Recombinant Proteins-Based Strategies in Bone Tissue Engineering
Abstract
1. Introduction
2. Bone Lesions and Regenerative Proposals
3. Tissue Engineering
4. Recombinant Proteins in Bone Tissue Engineering
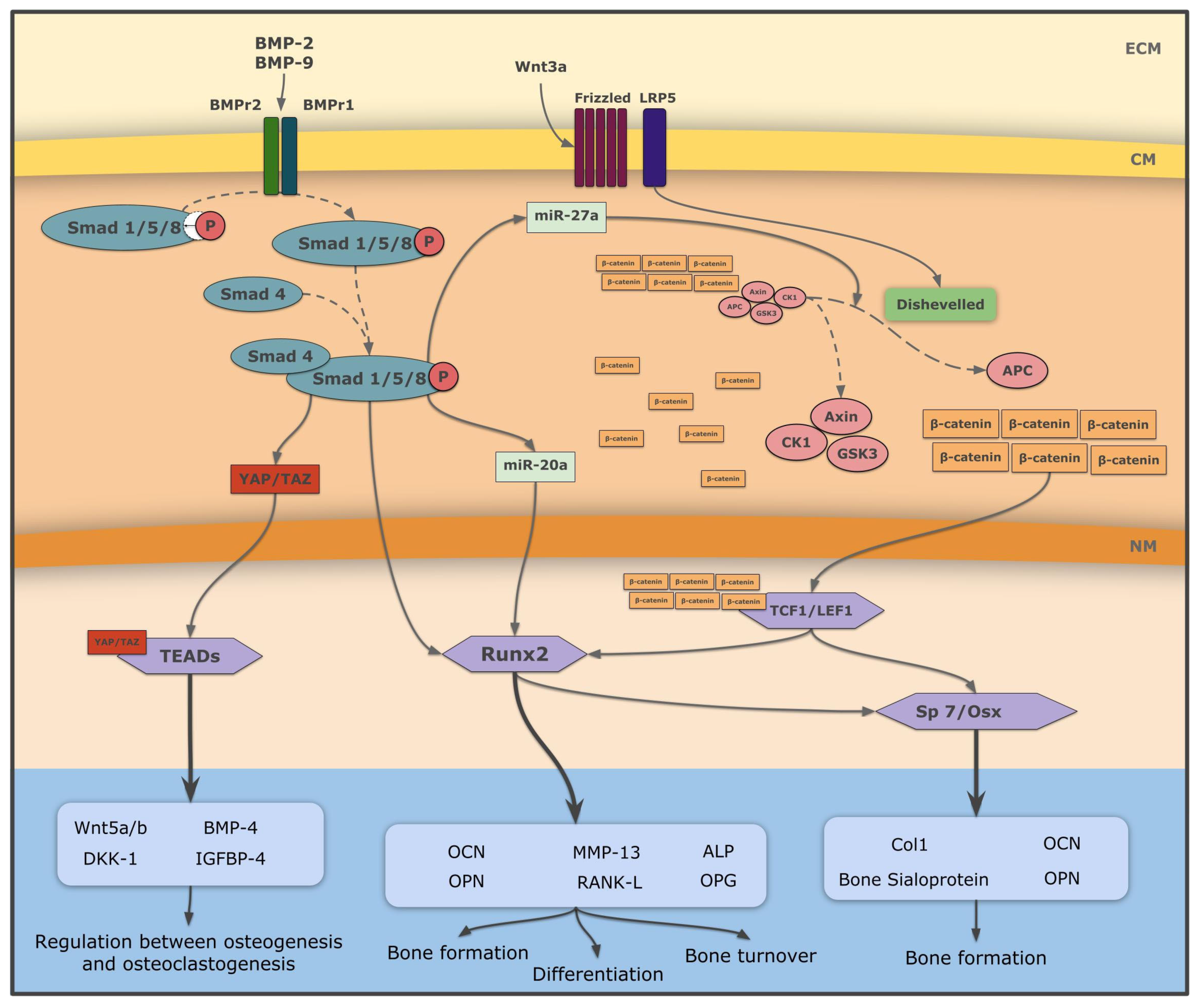
5. Recombinant Human BMPs in Bone Tissue Engineering
6. Collagen-Based Recombinants
7. Elastin-like Recombinamers (ELRs)
8. Recombinant Peptides Targeting Integrins
9. Recombinant Spider Silk
10. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tortora, G.; Derrickson, B. Principios de Anatomía y Fisiología, 11th ed.; Editorial Panamericana: Buenos Aires, Argentina, 2006. [Google Scholar]
- Cointry, G.; Capozza, R.; Feldman, S.; Reina, P. Los Huesos Son Estructuras Genéticas, Metabólicas, Biomecánicas, o Todo a La Vez? Actual. Osteol. 2009, 5, 185–195. [Google Scholar]
- Yelin, I.; Mortarino, P.; Capozza, R.; Cointry, G.; Feldman, S.; Reina, P.; Nocciolino, L.; Ferretti, J. Importancia del Entorno Mecánico en la Determinación Biológica de la Estructura Ósea. Actualización del Tema y Análisis Piloto de Datos de Individuos Sedentarios y Corredores. Actual. Osteol. 2012, 8, 86–100. [Google Scholar]
- Capozza, R.F.; Rittweger, J.; Reina, P.S.; Mortarino, P.; Nocciolino, L.M.; Feldman, S.; Ferretti, J.L.; Cointry, G.R. PQCT-Assessed Relationships between Diaphyseal Design and Cortical Bone Mass and Density in the Tibiae of Healthy Sedentary and Trained Men and Women. J. Musculoskelet Neuronal Interact. 2013, 13, 195–205. [Google Scholar]
- Kierszenbaum, A. Histologia e Biologia Celular: Uma Introdução à Patologia, 2nd ed.; Elsevier: Rio de Janeiro, Brazil, 2008. [Google Scholar]
- Sodek, K.L.; Tupy, J.H.; Sodek, J.; Grynpas, M.D. Relationships between Bone Protein and Mineral in Developing Porcine Long Bone and Calvaria. Bone 2000, 26, 189–198. [Google Scholar] [CrossRef]
- Bonewald, L.F. Osteocytes as Dynamic Multifunctional Cells. Ann. N. Y. Acad. Sci. 2007, 1116, 281–290. [Google Scholar] [CrossRef]
- Bonewald, L.F. Osteocytes: A Proposed Multifunctional Bone Cell. J. Musculoskelet Neuronal Interact. 2002, 2, 239–241. [Google Scholar]
- Bellido, T.; Saini, V.; Pajevic, P.D. Effects of PTH on Osteocyte Function. Bone 2013, 54, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Bellido, T. Osteocyte-Driven Bone Remodeling. Calcif. Tissue Int. 2014, 94, 25–34. [Google Scholar] [CrossRef]
- Plotkin, L.I. Apoptotic Osteocytes and the Control of Targeted Bone Resorption. Curr. Osteoporos. Rep. 2014, 12, 121–126. [Google Scholar] [CrossRef]
- Athanasiou, K.A.; Zhu, C.-F.; Lanctot, D.R.; Agrawal, C.M.; Wang, X. Fundamentals of Biomechanics in Tissue Engineering of Bone. Tissue Eng. 2000, 6, 361–381. [Google Scholar] [CrossRef]
- Romanos, G.; Froum, S.; Hery, C.; Cho, S.-C.; Tarnow, D. Survival Rate of Immediately vs Delayed Loaded Implants: Analysis of the Current Literature. J. Oral Implantol. 2010, 36, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Foss, N.B.; Kehlet, H. Mortality Analysis in Hip Fracture Patients: Implications for Design of Future Outcome Trials. Br. J. Anaesth. 2005, 94, 24–29. [Google Scholar] [CrossRef]
- Cointry, G.; Capozza, R.; Feldman, S.; Ferretti, J. Osteoporosis en iberoamérica. In Estructura y Biomecánica Ósea; Manual Moderno: Ciudad de México, Mexico, 2012. [Google Scholar]
- Compston, J. Osteoporosis: Social and Economic Impact. Radiol. Clin. N. Am. 2010, 48, 477–482. [Google Scholar] [CrossRef]
- Kremenetzky, A.; Kremenetzky, L.; Feldman, S. Aplicación de Aloinjerto óseo como cemento biológico. Rev. Asoc. Argent. Ortop. Y Traumatol. 2006, 71, 61–66. [Google Scholar]
- Chiapasco, M.; Colletti, G.; Coggiola, A.; Di Martino, G.; Anello, T.; Romeo, E. Clinical Outcome of the Use of Fresh Frozen Allogeneic Bone Grafts for the Reconstruction of Severely Resorbed Alveolar Ridges: Preliminary Results of a Prospective Study. Int. J. Oral Maxillofac. Implants 2015, 30, 450–460. [Google Scholar] [CrossRef][Green Version]
- Draenert, F.G.; Kämmerer, P.W.; Berthold, M.; Neff, A. Complications with Allogeneic, Cancellous Bone Blocks in Vertical Alveolar Ridge Augmentation: Prospective Clinical Case Study and Review of the Literature. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, e31–e43. [Google Scholar] [CrossRef]
- Wintermantel, E. Tissue Engineering Scaffolds Using Superstructures. Biomaterials 1996, 17, 83–91. [Google Scholar] [CrossRef]
- Stock, U.A.; Vacanti, J.P. Tissue Engineering: Current State and Prospects. Annu. Rev. Med. 2001, 52, 443–451. [Google Scholar] [CrossRef]
- Muschler, G.F.; Nakamoto, C.; Griffith, L.G. Engineering principles of clinical cell-based tissue engineering. J. Bone Jt. Surg. Am. Vol. 2004, 86, 1541–1558. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Kido, H.; Narita, T. Control of degradation rate of porous biodegradable polymers. In Proceedings of the 8th Polymer for Advanced Technologies International Symposium, Budapest, Hungary, 13–16 September 2005; pp. 13–16. [Google Scholar]
- Bar-Cohen, Y. Biomimetics—Using Nature to Inspire Human Innovation. Bioinspir. Biomim. 2006, 1, P1–P12. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Cai, Q.; Wang, B.; Deng, X.; Yang, X. Microfibrous β-TCP/Collagen Scaffolds Mimic Woven Bone in Structure and Composition. Biomed. Mater. 2010, 5, 065005. [Google Scholar] [CrossRef]
- Detsch, R.; Boccaccini, A.R. The Role of Osteoclasts in Bone Tissue Engineering: Role of Osteoclasts in Bone Tissue Engineering. J. Tissue Eng. Regen. Med. 2015, 9, 1133–1149. [Google Scholar] [CrossRef]
- Abarrategi, A.; Lópiz-Morales, Y.; Ramos, V.; Civantos, A.; López-Durán, L.; Marco, F.; López-Lacomba, J.L. Chitosan Scaffolds for Osteochondral Tissue Regeneration. J. Biomed. Mater. Res. 2010, 95A, 1132–1141. [Google Scholar] [CrossRef]
- Lyons, F.G.; Al-Munajjed, A.A.; Kieran, S.M.; Toner, M.E.; Murphy, C.M.; Duffy, G.P.; O’Brien, F.J. The Healing of Bony Defects by Cell-Free Collagen-Based Scaffolds Compared to Stem Cell-Seeded Tissue Engineered Constructs. Biomaterials 2010, 31, 9232–9243. [Google Scholar] [CrossRef]
- Lopez-Heredia, M.A.; Bongio, M.; Cuijpers, V.M.J.I.; van Dijk, N.W.M.; van den Beucken, J.J.J.P.; Wolke, J.G.C.; Jansen, J.A. Bone Formation Analysis: Effect of Quantification Procedures on the Study Outcome. Tissue Eng. Part C Methods 2012, 18, 369–373. [Google Scholar] [CrossRef]
- Thula, T.T.; Rodriguez, D.E.; Lee, M.H.; Pendi, L.; Podschun, J.; Gower, L.B. In Vitro Mineralization of Dense Collagen Substrates: A Biomimetic Approach toward the Development of Bone-Graft Materials. Acta Biomater. 2011, 7, 3158–3169. [Google Scholar] [CrossRef]
- Aho, A.J.; Tirri, T.; Kukkonen, J.; Strandberg, N.; Rich, J.; Seppälä, J.; Yli-Urpo, A. Injectable Bioactive Glass/Biodegradable Polymer Composite for Bone and Cartilage Reconstruction: Concept and Experimental Outcome with Thermoplastic Composites of Poly(ε-Caprolactone-Co-D,L-Lactide) and Bioactive Glass S53 P4. J. Mater. Sci. Mater. Med. 2004, 15, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Querido, W.; Abraçado, L.; Rossi, A.; Campos, A.; Rossi, A.; San Gil, R.; Borojevic, R.; Balduino, A.; Farina, M. Ultrastructural and Mineral Phase Characterization of the Bone-like Matrix Assembled in F-OST Osteoblast Cultures. Calcif. Tissue Int. 2011, 89, 358–371. [Google Scholar] [CrossRef]
- Yunoki, S.; Sugiura, H.; Ikoma, T.; Kondo, E.; Yasuda, K.; Tanaka, J. Effects of Increased Collagen-Matrix Density on the Mechanical Properties and in Vivo Absorbability of Hydroxyapatite–Collagen Composites as Artificial Bone Materials. Biomed. Mater. 2011, 6, 015012. [Google Scholar] [CrossRef]
- Lee, E.-J.; Jun, S.-H.; Kim, H.-E.; Koh, Y.-H. Collagen-Silica Xerogel Nanohybrid Membrane for Guided Bone Regeneration. J. Biomed. Mater. Res. 2012, 100A, 841–847. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, G.B.; Montesi, M.; Panseri, S.; Sprio, S.; Tampieri, A.; Sandri, M. Biomineralized Recombinant Collagen-Based Scaffold Mimicking Native Bone Enhances Mesenchymal Stem Cell Interaction and Differentiation. Tissue Eng. Part A 2017, 23, 1423–1435. [Google Scholar] [CrossRef] [PubMed]
- Werkmeister, J.A.; Ramshaw, J.A.M. Recombinant Protein Scaffolds for Tissue Engineering. Biomed. Mater. 2012, 7, 012002. [Google Scholar] [CrossRef]
- Gillman, C.E.; Jayasuriya, A.C. FDA-Approved Bone Grafts and Bone Graft Substitute Devices in Bone Regeneration. Mater. Sci. Eng. C 2021, 130, 112466. [Google Scholar] [CrossRef]
- Hu, K.; Olsen, B.R. The Roles of Vascular Endothelial Growth Factor in Bone Repair and Regeneration. Bone 2016, 91, 30–38. [Google Scholar] [CrossRef]
- Majidinia, M.; Sadeghpour, A.; Yousefi, B. The Roles of Signaling Pathways in Bone Repair and Regeneration. J. Cell Physiol. 2018, 233, 2937–2948. [Google Scholar] [CrossRef]
- Brooker, J.E.; Camison, L.B.; Bykowski, M.R.; Hurley, E.T.; Yerneni, S.S.; Campbell, P.G.; Weiss, L.E.; Mooney, M.P.; Cray, J.; Gilbert, J.R.; et al. Reconstruction of a Calvarial Wound Complicated by Infection: Comparing the Effects of Biopatterned Bone Morphogenetic Protein 2 and Vascular Endothelial Growth Factor. J. Craniofac. Surg. 2019, 30, 260–264. [Google Scholar] [CrossRef]
- Yang, C.; Hillas, P.J.; Báez, J.A.; Nokelainen, M.; Balan, J.; Tang, J.; Spiro, R.; Polarek, J.W. The Application of Recombinant Human Collagen in Tissue Engineering. BioDrugs 2004, 18, 103–119. [Google Scholar] [CrossRef]
- Mikos, A.G.; Herring, S.W.; Ochareon, P.; Elisseeff, J.; Lu, H.H.; Kandel, R.; Schoen, F.J.; Toner, M.; Mooney, D.; Atala, A.; et al. Engineering Complex Tissues. Tissue Eng. 2006, 12, 3307–3339. [Google Scholar] [CrossRef]
- Hou, L.-T.; Liu, C.-M.; Liu, B.-Y.; Chang, P.-C.; Chen, M.-H.; Ho, M.-H.; Jehng, S.-M.; Liu, H.-C. Tissue Engineering Bone Formation in Novel Recombinant Human Bone Morphogenic Protein 2–Atelocollagen Composite Scaffolds. J. Periodontol. 2007, 78, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.-C.; Liu, B.-Y.; Liu, C.-M.; Chou, H.-H.; Ho, M.-H.; Liu, H.-C.; Wang, D.-M.; Hou, L.-T. Bone Tissue Engineering with Novel RhBMP2-PLLA Composite Scaffolds. J. Biomed. Mater. Res. 2007, 81A, 771–780. [Google Scholar] [CrossRef]
- Zhou, J.; Guo, X.; Zheng, Q.; Wu, Y.; Cui, F.; Wu, B. Improving Osteogenesis of Three-Dimensional Porous Scaffold Based on Mineralized Recombinant Human-like Collagen via Mussel-Inspired Polydopamine and Effective Immobilization of BMP-2-Derived Peptide. Colloids Surf. B Biointerfaces 2017, 152, 124–132. [Google Scholar] [CrossRef]
- Fujioka-Kobayashi, M.; Sawada, K.; Kobayashi, E.; Schaller, B.; Zhang, Y.; Miron, R.J. Osteogenic Potential of RhBMP9 Combined with a Bovine-Derived Natural Bone Mineral Scaffold Compared to RhBMP2. Clin. Oral Impl. Res. 2017, 28, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Mottini, M.; Kobayashi, E.; Zhang, Y.; Schaller, B.; Miron, R.J. An in Vitro Study of Fibrin Sealant as a Carrier System for Recombinant Human Bone Morphogenetic Protein (RhBMP)–9 for Bone Tissue Engineering. J. Cranio-Maxillofac. Surg. 2017, 45, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Min, S.-H.; Kang, N.-E.; Song, S.-I.; Lee, J.-K. Regenerative Effect of Recombinant Human Bone Morphogenetic Protein-2/Absorbable Collagen Sponge (RhBMP-2/ACS) after Sequestrectomy of Medication-Related Osteonecrosis of the Jaw (MRONJ). JKAOMS 2020, 46, 191–196. [Google Scholar] [CrossRef]
- Andrews, S.; Cheng, A.; Stevens, H.; Logun, M.T.; Webb, R.; Jordan, E.; Xia, B.; Karumbaiah, L.; Guldberg, R.E.; Stice, S. Chondroitin Sulfate Glycosaminoglycan Scaffolds for Cell and Recombinant Protein-Based Bone Regeneration. Stem Cells Transl. Med. 2019, 8, 575–585. [Google Scholar] [CrossRef]
- Lienemann, P.S.; Vallmajo-Martin, Q.; Papageorgiou, P.; Blache, U.; Metzger, S.; Kiveliö, A.; Milleret, V.; Sala, A.; Hoehnel, S.; Roch, A.; et al. Smart Hydrogels for the Augmentation of Bone Regeneration by Endogenous Mesenchymal Progenitor Cell Recruitment. Adv. Sci. 2020, 7, 1903395. [Google Scholar] [CrossRef]
- Yang, L.; Hedhammar, M.; Blom, T.; Leifer, K.; Johansson, J.; Habibovic, P.; van Blitterswijk, C.A. Biomimetic Calcium Phosphate Coatings on Recombinant Spider Silk Fibres. Biomed. Mater. 2010, 5, 045002. [Google Scholar] [CrossRef]
- Pawelec, K.M.; Confalonieri, D.; Ehlicke, F.; van Boxtel, H.A.; Walles, H.; Kluijtmans, S.G.J.M. Osteogenesis and Mineralization of Mesenchymal Stem Cells in Collagen Type I-Based Recombinant Peptide Scaffolds: Stem Cells in Recombinant Peptide Scaffolds. J. Biomed. Mater. Res. 2017, 105, 1856–1866. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, F.Z.; Hu, K.; Zhu, X.D.; Fan, D.D. Bone Regeneration by Using Scaffold Based on Mineralized Recombinant Collagen. J. Biomed. Mater. Res. 2008, 86, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Suarez Muñoz, M.; Confalonieri, D.; Walles, H.; van Dongen, E.M.W.M.; Dandekar, G. Recombinant Collagen I Peptide Microcarriers for Cell Expansion and Their Potential Use As Cell Delivery System in a Bioreactor Model. JoVE 2018, 132, e57363. [Google Scholar] [CrossRef]
- Pal, P.; Nguyen, Q.C.; Benton, A.H.; Marquart, M.E.; Janorkar, A.V. Drug-Loaded Elastin-Like Polypeptide–Collagen Hydrogels with High Modulus for Bone Tissue Engineering. Macromol. Biosci. 2019, 19, 1900142. [Google Scholar] [CrossRef] [PubMed]
- Tytgat, L.; Dobos, A.; Markovic, M.; Van Damme, L.; Van Hoorick, J.; Bray, F.; Thienpont, H.; Ottevaere, H.; Dubruel, P.; Ovsianikov, A.; et al. High-Resolution 3D Bioprinting of Photo-Cross-Linkable Recombinant Collagen to Serve Tissue Engineering Applications. Biomacromolecules 2020, 21, 3997–4007. [Google Scholar] [CrossRef]
- Urry, D.; Parker, T.; Reid, M.; Gowda, D. Biocompatibility of the Bioelastic Materials, Poly(GVGVP) and Its γ-Irradiation Cross-Linked Matrix: Summary of Generic Biological Test Results. J. Bioact. Compat. Polym. 1991, 6, 263–282. [Google Scholar] [CrossRef]
- Rodríguez-Cabello, J.; Reguera, J.; Prieto, S.; Alonso, M. Chapter 6: Protein-based smart polymers. In Smart Polymers: Applications in Biotechnology and Biomedicine; CRC Press: Boca Raton, FL, USA, 2007; pp. 177–209. ISBN 978-0-8493-9161-3. [Google Scholar]
- Rodriguez-Cabello, J.; Ribeiro, A.; Reguera, J.; Girotti, A.; Testera, A. Elastin-like systems for tissue engineering. In Natural-Based Polymers for Biomedical Applications; CRC Press: Boca Raton, FL, USA, 2008; ISBN 978-1-84569-264-3. [Google Scholar]
- Sallach, R.E.; Wei, M.; Biswas, N.; Conticello, V.P.; Lecommandoux, S.; Dluhy, R.A.; Chaikof, E.L. Micelle Density Regulated by a Reversible Switch of Protein Secondary Structure. J. Am. Chem. Soc. 2006, 128, 12014–12019. [Google Scholar] [CrossRef]
- Dreher, M.R.; Simnick, A.J.; Fischer, K.; Smith, R.J.; Patel, A.; Schmidt, M.; Chilkoti, A. Temperature Triggered Self-Assembly of Polypeptides into Multivalent Spherical Micelles. J. Am. Chem. Soc. 2008, 130, 687–694. [Google Scholar] [CrossRef]
- Garcia, Y.; Hemantkumar, N.; Collighan, R.; Griffin, M.; Rodriguez-Cabello, J.C.; Pandit, A. In Vitro Characterization of a Collagen Scaffold Enzymatically Cross-Linked with a Tailored Elastin-like Polymer. Tissue Eng. Part A 2009, 15, 887–899. [Google Scholar] [CrossRef]
- Pinedo-Martín, G.; Castro, E.; Martín, L.; Alonso, M.; Rodríguez-Cabello, J.C. Effect of Surfactants on the Self-Assembly of a Model Elastin-like Block Corecombinamer: From Micelles to an Aqueous Two-Phase System. Langmuir 2014, 30, 3432–3440. [Google Scholar] [CrossRef]
- Martín, L.; Alonso, M.; Möller, M.; Rodríguez-Cabello, J.C.; Mela, P. 3D Microstructuring of Smart Bioactive Hydrogels Based on Recombinant Elastin-like Polymers. Soft. Matter. 2009, 5, 1591. [Google Scholar] [CrossRef]
- González de Torre, I.; Santos, M.; Quintanilla, L.; Testera, A.; Alonso, M.; Rodríguez Cabello, J.C. Elastin-like Recombinamer Catalyst-Free Click Gels: Characterization of Poroelastic and Intrinsic Viscoelastic Properties. Acta Biomater. 2014, 10, 2495–2505. [Google Scholar] [CrossRef]
- Coletta, D.J.; Ibáñez-Fonseca, A.; Missana, L.R.; Jammal, M.V.; Vitelli, E.J.; Aimone, M.; Zabalza, F.; Issa, J.P.M.; Alonso, M.; Rodríguez-Cabello, J.C.; et al. Bone Regeneration Mediated by a Bioactive and Biodegradable Extracellular Matrix-Like Hydrogel Based on Elastin-Like Recombinamers. Tissue Eng. Part A 2017, 23, 1361–1371. [Google Scholar] [CrossRef]
- Rodríguez-Cabello, J.C.; Girotti, A.; Ribeiro, A.; Arias, F.J. Synthesis of Genetically Engineered Protein Polymers (Recombinamers) as an Example of Advanced Self-Assembled Smart Materials. Methods Mol. Biol. 2012, 811, 17–38. [Google Scholar] [CrossRef]
- Hynes, R.O. Integrins: Bidirectional, Allosteric Signaling Machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Tatjana, F.; Gonzalez de Torre, I.; Quintanilla, L.; Rodriguez-Cabello, J.C. Spatial Control and Cell Adhesion Selectivity on Model Gold Surfaces Grafted with Elastin-like Recombinamers. Eur. Polym. J. 2018, 106, 19–29. [Google Scholar] [CrossRef]
- Ibáñez-Fonseca, A.; Ramos, T.L.; González de Torre, I.; Sánchez-Abarca, L.I.; Muntión, S.; Arias, F.J.; Del Cañizo, M.C.; Alonso, M.; Sánchez-Guijo, F.; Rodríguez-Cabello, J.C. Biocompatibility of Two Model Elastin-like Recombinamer-Based Hydrogels Formed through Physical or Chemical Cross-Linking for Various Applications in Tissue Engineering and Regenerative Medicine. J. Tissue Eng. Regen. Med. 2018, 12, e1450–e1460. [Google Scholar] [CrossRef]
- Flora, T.; de Torre, I.G.; Alonso, M.; Rodríguez-Cabello, J.C. Tethering QK Peptide to Enhance Angiogenesis in Elastin-like Recombinamer (ELR) Hydrogels. J. Mater. Sci. Mater. Med. 2019, 30, 30. [Google Scholar] [CrossRef]
- Contessotto, P.; Orbanić, D.; Da Costa, M.; Jin, C.; Owens, P.; Chantepie, S.; Chinello, C.; Newell, J.; Magni, F.; Papy-Garcia, D.; et al. Elastin-like Recombinamers-Based Hydrogel Modulates Post-Ischemic Remodeling in a Non-Transmural Myocardial Infarction in Sheep. Sci. Transl. Med. 2021, 13, eaaz5380. [Google Scholar] [CrossRef]
- Ibáñez-Fonseca, A.; Santiago Maniega, S.; Gorbenko Del Blanco, D.; Catalán Bernardos, B.; Vega Castrillo, A.; Álvarez Barcia, Á.J.; Alonso, M.; Aguado, H.J.; Rodríguez-Cabello, J.C. Elastin-Like Recombinamer Hydrogels for Improved Skeletal Muscle Healing Through Modulation of Macrophage Polarization. Front. Bioeng. Biotechnol. 2020, 8, 413. [Google Scholar] [CrossRef]
- Flora, T.; González de Torre, I.; Alonso, M.; Rodríguez-Cabello, J.C. Use of Proteolytic Sequences with Different Cleavage Kinetics as a Way to Generate Hydrogels with Preprogrammed Cell-Infiltration Patterns Imparted over Their given 3D Spatial Structure. Biofabrication 2019, 11, 035008. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez de Torre, I.; Alonso, M.; Rodriguez-Cabello, J.-C. Elastin-Based Materials: Promising Candidates for Cardiac Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 657. [Google Scholar] [CrossRef]
- de Torre, I.G.; Wolf, F.; Santos, M.; Rongen, L.; Alonso, M.; Jockenhoevel, S.; Rodríguez-Cabello, J.C.; Mela, P. Elastin-like Recombinamer-Covered Stents: Towards a Fully Biocompatible and Non-Thrombogenic Device for Cardiovascular Diseases. Acta Biomater. 2015, 12, 146–155. [Google Scholar] [CrossRef]
- Bessa, P.C.; Machado, R.; Nürnberger, S.; Dopler, D.; Banerjee, A.; Cunha, A.M.; Rodríguez-Cabello, J.C.; Redl, H.; van Griensven, M.; Reis, R.L.; et al. Thermoresponsive Self-Assembled Elastin-Based Nanoparticles for Delivery of BMPs. J. Control. Release 2010, 142, 312–318. [Google Scholar] [CrossRef]
- Cipriani, F.; Ariño Palao, B.; Gonzalez de Torre, I.; Vega Castrillo, A.; Aguado Hernández, H.J.; Alonso Rodrigo, M.; Àlvarez Barcia, A.J.; Sanchez, A.; García Diaz, V.; Lopez Peña, M.; et al. An Elastin-like Recombinamer-Based Bioactive Hydrogel Embedded with Mesenchymal Stromal Cells as an Injectable Scaffold for Osteochondral Repair. Regen. Biomater. 2019, 6, 335–347. [Google Scholar] [CrossRef]
- Pescador, D.; Ibáñez-Fonseca, A.; Sánchez-Guijo, F.; Briñón, J.G.; Arias, F.J.; Muntión, S.; Hernández, C.; Girotti, A.; Alonso, M.; Del Cañizo, M.C.; et al. Regeneration of Hyaline Cartilage Promoted by Xenogeneic Mesenchymal Stromal Cells Embedded within Elastin-like Recombinamer-Based Bioactive Hydrogels. J. Mater. Sci. Mater. Med. 2017, 28, 115. [Google Scholar] [CrossRef]
- Hughes, D.E.; Salter, D.M.; Dedhar, S.; Simpson, R. Integrin Expression in Human Bone. J. Bone Miner. Res. 2009, 8, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Shekaran, A.; García, J.R.; Clark, A.Y.; Kavanaugh, T.E.; Lin, A.S.; Guldberg, R.E.; García, A.J. Bone Regeneration Using an Alpha 2 Beta 1 Integrin-Specific Hydrogel as a BMP-2 Delivery Vehicle. Biomaterials 2014, 35, 5453–5461. [Google Scholar] [CrossRef]
- Martín-Moldes, Z.; Ebrahimi, D.; Plowright, R.; Dinjaski, N.; Perry, C.C.; Buehler, M.J.; Kaplan, D.L. Intracellular Pathways Involved in Bone Regeneration Triggered by Recombinant Silk–Silica Chimeras. Adv. Funct. Mater. 2018, 28, 1702570. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.Y.; Martin, K.E.; García, J.R.; Johnson, C.T.; Theriault, H.S.; Han, W.M.; Zhou, D.W.; Botchwey, E.A.; García, A.J. Integrin-Specific Hydrogels Modulate Transplanted Human Bone Marrow-Derived Mesenchymal Stem Cell Survival, Engraftment, and Reparative Activities. Nat. Commun. 2020, 11, 114. [Google Scholar] [CrossRef]
- Xin, S.; Gregory, C.A.; Alge, D.L. Interplay between Degradability and Integrin Signaling on Mesenchymal Stem Cell Function within Poly(Ethylene Glycol) Based Microporous Annealed Particle Hydrogels. Acta Biomater. 2020, 101, 227–236. [Google Scholar] [CrossRef]
- Ye, K.; Wang, X.; Cao, L.; Li, S.; Li, Z.; Yu, L.; Ding, J. Matrix Stiffness and Nanoscale Spatial Organization of Cell-Adhesive Ligands Direct Stem Cell Fate. Nano Lett. 2015, 15, 4720–4729. [Google Scholar] [CrossRef]
- Benitez, P.L.; Mascharak, S.; Proctor, A.C.; Heilshorn, S.C. Use of Protein-Engineered Fabrics to Identify Design Rules for Integrin Ligand Clustering in Biomaterials. Integr. Biol. 2016, 8, 50–61. [Google Scholar] [CrossRef]
- Rammensee, S.; Huemmerich, D.; Hermanson, K.D.; Scheibel, T.; Bausch, A.R. Rheological Characterization of Hydrogels Formed by Recombinantly Produced Spider Silk. Appl. Phys. A 2006, 82, 261–264. [Google Scholar] [CrossRef]
- Yan, H.; Saiani, A.; Gough, J.E.; Miller, A.F. Thermoreversible Protein Hydrogel as Cell Scaffold. Biomacromolecules 2006, 7, 2776–2782. [Google Scholar] [CrossRef] [PubMed]
- Humenik, M.; Magdeburg, M.; Scheibel, T. Influence of Repeat Numbers on Self-Assembly Rates of Repetitive Recombinant Spider Silk Proteins. J. Struct. Biol. 2014, 186, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Bini, E.; Foo, C.W.P.; Huang, J.; Karageorgiou, V.; Kitchel, B.; Kaplan, D.L. RGD-Functionalized Bioengineered Spider Dragline Silk Biomaterial. Biomacromolecules 2006, 7, 3139–3145. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.; Numata, K.; Leonor, I.B.; Mano, J.F.; Reis, R.L.; Kaplan, D.L. AFM Study of Morphology and Mechanical Properties of a Chimeric Spider Silk and Bone Sialoprotein Protein for Bone Regeneration. Biomacromolecules 2011, 12, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Torres-Rendon, J.; Leal-Egaña, A.; Walther, A.; Schlaad, H.; Cölfen, H.; Scheibel, T. Biomineralization of Engineered Spider Silk Protein-Based Composite Materials for Bone Tissue Engineering. Materials 2016, 9, 560. [Google Scholar] [CrossRef]
- Dinjaski, N.; Plowright, R.; Zhou, S.; Belton, D.J.; Perry, C.C.; Kaplan, D.L. Osteoinductive Recombinant Silk Fusion Proteins for Bone Regeneration. Acta Biomater. 2017, 49, 127–139. [Google Scholar] [CrossRef]
- dos Santos-Pinto, J.R.A.; Arcuri, H.A.; Esteves, F.G.; Palma, M.S.; Lubec, G. Spider Silk Proteome Provides Insight into the Structural Characterization of Nephila Clavipes Flagelliform Spidroin. Sci. Rep. 2018, 8, 14674. [Google Scholar] [CrossRef]
- Neubauer, V.J.; Scheibel, T. Spider Silk Fusion Proteins for Controlled Collagen Binding and Biomineralization. ACS Biomater. Sci. Eng. 2020, 6, 5599–5608. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, Z.; Zhou, S.; Martín-Moldes, Z.; Baugh, L.M.; Yeh, J.; Dinjaski, N.; Brown, L.T.; Garb, J.E.; Kaplan, D.L. Expanding Canonical Spider Silk Properties through a DNA Combinatorial Approach. Materials 2020, 13, 3596. [Google Scholar] [CrossRef]
- Agrawal, N.K.; Allen, P.; Song, Y.H.; Wachs, R.A.; Du, Y.; Ellington, A.D.; Schmidt, C.E. Oligonucleotide-Functionalized Hydrogels for Sustained Release of Small Molecule (Aptamer) Therapeutics. Acta Biomater. 2020, 102, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Raslan, A.A.; Yoon, J.K. R-Spondins: Multi-Mode WNT Signaling Regulators in Adult Stem Cells. Int. J. Biochem. Cell Biol. 2019, 106, 26–34. [Google Scholar] [CrossRef]
- Yoon, J.K.; Lee, J.-S. Cellular Signaling and Biological Functions of R-Spondins. Cellular Signalling 2012, 24, 369–377. [Google Scholar] [CrossRef]
- Shi, G.-X.; Mao, W.-W.; Zheng, X.-F.; Jiang, L.-S. The Role of R-Spondins and Their Receptors in Bone Metabolism. Prog. Biophys. Mol. Biol. 2016, 122, 93–100. [Google Scholar] [CrossRef]
- Levin, G.; Koga, B.A.A.; Belchior, G.G.; Carreira, A.C.O.; Sogayar, M.C. Production, Purification and Characterization of Recombinant Human R-Spondin1 (RSPO1) Protein Stably Expressed in Human HEK293 Cells. BMC Biotechnol. 2020, 20, 5. [Google Scholar] [CrossRef]
- Hsu, M.-N.; Yu, F.-J.; Chang, Y.-H.; Huang, K.-L.; Pham, N.N.; Truong, V.A.; Lin, M.-W.; Kieu Nguyen, N.T.; Hwang, S.-M.; Hu, Y.-C. CRISPR Interference-Mediated Noggin Knockdown Promotes BMP2-Induced Osteogenesis and Calvarial Bone Healing. Biomaterials 2020, 252, 120094. [Google Scholar] [CrossRef]
- Garcia, P.; Pieruschka, A.; Klein, M.; Tami, A.; Histing, T.; Holstein, J.H.; Scheuer, C.; Pohlemann, T.; Menger, M.D. Temporal and Spatial Vascularization Patterns of Unions and Nonunions: Role of Vascular Endothelial Growth Factor and Bone Morphogenetic Proteins. J. Bone Jt. Surg. 2012, 94, 49–58. [Google Scholar] [CrossRef]
- Kostenuik, P.; Mirza, F.M. Fracture Healing Physiology and the Quest for Therapies for Delayed Healing and Nonunion: Therapies for Delayed/Non-Union Fractures. J. Orthop. Res. 2017, 35, 213–223. [Google Scholar] [CrossRef]
- Tazawa, R.; Minehara, H.; Matsuura, T.; Kawamura, T.; Uchida, K.; Inoue, G.; Saito, W.; Takaso, M. Effect of Single Injection of Recombinant Human Bone Morphogenetic Protein-2-Loaded Artificial Collagen-Like Peptide in a Mouse Segmental Bone Transport Model. BioMed Res. Int. 2019, 2019, 1014594. [Google Scholar] [CrossRef]
- Singh, R.; Bleibleh, S.; Kanakaris, N.K.; Giannoudis, P.V. Upper Limb Non-Unions Treated with BMP-7: Efficacy and Clinical Results. Injury 2016, 47, S33–S39. [Google Scholar] [CrossRef]
- von Rüden, C.; Morgenstern, M.; Hierholzer, C.; Hackl, S.; Gradinger, F.L.; Woltmann, A.; Bühren, V.; Friederichs, J. The Missing Effect of Human Recombinant Bone Morphogenetic Proteins BMP-2 and BMP-7 in Surgical Treatment of Aseptic Forearm Nonunion. Injury 2016, 47, 919–924. [Google Scholar] [CrossRef]
- Hausbruck, P.; Tanner, M.; Vlachopoulos, W.; Hagelskamp, S.; Miska, M.; Ober, J.; Fischer, C.; Schmidmaier, G. Comparison of the Clinical Effectiveness of Bone Morphogenic Protein (BMP) -2 and -7 in the Adjunct Treatment of Lower Limb Nonunions. Orthop. Traumatol. Surg. Res. 2018, 104, 1241–1248. [Google Scholar] [CrossRef]
- Vaccaro, A.R.; Whang, P.G.; Patel, T.; Phillips, F.M.; Anderson, D.G.; Albert, T.J.; Hilibrand, A.S.; Brower, R.S.; Kurd, M.F.; Appannagari, A.; et al. The Safety and Efficacy of OP-1 (RhBMP-7) as a Replacement for Iliac Crest Autograft for Posterolateral Lumbar Arthrodesis: Minimum 4-Year Follow-up of a Pilot Study. Spine J. 2008, 8, 457–465. [Google Scholar] [CrossRef]
- Rogmark, C.; Kristensen, M.T.; Viberg, B.; Rönnquist, S.S.; Overgaard, S.; Palm, H. Hip Fractures in the Non-Elderly—Who, Why and Whither? Injury 2018, 49, 1445–1450. [Google Scholar] [CrossRef]
- Barquet, A.; Giannoudis, P.V.; Gelink, A. Femoral Neck Fractures after Removal of Hardware in Healed Trochanteric Fractures. Injury 2017, 48, 2619–2624. [Google Scholar] [CrossRef]
- Tall, M. Treatment of Aseptic Tibial Shaft Non-Union without Bone Defect. Orthop. Traumatol. Surg. Res. 2018, 104, S63–S69. [Google Scholar] [CrossRef]
| Type of Scaffold | In Vitro | In Vivo | Results | References |
|---|---|---|---|---|
| Collagen and BMP2 | hMSC | Cranial defects in rats | Satisfactory activity of alkaline phosphatase. Histopathological study and nuclear magnetic resonance imaging repair of the upper bone defect with the association of BMP-2. Insignificant inflammation. | [41] |
| Mineralized recombinant human-like collagen, nano-hydroxyapatite/recombinant human-like collagen/poly (lactic acid) nHA/RHLC/PLA scaffold with polydopamine (pDA)-assisted BMP-2-derived peptide (named as P24) as surface modification strategy | Rat MSC | Cranial defects in rats | Increased ALP activitiy and mRNA expression of osteo-specific markers of the nHA/RH)LC/PLA-P24 and non-P24-loaded nHA/RHLC/PLA groups. In vivo, it is demonstrated that the nHA/RHLC/PLA-pDA-P24 scaffolds significantly enhanced bone regeneration of rat cranial defects. | [45] |
| Atellocollagen and BMP-2 | No | Rats | Expressions of bone phenotypic markers, alkaline phosphatase, osteocalcin, osteopontin, and bone sialoprotein were detected by reverse transcription-polymerase chain reaction and immunohistochemistry. Mineralization and the expressions of key bone proteins were demonstrated in chondroblasts and osteoblasts at 7 to 14 days of culture. | [43] |
| NBM and BMP-2 | Mouse ST2 stromal bone marrow cells seeded on natural bone mineral of bovine origin | No | All concentrations of rhBMPs were able to significantly induce mRNA levels of Runx2, COL1a2 and OCN, but only rhBMP9 was able to significantly upregulate mRNA levels of ALP up to eight-fold, and ALP staining up to 25-fold, when compared to rhBMP2. | [47] |
| Two bioactive ELRs were developed, one including the osteogenic and osteoinductive bone morphogenetic protein-2 (BMP-2) and the other the Arg-Gly-Asp (RGD) cell adhesion motif. These two ELRs were mixed, obtaining a hydrogel scaffold | Bone marrow human MSC | Rabbit lateral distal metaphysic of the femur | In vitro, excellent cytocompatibility observed, and the culture of cells on RGD-containing ELRs resulted in optimal cell adhesion; in vivo, complete regeneration of the defect confirmed by radiography, computed tomography, and histology was demonstrated. | [51] |
| Protease-degradable poly(ethylene glycol) (PEG) synthetic hydrogel functionalized with a triple helical, α2β1 integrin-specific peptide (GFOGER) as a BMP-2 delivery scaffold | hMSC | Murine radial bone defect | These hydrogels promoted osteoprogenitor cell recruitment to the defect site and produced robust repair in a murine non-healing radial bone defect. These hydrogels displayed intrinsic osteogenic activity. | [52] |
| Glycosaminoglycan scaffolds (CS-GAG) hydrogelMSCs cells were modified to overexpress BMP-2 which are then seeded in a CS-GAG hydrogel. ectrospun polycaprolactone nanofiber meshes | Human umbilicas (uMSC) and bone marrow (bmMSC) | Nude rats; critically -sized defects in the mid-diaphysis of the femur | Extended release of rhBMP-2 from CS-GAG scaffolds and further extended release from CS-GAG gels seeded with BMP-2 MSC was demonstrated. In vivo, in bone injury, very good results were obtained, as measured by bone volume, strength, and stiffness. | [49] |
| Scaffolds | In Vitro | In Vivo | Results | References |
|---|---|---|---|---|
| Recombinant human type I collagen achieved by cross-linking followed by lyophilization, forming a 3-D porous structured scaffold | Yes | Cranial defects in rats | Osteogenic differentiation of stem cells. Mineralization increased with the scaffolds. High biocompatibility in vivo. | [41] |
| Recombinant human type I collagen–nanohydorxyapatite-poly(lactic acid) composite | Yes | Radial defects in rabbits | Osteogenic differentiation. Similar effects to collagen of animal origin, but without potential hazards. | [55] |
| Recombinant type I collagen-based scaffolds, obtained by three cross-linking procedures, using dehydrothermal, hesamethylene diisocyanate or genipin | Yes | No | Genipin crosslinking recombinant type I collagen scaffolds supported the lowest MSC adhesion. All the cross-linking methods produced scaffolds that support osteoblast differentiation and mineralization. | [35] |
| Recombinant collagen and elastin-like polypeptide (ELP)-based bone regenerative hydrogels loaded with recombinant human bone morphogenetic protein-2 (rhBMP-2) | Yes | No | Collagen-ELP hydrogels had a significantly higher modulus of 35 ± 5 kPa compared to collagen-only hydrogels. In vitro osteogenic markers, alkaline phosphatase and osteocalcin, were expressed. | [57] |
| Recombinant collagen type I was functionalized with photo-cross-linkable methacrylamide (RCPhC1-MA), norbornene (RCPhC1-NB), or thiol (RCPhC1-SH) functionalities to enable high-resolution 3D printing via two-photon polymerization (2PP) | Yes | No | Hydrogels developed were processable via 2PP and proved to be a perfect alternative to serve tissue engineering applications. | [58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paulini, M.; Camal Ruggieri, I.N.; Ramallo, M.; Alonso, M.; Rodriguez-Cabello, J.C.; Esbrit, P.; Mardegan Issa, J.P.; Feldman, S. Recombinant Proteins-Based Strategies in Bone Tissue Engineering. Biomolecules 2022, 12, 3. https://doi.org/10.3390/biom12010003
Paulini M, Camal Ruggieri IN, Ramallo M, Alonso M, Rodriguez-Cabello JC, Esbrit P, Mardegan Issa JP, Feldman S. Recombinant Proteins-Based Strategies in Bone Tissue Engineering. Biomolecules. 2022; 12(1):3. https://doi.org/10.3390/biom12010003
Chicago/Turabian StylePaulini, Marina, Iván Nadir Camal Ruggieri, Melina Ramallo, Matilde Alonso, José Carlos Rodriguez-Cabello, Pedro Esbrit, João Paulo Mardegan Issa, and Sara Feldman. 2022. "Recombinant Proteins-Based Strategies in Bone Tissue Engineering" Biomolecules 12, no. 1: 3. https://doi.org/10.3390/biom12010003
APA StylePaulini, M., Camal Ruggieri, I. N., Ramallo, M., Alonso, M., Rodriguez-Cabello, J. C., Esbrit, P., Mardegan Issa, J. P., & Feldman, S. (2022). Recombinant Proteins-Based Strategies in Bone Tissue Engineering. Biomolecules, 12(1), 3. https://doi.org/10.3390/biom12010003








