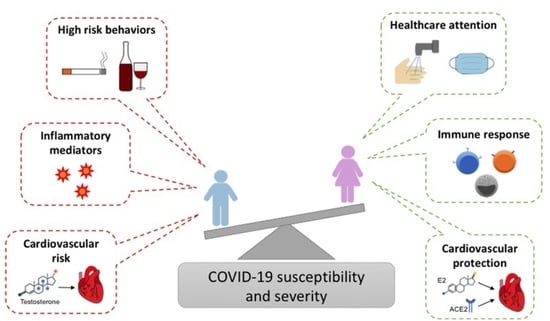
Sex-Related Factors in Cardiovascular Complications Associated to COVID-19
Abstract
:1. Introduction
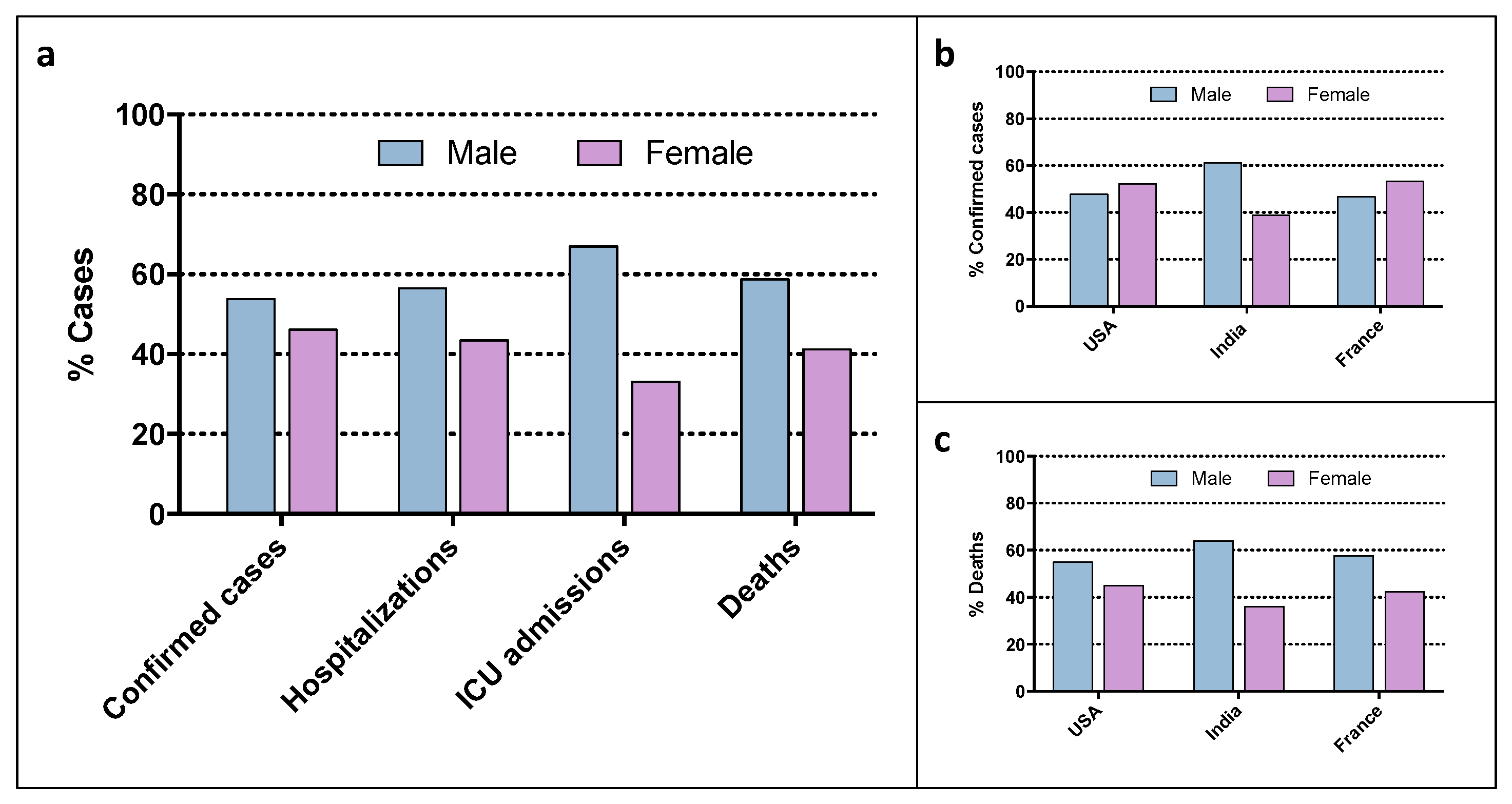
2. Sex and Gender Factors in COVID-19 Infection and Outcome
2.1. Genetic Factors
2.2. Sex Hormones
2.3. Behavior
2.4. Treatment and Vaccines
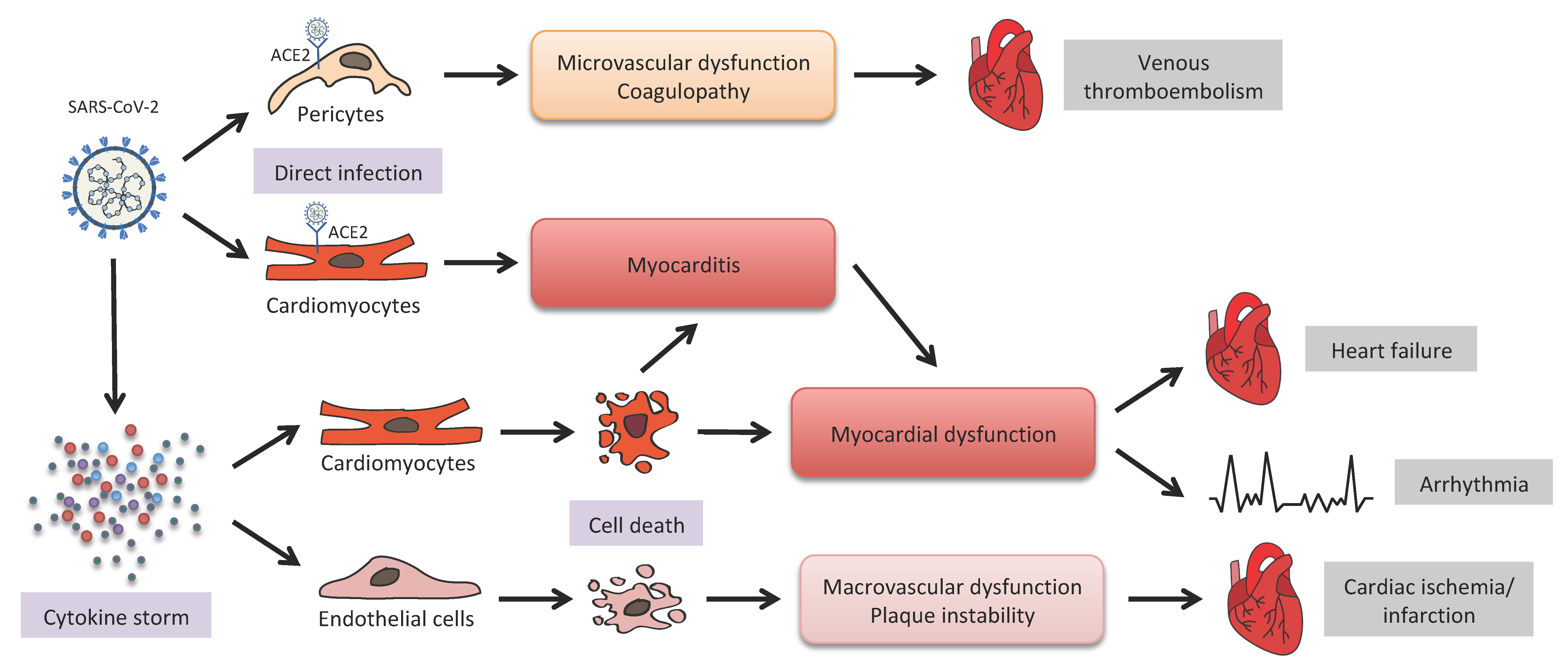
3. COVID-19 and Cardiovascular Diseases (CVDs)
3.1. ACE2-Mediated Viral Infection
3.2. Inflammation and Microthromboses
3.3. COVID-19 Therapies
3.4. Long COVID
4. Sex and Gender Factors in COVID-19-Related CVDs
4.1. Pre-Existing Cardiovascular Pathologies
4.2. COVID-19 Cardiovascular Complications
5. Sex-Related Biomarkers of COVID-19 Cardiovascular Complications
5.1. Cardiac Biomarkers
5.2. Thrombotic Markers
5.3. Inflammatory Response Markers
5.4. Serum Renin
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- World Health Organization. COVID-19 Weekly Epidemiological Update; World Health Organization: Geneva, Switzerland, 2021; pp. 1–23. [Google Scholar]
- Aggarwal, S.; Garcia-Telles, N.; Aggarwal, G.; Lavie, C.; Lippi, G.; Henry, B.M. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19): Early report from the United States. Diagnosis 2020, 7, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-U.; Kim, M.-J.; Ra, S.; Lee, J.; Bae, S.; Jung, J.; Kim, S.-H. Clinical characteristics of asymptomatic and symptomatic patients with mild COVID-19. Clin. Microbiol. Infect. 2020, 26, 948.e1–948.e3. [Google Scholar] [CrossRef] [PubMed]
- Lechien, J.R.; Chiesa-Estomba, C.M.; Place, S.; Van Laethem, Y.; Cabaraux, P.; Mat, Q.; Huet, K.; Plzak, J.; Horoi, M.; Hans, S.; et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J. Intern. Med. 2020, 288, 335–344. [Google Scholar] [CrossRef]
- Xu, T.; Huang, R.; Zhu, L.; Wang, J.; Cheng, J.; Zhang, B.; Zhao, H.; Chen, K.; Shao, H.; Zhu, C.; et al. Epidemiological and clinical features of asymptomatic patients with SARS-CoV-2 infection. J. Med. Virol. 2020, 92, 1884–1889. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases from the Chinese Center for Disease Control and Prevention. J. Am. Med. Assoc. 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, N.; Cao, Z.; Gundrum, J.; Sianis, J.; Safo, S. Risk Factors Associated With In-Hospital Mortality in a US National Sample of Patients With COVID-19. JAMA Netw. Open 2020, 3, e2029058. [Google Scholar] [CrossRef]
- Li, J.; Huang, D.Q.; Zou, B.; Yang, H.; Hui, W.Z.; Rui, F.; Yee, N.T.S.; Liu, C.; Nerurkar, S.N.; Kai, J.C.Y.; et al. Epidemiology of COVID-19: A systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J. Med. Virol. 2021, 93, 1449–1458. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Gupta, S.; Hayek, S.S.; Wang, W.; Chan, L.; Mathews, K.S.; Melamed, M.L.; Brenner, S.K.; Leonberg-Yoo, A.; Schenck, E.J.; Radbel, J.; et al. Factors Associated with Death in Critically Ill Patients with Coronavirus Disease 2019 in the US. JAMA Intern. Med. 2020, 180, 1436–1447. [Google Scholar] [CrossRef]
- Klein, F. Risikofaktor Komorbiditäten bei COVID-19-Erkrankung. Pneumologie 2020, 74, 640. [Google Scholar]
- Li, X.; Xu, S.; Yu, M.; Wang, K.; Tao, Y.; Zhou, Y.; Shi, J.; Zhou, M.; Wu, B.; Yang, Z.; et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J. Allergy Clin. Immunol. 2020, 146, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhu, D.S.; Chen, R.B.; Shi, N.N.; Liu, S.H.; Fan, Y.P.; Wu, G.H.; Yang, P.Y.; Bai, J.F.; Chen, H.; et al. Association of Overlapped and Un-overlapped Comorbidities with COVID-19 Severity and Treatment Outcomes: A Retrospective Cohort Study from Nine Provinces in China. Biomed. Environ. Sci. 2020, 33, 893–905. [Google Scholar]
- Shi, C.; Wang, L.; Ye, J.; Gu, Z.; Wang, S.; Xia, J.; Xie, Y.; Li, Q.; Xu, R.; Lin, N. Predictors of mortality in patients with coronavirus disease 2019: A systematic review and meta-analysis. BMC Infect. Dis. 2021, 21, 663. [Google Scholar] [CrossRef]
- Leulseged, T.W.; Alemahu, D.G.; Hassen, I.S.; Maru, E.H.; Zewde, W.C.; Chamiso, N.W.; Yegele, K.T.; Abebe, D.S.; Abdi, F.M.; Minyelshewa, E.Y.; et al. Factors associated with development of symptomatic disease in Ethiopian COVID-19 patients: A case-control study. BMC Infect. Dis. 2021, 21, 759. [Google Scholar] [CrossRef]
- Apicella, M.; Campopiano, M.C.; Mantuano, M.; Mazoni, L.; Coppelli, A.; Prato, S.D. COVID-19 in people with diabetes: Understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020, 8, 782. [Google Scholar] [CrossRef]
- Gomez, J.M.D.; Du-Fay-de-Lavallaz, J.M.; Fugar, S.; Sarau, A.; Simmons, J.A.; Clark, B.; Sanghani, R.M.; Aggarwal, N.T.; Williams, K.A.; Doukky, R.; et al. Sex Differences in COVID-19 Hospitalization and Mortality. J. Women’s Health 2021, 30, 646–653. [Google Scholar] [CrossRef]
- Karlberg, J.; Chong, D.S.Y.; Lai, W.Y.Y. Do Men Have a Higher Case Fatality Rate of Severe Acute Respiratory Syndrome than Women Do? Am. J. Epidemiol. 2004, 159, 229–231. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.M.; Bai, P.; He, W.; Wu, F.; Liu, X.F.; Han, D.M.; Liu, S.; Yang, J.K. Gender Differences in Patients With COVID-19: Focus on Severity and Mortality. Front. Public Health 2020, 8, 152. [Google Scholar] [CrossRef]
- The COVID-19 Sex-Disaggregated Data Tracker|Global Health 50/50 [Internet]. Available online: https://globalhealth5050.org/the-sex-gender-and-COVID-19-project/the-data-tracker/?explore=variable&variable=Confirmed+cases (accessed on 26 October 2021).
- Ahmed, S.B.; Dumanski, S.M. Sex, gender and COVID-19: A call to action. Can. J. Public Health 2020, 111, 980–983. [Google Scholar] [CrossRef]
- Wehbe, Z.; Hammoud, S.H.; Yassine, H.M.; Fardoun, M.; El-Yazbi, A.F.; Eid, A.H. Molecular and Biological Mechanisms Underlying Gender Differences in COVID-19 Severity and Mortality. Front. Immunol. 2021, 12, 659339. [Google Scholar] [CrossRef] [PubMed]
- Schurz, H.; Salie, M.; Tromp, G.; Hoal, E.G.; Kinnear, C.J.; Möller, M. The X chromosome and sex-specific effects in infectious disease susceptibility. Hum. Genom. 2019, 13, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadi, N.; Wu, S.C.; Spihlman, A.P.; Moulton, V.R. What’s Sex Got to Do With COVID-19? Gender-Based Differences in the Host Immune Response to Coronaviruses. Front. Immunol. 2020, 11, 2147. [Google Scholar] [CrossRef] [PubMed]
- Spolarics, Z. The X-files of inflammation: Cellular mosaicism of X-linked polymorphic genes and the female advantage in the host response to injury and infection. Shock 2007, 27, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Moulton, V.R. Sex Hormones in Acquired Immunity and Autoimmune Disease. Front. Immunol. 2018, 9, 2279. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.L.C.; Lely, A.T.; Navis, G.J.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Zou, X.; Chen, K.; Zou, J.; Han, P.; Hao, J.; Han, Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020, 14, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Chen, J.; Wang, X.; Zhang, F.; Liu, Y. Age- and gender-related difference of ACE2 expression in rat lung. Life Sci. 2006, 78, 2166–2171. [Google Scholar] [CrossRef]
- Stanić, B.M.; Maddox, S.; de Souza, A.M.A.; Wu, X.; Mehranfard, D.; Ji, H.; Speth, R.C.; Sandberg, K. Male bias in ACE2 basic science research: Missed opportunity for discovery in the time of COVID-19. Am. J. Physiol. Integr. Comp. Physiol. 2021, 320, R925–R937. [Google Scholar] [CrossRef]
- Pontecorvi, G.; Bellenghi, M.; Ortona, E.; Carè, A. microRNAs as new possible actors in gender disparities of COVID-19 pandemic. Acta Physiol. 2020, 230, e13538. [Google Scholar] [CrossRef]
- Erfinanda, L.; Ravindran, K.; Kohse, F.; Gallo, K.; Preissner, R.; Walther, T.; Kuebler, W.M. Estrogen-mediated upregulation of the Mas receptor contributes to sex differences in acute lung injury and lung vascular barrier regulation. Eur. Respir. J. 2020, 57, 2000921. [Google Scholar] [CrossRef] [PubMed]
- Ciarambino, T.; Sansone, G.; Menna, G.; Para, O.; Giordano, M. What is known in male gender differences, comorbidity and age for COVID-19 pandemia? A narrative minireview. J. Gerontol. Geriatr. 2020, 68, 216–223. [Google Scholar] [CrossRef]
- Takahashi, T.; Iwasaki, A. Sex differences in immune responses. Science 2021, 371, 347–348. [Google Scholar] [CrossRef] [PubMed]
- Ya’qoub, L.; Elgendy, I.Y.; Pepine, C.J. Sex and gender differences in COVID-19: More to be learned! Am. Hear J. Plus Cardiol. Res. Pract. 2021, 3, 100011. [Google Scholar] [CrossRef] [PubMed]
- Raza, H.A.; Sen, P.; Bhatti, O.A.; Gupta, L. Sex hormones, autoimmunity and gender disparity in COVID-19. Rheumatol. Int. 2021, 41, 1375–1386. [Google Scholar] [CrossRef]
- Spini, A.; Giudice, V.; Brancaleone, V.; Morgese, M.G.; De Francia, S.; Filippelli, A.; Ruggieri, A.; Ziche, M.; Ortona, E.; Cignarella, A.; et al. Sex-tailored pharmacology and COVID-19: Next steps towards appropriateness and health equity. Pharmacol. Res. 2021, 173, 105848. [Google Scholar] [CrossRef]
- Costeira, R.; Lee, K.A.; Murray, B.; Christiansen, C.; Castillo-Fernandez, J.; Lochlainn, M.N.; Pujol, J.C.; Macfarlane, H.; Kenny, L.C.; Buchan, I.; et al. Estrogen and COVID-19 symptoms: Associations in women from the COVID Symptom Study. PLoS ONE 2021, 16, e0257051. [Google Scholar] [CrossRef]
- Galasso, V.; Pons, V.; Profeta, P.; Becher, M.; Brouard, S.; Foucault, M. Gender differences in COVID-19 attitudes and behavior: Panel evidence from eight countries. Proc. Natl. Acad. Sci. USA 2020, 117, 27285–27291. [Google Scholar] [CrossRef]
- Pivonello, R.; Auriemma, R.S.; Pivonello, C.; Isidori, A.M.; Corona, G.; Colao, A.; Millar, R.P. Sex Disparities in COVID-19 Severity and Outcome: Are Men Weaker or Women Stronger? Neuroendocrinology 2021, 111, 1066–1085. [Google Scholar] [CrossRef]
- Varì, R.; Scazzocchio, B.; Del Papa, S. Dietary habits and gender difference. Ital. J. Gender-Specif. Med. 2017, 3, 55–58. [Google Scholar]
- Detopoulou, P.; Demopoulos, C.A.; Antonopoulou, S. Micronutrients, Phytochemicals and Mediterranean Diet: A Potential Protective Role against COVID-19 through Modulation of PAF Actions and Metabolism. Nutrients 2021, 13, 462. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, J.; Safdar, B.; Madsen, T.E.; Sethuraman, K.N.; Becker, B.; Greenberg, M.R.; McGregor, A.J. Sex- or Gender-specific Differences in the Clinical Presentation, Outcome, and Treatment of SARS-CoV-2. Clin. Ther. 2021, 43, 557–571.e1. [Google Scholar] [CrossRef]
- Bignucolo, A.; Scarabel, L.; Mezzalira, S.; Polesel, J.; Cecchin, E.; Toffoli, G. Sex disparities in efficacy in COVID-19 vaccines: A systematic review and meta-analysis. Vaccines 2021, 9, 825. [Google Scholar] [CrossRef] [PubMed]
- Hendren, N.S.; Drazner, M.H.; Bozkurt, B.; Cooper, L.T., Jr. Description and Proposed Management of the Acute COVID-19 Cardiovascular Syndrome. Circulation 2020, 141, 1903. [Google Scholar] [CrossRef] [PubMed]
- Clerkin, K.J.; Fried, J.A.; Raikhelkar, J.; Sayer, G.; Griffin, J.M.; Masoumi, A.; Jain, S.S.; Burkhoff, D.; Kumaraiah, D.; Rabbani, L.; et al. COVID-19 and Cardiovascular Disease. Circulation 2020, 141, 1648–1655. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.S.; Shahid, I.; Anker, S.D.; Solomon, S.D.; Vardeny, O.; Michos, E.D.; Fonarow, G.C.; Butler, J. Cardiovascular implications of COVID-19 versus influenza infection: A review. BMC Med. 2020, 18, 403. [Google Scholar] [CrossRef] [PubMed]
- Duffy, E.Y.; Cainzos-Achirica, M.; Michos, E.D. Primary and Secondary Prevention of Cardiovascular Disease in the Era of the Coronavirus Pandemic. Circulation 2020, 141, 1943–1945. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, R.; Goharrizi, M.A.S.B.; Bahardoust, M.; Alvanegh, A.G.; Ataee, M.R.; Bagheri, M.; Navidiyan, E.S.; Zijoud, S.R.H.; Heiat, M. Should all patients with hypertension be worried about developing severe coronavirus disease 2019 (COVID-19)? Clin. Hypertens. 2021, 27, 3. [Google Scholar] [CrossRef]
- Hendren, N.S.; de Lemos, J.A.; Ayers, C.; Das, S.R.; Rao, A.; Carter, S.; Rosenblatt, A.; Walchok, J.; Omar, W.; Khera, R.; et al. Association of Body Mass Index and Age with Morbidity and Mortality in Patients Hospitalized with COVID-19: Results from the American Heart Association COVID-19 Cardiovascular Disease Registry. Circulation 2021, 143, 135–144. [Google Scholar] [CrossRef]
- Lala, A.; Johnson, K.W.; Januzzi, J.L.; Russak, A.J.; Paranjpe, I.; Richter, F.; Zhao, S.; Somani, S.; Van Vleck, T.; Vaid, A.; et al. Prevalence and Impact of Myocardial Injury in Patients Hospitalized With COVID-19 Infection. J. Am. Coll. Cardiol. 2020, 76, 533. [Google Scholar] [CrossRef] [PubMed]
- Bavishi, C.; Bonow, R.O.; Trivedi, V.; Abbott, J.D.; Messerli, F.H.; Bhatt, D.L. Special Article—Acute myocardial injury in patients hospitalized with COVID-19 infection: A review. Prog. Cardiovasc. Dis. 2020, 63, 682. [Google Scholar] [CrossRef] [PubMed]
- Fried, J.A.; Ramasubbu, K.; Bhatt, R.; Topkara, V.K.; Clerkin, K.J.; Horn, E.; Rabbani, L.; Brodie, D.; Jain, S.S.; Kirtane, A.J.; et al. The Variety of Cardiovascular Presentations of COVID-19. Circulation 2020, 141, 1930–1936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dherange, P.; Lang, J.; Qian, P.; Oberfeld, B.; Sauer, W.H.; Koplan, B.; Tedrow, U. Arrhythmias and COVID-19. JACC Clin. Electrophysiol. 2020, 6, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Castiello, T.; Georgiopoulos, G.; Finocchiaro, G.; Claudia, M.; Gianatti, A.; Delialis, D.; Aimo, A.; Prasad, S. COVID-19 and myocarditis: A systematic review and overview of current challenges. Heart Fail. Rev. 2021, 1, 1–11. [Google Scholar] [CrossRef]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus—Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef] [Green Version]
- Inciardi, R.M.; Adamo, M.; Lupi, L.; Cani, D.S.; Di Pasquale, M.; Tomasoni, D.; Italia, L.; Zaccone, G.; Tedino, C.; Fabbricatore, D.; et al. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur. Heart J. 2020, 41, 1821. [Google Scholar] [CrossRef]
- Flores, D.; Walter, J.; Wussler, D.; Kozhuharov, N.; Nowak, A.; Dinort, J.; Badertscher, P.; Martin, J.; Sabti, Z.; du Fay de Lavallaz, J.; et al. Direct Comparison of High-Sensitivity Cardiac Troponin T and I for Prediction of Mortality in Patients with Pneumonia. J. Clin. Chem. Lab. Med. 2020, 2, 131. [Google Scholar]
- Chen, L.; Li, X.; Chen, M.; Feng, Y.; Xiong, C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc. Res. 2020, 116, 1097–1100. [Google Scholar] [CrossRef] [Green Version]
- Atri, D.; Siddiqi, H.K.; Lang, J.P.; Nauffal, V.; Morrow, D.A.; Bohula, E.A. COVID-19 for the Cardiologist: Basic Virology, Epidemiology, Cardiac Manifestations, and Potential Therapeutic Strategies. JACC Basic Transl. Sci. 2020, 5, 518–536. [Google Scholar] [CrossRef] [PubMed]
- Bos, J.M.; Hebl, V.B.; Oberg, A.L.; Sun, Z.; Herman, D.S.; Teekakirikul, P.; Seidman, J.G.; Seidman, C.E.; Dos Remedios, C.G.; Maleszewski, J.J.; et al. Marked Up-Regulation of ACE2 in Hearts of Patients With Obstructive Hypertrophic Cardiomyopathy: Implications for SARS-CoV-2–Mediated COVID-19. Mayo Clin. Proc. 2020, 95, 1354–1368. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Yin, H.Q.; Yu, Q.T.; Qiao, Y.; Dai, H.Y.; Zhang, M.X.; Zhang, L.; Liu, Y.F.; Wang, L.C.; Liu, D.S.; et al. ACE2 Overexpression Ameliorates Left Ventricular Remodeling and Dysfunction in a Rat Model of Myocardial Infarction. Hum. Gene Ther. 2010, 21, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Burrell, L.M.; Risvanis, J.; Kubota, E.; Dean, R.G.; Macdonald, P.S.; Lu, S.; Tikellis, C.; Grant, S.L.; Lew, R.A.; Smith, A.I.; et al. Myocardial infarction increases ACE2 expression in rat and humans. Eur. Heart J. 2005, 26, 369–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zisman, L.S.; Keller, R.S.; Weaver, B.; Lin, Q.; Speth, R.; Bristow, M.R.; Canver, C.C. Increased angiotensin-(1-7)-forming activity in failing human heart ventricles: Evidence for upregulation of the angiotensin-converting enzyme Homologue ACE2. Circulation 2003, 108, 1707–1712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metkus, T.S.; Sokoll, L.J.; Barth, A.S.; Czarny, M.J.; Hays, A.G.; Lowenstein, C.J.; Michos, E.D.; Nolley, E.P.; Post, W.S.; Resar, J.R.; et al. Myocardial Injury in Severe COVID-19 Compared with Non-COVID-19 Acute Respiratory Distress Syndrome. Circulation 2021, 143, 553–565. [Google Scholar] [CrossRef]
- Musher, D.M.; Abers, M.S.; Corrales-Medina, V.F. Acute Infection and Myocardial Infarction. N. Engl. J. Med. 2019, 380, 171–176. [Google Scholar] [CrossRef]
- Bois, M.C.; Boire, N.A.; Layman, A.J.; Aubry, M.C.; Alexander, M.P.; Roden, A.C.; Hagen, C.E.; Quinton, R.A.; Larsen, C.; Erben, Y.; et al. COVID-19-Associated Nonocclusive Fibrin Microthrombi in the Heart. Circulation 2021, 143, 230–243. [Google Scholar] [CrossRef]
- Perez, A.L.; Grodin, J.L.; Chaikijurajai, T.; Wu, Y.; Hernandez, A.F.; Butler, J.; Metra, M.; Felker, G.M.; Voors, A.A.; McMurray, J.J.; et al. Interleukin-6 and Outcomes in Acute Heart Fail-ure: An ASCEND-HF Substudy. J. Card. Fail. 2021, 27, 670–676. [Google Scholar] [CrossRef]
- Evans, P.C.; Rainger, G.E.; Mason, J.C.; Guzik, T.J.; Osto, E.; Stamataki, Z.; Neil, D.; Hoefer, I.E.; Fragiadaki, M.; Waltenberger, J.; et al. Endothelial dysfunction in COVID-19: A position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc. Res. 2020, 116, 2177–2184. [Google Scholar] [CrossRef]
- Demopoulos, C.; Antonopoulou, S.; Theoharides, T.C. COVID-19, microthromboses, inflammation, and platelet activating factor. BioFactors 2020, 46, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Detopoulou, P.; Nomikos, T.; Fragopoulou, E.; Panagiotakos, D.B.; Pitsavos, C.; Stefanadis, C.; Antonopoulou, S. Lipoprotein-associated phospholipase A2 (Lp-PLA2) activity, platelet-activating factor acetylhydrolase (PAF-AH) in leukocytes and body composition in healthy adults. Lipids Health Dis. 2009, 8, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, M.; Dao, V.; Khan, F. A Review of Platelet-Activating Factor As a Potential Contributor to Morbidity and Mortality Associated with Severe COVID-19. Clin. Appl. Thromb. Hemost. 2021, 27, 10760296211051764. [Google Scholar] [CrossRef] [PubMed]
- Malek, R.J.; Bill, C.A.; Vines, C.M. Clinical drug therapies and biologicals currently used or in clinical trial to treat COVID-19. Biomed Pharmacother. 2021, 144, 112276. [Google Scholar] [CrossRef]
- Martínez, V.G.; Salas, A.A.; Ballestín, S.S. Antiviral Therapeutic Approaches for SARS-CoV-2 Infection: A Systematic Review. Pharmaceuticals 2021, 14, 736. [Google Scholar] [CrossRef]
- Eastman, R.T.; Roth, J.S.; Brimacombe, K.R.; Simeonov, A.; Shen, M.; Patnaik, S.; Hall, M.D. Remdesivir: A Review of Its Discovery and Development Leading to Emergency Use Authorization for Treatment of COVID-19. ACS Cent. Sci. 2020, 6, 672. [Google Scholar] [CrossRef]
- Gubitosa, J.C.; Kakar, P.; Gerula, C.; Nossa, H.; Finkel, D.; Wong, K.; Khatri, M.; Ali, H. Marked Sinus Bradycardia Associated With Remdesivir in COVID-19: A Case and Literature Review. JACC Case Rep. 2020, 2, 2260–2264. [Google Scholar] [CrossRef]
- Gupta, A.K.; Parker, B.M.; Priyadarshi, V.; Parker, J. Cardiac Adverse Events With Remdesivir in COVID-19 Infection. Cureus 2020, 12, e11132. [Google Scholar] [CrossRef]
- Touafchia, A.; Bagheri, H.; Carrié, D.; Durrieu, G.; Sommet, A. Serious bradycardia and remdesivir for coronavirus 2019 (COVID-19): A new safety concerns. Clin. Microbiol. Infect. 2021, 27, 791.e5–791.e8. [Google Scholar] [CrossRef]
- Mehta, N.; Mazer-Amirshahi, M.; Alkindi, N.; Pourmand, A. Pharmacotherapy in COVID-19; A narrative review for emergency providers. Am. J. Emerg. Med. 2020, 38, 1488–1493. [Google Scholar] [CrossRef]
- Perazzolo, S.; Zhu, L.; Lin, W.; Nguyen, A.; Ho, R.J. Systems and Clinical Pharmacology of COVID-19 Therapeutic Candidates: A Clinical and Translational Medicine Perspective. J. Pharm. Sci. 2021, 110, 1002–1017. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, L. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef]
- Alattar, R.; Ibrahim, T.B.; Shaar, S.H.; Abdalla, S.; Shukri, K.; Daghfal, J.N.; Khatib, M.Y.; Aboukamar, M.; Abukhattab, M.; Alsoub, H.A.; et al. Tocilizumab for the treatment of severe coronavirus disease 2019. J. Med. Virol. 2020, 92, 2042–2049. [Google Scholar] [CrossRef]
- Gabay, C.; Riek, M.; Hetland, M.L.; Hauge, E.M.; Pavelka, K.; Tomšič, M.; Canhao, H.; Chatzidionysiou, K.; Lukina, G.; Nordström, D.C.; et al. Effectiveness of tocilizumab with and without synthetic disease-modifying antirheumatic drugs in rheumatoid arthritis: Results from a European collaborative study. Ann. Rheum. Dis. 2016, 75, 1336–1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keyaerts, E.; Vijgen, L.; Maes, P.; Neyts, J.; Van Ranst, M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem. Biophys. Res. Commun. 2004, 323, 264–268. [Google Scholar] [CrossRef]
- Liu, J.; Cao, R.; Xu, M.; Wang, X.; Zhang, H.; Hu, H.; Li, Y.; Hu, Z.; Zhong, W.; Wang, M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020, 6, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatre, C.; Roubille, F.; Vernhet, H.; Jorgensen, C.; Pers, Y.M. Cardiac Complications Attributed to Chloroquine and Hydroxychloroquine: A Systematic Review of the Literature. Drug Saf. 2018, 41, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Jenista, E.R.; Wendell, D.C.; Azevedo, C.F.; Campbell, M.J.; Darty, S.N.; Parker, M.A.; Kim, R.J. Patients With Acute Myocarditis Following mRNA COVID-19 Vaccination. JAMA Cardiol. 2021, 6, 1196. [Google Scholar] [CrossRef]
- Verma, A.K.; Lavine, K.J.; Lin, C.-Y. Myocarditis after COVID-19 mRNA Vaccination. N. Engl. J. Med. 2021, 385, 1332–1334. [Google Scholar] [CrossRef]
- Montgomery, J.; Ryan, M.; Engler, R.; Hoffman, D.; McClenathan, B.; Collins, L.; Loran, D.; Hrncir, D.; Herring, K.; Platzer, M.; et al. Myocarditis Following Immunization With mRNA COVID-19 Vaccines in Members of the US Military. JAMA Cardiol. 2021, 6, 1202. [Google Scholar] [CrossRef]
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis With COVID-19 mRNA Vaccines. Circulation 2021, 144, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wong, C.K.; Un, K.C.; Lau, Y.M.; Lee, J.C.Y.; Tam, F.C.C.; Lau, Y.M.; Lai, W.H.; Tam, A.R.; Lam, Y.Y.; et al. Cardiovascular sequalae in uncomplicated COVID-19 survivors. PLoS ONE 2021, 16, e0246732. [Google Scholar] [CrossRef] [PubMed]
- Satterfield, B.A.; Bhatt, D.L.; Gersh, B.J. Cardiac involvement in the long-term implications of COVID-19. Nat. Rev. Cardiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Li, H. Loneliness, Social Isolation, and Cardiovascular Health. Antioxid. Redox Signal. 2018, 28, 837–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vigorito, C.; Giallauria, F. Loneliness, social isolation and risk of cardiovascular disease in the English Longitudinal Study of Ageing. Eur. J. Prev. Cardiol. 2018, 25, 1384–1386. [Google Scholar] [CrossRef]
- Valtorta, N.K.; Kanaan, M.; Gilbody, S.; Ronzi, S.; Hanratty, B. Loneliness and social isolation as risk factors for coronary heart disease and stroke: Systematic review and meta-analysis of longitudinal observational studies. Heart 2016, 102, 1009–1016. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Bai, H.; Liu, J.; Chen, G.; Liao, Q.; Yang, J.; Wu, P.; Wei, J.; Ma, D.; Chen, G.; et al. Distinct Clinical Characteristics and Risk Factors for Mortality in Female Inpatients With Coronavirus Disease 2019 (COVID-19): A Sex-stratified, Large-scale Cohort Study in Wuhan, China. Clin. Infect. Dis. 2020, 71, 3188–3195. [Google Scholar] [CrossRef] [PubMed]
- Team TNCPERE. The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19)—China, 2020. China CDC Wkly. 2020, 2, 113. [Google Scholar] [CrossRef]
- Ruzzenenti, G.; Maloberti, A.; Giani, V.; Biolcati, M.; Leidi, F.; Monticelli, M.; Grasso, E.; Cartella, I.; Palazzini, M.; Garatti, L.; et al. COVID and Cardiovascular Diseases: Direct and Indirect Damages and Future Perspective. High Blood Press. Cardiovasc. Prev. 2021, 28, 439–445. [Google Scholar] [CrossRef]
- Cenko, E.; Badimon, L.; Bugiardini, R.; Claeys, M.J.; De Luca, G.; de Wit, C.; Derumeaux, G.; Dorobantu, M.; Duncker, D.J.; Eringa, E.C.; et al. Cardiovascular disease and COVID-19: A consensus paper from the ESC Working Group on Coronary Pathophysiology & Microcirculation, ESC Working Group on Thrombosis and the Association for Acute CardioVascular Care (ACVC), in collaboration with the European Heart Rhythm Association (EHRA). Cardiovasc. Res. 2021, 11, 2705–2729. [Google Scholar]
- Galiuto, L.; Locorotondo, G. Gender differences in cardiovascular disease. J. Integr. Cardiol. 2015, 1, 20–22. [Google Scholar]
- Raparelli, V.; Romiti, G.F.; Spugnardi, V.; Borgi, M.; Cangemi, R.; Basili, S.; Proietti, M. The EVA Collaborative Group Gender-Related Determinants of Adherence to the Mediterranean Diet in Adults with Ischemic Heart Disease. Nutrients 2020, 12, 759. [Google Scholar] [CrossRef] [Green Version]
- Regitz-Zagrosek, V.; Oertelt-Prigione, S.; Seeland, U.; Hetzer, R. Sex and Gender Differences in Myocardial Hypertrophy and Heart Failure. Circ. J. 2010, 74, 1265–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regitz-Zagrosek, V.; Kararigas, G. Mechanistic Pathways of Sex Differences in Cardiovascular Disease. Physiol. Rev. 2017, 97, 1–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chilazi, M.; Duffy, E.Y.; Thakkar, A.; Michos, E.D. COVID and Cardiovascular Disease: What We Know in 2021. Curr. Atheroscler. Rep. 2021, 23, 37. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, Y.; Wang, D.W. SARS-CoV-2: A potential novel etiology of fulminant myocarditis. Herz 2020, 45, 230–232. [Google Scholar] [CrossRef] [Green Version]
- Madjid, M.; Safavi-Naeini, P.; Solomon, S.D.; Vardeny, O. Potential Effects of Coronaviruses on the Cardiovascular System: A Review. JAMA Cardiol. 2020, 5, 831–840. [Google Scholar] [CrossRef] [Green Version]
- Ritter, O.; Kararigas, G. Sex-Biased Vulnerability of the Heart to COVID-19. Proc. Mayo Clin. Proc. 2020, 95, 2332–2335. [Google Scholar] [CrossRef]
- Wang, K.; Gheblawi, M.; Oudit, G.Y. Angiotensin Converting Enzyme 2: A Double-Edged Sword. Circulation 2020, 142, 426–428. [Google Scholar] [CrossRef]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Brojakowska, A.; Narula, J.; Shimony, R.; Bander, J. Clinical Implications of SARS-CoV-2 Interaction With Renin Angiotensin System: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 75, 3085–3095. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Liu, C.; Yang, J.; Yang, Y.; Chen, R.; Ding, X.; Gao, X.; Ke, J.; Yuan, F.; He, C.; et al. Sex Differences in the Incidence and Risk Factors of Myocardial Injury in COVID-19 Patients: A Retrospective Cohort Study. Front. Physiol. 2021, 12, 632123. [Google Scholar] [CrossRef] [PubMed]
- Pullen, A.B.; Kain, V.; Serhan, C.N.; Halade, G.V. Molecular and Cellular Differences in Cardiac Repair of Male and Female Mice. J. Am. Heart Assoc. 2020, 9, e015672. [Google Scholar] [CrossRef] [PubMed]
- Stefanini, G.G.; Montorfano, M.; Trabattoni, D.; Andreini, D.; Ferrante, G.; Ancona, M.; Metra, M.; Curello, S.; Maffeo, D.; Pero, G.; et al. ST-Elevation Myocardial Infarction in Patients With COVID-19: Clinical and Angiographic Outcomes. Circulation 2020, 141, 2113–2116. [Google Scholar] [CrossRef]
- Piazza, G.; Campia, U.; Hurwitz, S.; Snyder, J.E.; Rizzo, S.M.; Pfeferman, M.B.; Morrison, R.B.; Leiva, O.; Fanikos, J.; Nauffal, V.; et al. Registry of Arterial and Venous Thromboembolic Complications in Patients With COVID-19. J. Am. Coll. Cardiol. 2020, 76, 2060–2072. [Google Scholar] [CrossRef]
- Shah, A.; Donovan, K.; McHugh, A.; Pandey, M.; Aaron, L.; Bradbury, C.A.; Stanworth, S.J.; Alikhan, R.; Von Kier, S.; Maher, K.; et al. Thrombotic and haemorrhagic complications in critically ill patients with COVID-19: A multicentre observational study. Crit. Care 2020, 24, 1–10. [Google Scholar] [CrossRef]
- Kyrle, P.A.; Minar, E.; Bialonczyk, C.; Hirschl, M.; Weltermann, A.; Eichinger, S. The Risk of Recurrent Venous Thromboembolism in Men and Women. N. Engl. J. Med. 2004, 350, 2558–2563. [Google Scholar] [CrossRef]
- La Vignera, S.; Cannarella, R.; Condorelli, R.A.; Torre, F.; Aversa, A.; Calogero, A.E. Sex-Specific SARS-CoV-2 Mortality: Among Hormone-Modulated ACE2 Expression, Risk of Venous Thromboembolism and Hypovitaminosis D. Int. J. Mol. Sci. 2020, 21, 2948. [Google Scholar] [CrossRef]
- Lete, I. Combined hormonal contraception and COVID-19. Eur. J. Contracept. Reprod. Health Care 2021, 26, 128–131. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, H.; Zeng, Q. Clinical characteristics of COVID-19 patients with complication of cardiac arrhythmia. J. Infect. 2020, 81, e6–e8. [Google Scholar] [CrossRef]
- Klein, S.L. Sex influences immune responses to viruses, and efficacy of prophylaxis and therapeutic treatments for viral diseases. Bioessays 2012, 34, 1050. [Google Scholar] [CrossRef] [Green Version]
- Rivera, D.R.; Peters, S.; Panagiotou, O.A.; Shah, D.P.; Kuderer, N.M.; Hsu, C.-Y.; Rubinstein, S.M.; Lee, B.J.; Choueiri, T.K.; de Lima Lopes, G., Jr.; et al. Utilization of COVID-19 treatments and clinical outcomes among patients with cancer: A COVID-19 and Cancer Consortium (CCC19) cohort study. Cancer Discov. 2020, 10, 1514–1527. [Google Scholar] [CrossRef]
- Vernaz-Hegi, N.; Agoritsas, T.; Calmy, A.; Gayet-Ageron, A.; Gold, G.; Perrier, A.; Picard, F.; Prendki, V.; Reny, J.L.; Samer, C.F.; et al. Early experimental COVID-19 therapies: Associations with length of hospital stay, mortality and related costs. Swiss Med. Wkly. 2020, 150, w20446. [Google Scholar]
- Taquet, M.; Dercon, Q.; Luciano, S.; Geddes, J.R.; Husain, M.; Harrison, P.J. Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021, 18, e1003773. [Google Scholar] [CrossRef]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; Macary, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Ng, S.C.; Tilg, H. COVID-19 and the gastrointestinal tract: More than meets the eye. Gut 2020, 69, 973–974. [Google Scholar] [CrossRef] [Green Version]
- Ronco, C.; Reis, T.; Husain-Syed, F. Management of acute kidney injury in patients with COVID-19. Lancet Respir. Med. 2020, 8, 738–742. [Google Scholar] [CrossRef]
- Zheng, Y.Y.; Ma, Y.T.; Zhang, J.Y.; Xie, X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020, 17, 259–260. [Google Scholar] [CrossRef] [Green Version]
- Feng, G.; Zheng, K.; Yan, Q.-Q.; Rios, R.S.; Targher, G.; Byrne, C.D.; Van Poucke, S.; Liu, W.-Y.; Zheng, M.-H. COVID-19 and Liver Dysfunction: Current Insights and Emergent Therapeutic Strategies. J. Clin. Transl. Hepatol. 2020, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- Gebhard, C.; Regitz-Zagrosek, V.; Neuhauser, H.K.; Morgan, R.; Klein, S.L. Impact of sex and gender on COVID-19 outcomes in Europe. Biol. Sex Differ. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Haitao, T.; Vermunt, J.; Abeykoon, J.; Ghamrawi, R.; Gunaratne, M.; Jayachandran, M.; Narang, K.; Parashuram, S.; Suvakov, S.; Garovic, V. COVID-19 and Sex Differences: Mechanisms and Biomarkers. Mayo Clin. Proc. 2020, 95, 2189–2203. [Google Scholar] [CrossRef]
- Shi, S.; Qin, M.; Cai, Y.; Liu, T.; Shen, B.; Yang, F.; Cao, S.; Liu, X.; Xiang, Y.; Zhao, Q.; et al. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur. Heart J. 2020, 41, 2070–2079. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Cunningham, J.W.; Claggett, B.L.; Jering, K.S.; Vaduganathan, M.; Bhatt, A.S.; Rosenthal, N.; Solomon, S.D. Prognostic Value of Natriuretic Peptides & Cardiac Troponins in COVID-19. Circulation 2021, 144, 177–179. [Google Scholar] [CrossRef]
- Sandoval, Y.; Januzzi, J.L., Jr.; Jaffe, A.S. Cardiac Troponin for Assessment of Myocardial Injury in COVID-19: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 76, 1244–1258. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.; Giannitsis, E.; Jaffe, A.S.; Huber, K.; Mair, J.; Cullen, L.; Hammarsten, O.; Mills, N.L.; Möckel, M.; Krychtiuk, K.; et al. Cardiovascular biomarkers in patients with COVID-19. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Ten-Caten, F.; Gonzalez-Dias, P.; Castro, Í.; Ogava, R.L.; Giddaluru, J.; Silva, J.C.S.; Martins, F.; Gonçalves, A.N.; Costa-Martins, A.G.; Araujo, J.D.; et al. In-depth analysis of laboratory parameters reveals the interplay between sex, age, and systemic inflammation in individuals with COVID-19. Int. J. Infect. Dis. 2021, 105, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, M.U.; Ikram, M.; Shafiq, Z.; Sarfraz, A.; Sarfraz, Z.; Jaiswal, V.; Sarfraz, M.; Chérrez-Ojeda, I. COVID-19 Vaccine-Associated Thrombosis With Thrombocytopenia Syndrome (TTS): A Systematic Review and Post Hoc Analysis. Clin. Appl. Thromb. 2021, 27, 10760296211048815. [Google Scholar] [CrossRef] [PubMed]
- Khera, A.; McGuire, D.K.; Murphy, S.A.; Stanek, H.G.; Das, S.R.; Vongpatanasin, W.; Wians, F.H.; Grundy, S.M.; de Lemos, J.A. Race and gender differences in C-reactive protein levels. J. Am. Coll. Cardiol. 2005, 46, 464–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ershler, W.B.; Keller, E.T. Age-Associated Increased Interleukin-6 Gene Expression, Late-Life Diseases, and Frailty. Annu. Rev. Med. 2000, 51, 245–270. [Google Scholar] [CrossRef]
- Wener, M.H.; Daum, P.R.; McQuillan, G.M. The influence of age, sex, and race on the upper reference limit of serum C-reactive protein concentration. J. Rheumatol. 2000, 27, 2351–2359. [Google Scholar]
- Lau, E.S.; McNeill, J.N.; Paniagua, S.M.; Liu, E.E.; Wang, J.K.; Bassett, I.V.; Selvaggi, C.A.; Lubitz, S.A.; Foulkes, A.S.; Ho, J.E. Sex differences in inflammatory markers in patients hospitalized with COVID-19 infection: Insights from the MGH COVID-19 patient registry. PLoS ONE 2021, 16, e0250774. [Google Scholar] [CrossRef] [PubMed]
- Risitano, A.M.; Mastellos, D.C.; Huber-Lang, M.; Yancopoulou, D.; Garlanda, C.; Ciceri, F.; Lambris, J.D. Complement as a target in COVID-19? Nat. Rev. Immunol. 2020, 20, 343–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Channappanavar, R.; Fett, C.; Mack, M.; Eyck PPTen Meyerholz, D.K.; Perlman, S. Sex-Based Differences in Susceptibility to Severe Acute Respiratory Syndrome Coronavirus Infection. J. Immunol. 2017, 198, 4046–4053. [Google Scholar] [CrossRef] [PubMed]
- Kadel, S.; Kovats, S. Sex Hormones Regulate Innate Immune Cells and Promote Sex Differences in Respiratory Virus Infection. Front. Immunol. 2018, 9, 1653. [Google Scholar] [CrossRef]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef] [Green Version]
- Guan, W.-J.; Liang, W.-H.; Zhao, Y.; Liang, H.-R.; Chen, Z.-S.; Li, Y.-M.; Liu, X.-Q.; Chen, R.-C.; Tang, C.-L.; Wang, T.; et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis. Eur. Respir. J. 2020, 55, 2000547. [Google Scholar] [CrossRef] [Green Version]
- Oudit, G.Y.; Pfeffer, M.A. Plasma angiotensin-converting enzyme 2: Novel biomarker in heart failure with implications for COVID-19. Eur. Heart J. 2020, 41, 1818–1820. [Google Scholar] [CrossRef]
- Verdecchia, P.; Cavallini, C.; Spanevello, A.; Angeli, F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur. J. Intern. Med. 2020, 76, 14–20. [Google Scholar] [CrossRef]
- Schunkert, H.; Danser, A.J.; Hense, H.-W.; Derkx, F.H.; Kürzinger, S.; Riegger, G.A. Effects of Estrogen Replacement Therapy on the Renin-Angiotensin System in Postmenopausal Women. Circulation 1997, 95, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Jehpsson, L.; Sun, J.; Nilsson, P.M.; Edsfeldt, A.; Swärd, P. Serum Renin Levels Increase With Age in Boys Resulting in Higher Renin Levels in Young Men Compared to Young Women, and Soluble Angiotensin-Converting Enzyme 2 Correlates With Renin and Body Mass Index. Front. Physiol. 2021, 11, 622179. [Google Scholar] [CrossRef] [PubMed]
- Straub, R.H. The Complex Role of Estrogens in Inflammation. Endocr. Rev. 2007, 28, 521–574. [Google Scholar] [CrossRef] [Green Version]
- Furman, D.; Hejblum, B.P.; Simon, N.; Jojic, V.; Dekker, C.L.; Thiébaut, R.; Tibshirani, R.J.; Davis, M.M. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 869–874. [Google Scholar] [CrossRef] [Green Version]
- White, M.C.; Fleeman, R.; Arnold, A.C. Sex differences in the metabolic effects of the renin-angiotensin system. Biol. Sex Differ. 2019, 10, 31. [Google Scholar] [CrossRef] [Green Version]
- Kornilov, S.A.; Lucas, I.; Jade, K.; Dai, C.; Lovejoy, J.C.; Magis, A. Plasma levels of soluble ACE2 are associated with sex, Metabolic Syndrome, and its biomarkers in a large cohort, pointing to a possible mechanism for increased severity in COVID-19. Crit. Care 2020, 24, 452. [Google Scholar] [CrossRef] [PubMed]
- Sama, I.E.; Ravera, A.; Santema, B.T.; Van Goor, H.; Ter Maaten, J.M.; Cleland, J.G.; Rienstra, M.; Friedrich, A.W.; Samani, N.J.; Ng, L.L.; et al. Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin-angiotensin-aldosterone inhibitors. Eur. Heart J. 2020, 41, s1810–s1817. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Megiorni, F.; Pontecorvi, P.; Gerini, G.; Anastasiadou, E.; Marchese, C.; Ceccarelli, S. Sex-Related Factors in Cardiovascular Complications Associated to COVID-19. Biomolecules 2022, 12, 21. https://doi.org/10.3390/biom12010021
Megiorni F, Pontecorvi P, Gerini G, Anastasiadou E, Marchese C, Ceccarelli S. Sex-Related Factors in Cardiovascular Complications Associated to COVID-19. Biomolecules. 2022; 12(1):21. https://doi.org/10.3390/biom12010021
Chicago/Turabian StyleMegiorni, Francesca, Paola Pontecorvi, Giulia Gerini, Eleni Anastasiadou, Cinzia Marchese, and Simona Ceccarelli. 2022. "Sex-Related Factors in Cardiovascular Complications Associated to COVID-19" Biomolecules 12, no. 1: 21. https://doi.org/10.3390/biom12010021
APA StyleMegiorni, F., Pontecorvi, P., Gerini, G., Anastasiadou, E., Marchese, C., & Ceccarelli, S. (2022). Sex-Related Factors in Cardiovascular Complications Associated to COVID-19. Biomolecules, 12(1), 21. https://doi.org/10.3390/biom12010021