Automatic Activity Arising in Cardiac Muscle Sleeves of the Pulmonary Vein
Abstract
:1. Introduction
2. Pulmonary Veins: An Anatomical Substrate Favorable to the Initiation and Conduction of Ectopic Electrical Activities
2.1. Embryological Development of Pulmonary Veins Cardiac Muscle Sleeves
2.2. The Organization of Cardiac Muscle in the Pulmonary Veins and Left Atria
2.3. Innervation of the Pulmonary Veins
3. Pulmonary Vein Electrophysiology
3.1. Resting Membrane Potential in the Pulmonary Vein Myocytes
3.2. Conduction of Electrical Activity in the Pulmonary Veins
4. Excitation–Contraction Coupling
5. Pulmonary Veins: A Source of Spontaneous, Triggered and Catecholaminergic Automatic Activity
5.1. Spontaneous Electrical Activity
5.2. Triggered Activity
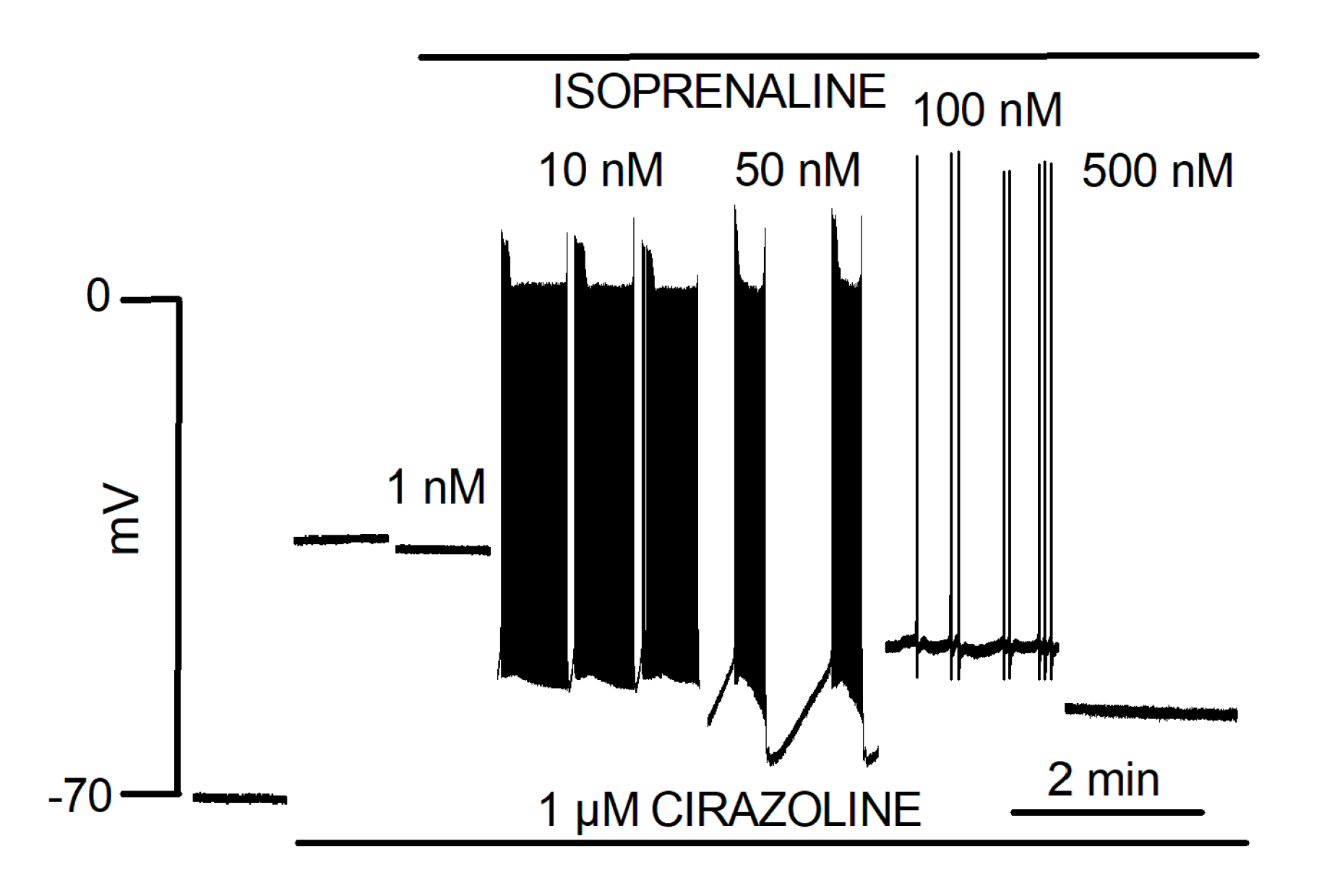
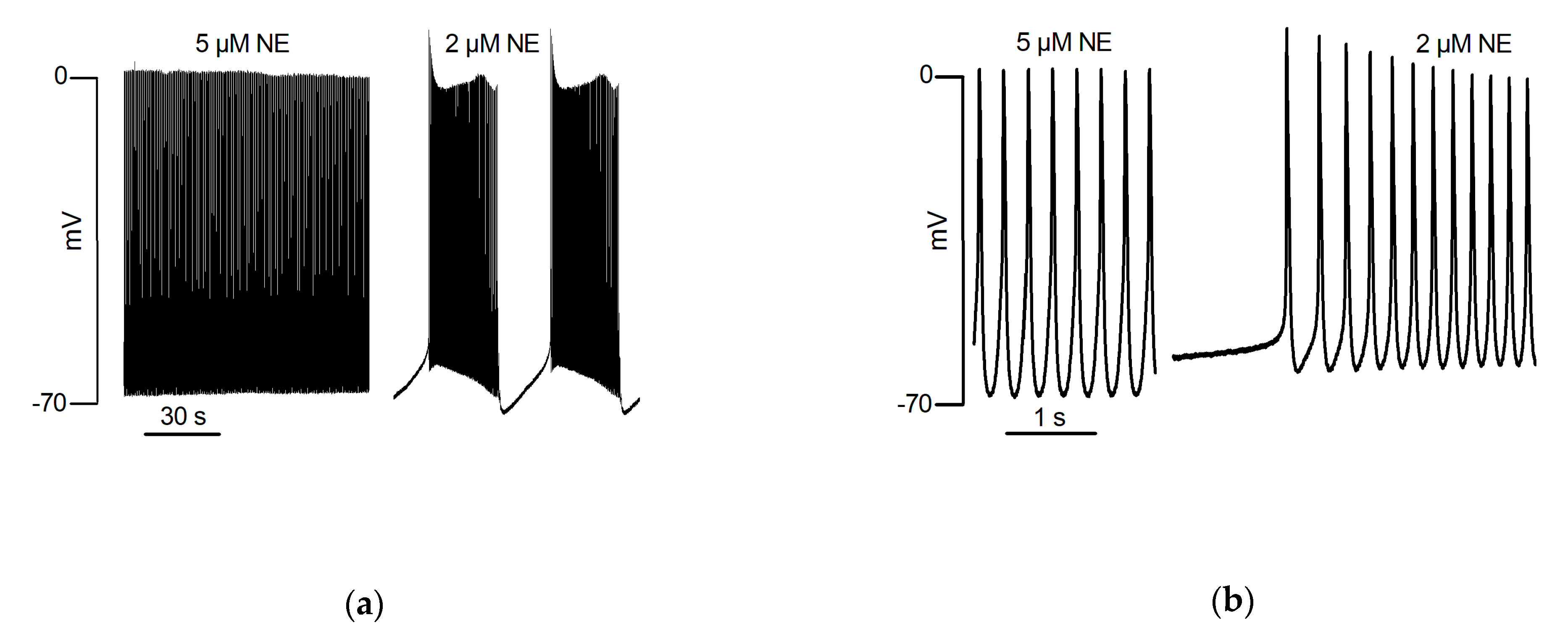
5.3. Catecholaminergic Automatic Activity
5.4. Other Thoracic Veins
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brunton, T.L.; Fayrer, J. Note on Independent Pulsation of the Pulmonary Veins and Vena Cava. Proc. R. Soc. Lond. 1877, 25, 174–176. [Google Scholar] [CrossRef] [Green Version]
- Haïssaguerre, M.; Jaïs, P.; Shah, D.C.; Takahashi, A.; Hocini, M.; Quiniou, G.; Garrigue, S.; le Mouroux, A.; le Métayer, P.; Clémenty, J. Spontaneous Initiation of Atrial Fibrillation by Ectopic Beats Originating in the Pulmonary Veins. N. Engl. J. Med. 1998, 339, 659–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashizume, H.; Tango, M.; Ushiki, T. Three-Dimensional Cytoarchitecture of Rat Pulmonary Venous Walls: A Light and Scanning Electron Microscopic Study. Anat. Embryol. 1998, 198, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Ellinor, P.T. Compendium on Atrial Fibrillation. Circ. Res. 2020, 127, 1–3. [Google Scholar] [CrossRef]
- Guzik, T.J. (Ed.) Spotlight Issue on atrial fibrillation from bench to community: Issues in translating new basic ideas to clinical practice. Cardiovasc. Res. 2021, 117, e88–e90. [Google Scholar]
- Mommersteeg, M.T.M.; Hoogaars, W.M.H.; Prall, O.W.J.; de Gier-de Vries, C.; Wiese, C.; Clout, D.E.W.; Papaioannou, V.E.; Brown, N.A.; Harvey, R.P.; Moorman, A.F.M.; et al. Molecular Pathway for the Localized Formation of the Sinoatrial Node. Circ. Res. 2007, 100, 354–362. [Google Scholar] [CrossRef] [Green Version]
- Mommersteeg, M.T.M.; Domínguez, J.N.; Wiese, C.; Norden, J.; de Gier-de Vries, C.; Burch, J.B.E.; Kispert, A.; Brown, N.A.; Moorman, A.F.M.; Christoffels, V.M. The Sinus Venosus Progenitors Separate and Diversify from the First and Second Heart Fields Early in Development. Cardiovasc. Res. 2010, 87, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Douglas, Y.L.; Jongbloed, M.R.M.; Gittenberger-de Groot, A.C.; Evers, D.; Dion, R.A.E.; Voigt, P.; Bartelings, M.M.; Schalij, M.J.; Ebels, T.; DeRuiter, M.C. Histology of Vascular Myocardial Wall of Left Atrial Body after Pulmonary Venous Incorporation. Am. J. Cardiol. 2006, 97, 662–670. [Google Scholar] [CrossRef]
- Sizarov, A.; Anderson, R.H.; Christoffels, V.M.; Moorman, A.F.M. Three-Dimensional and Molecular Analysis of the Venous Pole of the Developing Human Heart. Circulation 2010, 122, 798–807. [Google Scholar] [CrossRef]
- Sánchez-Quintana, D.; López-Mínguez, J.R.; Pizarro, G.; Murillo, M.; Cabrera, J.A. Triggers and Anatomical Substrates in the Genesis and Perpetuation of Atrial Fibrillation. Curr. Cardiol. Rev. 2012, 8, 310–326. [Google Scholar] [CrossRef] [Green Version]
- Hocini, M.; Ho, S.Y.; Kawara, T.; Linnenbank, A.C.; Potse, M.; Shah, D.; Jaïs, P.; Janse, M.J.; Haïssaguerre, M.; de Bakker, J.M.T. Electrical Conduction in Canine Pulmonary Veins: Electrophysiological and Anatomic Correlation. Circulation 2002, 105, 2442–2448. [Google Scholar] [CrossRef] [Green Version]
- Kracklauer, M.P.; Feng, H.-Z.; Jiang, W.; Lin, J.L.-C.; Lin, J.J.-C.; Jin, J.-P. Discontinuous Thoracic Venous Cardiomyocytes and Heart Exhibit Synchronized Developmental Switch of Troponin Isoforms. FEBS J. 2013, 280, 880–891. [Google Scholar] [CrossRef] [Green Version]
- Pasqualin, C.; Yu, A.; Malécot, C.O.; Gannier, F.; Cognard, C.; Godin-Ribuot, D.; Morand, J.; Bredeloux, P.; Maupoil, V. Structural Heterogeneity of the Rat Pulmonary Vein Myocardium: Consequences on Intracellular Calcium Dynamics and Arrhythmogenic Potential. Sci. Rep. 2018, 8, 3244. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Chen, S.-A.; Chen, Y.-C.; Yeh, H.-I.; Chang, M.-S.; Lin, C.-I. Electrophysiology of Single Cardiomyocytes Isolated from Rabbit Pulmonary Veins: Implication in Initiation of Focal Atrial Fibrillation. Basic Res. Cardiol. 2002, 97, 26–34. [Google Scholar] [CrossRef]
- Ehrlich, J.R.; Cha, T.-J.; Zhang, L.; Chartier, D.; Melnyk, P.; Hohnloser, S.H.; Nattel, S. Cellular Electrophysiology of Canine Pulmonary Vein Cardiomyocytes: Action Potential and Ionic Current Properties. J. Physiol. 2003, 551, 801–813. [Google Scholar] [CrossRef]
- Bond, R.C.; Choisy, S.C.; Bryant, S.M.; Hancox, J.C.; James, A.F. Ion Currents, Action Potentials, and Noradrenergic Responses in Rat Pulmonary Vein and Left Atrial Cardiomyocytes. Physiol. Rep. 2020, 8, e14432. [Google Scholar] [CrossRef]
- Malécot, C.O.; Bredeloux, P.; Findlay, I.; Maupoil, V. A TTX-Sensitive Resting Na+ Permeability Contributes to the Catecholaminergic Automatic Activity in Rat Pulmonary Vein. J. Cardiovasc. Electrophysiol. 2015, 26, 311–319. [Google Scholar] [CrossRef]
- Okamoto, Y.; Takano, M.; Ohba, T.; Ono, K. Arrhythmogenic Coupling between the Na+-Ca2+ Exchanger and Inositol 1,4,5-Triphosphate Receptor in Rat Pulmonary Vein Cardiomyocytes. J. Mol. Cell. Cardiol. 2012, 52, 988–997. [Google Scholar] [CrossRef]
- Blatter, L.A.; Kanaporis, G.; Martinez-Hernandez, E.; Oropeza-Almazan, Y.; Banach, K. Excitation-Contraction Coupling and Calcium Release in Atrial Muscle. Pflüg. Arch.-Eur. J. Physiol. 2021, 473, 317–329. [Google Scholar] [CrossRef]
- Dibb, K.M.; Clarke, J.D.; Eisner, D.A.; Richards, M.A.; Trafford, A.W. A Functional Role for Transverse (t-) Tubules in the Atria. J. Mol. Cell. Cardiol. 2013, 58, 84–91. [Google Scholar] [CrossRef]
- Richards, M.A.; Clarke, J.D.; Saravanan, P.; Voigt, N.; Dobrev, D.; Eisner, D.A.; Trafford, A.W.; Dibb, K.M. Transverse Tubules Are a Common Feature in Large Mammalian Atrial Myocytes Including Human. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1996–H2005. [Google Scholar] [CrossRef] [Green Version]
- Brandenburg, S.; Pawlowitz, J.; Fakuade, F.E.; Kownatzki-Danger, D.; Kohl, T.; Mitronova, G.Y.; Scardigli, M.; Neef, J.; Schmidt, C.; Wiedmann, F.; et al. Axial Tubule Junctions Activate Atrial Ca2+ Release Across Species. Front. Physiol. 2018, 9, 1227. [Google Scholar] [CrossRef] [Green Version]
- Orts Llorca, F.; Domenech Mateu, J.M.; Puerta Fonolla, J. Innervation of the Sinu-Atrial Node and Neighbouring Regions in Two Human Embryos. J. Anat. 1979, 128, 365–375. [Google Scholar]
- Gardner, E.; O’Rahilly, R. The Nerve Supply and Conducting System of the Human Heart at the End of the Embryonic Period Proper. J. Anat. 1976, 121, 571–587. [Google Scholar]
- Tan, A.Y.; Li, H.; Wachsmann-Hogiu, S.; Chen, L.S.; Chen, P.-S.; Fishbein, M.C. Autonomic Innervation and Segmental Muscular Disconnections at the Human Pulmonary Vein-Atrial Junction: Implications for Catheter Ablation of Atrial-Pulmonary Vein Junction. J. Am. Coll. Cardiol. 2006, 48, 132–143. [Google Scholar] [CrossRef] [Green Version]
- Vaitkevicius, R.; Saburkina, I.; Rysevaite, K.; Vaitkeviciene, I.; Pauziene, N.; Zaliunas, R.; Schauerte, P.; Jalife, J.; Pauza, D.H. Nerve Supply of the Human Pulmonary Veins: An Anatomical Study. Heart Rhythm 2009, 6, 221–228. [Google Scholar] [CrossRef]
- Tan, A.Y.; Chen, P.-S.; Chen, L.S.; Fishbein, M.C. Autonomic Nerves in Pulmonary Veins. Heart Rhythm 2007, 4, S57–S60. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.; Wang, F.; Jiang, R.; Zhang, J.; Mou, H.; Yin, Y. A Region-Specific Quantitative Profile of Autonomic Innervation of the Canine Left Atrium and Pulmonary Veins. Auton. Neurosci. Basic Clin. 2011, 162, 42–47. [Google Scholar] [CrossRef]
- Chevalier, P.; Tabib, A.; Meyronnet, D.; Chalabreysse, L.; Restier, L.; Ludman, V.; Aliès, A.; Adeleine, P.; Thivolet, F.; Burri, H.; et al. Quantitative Study of Nerves of the Human Left Atrium. Heart Rhythm 2005, 2, 518–522. [Google Scholar] [CrossRef]
- Potekhina, V.M.; Averina, O.A.; Razumov, A.A.; Kuzmin, V.S.; Rozenshtraukh, L.V. The Local Repolarization Heterogeneity in the Murine Pulmonary Veins Myocardium Contributes to the Spatial Distribution of the Adrenergically Induced Ectopic Foci. J. Physiol. Sci. JPS 2019, 69, 1041–1055. [Google Scholar] [CrossRef]
- Kuzmin, V.S.; Ivanova, A.D.; Potekhina, V.M.; Samoilova, D.V.; Ushenin, K.S.; Shvetsova, A.A.; Petrov, A.M. The Susceptibility of the Rat Pulmonary and Caval Vein Myocardium to the Catecholamine-Induced Ectopy Changes Oppositely in Postnatal Development. J. Physiol. 2021, 599, 2803–2821. [Google Scholar] [CrossRef] [PubMed]
- Po, S.S.; Li, Y.; Tang, D.; Liu, H.; Geng, N.; Jackman, W.M.; Scherlag, B.; Lazzara, R.; Patterson, E. Rapid and Stable Re-Entry within the Pulmonary Vein as a Mechanism Initiating Paroxysmal Atrial Fibrillation. J. Am. Coll. Cardiol. 2005, 45, 1871–1877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doisne, N.; Maupoil, V.; Cosnay, P.; Findlay, I. Catecholaminergic Automatic Activity in the Rat Pulmonary Vein: Electrophysiological Differences between Cardiac Muscle in the Left Atrium and Pulmonary Vein. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H102–H108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egorov, Y.V.; Kuz’min, V.S.; Glukhov, A.V.; Rosenshtraukh, L.V. Electrophysiological Characteristics, Rhythm, Disturbances and Conduction Discontinuities Under Autonomic Stimulation in the Rat Pulmonary Vein Myocardium. J. Cardiovasc. Electrophysiol. 2015, 26, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Tsuneoka, Y.; Kobayashi, Y.; Honda, Y.; Namekata, I.; Tanaka, H. Electrical Activity of the Mouse Pulmonary Vein Myocardium. J. Pharmacol. Sci. 2012, 119, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Namekata, I.; Tsuneoka, Y.; Takahara, A.; Shimada, H.; Sugimoto, T.; Takeda, K.; Nagaharu, M.; Shigenobu, K.; Kawanishi, T.; Tanaka, H. Involvement of the Na(+)/Ca(2+) Exchanger in the Automaticity of Guinea-Pig Pulmonary Vein Myocardium as Revealed by SEA0400. J. Pharmacol. Sci. 2009, 110, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Tsuneoka, Y.; Irie, M.; Tanaka, Y.; Sugimoto, T.; Kobayashi, Y.; Kusakabe, T.; Kato, K.; Hamaguchi, S.; Namekata, I.; Tanaka, H. Permissive Role of Reduced Inwardly-Rectifying Potassium Current Density in the Automaticity of the Guinea Pig Pulmonary Vein Myocardium. J. Pharmacol. Sci. 2017, 133, 195–202. [Google Scholar] [CrossRef]
- Melnyk, P.; Ehrlich, J.R.; Pourrier, M.; Villeneuve, L.; Cha, T.-J.; Nattel, S. Comparison of Ion Channel Distribution and Expression in Cardiomyocytes of Canine Pulmonary Veins versus Left Atrium. Cardiovasc. Res. 2005, 65, 104–116. [Google Scholar] [CrossRef]
- Egorov, Y.V.; Lang, D.; Tyan, L.; Turner, D.; Lim, E.; Piro, Z.D.; Hernandez, J.J.; Lodin, R.; Wang, R.; Schmuck, E.G.; et al. Caveolae-Mediated Activation of Mechanosensitive Chloride Channels in Pulmonary Veins Triggers Atrial Arrhythmogenesis. J. Am. Heart Assoc. 2019, 8, e012748. [Google Scholar] [CrossRef]
- Okamoto, Y.; Kawamura, K.; Nakamura, Y.; Ono, K. Pathological Impact of Hyperpolarization-Activated Chloride Current Peculiar to Rat Pulmonary Vein Cardiomyocytes. J. Mol. Cell. Cardiol. 2014, 66, 53–62. [Google Scholar] [CrossRef]
- Takagi, D.; Okamoto, Y.; Ohba, T.; Yamamoto, H.; Ono, K. Comparative Study of Hyperpolarization-Activated Currents in Pulmonary Vein Cardiomyocytes Isolated from Rat, Guinea Pig, and Rabbit. J. Physiol. Sci. JPS 2020, 70, 6. [Google Scholar] [CrossRef] [Green Version]
- Cheung, D.W. Electrical Activity of the Pulmonary Vein and Its Interaction with the Right Atrium in the Guinea-Pig. J. Physiol. 1981, 314, 445–456. [Google Scholar] [CrossRef] [Green Version]
- Miyauchi, Y.; Hayashi, H.; Miyauchi, M.; Okuyama, Y.; Mandel, W.J.; Chen, P.-S.; Karagueuzian, H.S. Heterogeneous Pulmonary Vein Myocardial Cell Repolarization Implications for Reentry and Triggered Activity. Heart Rhythm 2005, 2, 1339–1345. [Google Scholar] [CrossRef]
- Bredeloux, P.; Findlay, I.; Pasqualin, C.; Hocini, M.; Bernus, O.; Maupoil, V. Selective Inhibition of Electrical Conduction within the Pulmonary Veins by A1-Adrenergic Receptors Activation in the Rat. Sci. Rep. 2020, 10, 5390. [Google Scholar] [CrossRef]
- Ivanova, A.D.; Filatova, T.S.; Abramochkin, D.V.; Atkinson, A.; Dobrzynski, H.; Kokaeva, Z.G.; Merzlyak, E.M.; Pustovit, K.B.; Kuzmin, V.S. Attenuation of Inward Rectifier Potassium Current Contributes to the A1-Adrenergic Receptor-Induced Proarrhythmicity in the Caval Vein Myocardium. Acta Physiol. Oxf. Engl. 2021, 231, e13597. [Google Scholar] [CrossRef]
- Fabiato, A.; Fabiato, F. Calcium and Cardiac Excitation-Contraction Coupling. Annu. Rev. Physiol. 1979, 41, 473–484. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chen, S.-A.; Chen, Y.-J.; Tai, C.-T.; Chan, P.; Lin, C.-I. T-Type Calcium Current in Electrical Activity of Cardiomyocytes Isolated from Rabbit Pulmonary Vein. J. Cardiovasc. Electrophysiol. 2004, 15, 567–571. [Google Scholar] [CrossRef]
- Malécot, C.O. Low Voltage-Activated Channels in Rat Pulmonary Vein Cardiomyocytes: Coexistence of a Non-Selective Cationic Channel and of T-Type Ca Channels. Pflugers Arch. 2020, 472, 1019–1029. [Google Scholar] [CrossRef]
- Coutu, P.; Chartier, D.; Nattel, S. Comparison of Ca2+-Handling Properties of Canine Pulmonary Vein and Left Atrial Cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H2290–H2300. [Google Scholar] [CrossRef] [Green Version]
- Bootman, M.D.; Smyrnias, I.; Thul, R.; Coombes, S.; Roderick, H.L. Atrial Cardiomyocyte Calcium Signalling. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2011, 1813, 922–934. [Google Scholar] [CrossRef] [Green Version]
- Logantha, S.J.R.J.; Cruickshank, S.F.; Rowan, E.G.; Drummond, R.M. Spontaneous and Electrically Evoked Ca2+ Transients in Cardiomyocytes of the Rat Pulmonary Vein. Cell Calcium 2010, 48, 150–160. [Google Scholar] [CrossRef]
- Patterson, E.; Po, S.S.; Scherlag, B.J.; Lazzara, R. Triggered Firing in Pulmonary Veins Initiated by in Vitro Autonomic Nerve Stimulation. Heart Rhythm 2005, 2, 624–631. [Google Scholar] [CrossRef]
- Yamamoto, M.; Dobrzynski, H.; Tellez, J.; Niwa, R.; Billeter, R.; Honjo, H.; Kodama, I.; Boyett, M.R. Extended Atrial Conduction System Characterised by the Expression of the HCN4 Channel and Connexin45. Cardiovasc. Res. 2006, 72, 271–281. [Google Scholar] [CrossRef]
- Jones, S.A.; Yamamoto, M.; Tellez, J.O.; Billeter, R.; Boyett, M.R.; Honjo, H.; Lancaster, M.K. Distinguishing Properties of Cells From the Myocardial Sleeves of the Pulmonary Veins. Circ. Arrhythm. Electrophysiol. 2008, 1, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.-L.; Chen, Y.-C.; Chen, Y.-J.; Wangcharoen, W.; Lee, S.-H.; Lin, C.-I.; Chen, S.-A. Mechanoelectrical Feedback Regulates the Arrhythmogenic Activity of Pulmonary Veins. Heart Br. Card. Soc. 2007, 93, 82–88. [Google Scholar] [CrossRef] [Green Version]
- Hamaguchi, S.; Hikita, K.; Tanaka, Y.; Tsuneoka, Y.; Namekata, I.; Tanaka, H. Enhancement of Automaticity by Mechanical Stretch of the Isolated Guinea Pig Pulmonary Vein Myocardium. Biol. Pharm. Bull. 2016, 39, 1216–1219. [Google Scholar] [CrossRef] [Green Version]
- Patterson, E.; Jackman, W.M.; Beckman, K.J.; Lazzara, R.; Lockwood, D.; Scherlag, B.J.; Wu, R.; Po, S. Spontaneous Pulmonary Vein Firing in Man: Relationship to Tachycardia-Pause Early Afterdepolarizations and Triggered Arrhythmia in Canine Pulmonary Veins in Vitro. J. Cardiovasc. Electrophysiol. 2007, 18, 1067–1075. [Google Scholar] [CrossRef]
- Patterson, E.; Lazzara, R.; Szabo, B.; Liu, H.; Tang, D.; Li, Y.-H.; Scherlag, B.J.; Po, S.S. Sodium-Calcium Exchange Initiated by the Ca2+ Transient: An Arrhythmia Trigger within Pulmonary Veins. J. Am. Coll. Cardiol. 2006, 47, 1196–1206. [Google Scholar] [CrossRef] [Green Version]
- Honjo, H.; Boyett, M.R.; Niwa, R.; Inada, S.; Yamamoto, M.; Mitsui, K.; Horiuchi, T.; Shibata, N.; Kamiya, K.; Kodama, I. Pacing-Induced Spontaneous Activity in Myocardial Sleeves of Pulmonary Veins after Treatment with Ryanodine. Circulation 2003, 107, 1937–1943. [Google Scholar] [CrossRef]
- Takahara, A.; Sugimoto, T.; Kitamura, T.; Takeda, K.; Tsuneoka, Y.; Namekata, I.; Tanaka, H. Electrophysiological and Pharmacological Characteristics of Triggered Activity Elicited in Guinea-Pig Pulmonary Vein Myocardium. J. Pharmacol. Sci. 2011, 115, 176–181. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki, Y.-K.; Kato, T.; Xiong, F.; Shi, Y.-F.; Naud, P.; Maguy, A.; Mizuno, K.; Tardif, J.-C.; Comtois, P.; Nattel, S. Atrial Fibrillation Promotion with Long-Term Repetitive Obstructive Sleep Apnea in a Rat Model. J. Am. Coll. Cardiol. 2014, 64, 2013–2023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwasaki, Y.-K.; Yamashita, T.; Sekiguchi, A.; Hayami, N.; Shimizu, W. Importance of Pulmonary Vein Preferential Fibrosis for Atrial Fibrillation Promotion in Hypertensive Rat Hearts. Can. J. Cardiol. 2016, 32, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Wang, X.; Yin, S.; Zhang, Y.; Han, Y.; Yang, N.; Xu, J.; Sun, L.; Yuan, Y.; Sheng, L.; et al. Atrial Fibrillation Promotion in a Rat Model of Rheumatoid Arthritis. J. Am. Heart Assoc. 2017, 6, e007320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, T.; Liu, X.; Qu, C.; Zhang, C.; Fo, Y.; Guo, Y.; Chen, X.; Shi, S.; Yang, B. Chronic Inhibition of the Sigma-1 Receptor Exacerbates Atrial Fibrillation Susceptibility in Rats by Promoting Atrial Remodeling. Life Sci. 2019, 235, 116837. [Google Scholar] [CrossRef]
- Stavrakis, S.; Po, S. Ganglionated Plexi Ablation: Physiology and Clinical Applications. Arrhythmia Electrophysiol. Rev. 2017, 6, 186–190. [Google Scholar] [CrossRef] [Green Version]
- Kampaktsis, P.N.; Oikonomou, E.K.; Choi, D.Y.; Cheung, J.W. Efficacy of Ganglionated Plexi Ablation in Addition to Pulmonary Vein Isolation for Paroxysmal versus Persistent Atrial Fibrillation: A Meta-Analysis of Randomized Controlled Clinical Trials. J. Interv. Card. Electrophysiol. Int. J. Arrhythm. Pacing 2017, 50, 253–260. [Google Scholar] [CrossRef]
- Hanna, P.; Dacey, M.J.; Brennan, J.; Moss, A.; Robbins, S.; Achanta, S.; Biscola, N.P.; Swid, M.A.; Rajendran, P.S.; Mori, S.; et al. Innervation and Neuronal Control of the Mammalian Sinoatrial Node a Comprehensive Atlas. Circ. Res. 2021, 128, 1279–1296. [Google Scholar] [CrossRef]
- Maupoil, V.; Bronquard, C.; Freslon, J.-L.; Cosnay, P.; Findlay, I. Ectopic Activity in the Rat Pulmonary Vein Can Arise from Simultaneous Activation of Alpha1- and Beta1-Adrenoceptors. Br. J. Pharmacol. 2007, 150, 899–905. [Google Scholar] [CrossRef] [Green Version]
- Egorov, Y.V.; Rosenshtraukh, L.V.; Glukhov, A.V. Arrhythmogenic Interaction Between Sympathetic Tone and Mechanical Stretch in Rat Pulmonary Vein Myocardium. Front. Physiol. 2020, 11, 237. [Google Scholar] [CrossRef]
- Umehara, S.; Tan, X.; Okamoto, Y.; Ono, K.; Noma, A.; Amano, A.; Himeno, Y. Mechanisms Underlying Spontaneous Action Potential Generation Induced by Catecholamine in Pulmonary Vein Cardiomyocytes: A Simulation Study. Int. J. Mol. Sci. 2019, 20, E2913. [Google Scholar] [CrossRef] [Green Version]
- Irie, M.; Tsuneoka, Y.; Shimobayashi, M.; Hasegawa, N.; Tanaka, Y.; Mochizuki, S.; Ichige, S.; Hamaguchi, S.; Namekata, I.; Tanaka, H. Involvement of Alpha- and Beta-Adrenoceptors in the Automaticity of the Isolated Guinea Pig Pulmonary Vein Myocardium. J. Pharmacol. Sci. 2017, 133, 247–253. [Google Scholar] [CrossRef]
- Ivanova, A.D.; Kuzmin, V.S. Electrophysiological Characteristics of the Rat Azygos Vein under Electrical Pacing and Adrenergic Stimulation. J. Physiol. Sci. JPS 2018, 68, 617–628. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bredeloux, P.; Pasqualin, C.; Bordy, R.; Maupoil, V.; Findlay, I. Automatic Activity Arising in Cardiac Muscle Sleeves of the Pulmonary Vein. Biomolecules 2022, 12, 23. https://doi.org/10.3390/biom12010023
Bredeloux P, Pasqualin C, Bordy R, Maupoil V, Findlay I. Automatic Activity Arising in Cardiac Muscle Sleeves of the Pulmonary Vein. Biomolecules. 2022; 12(1):23. https://doi.org/10.3390/biom12010023
Chicago/Turabian StyleBredeloux, Pierre, Come Pasqualin, Romain Bordy, Veronique Maupoil, and Ian Findlay. 2022. "Automatic Activity Arising in Cardiac Muscle Sleeves of the Pulmonary Vein" Biomolecules 12, no. 1: 23. https://doi.org/10.3390/biom12010023
APA StyleBredeloux, P., Pasqualin, C., Bordy, R., Maupoil, V., & Findlay, I. (2022). Automatic Activity Arising in Cardiac Muscle Sleeves of the Pulmonary Vein. Biomolecules, 12(1), 23. https://doi.org/10.3390/biom12010023





