Chemistry and Biochemistry Aspects of the 4-Hydroxy-2,3-trans-nonenal
Abstract
:1. Introduction
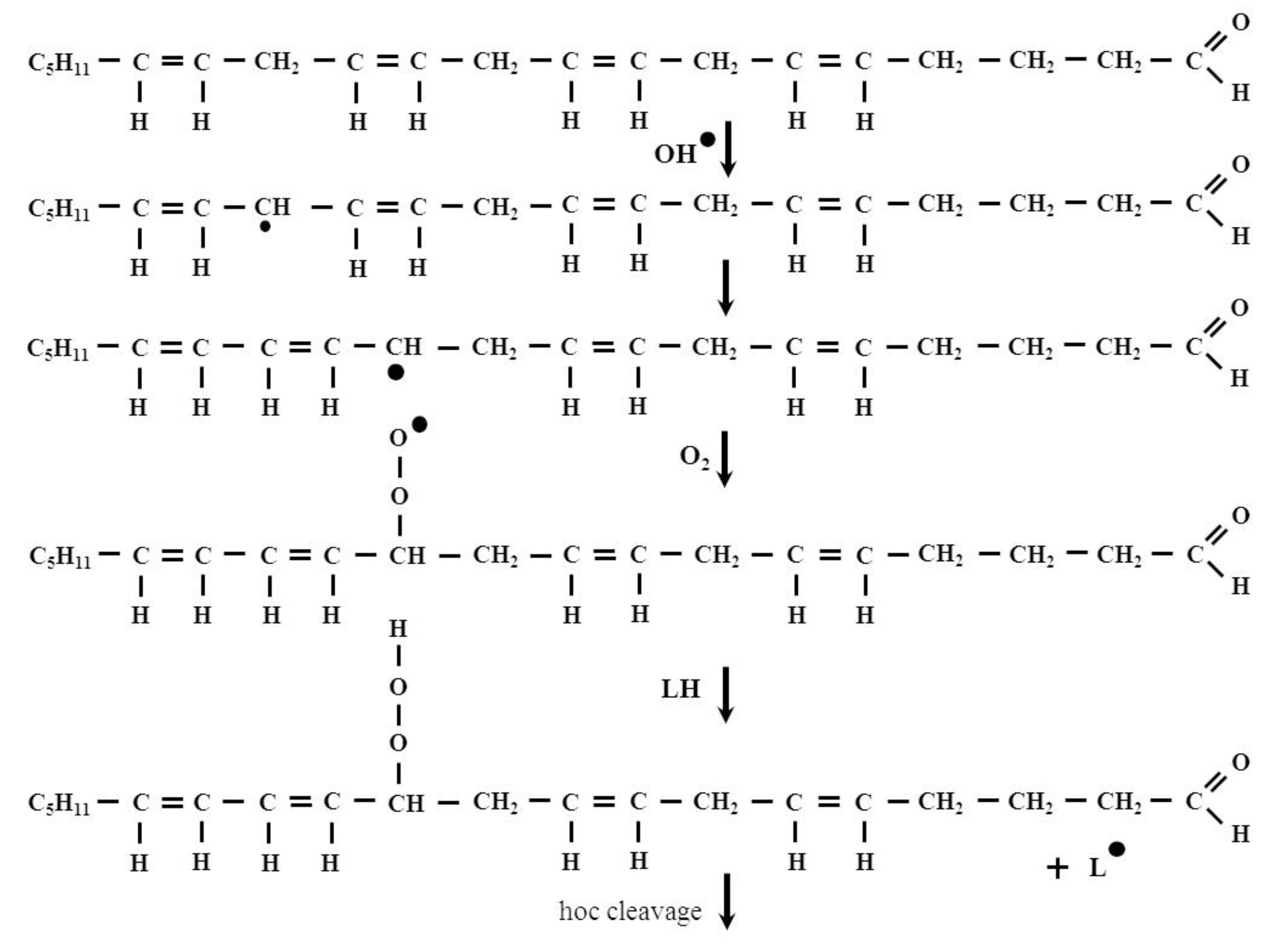
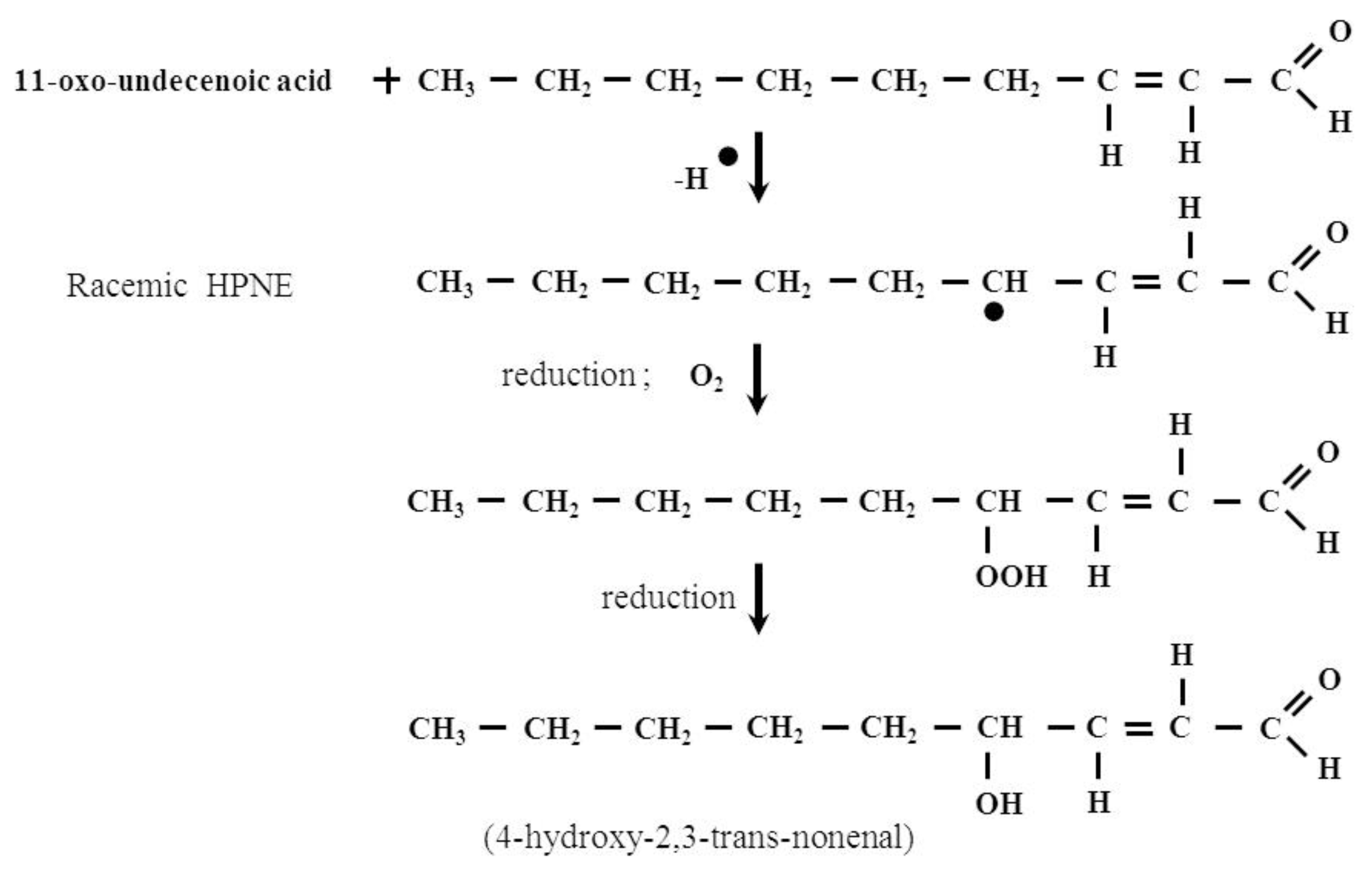
2. The Formation and Removal of HNE
- Initiation:
- LH + •OH → L• + H2O
- Propagation:
- L• + O2 → LOO•
- Propagation:
- LOO• + LH → LOOH + L•
- Propagation:
- LOOH → LO•, LOO•, •OH, aldehydes, ketones, alcohols
- Termination:
- L• + L• → L-L (lipid dimer; stable nonradical product)
- Termination:
- LOO• + LOO• → LOOL (stable nonradical product) + O2
- Termination:
- L• + LOO• → LOOL (stable nonradical product)
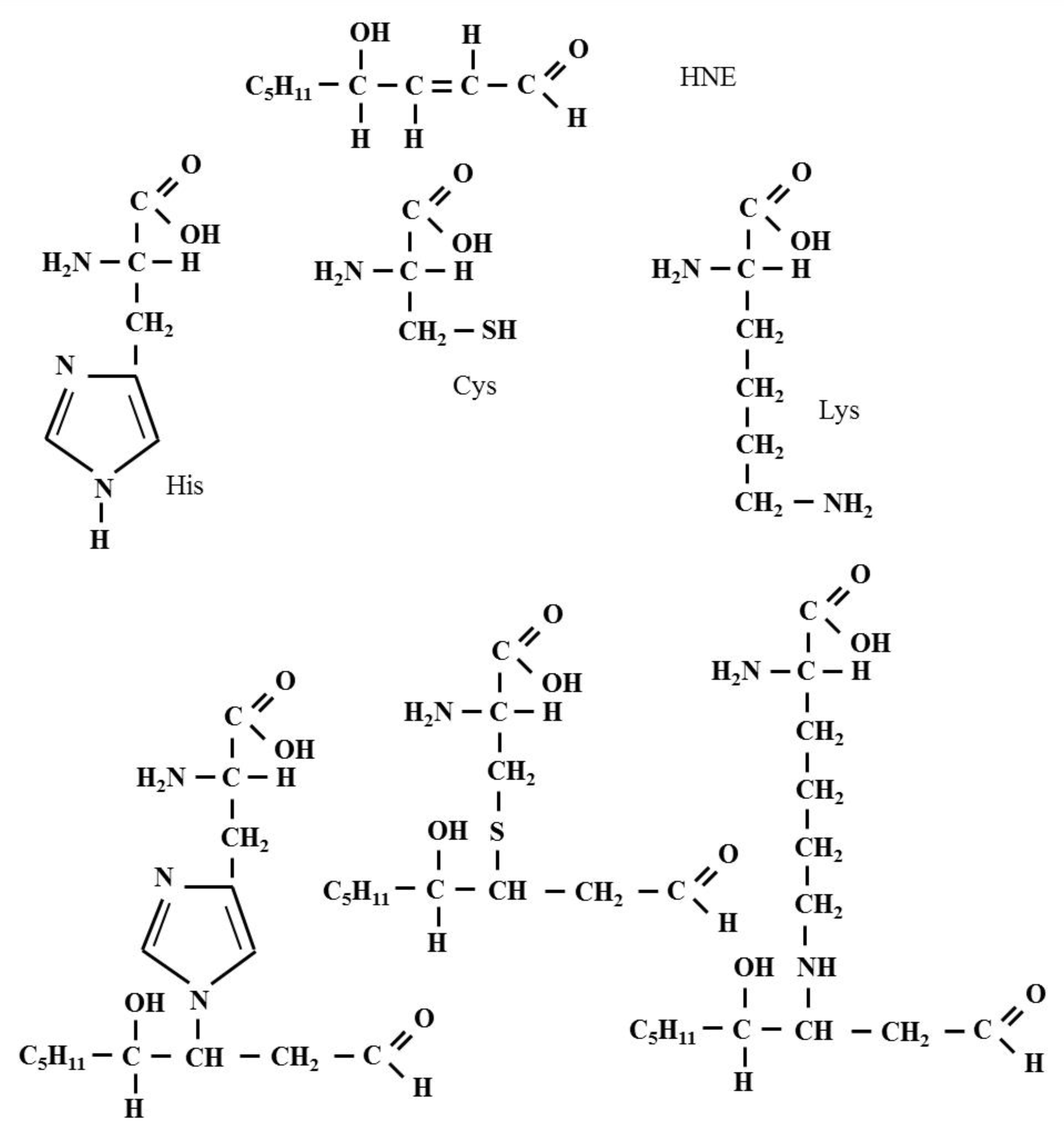
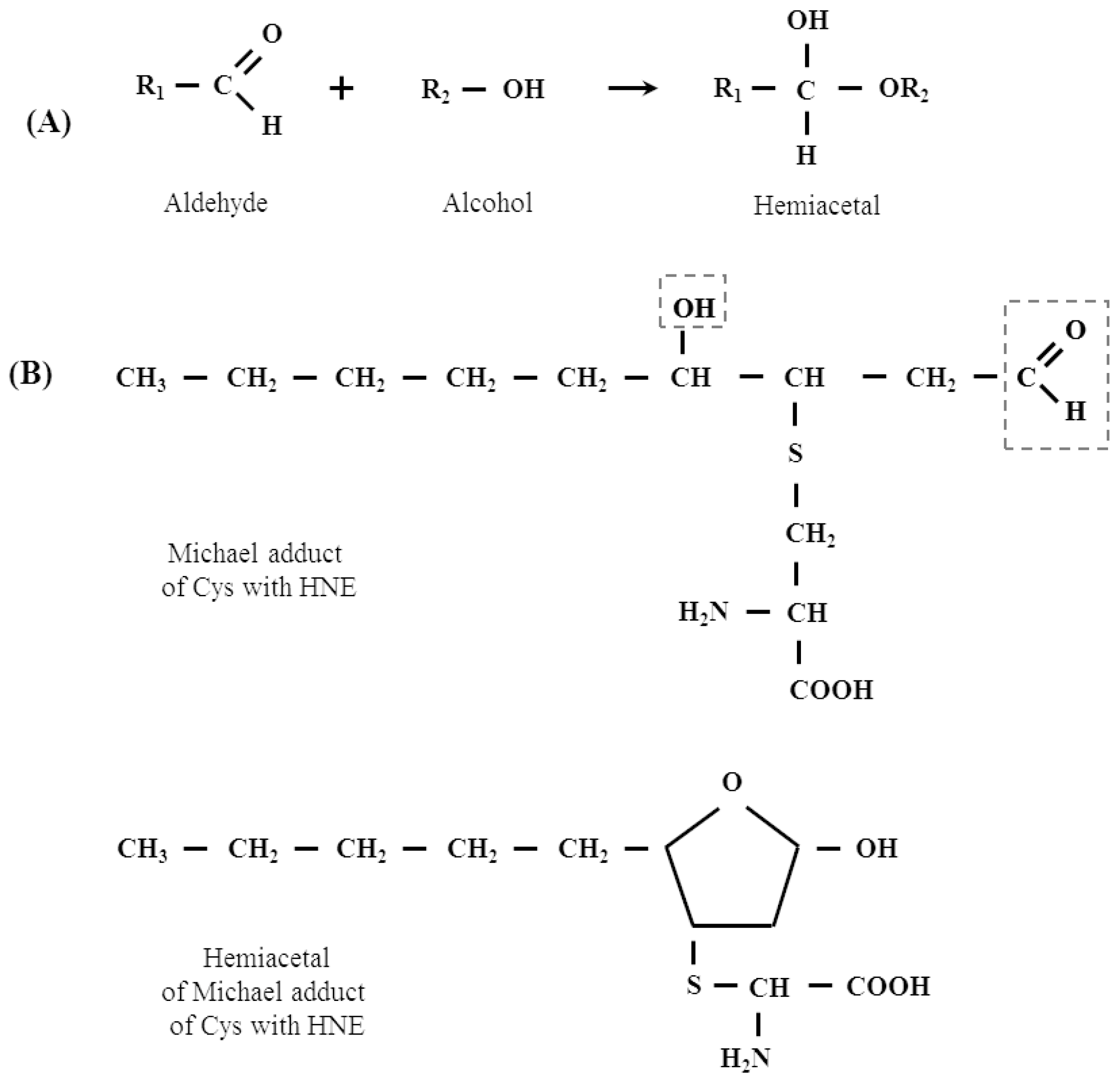
2.1. Protein Adducts
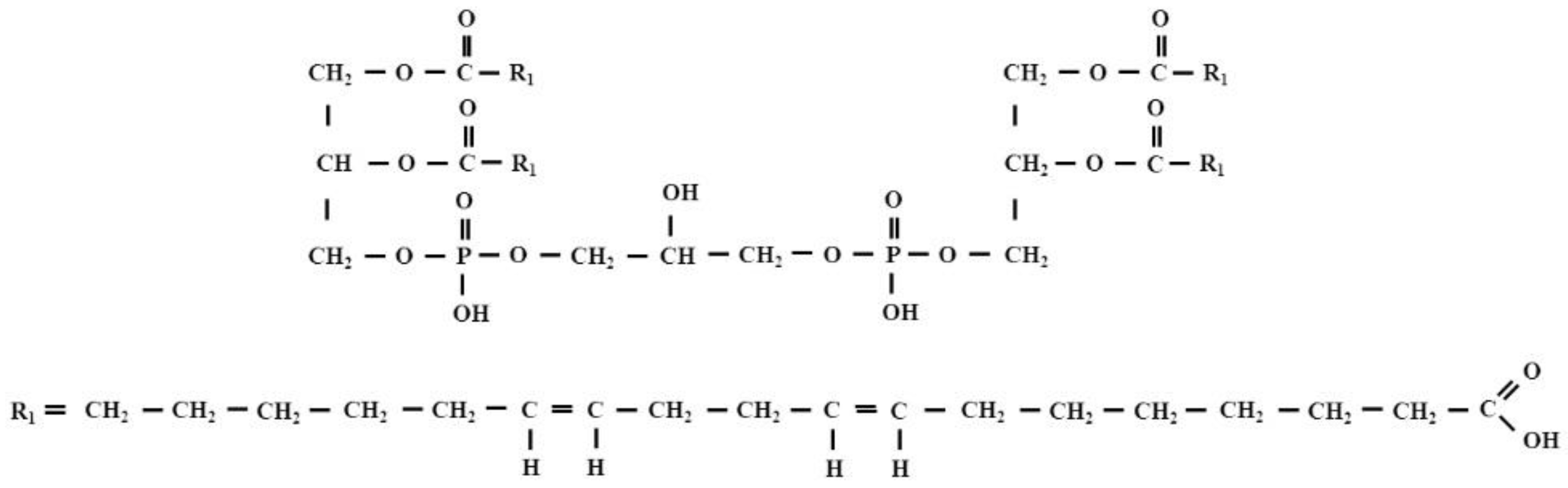
2.2. Lipid Adducts
2.3. Adducts with Nucleic Acids
3. HNE in Pathology
A Dual Role of HNE in Cancer
4. Conclusions
HNE Is Not Only Harmful but Also Beneficial
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benedetti, A.; Comporti, M.; Esterbauer, H. Identification of 4-hydroxynonenal as a cytotoxic product originating from the peroxidation of liver microsomal lipids. Biochim. Biophys. Acta 1980, 620, 281–296. [Google Scholar] [CrossRef]
- Cadenas, E.; Muller, A.; Brigelius, R.; Esterbauer, H.; Sies, H. Effects of 4-hydroxynonenal on isolated hepatocytes. Studies on chemiluminescence response, alkane production and glutathione status. Biochem. J. 1983, 214, 479–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esterbauer, H.; Benedetti, A.; Lang, J.; Fulceri, R.; Fauler, G.; Comporti, M. Studies on the mechanism of formation of 4-hydroxynonenal during microsomal lipid peroxidation. Biochim. Biophys. Acta Lipids Lipid Metab. 1986, 876, 154–166. [Google Scholar] [CrossRef]
- Schauenstein, E.; Esterbauer, H. Formation and properties of reactive aldehydes. Ciba Found. Symp. 1978, 67, 225–244. [Google Scholar]
- Winkler, P.; Lindner, W.; Esterbauer, H.; Schauenstein, E.; Schaur, R.J.; Khoschsorur, G.A. Detection of 4-hydroxynonenal as a product of lipid peroxidation in native Ehrlich ascites tumor cells. Biochim. Biophys. Acta Lipids Lipid Metab. 1984, 796, 232–237. [Google Scholar] [CrossRef]
- Schaur, R.J.; Siems, W.; Bresgen, N.; Eckl, P.M. 4-hydroxy-nonenal—A bioactive lipid peroxidation product. Biomolecules 2015, 5, 2247–2337. [Google Scholar] [CrossRef] [Green Version]
- Esterbauer, H.; Eckl, P.; Ortner, A. Possible mutagens derived from lipids and lipid precursors. Mutat. Res. 1990, 238, 223–233. [Google Scholar] [CrossRef]
- Schaur, R.J. Basic aspects of the biochemical reactivity of 4-hydroxynonenal. Mol. Asp. Med. 2003, 24, 149–159. [Google Scholar] [CrossRef]
- Dix, T.A.; Aikens, J. Mechanisms and biological relevance of lipid peroxidation initiation. Chem. Res. Toxicol. 1993, 6, 2–18. [Google Scholar] [CrossRef]
- Moore, K.; Roberts, L.J. Measurement of lipid peroxidation. Free Radic. Res. 1998, 28, 659–671. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Leake, D.; Bruckdorfer, K.R.; Diplock, A.T. Practical approaches to low density lipoprotein oxidation: Whys, wherefores and pitfalls. Free Radic. Res. 1996, 25, 285–1311. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Role of free radicals and catalytic metal ions in human disease: An overview. Methods Enzymol. 1990, 186, 1–85. [Google Scholar]
- Hicks, M.; Delbridge, L.; Yue, D.K.; Reeve, T.S. Catalysis of lipid peroxidation by glucose and glycosylated collagen. Biochem. Biophys. Res. Commun. 1988, 151, 649–655. [Google Scholar] [CrossRef]
- Csala, M.; Kardon, T.; Legeza, B.; Lizák, B.; Mandl, J.; Margittai, É.; Puskás, F.; Száraz, P.; Szelényi, P.; Bánhegyi, G. On the role of 4-hydroxynonenal in health and disease. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 826–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, M.; Ishida, N. β-Scission of alkoxy radicals in synthetic transformations. Chem. Lett. 2017, 46, 1692–1700. [Google Scholar] [CrossRef]
- Haze, S.; Gozu, Y.; Nakamura, S.; Kohno, Y.; Sawano, K.; Ohta, H.; Yamazaki, K. 2-Nonenal Newly Found in Human Body Odor Tends to Increase with Aging. J. Investig. Dermatol. 2001, 116, 520–524. [Google Scholar] [CrossRef] [Green Version]
- Mol, M.; Regazzoni, L.; Altomare, A.; Degani, G.; Carini, M.; Vistoli, G.; Aldini, G. Enzymatic and non-enzymatic detoxification of 4-hydroxynonenal: Methodological aspects and biological consequences. Free Radic. Biol. Med. 2017, 111, 328–344. [Google Scholar] [CrossRef] [PubMed]
- Shappell, S.B.; Boeglin, W.E.; Olson, S.J.; Kasper, S.; Brash, A.R. 15-lipoxygenase-2 (15-LOX-2) is expressed in benign prostatic epithelium and reduced in prostate adenocarcinoma. Am. J. Pathol. 1999, 155, 235–245. [Google Scholar] [CrossRef] [Green Version]
- Mashima, R.; Okuyama, T. The role of lipoxygenases in pathophysiology; new insights and future perspectives. Redox Biol. 2015, 6, 297–310. [Google Scholar] [CrossRef] [Green Version]
- Riahi, Y.; Cohen, G.; Shamni, O.; Sasson, S. Signaling and cytotoxic functions of 4-hydroxyalkenals. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E879–E886. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Allen, T.D.; Yang, Y.; Moore, D.R.; Huycke, M.M. Cyclooxygenase-2 generates the endogenous mutagen trans-4-hydroxy-2-nonenal in Enterococcus faecalis–infected macrophages. Cancer Prev. Res. 2013, 6, 206–216. [Google Scholar] [CrossRef] [Green Version]
- Dalleau, S.; Baradat, M.; Guéraud, F.; Huc, L. Cell death and diseases related to oxidative stress: 4-hydroxynonenal (HNE) in the balance. Cell Death Differ. 2013, 20, 1615–1630. [Google Scholar] [CrossRef] [Green Version]
- Ferro, M.; Marinari, U.M.; Poli, G.; Dianzani, M.U.; Fauler, G.N.; Zollner, H.; Esterbauer, H. Metabolism of 4-hydroxynonenal by the rat hepatoma cell line MH1C1. Cell Biochem. Funct. 1988, 6, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Gasparovic, A.C.; Milkovic, L.; Sunjic, S.B.; Zarkovic, N. Cancer growth regulation by 4-hydroxynonenal. Free Radic. Biol. Med. 2017, 111, 226–234. [Google Scholar] [CrossRef]
- Doorn, J.A.; Petersen, D.R. Covalent adduction of nucleophilic amino acids by 4-hydroxynonenal and 4-oxononenal. Chem. Biol. Interact. 2003, 143–144, 93–100. [Google Scholar] [CrossRef]
- Altomare, A.; Baron, G.; Gianazza, E.; Banfi, C.; Carini, M.; Aldini, G. Lipid peroxidation derived reactive carbonyl species in free and conjugated forms as an index of lipid peroxidation: Limits and perspectives. Redox Biol. 2021, 42, 101899. [Google Scholar] [CrossRef]
- Maier, C.S.; Chavez, J.; Wang, J.; Wu, J. Protein adducts of aldehydic lipid peroxidation products: Identification and characterization of protein adducts using an aldehyde/keto-reactive probe in combination with mass spectrometry. Meth. Enzymol. 2010, 473, 305–330. [Google Scholar]
- Mendez, D.; Hernáez, M.L.; Diez, A.; Puyet, A.; Bautista, J.M. Combined proteomic approaches for the identification of specific amino acid residues modified by 4-hydroxy-2-nonenal under physiological conditions. J. Proteome Res. 2010, 9, 5770–5781. [Google Scholar] [CrossRef]
- Sayre, L.M.; Lin, D.; Yuan, Q.; Zhu, X.; Tang, X. Protein adducts generated from products of lipid oxidation: Focus on HNE and one. Drug Metab. Rev. 2006, 38, 651–675. [Google Scholar] [CrossRef] [PubMed]
- Michael, A. Ueber die Addition von Natriumacetessig- und Natriummalonsäureäthern zu den Aethern ungesättigter Säuren. J. Prakt. Chem. 1887, 35, 349–356. [Google Scholar] [CrossRef] [Green Version]
- Tokoroyama, T. Discovery of the Michael Reaction. Eur. J. Org. Chem. 2010, 10, 2009–2016. [Google Scholar] [CrossRef]
- Schiff, H. Mittheilungen aus dem Universitätslaboratorium in Pisa: Eine neue Reihe organischer Basen. Justus Liebigs Ann. Chem. 1864, 131, 118–119. [Google Scholar] [CrossRef] [Green Version]
- Uchida, K. Cellular response to bioactive lipid peroxidation products. Free Radic. Res. 2000, 6, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Poli, G.; Biasi, F.; Leonarduzzi, G. 4-Hydroxynonenal–protein adducts: A reliable biomarker of lipid oxidation in liver diseases. Mol. Asp. Med. 2008, 29, 67–71. [Google Scholar] [CrossRef]
- Soulage, C.O.; Pelletier, C.C.; Florens, N.; Lemoine, S.; Dubourg, L.; Juillard, L.; Guebre-Egziabher, F. Two Toxic Lipid Aldehydes, 4-hydroxy-2-hexenal (4-HHE) and 4-hydroxy-2-nonenal (4-HNE), Accumulate in Patients with Chronic Kidney Disease. Toxins 2020, 12, 567. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Sultana, R. Redox proteomics: Understanding oxidative stress in the progression of age-related neurodegenerative disorders. Expert Rev. Proteom. 2008, 5, 157–160. [Google Scholar] [CrossRef] [Green Version]
- Beckman, K.B.; Ames, B.N. The free radical theory of aging matures. Physiol. Rev. 1998, 78, 547–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montine, T.J.; Neely, M.D.; Quinn, J.F.; Beal, M.F.; Markesbery, W.R.; Roberts, L.J.; Morrow, J.D. Lipid peroxidation in aging brain and Alzheimer’s disease. Free Radic. Biol. Med. 2002, 33, 620–626. [Google Scholar] [CrossRef]
- Ambrożewicz, E.; Bielawska, K. Protein carbonylation-reasons, effects and determination. Postepy Biochem. 2016, 62, 495–505. (In Polish) [Google Scholar]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Castro, J.P.; Jung, T.; Grune, T.; Siems, W. 4-Hydroxynonenal (HNE) modified proteins in metabolic diseases. Free Radic. Biol. Med. 2017, 111, 309–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elrayess, M.A.; Almuraikhy, S.; Kafienah, W.; Al-Menhali, A.; Al-Khelaifi, F.; Bashah, M.; Zarkovic, K.; Zarkovic, N.; Waeg, G.; Alsayrafi, M.; et al. 4-hydroxynonenal causes impairment of human subcutaneous adipogenesis and induction of adipocyte insulin resistance. Free Radic. Biol. Med. 2017, 104, 129–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaganjac, M.; Milkovic, L.; Gegotek, A.; Cindric, M.; Zarkovic, K.; Skrzydlewska, E.; Zarkovic, N. The relevance of pathophysiological alterations in redox signaling of 4-hydroxynonenal for pharmacological therapies of major stress-associated diseases. Free Radic. Biol. Med. 2020, 157, 128–153. [Google Scholar] [CrossRef] [PubMed]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Mel’nikova, T.I.; Porozov, Y.B.; Terentiev, A.A. Oxidative stress and advanced lipoxidation and glycation end products (ALEs and AGEs) in aging and age-related diseases. Oxidative Med. Cell. Longev. 2019, 2019, 3085756. [Google Scholar] [CrossRef] [Green Version]
- Sonowal, H.; Ramana, K.V. 4-Hydroxy-Trans-2-Nonenal in the Regulation of Anti-Oxidative and Pro-Inflammatory Signaling Pathways. Oxidative Med. Cell. Longev. 2019, 2019, 5937326. [Google Scholar] [CrossRef] [Green Version]
- Cleary, J.A.; Doherty, W.; Evans, P.; Malthouse, J.P. Quantifying tetrahedral adduct formation and stabilization in the cysteine and the serine proteases. Biochim. Biophys. Acta 2015, 185, 1382–1391. [Google Scholar] [CrossRef] [Green Version]
- Guichardant, M.; Taibi-Tronche, P.; Fay, L.B.; Lagarde, M. Covalent modifications of aminophospholipids by 4-hydroxynonenal. Free Radic. Biol. Med. 1998, 25, 1049–1056. [Google Scholar] [CrossRef]
- Bacot, S.; Bernoud-Hubac, N.; Chantegrel, B.; Deshayes, C.; Doutheau, A.; Ponsin, G.; Lagarde, M.; Guichardant, M. Evidence for in situ ethanolamine phospholipid adducts with hydroxy-alkenals. J. Lipid. Res. 2007, 48, 816–825. [Google Scholar] [CrossRef] [Green Version]
- Vazdar, K.; Vojta, D.; Margetić, D.; Vazdar, M. Reaction Mechanism of Covalent Modification of Phosphatidylethanolamine Lipids by Reactive Aldehydes 4-Hydroxy-2-nonenal and 4-Oxo-2-nonenal. Chem. Res. Toxicol. 2017, 30, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Sodum, R.S.; Chung, F.L. Stereoselective formation of in vitro nucleic acid adducts by 2,3-epoxy-4-hydroxynonanal. Cancer Res. 1991, 51, 137–143. [Google Scholar] [PubMed]
- Chung, F.L.; Nath, R.G.; Ocando, J.; Nishikawa, A.; Zhang, L. Deoxyguanosine Adducts of T-4-Hydroxy-2-Nonenal Are Endogenous DNA Lesions in Rodents and Humans: Detection and Potential Sources. Cancer Res. 2000, 60, 1507–1511. [Google Scholar] [PubMed]
- Hu, W.; Feng, Z.; Eveleigh, J.; Iyer, G.; Pan, J.; Amin, S.; Chung, F.L.; Tang, M.S. The major lipid peroxidation product, trans-4-hydroxy-2-nonenal, preferentially forms dna adducts at codon 249 of human p53 gene, a unique mutational hotspot in hepatocellular carcinoma. Carcinogenesis 2002, 23, 1781–1789. [Google Scholar] [CrossRef]
- Dyba, M.; da Silva, B.; Coia, H.; Hou, Y.; Noguchi, S.; Pan, J.; Berry, D.; Creswell, K.; Krzeminski, J.; Desai, D.; et al. Monoclonal Antibodies for the Detection of a Specific Cyclic DNA Adduct Derived from ω-6 Polyunsaturated Fatty Acids. Chem. Res. Toxicol. 2018, 31, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Teufel, U.; Peccerella, T.; Engelmann, G.; Bruckner, T.; Flechtenmacher, C.; Millonig, G.; Stickel, F.; Hoffmann, G.F.; Schirmacher, P.; Mueller, S.; et al. Detection of carcinogenic etheno-DNA adducts in children and adolescents with non-alcoholic steatohepatitis (NASH). Hepatobiliary Surg. Nutr. 2015, 4, 426–435. [Google Scholar] [PubMed]
- Feng, Z.; Hu, W.; Tang, M.S. Trans-4-hydroxy-2-nonenal inhibits nucleotide excision repair in human cells: A possible mechanism for lipid peroxidation-induced carcinogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 8598–8602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil, L.; Siems, W.; Mazurek, B.; Gross, J.; Schroeder, P.; Voss, P.; Grune, T. Age-associated analysis of oxidative stress parameters in human plasma and erythrocytes. Free Radic. Res. 2006, 40, 495–505. [Google Scholar] [CrossRef]
- Aschner, M.; Nguyen, T.T.; Sinitskii, A.I.; Santamaría, A.; Bornhorst, J.; Ajsuvakova, O.P.; da Rocha, J.B.T.; Skalny, A.V.; Tinkov, A.A. Isolevuglandins (isoLGs) as toxic lipid peroxidation byproducts and their pathogenetic role in human diseases. Free Radic Biol. Med. 2021, 162, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical relevance of biomarkers of oxidative stress. Antioxid. Redox Signal. 2015, 23, 1144–1170. [Google Scholar] [CrossRef] [Green Version]
- Jaganjac, M.; Cindrić, M.; Jakovčević, A.; Žarković, K.; Žarković, N. Lipid peroxidation in brain tumors. Neurochem. Int. 2021, 149, 105118. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Zhang, J.; Decker, E.A.; Zhang, G. Roles of Lipid Peroxidation-Derived Electrophiles in Pathogenesis of Colonic Inflammation and Colon Cancer. Front. Cell Dev. Biol. 2021, 9, 665591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fu, L. The role of ALDH2 in tumorigenesis and tumor progression: Targeting ALDH2 as a potential cancer treatment. Acta Pharm. Sin. B 2021, 11, 1400–1411. [Google Scholar] [CrossRef]
- Ramana, K.V.; Bhatnagar, A.; Srivastava, S.; Yadav, U.C.; Awasthi, S.; Awasthi, Y.C.; Srivastava, S.K. Mitogenic responses of vascular smooth muscle cells to lipid peroxidation-derived aldehyde 4-hydroxy-trans-2-nonenal (HNE): Role of aldose reductase-catalyzed reduction of the HNE-glutathione conjugates in regulating cell growth. J. Biol. Chem. 2006, 281, 17652–17660. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Sung, B.; Jang, E.J.; Kim, D.H.; Park, C.H.; Choi, Y.J.; Ha, Y.M.; Kim, M.K.; Kim, N.D.; Yu, P.B.; et al. Inhibitory action of salicylideneamino-2-thiophenol on NF-κB signaling cascade and cyclooxygenase-2 in HNE-treated endothelial cells”. Arch Pharm. Res. 2013, 36, 880–889. [Google Scholar] [CrossRef]
- Jang, E.J.; Jeong, H.O.; Park, D.; Kim, D.H.; Choi, Y.J.; Chung, K.W.; Park, M.H.; Yu, B.P.; Chung, H.Y. Src Tyrosine Kinase Activation by 4-Hydroxynonenal Upregulates p38, ERK/AP-1 Signaling and COX-2 Expression in YPEN-1 Cells. PLoS ONE 2015, 10, e0129244. [Google Scholar] [CrossRef] [Green Version]
- Jang, E.J.; Kim, D.H.; Lee, B.; Lee, E.K.; Chung, K.W.; Moon, K.M.; Kim, M.J.; An, H.J.; Jeong, J.W.; Kim, Y.R.; et al. Activation of proinflammatory signaling by 4-hydroxynonenal-Src adducts in aged kidneys. Oncotarget 2016, 7, 50864–50874. [Google Scholar] [CrossRef] [Green Version]
- Grimsrud, P.A.; Picklo, M.J.; Griffin, T.J.; Bernlohr, D.A. Carbonylation of adipose proteins in obesity and insulin resistance: Identification of adipocyte fatty acid-binding protein as a cellular target of 4-hydroxynonenal. Mol. Cell. Proteom. 2007, 6, 624–637. [Google Scholar] [CrossRef] [Green Version]
- Khatoon, F.; Alam, K.M.; Ali, A. Physicochemical and immunological studies on 4-hydroxynonenal modified HSA: Implications of protein damage by lipid peroxidation products in the etiopathogenesis of SLE. Hum. Immunol. 2012, 73, 1132–1139. [Google Scholar] [CrossRef]
- Nègre-Salvayre, A.; Garoby-Salom, S.; Swiader, A.; Rouahi, M.; Pucelle, M.; Salvayre, R. Proatherogenic effects of 4-hydroxynonenal. Free Radic. Biol. Med. 2017, 111, 127–139. [Google Scholar] [CrossRef]
- Rosen, M.; Chan, P.; Saleem, M.; Herrmann, N.; Adibfar, A.; Andreazza, A.; Oh, P.I.; Lanctôt, K.L. Longitudinal associations between 4-hydroxynonenal and depression in coronary artery disease patients. Psychiatry Res. 2018, 270, 219–224. [Google Scholar] [CrossRef]
- Perković, M.N.; Milković, L.; Uzun, S.; Mimica, N.; Pivac, N.; Waeg, G.; Žarković, N. Association of Lipid Peroxidation Product 4-Hydroxynonenal with Post-Traumatic Stress Disorder. Biomolecules 2021, 11, 1365. [Google Scholar] [CrossRef]
- Pecorelli, A.; Leoncini, S.; De Felice, C.; Signorini, C.; Cerrone, C.; Valacchi, G.; Ciccoli, L.; Hayek, J. Non-protein-bound iron and 4-protein adducts in classic autism. Brain Dev. 2013, 35, 146–154. [Google Scholar] [CrossRef]
- Solmi, M.; Veronese, N.; Manzato, E.; Sergi, G.; Favaro, A.; Santonastaso, P.; Correll, C.U. Oxidative stress and antioxidant levels in patients with anorexia nervosa: A systematic review and exploratory meta-analysis. Int. J. Eat. Disord. 2015, 48, 826–841. [Google Scholar] [CrossRef]
- Zimniak, P. 4-Hydroxynonenal and fat storage: A paradoxical proobesity mechanism? Cell Cycle 2010, 9, 3393–3394. [Google Scholar] [CrossRef] [Green Version]
- Biasi, F.; Vizio, B.; Mascia, C.; Gaia, E.; Zarkovic, N.; Chiarpotto, E.; Leonarduzzi, G.; Poli, G. c-Jun N-terminal kinase upregulation as a key event in the proapoptotic interaction between transforming growth factor-beta1 and 4-hydroxynonenal in colon mucosa. Free Radic. Biol. Med. 2006, 41, 443–454. [Google Scholar] [CrossRef]
- Su, E.; Han, X.; Jiang, G. The transforming growth factor beta 1/SMAD signaling pathway involved in human chronic myeloid leukemia. Tumori 2010, 96, 659–666. [Google Scholar] [CrossRef]
- Chaudhary, P.; Sharma, R.; Sharma, A.; Vatsyayan, R.; Yadav, S.; Singhal, S.S.; Rauniyar, N.; Prokai, L.; Awasthi, S.; Awasthi, Y.C. Mechanisms of 4-hydroxy-2-nonenal induced pro- and anti-apoptotic signaling. Biochemistry 2010, 49, 6263–6275. [Google Scholar] [CrossRef] [Green Version]
- Carpten, J.D.; Faber, A.L.; Horn, C.; Donoho, G.P.; Briggs, S.L.; Robbins, C.M.; Hostetter, G.; Boguslawski, S.; Moses, T.Y.; Savage, S.; et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature 2007, 448, 439–444. [Google Scholar] [CrossRef]
- Vatsyayan, R.; Lelsani, P.C.R.; Chaudhary, P.; Kumar, S.; Awasthi, S.; Awasthi, Y.C. The expression and function of vascular endothelial growth factor in retinal pigment epithelial (RPE) cells is regulated by 4-hydroxynonenal (HNE) and glutathione S-transferaseA4-4. Biochem. Biophys. Res. Commun. 2012, 417, 346–351. [Google Scholar] [CrossRef] [Green Version]
- Niki, E. Lipid peroxidation: Physiological levels and dual biological effects. Free Radic. Biol. Med. 2009, 47, 469–484. [Google Scholar] [CrossRef]
- Zhang, H.; Forman, H.J. Signaling by 4-hydroxy-2-nonenal: Exposure protocols, target selectivity and degradation. Arch. Biochem. Biophys. 2017, 617, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Doorn, J.A.; Hurley, T.D.; Petersen, D.R. Inhibition of human mitochondrial aldehyde dehydrogenase by 4-hydroxynon-2-enal and 4-oxonon-2-enal. Chem. Res. Toxicol. 2006, 19, 102–110. [Google Scholar] [CrossRef]
- Zhong, H.; Yin, H. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: Focusing on mitochondria. Redox Biol. 2015, 4, 193–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Porter, N.A.; Schneider, C.; Brash, A.R.; Yin, H. Formation of 4-hydroxynonenal from cardiolipin oxidation: Intramolecular peroxyl radical addition and decomposition. Free Radic. Biol. Med. 2011, 50, 166–178. [Google Scholar] [CrossRef] [Green Version]
- Kagan, V.E.; Tyurin, V.A.; Jiang, J.; Tyurina, Y.Y.; Ritov, V.B.; Amoscato, A.A.; Osipov, A.N.; Belikova, N.A.; Kapralov, A.A.; Kini, V.; et al. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat. Chem. Biol. 2005, 1, 223–232. [Google Scholar] [CrossRef]
- Zhong, J.; Lu, L.; Xia, M.; Zhu, H. Yin, Formation of electrophilic oxidation products from mitochondrial cardiolipin in vitro and in vivo in the context of apoptosis and atherosclerosis. Redox Biol. 2014, 2, 878–888. [Google Scholar] [CrossRef] [Green Version]
- Pizzimenti, S.; Ciamporcero, E.; Pettazzoni, P.; Osella-Abate, S.; Novelli, M.; Toaldo, C.; Husse, M.; Daga, M.; Minelli, R.; Bisazza, A.; et al. The inclusion complex of 4-hydroxynonenal with a polymeric derivative of β-cyclodextrin enhances the antitumoral efficacy of the aldehyde in several tumor cell lines and in a three-dimensional human melanoma model. Free Radic. Biol. Med. 2013, 65, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Brown, D.; Awasthi, S.; Yang, Y.; Sharma, A.; Patrick, B.; Saini, M.K.; Singh, S.P.; Zimniak, P.; Singh, S.V.; et al. Transfection with 4-hydroxynonenal-metabolizing glutathione S-transferase isozymes leads to phenotypic transformation and immortalization of adherent cells. Eur. J. Biochem. 2004, 271, 1690–1701. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.S.; Singh, S.P.; Singhal, P.; Horne, D.; Singhal, J.; Awasthi, S. Antioxidant role of glutathione S-transferases: 4-Hydroxynonenal, a key molecule in stress-mediated signaling. Toxicol. Appl. Pharmacol. 2015, 289, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Zarkovic, N. 4-Hydroxynonenal as a bioactive marker of pathophysiological processes. Mol. Asp. Med. 2003, 24, 281–291. [Google Scholar] [CrossRef]
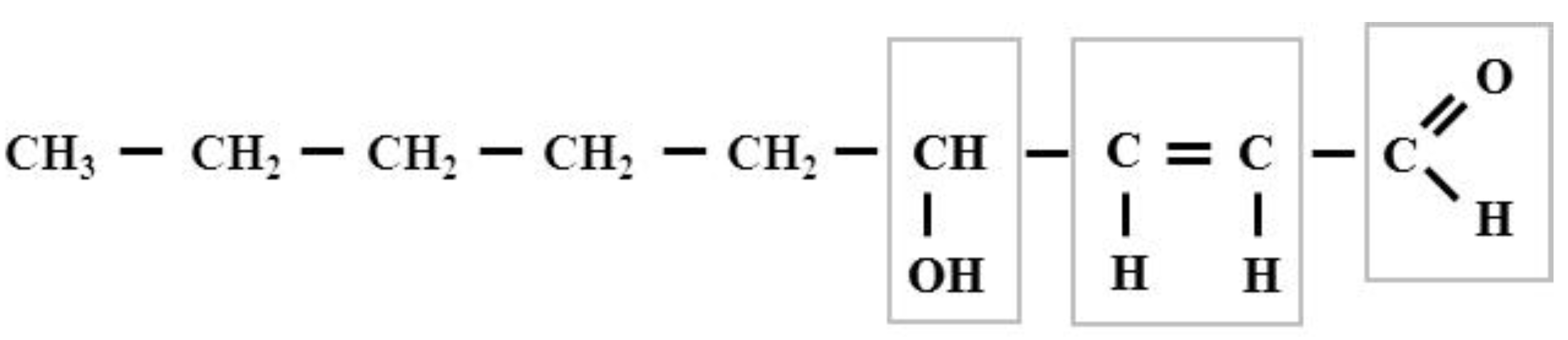


| HNE-Modified Molecules | Mode of Action | Medical Aspects | Some References |
|---|---|---|---|
| Activator protein 1 (AP-1) transcription factor | HNE alters AP-1 transcriptional activity | Vascular complications | [62] |
| Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) | HNE can induce either activation or inhibition of NF-κB | Increase the NF-κB activity is nvolved in inflammatory signaling in rheumatoid arthritis | [45,63] |
| A non-receptor protein tyrosine kinases family; Src kinases | HNE induces the activation and phosphorylation of these kinases Activation of Src by HNE leads to the expression of inflammatory mediator Cox2 and transcription factor AP-1, via activation of p38MAPK, JNK and ERK1/2 | The HNE-Src adduct is pro-inflammatory in aged kidneys | [64,65] |
| Peroxisome proliferator-activated receptors delta (PPARδ) | HNE and its derivatives are the endogenous ligands for PPARδ | HNE and its derivatives as ligands of PPAR are proposed as drugs (or they serve as prototype molecules for the development of such therapeutic agents) in the treatment of metabolic syndrome diseases. Metabolic syndrome is associated with the risk of developing cardiovascular disease and type 2 diabetes. | [20] |
| Adipose proteins | HNE can form adduct with these proteins | Insulin resistant obesity | [66] |
| Human serum albumin (HSA) | HNE can form adduct with HSA, making it highly immunogenic | Systemic lupus erythematosus (SLE), a chronic autoimmune disease, | [67] |
| Apolipoprotein B (ApoB) | HNE can form adduct with ApoB | Atherosclerosis; increasing the risk of athero-thrombotic events. | [68] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilska-Wilkosz, A.; Iciek, M.; Górny, M. Chemistry and Biochemistry Aspects of the 4-Hydroxy-2,3-trans-nonenal. Biomolecules 2022, 12, 145. https://doi.org/10.3390/biom12010145
Bilska-Wilkosz A, Iciek M, Górny M. Chemistry and Biochemistry Aspects of the 4-Hydroxy-2,3-trans-nonenal. Biomolecules. 2022; 12(1):145. https://doi.org/10.3390/biom12010145
Chicago/Turabian StyleBilska-Wilkosz, Anna, Małgorzata Iciek, and Magdalena Górny. 2022. "Chemistry and Biochemistry Aspects of the 4-Hydroxy-2,3-trans-nonenal" Biomolecules 12, no. 1: 145. https://doi.org/10.3390/biom12010145
APA StyleBilska-Wilkosz, A., Iciek, M., & Górny, M. (2022). Chemistry and Biochemistry Aspects of the 4-Hydroxy-2,3-trans-nonenal. Biomolecules, 12(1), 145. https://doi.org/10.3390/biom12010145