 53, 287–302. [Google Scholar] [CrossRef]
- Spayd, S.E.; Tarara, J.M.; Mee, D.L.; Ferguson, J.C. Separation of Sunlight and Temperature Effects on the Composition of Vitis Vinifera Cv. Merlot Berries. Am. J. Enol. Vitic. 2002, 53, 171–182. [Google Scholar]
- Martínez-Lüscher, J.; Brillante, L.; Kurtural, S.K. Flavonol Profile Is a Reliable Indicator to Assess Canopy Architecture and the Exposure of Red Wine Grapes to Solar Radiation. Front. Plant Sci. 2019, 10, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koyama, K.; Ikeda, H.; Poudel, P.R.; Goto-Yamamoto, N. Light Quality Affects Flavonoid Biosynthesis in Young Berries of Cabernet Sauvignon Grape. Phytochemistry 2012, 78, 54–64. [Google Scholar] [CrossRef]
- Reshef, N.; Walbaum, N.; Agam, N.; Fait, A. Sunlight Modulates Fruit Metabolic Profile and Shapes the Spatial Pattern of Compound Accumulation within the Grape Cluster. Front. Plant Sci. 2017, 8, 70. [Google Scholar] [CrossRef] [Green Version]
- Hixson, J.; Grebneva, Y.; Glameyer, N.; Vollmer, K.; Black, C.; Krstic, M.; Herderich, M. Shedding Light on the Modulation of Key Riesling Wine Aroma Compounds in a Changing Climate. In Proceedings of the XV Weurman Flavour Research Symposium, Graz, Austria, 18–22 September 2017; pp. 19–24. [Google Scholar]
- Kwasniewski, M.T.; Vanden Heuvel, J.E.; Pan, B.S.; Sacks, G.L. Timing of Cluster Light Environment Manipulation during Grape Development Affects C13 Norisoprenoid and Carotenoid Concentrations in Riesling. J. Agric. Food Chem. 2010, 58, 6841–6849. [Google Scholar] [CrossRef]
- Gambetta, J.M.; Bastian, S.E.P.; Cozzolino, D.; Jeffery, D.W. Factors Influencing the Aroma Composition of Chardonnay Wines. J. Agric. Food Chem. 2014, 62, 6512–6534. [Google Scholar] [CrossRef]
- Río Segade, S.; Vilanova, M.; Giacosa, S.; Perrone, I.; Chitarra, W.; Pollon, M.; Torchio, F.; Boccacci, P.; Gambino, G.; Gerbi, V.; et al. Ozone Improves the Aromatic Fingerprint of White Grapes. Sci. Rep. 2017, 7, 16301. [Google Scholar] [CrossRef] [Green Version]
- Jud, W.; Vanzo, E.; Li, Z.; Ghirardo, A.; Zimmer, I.; Sharkey, T.D.; Hansel, A.; Schnitzler, J.P. Effects of Heat and Drought Stress on Post-Illumination Bursts of Volatile Organic Compounds in Isoprene-Emitting and Non-Emitting Poplar. Plant Cell Environ. 2016, 39, 1204–1215. [Google Scholar] [CrossRef] [Green Version]
- Sweetman, C.; Sadras, V.O.; Hancock, R.D.; Soole, K.L.; Ford, C.M. Metabolic Effects of Elevated Temperature on Organic Acid Degradation in Ripening Vitis vinifera Fruit. J. Exp. Bot. 2014, 65, 5975–5988. [Google Scholar] [CrossRef]
- Mori, K.; Goto-Yamamoto, N.; Kitayama, M.; Hashizume, K. Loss of Anthocyanins in Red-Wine Grape under High Temperature. J. Exp. Bot. 2007, 58, 1935–1945. [Google Scholar] [CrossRef]
- Vickers, C.E.; Gershenzon, J.; Lerdau, M.T.; Loreto, F. A Unified Mechanism of Action for Volatile Isoprenoids in Plant Abiotic Stress. Nat. Chem. Biol. 2009, 5, 283–291. [Google Scholar] [CrossRef]
- Copolovici, L.; Kännaste, A.; Pazouki, L.; Niinemets, Ü. Emissions of Green Leaf Volatiles and Terpenoids from Solanum lycopersicum Are Quantitatively Related to the Severity of Cold and Heat Shock Treatments. J. Plant Physiol. 2012, 169, 664–672. [Google Scholar] [CrossRef]
- Pazouki, L.; Kanagendran, A.; Li, S.; Kännaste, A.; Memari, H.R.; Bichele, R.; Niinemets, Ü. Mono- and Sesquiterpene Release from Tomato (Solanum lycopersicum) Leaves upon Mild and Severe Heat Stress and through Recovery: From Gene Expression to Emission Responses. Environ. Exp. Bot. 2016, 132, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Turan, S.; Kask, K.; Kanagendran, A.; Li, S.; Anni, R.; Talts, E.; Rasulov, B.; Kännaste, A.; Niinemets, Ü. Lethal Heat Stress-Dependent Volatile Emissions from Tobacco Leaves: What Happens beyond the Thermal Edge? J. Exp. Bot 2019, 70, 5017–5030. [Google Scholar] [CrossRef]
- Rustioni, L.; Fracassetti, D.; Prinsi, B.; Geuna, F.; Ancelotti, A.; Fauda, V.; Tirelli, A.; Espen, L.; Failla, O. Oxidations in White Grape (Vitis vinifera L.) Skins: Comparison between Ripening Process and Photooxidative Sunburn Symptoms. Plant Physiol. Biochem. 2020, 150, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Deluc, L.G.; Grimplet, J.; Wheatley, M.D.; Tillett, R.L.; Quilici, D.R.; Osborne, C.; Schooley, D.A.; Schlauch, K.A.; Cushman, J.C.; Cramer, G.R. Transcriptomic and Metabolite Analyses of Cabernet Sauvignon Grape Berry Development. BMC Genom. 2007, 8, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diago, M.P.; Ayestarán, B.; Guadalupe, Z.; Poni, S.; Tardáguila, J. Impact of Prebloom and Fruit-Set Basal Leaf Removal on the Flavonol and Anthocyanin Composition of Tempranillo Grapes. Am. J. Enol. Vitic. 2012, 63, 367–376. [Google Scholar] [CrossRef]
- Bureau of Meteorology. Summer 2019 Sets New Benchmarks for Australian Temperatures. Available online: http://media.bom.gov.au/social/blog/2020/summer-2019-sets-new-benchmarks-for-australian-temperatures/?utm_source=edm&utm_medium=org&utm_campaign=sm-003-0187&utm_content=Nbr%C2%A0 (accessed on 8 February 2019).
- Delaunois, B.; Cordelier, S.; Conreux, A.; Clément, C.; Jeandet, P. Molecular Engineering of Resveratrol in Plants. Plant Biotechnol. J. 2009, 7, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Zhan, J.-C.; Yang, H.-R.; Huang, W.-D. Changes of Resveratrol and Antioxidant Enzymes during UV-Induced Plant Defense Response in Peanut Seedlings. J. Plant Physiol. 2010, 167, 95–102. [Google Scholar] [CrossRef] [PubMed]


| Véraison | Harvest | ||||||
|---|---|---|---|---|---|---|---|
| BAL | CUM | p | BAL | CUM | p | ||
| CAR 1 | Carotenoids | ||||||
| Neoxanthin | 0.68 ± 0.06 | 0.76 ± 0.18 | ns 2 | 0.29 ± 0.04 | 0.23 ± 0.06 | ns | |
| Violaxanthin | 0.60 ± 0.04 | 0.77 ± 0.11 | ns | 0.39 ± 0.08 | 0.30 ± 0.10 | ns | |
| Zeaxanthin | 0.50 ± 0.04 | 1.2 ± 0.2 | * | 0.47 ± 0.06 | 2.0 ± 0.6 | * | |
| Lutein | 8.6 ± 0.2 | 11 ± 2 | ns | 4.0 ± 0.7 | 4.0 ± 1.0 | ns | |
| β-Carotene | 2.6 ± 0.5 | 4.9 ± 1.2 | * | 2.1 ± 0.3 | 2.7 ± 0.5 | ns | |
| Chlorophylls | |||||||
| Chlorophyll a | 13 ± 0.4 | 19 ± 0.7 | ** | 4.4 ± 0.9 | 6.3 ± 2.6 | ns | |
| Chlorophyll b | 6.6 ± 0.7 | 7.2 ± 1.5 | ns | 2.9 ± 0.4 | 1.8 ± 0.5 | * | |
| PP | Flavonoids | ||||||
| Quercetin 3-O-glc (µg/g) 3 | 11 ± 2 | 31 ± 5 | * | 48 ± 8 | 41 ± 10 | ns | |
| Quercetin 3-O-gal (µg/g) | 1.0 ± 0.3 | 4.1 ± 0.6 | * | 8.1 ± 1.3 | 6.5 ± 1.4 | ns | |
| Quercetin 3-O-gln (µg/g) | 21 ± 5 | 32 ± 5 | * | 7.7 ± 1.4 | 10 ± 1 | ns | |
| Laricitrin 3-O-glc | 9.3 ± 0.8 | 21 ± 0.2 | *** | 2.8 ± 0.2 | 7.6 ± 1.7 | * | |
| Myricetin 3-O-glc | 78 ± 0.6 | 165 ± 35 | * | 22 ± 3 | 80 ± 14 | * | |
| Myricetin 3-O-gal | 7.5 ± 0.9 | 17 ± 4 | ** | 3.7 ± 0.3 | 6.3 ± 2.0 | ns | |
| Myricetin 3-O-gln | 48 ± 7 | 123 ± 27 | * | 24 ± 2 | 28 ± 4 | ns | |
| Isorhamnetin 3-O-glc | 229 ± 49 | 321 ± 41 | ns | 95 ± 13 | 112 ± 20 | ns | |
| Isorhamnetin 3-O-gln | 4.1 ± 0.4 | 13 ± 3 | * | 9.2 ± 2.1 | 17 ± 3 | * | |
| Kaempferol 3-O-glc (µg/g) | 0.20 ± 0.06 | 0.64 ± 0.07 | ** | 7.3 ± 1.5 | 6.5 ± 0.9 | ns | |
| Kaempferol 3-O-gal (µg/g) | 0.07 ± 0.02 | 0.19 ± 0.00 | ** | 1.6 ± 0.4 | 1.4 ± 0.3 | ns | |
| Kaempferol 3-O-gln (µg/g) | 0.18 ± 0.03 | 0.31 ± 0.03 | * | 0.29 ± 0.02 | 0.29 ± 0.05 | ns | |
| Astilbin (µg/g) | 0.13 ± 0.02 | 0.31 ± 0.05 | * | 1.3 ± 0.09 | 0.84 ± 0.11 | * | |
| Dihydroquercetin | 122 ± 9 | 42 ± 6 | *** | 38 ± 4 | 17 ± 3 | ** | |
| Rutin | 55 ± 3 | 74 ± 10 | * | 200 ± 8 | 149 ± 20 | * | |
| Procyanidin Dimer B1 (µg/g) | 2.0 ± 0.1 | 1.5 ± 0.1 | * | 0.77 ± 0.15 | 0.98 ± 0.11 | ns | |
| Flavan-3-ols | |||||||
| (+)-Gallocatechin (µg/g) | 10 ± 1 | 11 ± 2 | ns | 2.6 ± 0.3 | 4.6 ± 0.8 | * | |
| (−)-Epigallocatechin (µg/g) | 0.43 ± 0.08 | 0.49 ± 0.06 | ns | 0.26 ± 0.03 | 0.95 ± 0.11 | ** | |
| (+)-Catechin (µg/g) | 19 ± 2 | 12 ± 1 | * | 5.9 ± 1.0 | 4.2 ± 0.7 | ns | |
| (−)-Epicatechin (µg/g) | 15 ± 1 | 9.3 ± 1.2 | * | 4.7 ± 0.8 | 3.5 ± 0.5 | ns | |
| (−)-Epicatechin gallate | 24 ± 5 | 15 ± 2 | * | 10 ± 2 | 12 ± 1 | ns | |
| Stilbenoids and chalcones | |||||||
| Z-Resveratrol | 74 ± 6 | 63 ± 11 | ns | 140 ± 11 | 47 ± 3 | *** | |
| E-Resveratrol (µg/g) | 4.7 ± 0.3 | 3.6 ± 0.3 | * | 1.8 ± 0.3 | 2.3 ± 0.3 | ns | |
| Trimer | 140 ± 10 | 60 ± 7 | ** | 50 ± 7 | 39 ± 3 | ns | |
| Z-Piceid | 2.3 ± 0.09 | 2.2 ± 0.4 | ns | 5.4 ± 0.4 | 3.7 ± 0.5 | * | |
| Naringenin chalcone | 0.82 ± 0.05 | 0.73 ± 0.04 | ns | 2.5 ± 0.2 | 1.5 ± 0.3 | * | |
| Amino Acids | |||||||
| Tyrosine | 44 ± 7 | 71 ± 4 | * | 236 ± 41 | 446 ± 38 | * | |
| Tryptophan | 0.73 ± 0.03 | 0.82 ± 0.1 | ns | 0.48 ± 0.05 | 0.74 ± 0.11 | * | |
| Phenylalanine (µg/g) | 1.1 ± 0.1 | 1.2 ± 0.1 | ns | 3.0 ± 0.4 | 3.8 ± 0.3 | ns | |
| Glutathione (reduced) | 171 ± 40 | 723 ± 42 | *** | 595 ± 141 | 1641 ± 3 | *** | |
| Glutathione (oxidized) | 9.3 ± 0.8 | 2.6 ± 0.3 | *** | 3.6 ± 1.3 | 1.9 ± 0.2 | ns | |
| Hydroxycinnamic Acids | |||||||
| Z-Caftaric acid (µg/g) | 204 ± 13 | 202 ± 13 | ns | 48 ± 2 | 81 ± 8 | * | |
| E-Caftaric acid (µg/g) | 124 ± 12 | 119 ± 4 | ns | 40 ± 3 | 62 ± 3 | ** | |
| p-Coumaric acid | 2.1 ± 0.3 | 1.9 ± 0.4 | ns | 0.46 ± 0.11 | 1.1 ± 0.0 | * | |
| Z- and E-Coutaric acid (µg/g) | 757 ± 48 | 689 ± 62 | ns | 166 ± 23 | 336 ± 36 | * | |
| Z-Fertaric acid | 873 ± 63 | 765 ± 156 | ns | 15 ± 4 | 71 ± 13 | * | |
| E-Fertaric acid (µg/g) | 17 ± 2 | 16 ± 2 | ns | 7.1 ± 0.6 | 17 ± 2 | * | |
| Syringic acid | 230 ± 6 | 296 ± 10 | ** | 113 ± 8 | 220 ± 27 | * | |
| FV | Aldehydes | ||||||
| Hexanal (µg/g) | 6.3 ± 0.7 | 10 ± 1 | * | 34 ± 1 | 49 ± 2 | *** | |
| E-2-Hexenal (µg/g) | 1.8 ± 0.1 | 3.6 ± 0.5 | * | 11 ± 0 | 20 ± 1 | *** | |
| 1-Heptanal | 4.8 ± 0.7 | 6.4 ± 0.3 | * | 28 ± 3 | 52 ± 5 | * | |
| E-2-Octenal | 2.1 ± 0.2 | 3.1 ± 0.4 | * | 13 ± 1 | 16 ± 3 | ns | |
| Nonanal | 5.8 ± 0.4 | 5.6 ± 0.1 | ns | 40 ± 8 | 93 ± 13 | * | |
| E-2-Nonenal | 14 ± 0 | 14 ± 0 | * | 15 ± 0 | 14 ± 0 | ** | |
| Benzaldehyde | 0.79 ± 0.09 | 0.73 ± 0.1 | ns | 5.7 ± 0.2 | 3.4 ± 0.4 | ** | |
| Furfural | 45 ± 0.1 | 190 ± 58 | * | 128 ± 24 | 237 ± 7 | * | |
| Isoprenoids | |||||||
| 1,4-Cineole | 0.29 ± 0.06 | 0.90 ± 0.05 | ** | 0.048 ± 0.007 | 0.065 ± 0.005 | * | |
| α-Terpinene | 0.60 ± 0.19 | 0.80 ± 0.02 | ns | 0.17 ± 0.04 | 0.25 ± 0.06 | ns | |
| γ-Terpinene | 0.066 ± 0.008 | 0.082 ± 0.005 | * | 0.027 ± 0.002 | 0.026 ± 0.006 | ns | |
| Terpinen-4-ol | 0.38 ± 0.01 | 1.1 ± 0.1 | ** | 0.17 ± 0.03 | 0.12 ± 0.03 | ns | |
| α-Terpineol | 7.1 ± 0.9 | 14 ± 3 | * | 4.3 ± 0.9 | 2.1 ± 0.3 | ** | |
| β-Citronellol | 0.40 ± 0.05 | 0.31 ± 0.07 | ns | 0.045 ± 0.001 | 0.025 ± 0.001 | * | |
| Linalool | 4.2 ± 0.2 | 4.0 ± 0.1 | ns | 5.7 ± 0.0 | 5.1 ± 0.3 | * | |
| Nerol | 0.048 ± 0.007 | 0.033 ± 0.003 | * | 0.072 ± 0.003 | 0.043 ± 0.009 | * | |
| Geraniol | 2.3 ± 0.5 | 0.85 ± 0.03 | * | 0.88 ± 0.10 | 0.51 ± 0.00 | * | |
| C13-apocarotenoids | |||||||
| β-Damascenone | 0.52 ± 0.00 | 0.47 ± 0.08 | ns | 0.024 ± 0.003 | 0.016 ± 0.002 | * | |
| α-Ionone | 0.025 ± 0.001 | 0.044 ± 0.001 | * | 0.018 ± 0.001 | 0.021 ± 0.001 | * | |
| β-Ionone | 1.4 ± 0.3 | 1.2 ± 0.3 | ns | 0.41 ± 0.02 | 0.30 ±0.06 | * | |
| Alcohols | |||||||
| 1-Pentanol | 24 ± 1 | 36 ± 10 | ns | 31 ± 1 | 41 ± 7 | ns | |
| 1-Hexanol | 25 ± 5 | 11 ± 0.7 | * | 64 ± 13 | 54 ± 5 | ns | |
| E-2-Hexenol | 19 ± 2 | 7.4 ± 0.3 | ** | 38 ± 4 | 21 ± 1 | * | |
| Z-2-Hexenol | 0.35 ± 0.03 | 0.22 ± 0.02 | * | 0.36 ± 0.07 | 0.11 ± 0.01 | * | |
| E-3-Hexenol | 1.9 ± 0.4 | 1.4 ± 0.4 | ns | 1.2 ± 0.0 | 1.4 ± 0.3 | ns | |
| Z-3-Hexenol | 48 ± 10 | 18 ± 2 | * | 16 ± 1 | 3.2 ± 0.2 | *** | |
| 2-Ethyl-1-hexanol | 4.3 ± 0.6 | 3.6 ± 0.5 | ns | 6.9 ± 0.9 | 5.8 ± 0.5 | ns | |
| 1-Heptanol | 1.5 ± 0.1 | 1.3 ± 0.1 | * | 2.1 ± 0.4 | 3.3 ± 0.5 | * | |
| 1-Octanol | 1.6 ± 0.2 | 1.5 ± 0.2 | ns | 13 ± 0 | 7.3 ± 1.0 | ** | |
| Benzyl alcohol | 2.9 ± 0.2 | 1.6 ± 0.2 | * | 1.2 ± 0.1 | 1.1 ± 0.1 | ns | |
| Phenylethanol | 15 ± 1 | 8.9 ± 1.8 | * | 4.4 ± 0.0 | 3.1 ± 0.1 | *** | |
| Others | |||||||
| Hexyl acetate | 0.24 ± 0.04 | 0.23 ± 0.05 | ns | 0.42 ± 0.12 | 0.077 ± 0.015 | * | |
| Z-3-Hexenyl acetate | 8.1 ± 0.8 | 2.9 ± 0.5 | ** | 1.6 ± 0.1 | 0.26 ± 0.04 | *** | |
| Hexanoic acid | 93 ± 21 | 203 ± 8 | * | 72 ± 17 | 82 ±8 | ns | |
| Vineyard | Damage Level | ND 1 | ED | LD |
|---|---|---|---|---|
| BAL | SB1 2 | 10 ± 0 a 3 | 28 ± 15 c | 19 ± 8 b |
| SB2 | 2 ± 4 a | 9 ± 9 ab | 26 ± 10 c | |
| SN | 4 ± 6 | 3 ± 5 | 4 ± 3 | |
| CUM | SB1 | 3 ± 4 a | 8 ± 5 b | 8 ± 6 b |
| SB2 | 3 ± 4 | 11 ± 9 | 9 ± 9 | |
| SN | 4 ± 6 a | 8 ± 3 a | 22 ± 14 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gambetta, J.M.; Romat, V.; Schmidtke, L.M.; Holzapfel, B.P. Secondary Metabolites Coordinately Protect Grapes from Excessive Light and Sunburn Damage during Development. Biomolecules 2022, 12, 42. https://doi.org/10.3390/biom12010042
Gambetta JM, Romat V, Schmidtke LM, Holzapfel BP. Secondary Metabolites Coordinately Protect Grapes from Excessive Light and Sunburn Damage during Development. Biomolecules. 2022; 12(1):42. https://doi.org/10.3390/biom12010042
Chicago/Turabian StyleGambetta, Joanna M., Valentina Romat, Leigh M. Schmidtke, and Bruno P. Holzapfel. 2022. "Secondary Metabolites Coordinately Protect Grapes from Excessive Light and Sunburn Damage during Development" Biomolecules 12, no. 1: 42. https://doi.org/10.3390/biom12010042
APA StyleGambetta, J. M., Romat, V., Schmidtke, L. M., & Holzapfel, B. P. (2022). Secondary Metabolites Coordinately Protect Grapes from Excessive Light and Sunburn Damage during Development. Biomolecules, 12(1), 42. https://doi.org/10.3390/biom12010042