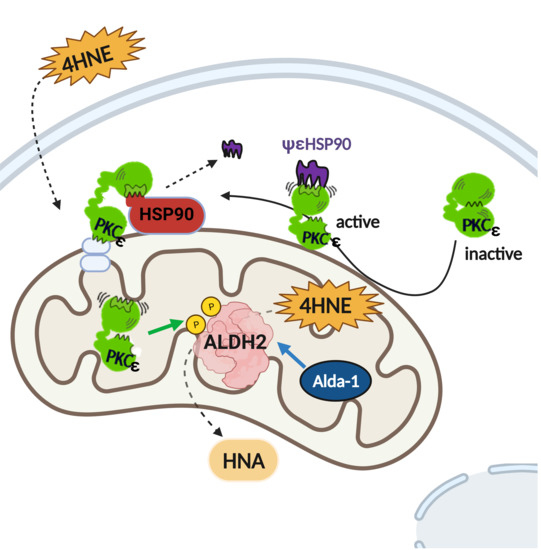
Activation of PKCε-ALDH2 Axis Prevents 4-HNE-Induced Pain in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Reagents
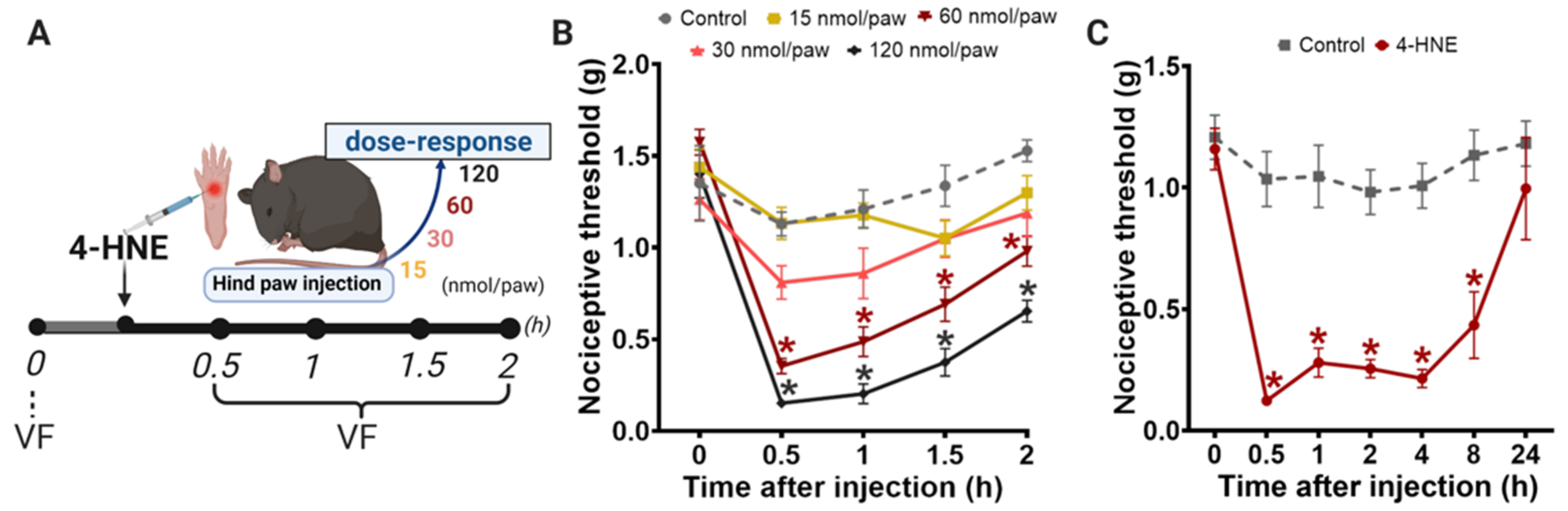
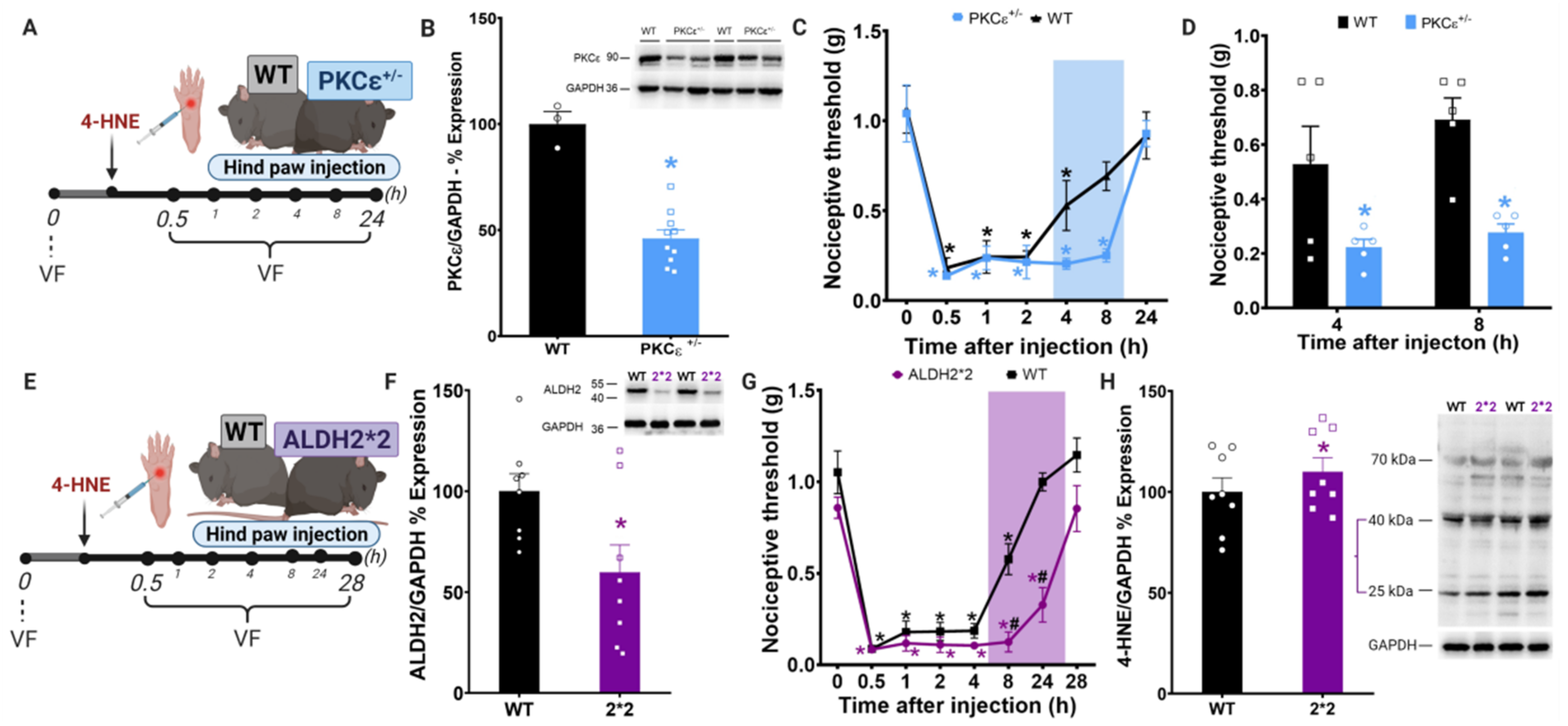
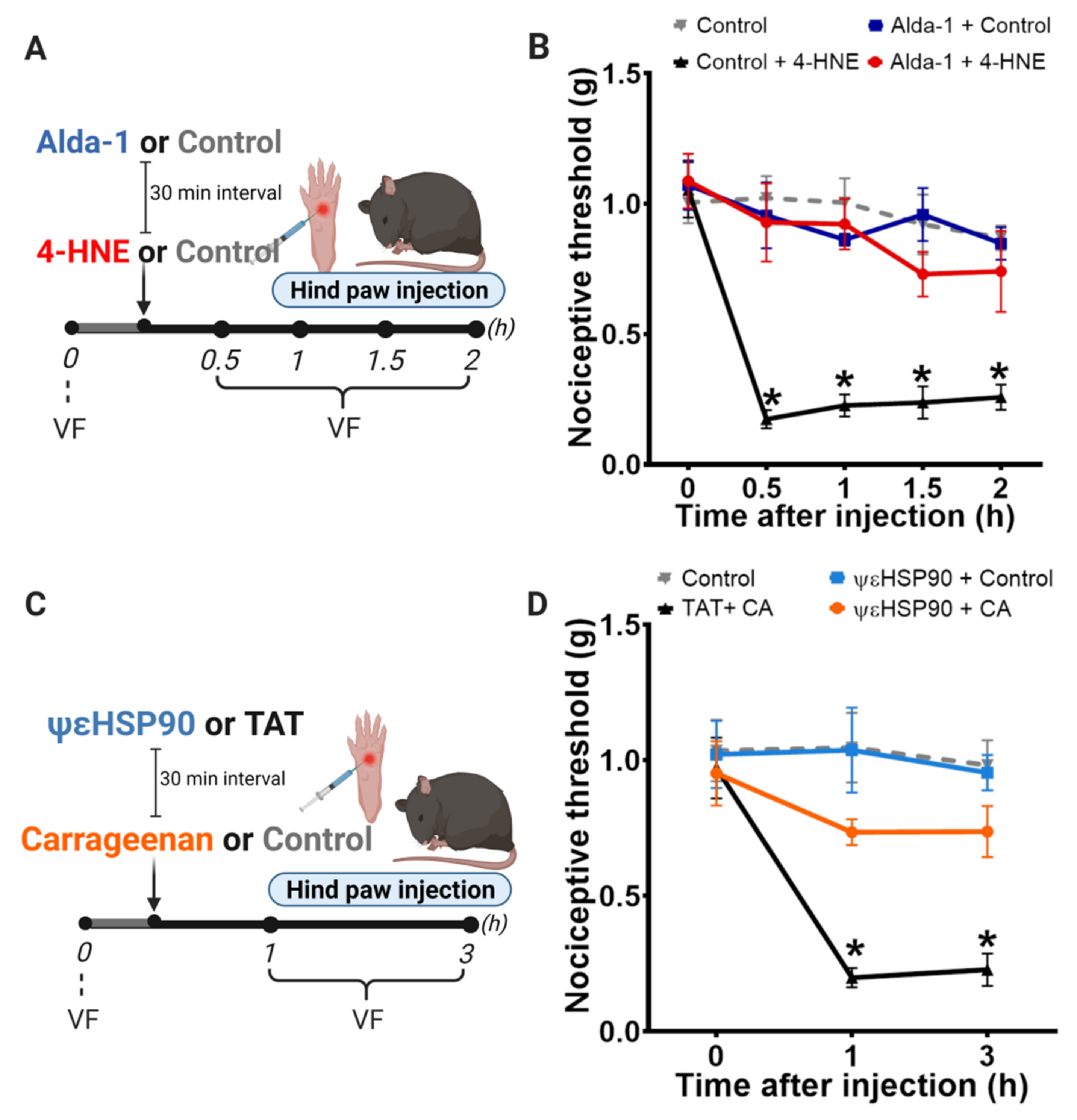
2.3. Drug Administration
2.4. Behavioral Testing
2.5. Western Blot
2.6. Statistical Analysis
3. Results
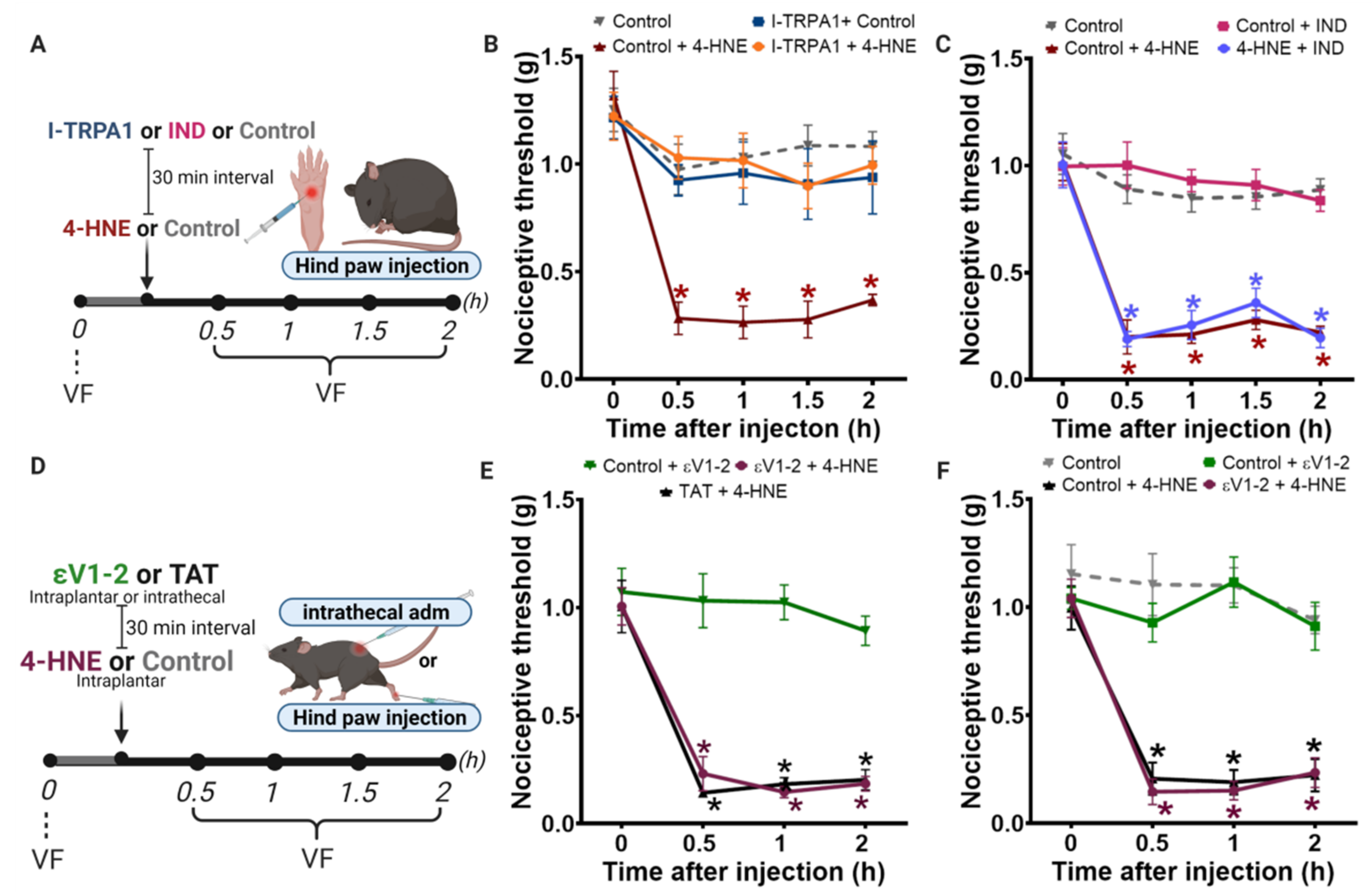
3.1. Disruption of PKCε-ALDH2 Axis Contributes to 4-HNE-Induced Mechanical Hypersensitivity
3.2. Selective Activation of PKCε in Mitochondria Blocks 4-HNE-Induced Hypersensitivity
3.3. Selective Activation of PKCε in Mitochondria Prevents Carrageenan-Induced Hypersensitivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Goldberg, D.S.; McGee, S.J. Pain as a Global Public Health Priority. BMC Public Health 2011, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D. The pain drain. Nature 2016, 535, S2–S3. [Google Scholar] [CrossRef] [PubMed]
- Disatnik, M.H.; Jones, S.N.; Mochly-Rosen, D. Stimulus-dependent subcellular localization of activated protein kinase C; a study with acidic fibroblast growth factor and transforming growth factor-β1 in cardiac myocytes. J. Mol. Cell. Cardiol. 1995, 27, 2473–2481. [Google Scholar] [CrossRef]
- Cesare, P.; Dekker, L.V.; Sardini, A.; Parker, P.J.; Mcnaughton, P.A. Specific Involvement of PKC-ε in Sensitization of the Neuronal Response to Painful Heat plaining the lowering of the threshold for heat pain that. Neuron 1999, 23, 617–624. [Google Scholar] [CrossRef]
- Khasar, S.G.; Lin, Y.-H.; Martin, A.; Dadgar, J.; McMahon, T.; Wang, D.; Hundle, B.; Aley, K.; Isenberg, W.; McCarter, G.; et al. A novel nociceptor signaling pathway revealed in protein kinase c ε mutant mice. Neuron 1999, 24, 253–260. [Google Scholar] [CrossRef]
- Wu, D.-F.; Chandra, D.; McMahon, T.; Wang, D.; Dadgar, J.; Kharazia, V.N.; Liang, Y.-J.; Waxman, S.G.; Dib-Hajj, S.D.; Messing, R. PKCε phosphorylation of the sodium channel Na V1.8 increases channel function and produces mechanical hyperalgesia in mice. J. Clin. Investig. 2012, 122, 1306–1315. [Google Scholar] [CrossRef]
- Cousins, M.J.; Pickthorn, K.; Huang, S.; Critchley, L.; Bell, G. The Safety and Efficacy of KAI-1678- An Inhibitor of Epsilon Protein Kinase C (εPKC)-Versus Lidocaine and Placebo for the Treatment of Postherpetic Neuralgia: A Crossover Study Design. Pain Med. 2013, 14, 533–540. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moodie, J.E.; Bisley, E.J.; Huang, S.; Pickthorn, K.; Bell, G. A Single-Center, Randomized, Double-Blind, Active, and Placebo-Controlled Study of KAI-1678, a Novel PKC-Epsilon Inhibitor, in the Treatment of Acute Postoperative Orthopedic Pain. Pain Med. 2013, 916–924. [Google Scholar] [CrossRef]
- Zambelli, V.O.; Gross, E.R.; Chen, C.H.; Gutierrez, V.P.; Cury, Y.; Mochly-Rosen, D. Aldehyde dehydrogenase-2 regulates nociception in rodent models of acute inflammatory pain. Sci. Transl. Med. 2014, 6, 251ra118. [Google Scholar] [CrossRef]
- Chen, C.H.; Ferreira, J.C.B.; Gross, E.R.; Mochly-Rosen, D. Targeting aldehyde dehydrogenase 2: New therapeutic opportunities. Physiol. Rev. 2014, 94, 1–34. [Google Scholar] [CrossRef]
- Dalleau, S.; Baradat, M.; Guéraud, F.; Huc, L. Cell death and diseases related to oxidative stress:4-hydroxynonenal (HNE) in the balance. Cell Death Differ. 2013, 20, 1615–1630. [Google Scholar] [CrossRef] [PubMed]
- McAllister, S.L.; Sinharoy, P.; Vasu, M.; Gross, E.R. Aberrant reactive aldehyde detoxification by aldehyde dehydrogenase-2 influences endometriosis development and pain-associated behaviors. Pain 2021, 162, 71–83. [Google Scholar] [CrossRef]
- Khan, M.; Qiao, F.; Kumar, P.; Islam, S.M.T.; Singh, A.K.; Won, J.; Singh, I. Neuroprotective effects of Alda-1 mitigate spinal cord injury in mice: Involvement of Alda-1-induced ALDH2 activation-mediated suppression of reactive aldehyde mechanisms. Neural Regen. Res. 2022, 17, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Ritter, C.; Dalenogare, D.P.; de Almeida, A.S.; Pereira, V.L.; Pereira, G.C.; Fialho, M.F.P.; Lückemeyer, D.D.; Antoniazzi, C.; Kudsi, S.Q.; Ferreira, J.; et al. Nociception in a Progressive Multiple Sclerosis Model in Mice Is Dependent on Spinal TRPA1 Channel Activation. Mol. Neurobiol. 2020, 57, 2420–2435. [Google Scholar] [CrossRef] [PubMed]
- Marone, I.M.; De Logu, F.; Nassini, R.; Goncalves, M.D.C.; Benemei, S.; Ferreira, J.; Jain, P.; Puma, S.L.; Bunnett, N.W.; Geppetti, P.; et al. TRPA1/NOX in the soma of trigeminal ganglion neurons mediates migraine-related pain of glyceryl trinitrate in mice. Brain 2018, 141, 2312–2328. [Google Scholar] [CrossRef]
- Trevisani, M.; Siemens, J.; Materazzi, S.; Bautista, D.M.; Nassini, R.; Campi, B.; Imamachi, N.; Andre, E.; Patacchini, R.; Cottrell, G.S.; et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Anticancer Res. 2007, 104, 13519–13524. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Aldini, G.; Carini, M.; Colombo, R.; Rossi, R.; Milzani, A. Protein carbonylation, cellular dysfunction, and disease progression. J. Cell Mol. Med. 2006, 10, 389–406. [Google Scholar] [CrossRef]
- Castrillo, A.; Pennington, D.J.; Otto, F.; Parker, P.J.; Owen, M.J.; Boscá, L. Protein kinase Cε is required for macrophage activation and defense against bacterial infection. J. Exp. Med. 2001, 194, 1231–1242. [Google Scholar] [CrossRef]
- da Costa, D.S.M.; Meotti, F.C.; Andrade, E.L.; Leal, P.C.; Motta, E.M.; Emerson Marcelo Motta, J.B.C. The involvement of the transient receptor potential A1 (TRPA1) in the maintenance of mechanical and cold hyperalgesia in persistent inflammation. Pain 2010, 148, 431–437. [Google Scholar] [CrossRef]
- Trevisan, G.; Hoffmeisterc, C.; Rossatoa, M.F.; Oliveira, S.M.; Silva, M.A.; Silva, C.R.; Fusi, C.; Tonello, R.; Minocci, D.; Guerra, G.P. TRPA1 receptor stimulation by hydrogen peroxide is critical to triggerhyperalgesia and inflammation in a model of acute gout. Free Radic. Biol. Med. 2014, 72, 200–209. [Google Scholar] [CrossRef]
- Sant’Anna, M.; Kusuda, R.; Bozzo, T.A.; Bassi, G.S.; Alves-Filho, J.C.; Cunha, F.Q.; Ferreira, S.H.; Souza, G.R.; Cunha, T. Medial plantar nerve ligation as a novel model of neuropathic pain in mice: Pharmacological and molecular characterization. Sci. Rep. 2016, 6, 26955. [Google Scholar] [CrossRef]
- Sweitzer, S.; Wong, S. Protein Kinase C ϵ and γ Involvement in Formalin-Induced Nociception in Neonatal Rats. J. Pharmacol. Exp. Ther. 2004, 309, 616–625. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, Z.J. Nociceptor beta II, delta, and epsilon isoforms of PKC differentially mediate paclitaxel-induced spontaneous and evoked pain. J. Neurosci. 2015, 35, 4614–4625. [Google Scholar] [CrossRef]
- Joseph, E.K.; Reichling, D.B.; Levine, J.D. Shared Mechanisms for Opioid Tolerance and a Transition to Chronic Pain. J. Neurosci. 2010, 30, 4660–4666. [Google Scholar] [CrossRef]
- Sun, X.; Budas, G.R.; Xu, L.; Barreto, G.E.; Mochly-Rosen, D.; Sun, X.; Budas, G.R.; Xu, L.; Barreto, G.E.; Daria Mochly-Rosen, R.G.G. Selective activation of protein kinase C∊ in mitochondria is neuroprotective in vitro and reduces focal ischemic brain injury in mice. J. Neurosci. Res. 2013, 91, 799–807. [Google Scholar] [CrossRef]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Dalenogare, D.P.; Theisen, M.C.; Peres, D.S.; Fialho, M.F.P.; Lückemeyer, D.D.; Antoniazzi, C.T.d.D.; Kudsi, S.Q.; Ferreira, M.D.A.; Ritter, C.D.S.; Ferreira, J.; et al. TRPA1 activation mediates nociception behaviors in a mouse model of relapsing-remitting experimental autoimmune encephalomyelitis. Exp. Neurol. 2020, 328, 113241. [Google Scholar] [CrossRef] [PubMed]
- Brooks, P.J.; Enoch, M.; Goldman, D.; Li, T.; Yokoyama, A. The Alcohol Flushing Response: An Unrecognized Risk Factor for Esophageal Cancer from Alcohol Consumption. PLoS Med. 2009, 6, 258–263. [Google Scholar] [CrossRef]
- Chen, C.-H.; Budas, G.R.; Churchill, E.N.; Disatnik, M.-H.; Hurley, T.D.; Mochly-Rosen, D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Supporting Online Mater. Sci. 2008, 321, 1493–1495. [Google Scholar] [CrossRef] [PubMed]
- Budas, G.R.; Churchill, E.N.; Disatnik, M.H.; Sun, L.; Mochly-Rosen, D. Mitochondrial import of PKC is mediated by HSP90: A role in cardioprotection from ischaemia and reperfusion injury. Cardiovasc. Res. 2010, 88, 83–92. [Google Scholar] [CrossRef]
- Parada, C.A.; Yeh, J.J.; Reichling, D.B.; Levine, J.D. Transient attenuation of protein kinase Cε can terminate a chronic hyperalgesic state in the rat. Neuroscience 2003, 120, 219–226. [Google Scholar] [CrossRef]
- Numazaki, M.; Tominaga, T.; Toyooka, H.; Tominaga, M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cε and identification of two target serine residues. J. Biol. Chem. 2002, 277, 13375–13378. [Google Scholar] [CrossRef]
- Srinivasan, R.; Wolfe, D.; Goss, J.; Watkins, S.; de Groat, W.C.; Sculptoreanu, A.; Glorioso, J.C. Protein kinase C epsilon contributes to basal and sensitizing responses of TRPV1 to capsaicin in rat dorsal root ganglion neurons. Eur. J. Neurosci. 2008, 28, 1241–1254. [Google Scholar] [CrossRef] [PubMed]
- Sachs, D.; Villarreal, C.; Cunha, F.; Parada, C.; Ferreira, S. The role of PKA and PKCe pathways in prostaglandin E2-mediated hypernociception. Br. J. Pharmacol. 2009, 156, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Strohmaier, H.; Highofer-Szalkay, H.; Schaur, R.J. Detection of 4-hydroxynonenal (HNE) as a physiological component in human plasma. J. Lipid Mediat. Cell Signal. 1995, 11, 51–61. [Google Scholar] [CrossRef]
- Zhong, H.; Yin, H. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: Focusing on mitochondria. Redox Biol. 2015, 4, 193–199. [Google Scholar] [CrossRef] [PubMed]
- El-Brolosy, M.A.; Stainier, D.Y.R. Genetic compensation: A phenomenon in search of mechanisms. PLoS Genet. 2017, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Neupert, W.; Herrmann, J.M. Translocation of proteins into mitochondria. Annu. Rev. Biochem. 2007, 76, 723–749. [Google Scholar] [CrossRef] [PubMed]
- Perez-Miller, S.; Younus, H.; Vanam, R.; Chen, C.; Mochly-Rosen, D.; Hurley, T.D. Alda-1 is an agonist and chemical chaperone for the common human aldehyde dehydrogenase 2 variant. Nat. Struct Mol. Biol. 2010, 17, 159–164. [Google Scholar] [CrossRef]
- Dray, A.; Bettaney, J.; Forster, P.; Perkins, M.N. Bradykinin-induced stimulation of afferent fibres is mediated through protein kinase C. Neurosci. Lett. 1988, 91, 301–307. [Google Scholar] [CrossRef]
- Rang, H.P.; Ritchie, J.M. Depolarization of Nonmyelinated Fibers of the Rat Vagus Nerve Produced by Activation of Protein Kinase C. J. Neurosci. 1988, 8, 2606–2617. [Google Scholar] [CrossRef] [PubMed]
- Mefllinger, K.; Schmidt, R.F. The effects of phorbol ester on slowly conducting afferents of the cat’s knee joint. Exp. Brain Res. 1993, 92, 391–398. [Google Scholar]
- De Logu, F.; De Prá, S.D.-T.; Antoniazzi, C.T.D.D.; Kudsi, S.Q.; Ferro, P.R.; Landini, L.; Rigo, F.K.; Silveira, G.D.B.; Silveira, P.C.L.; Oliveira, S.M.; et al. Macrophages and Schwann cell TRPA1 mediate chronic allodynia in a mouse model of complex regional pain syndrome type I. Brain Behav. Immun. 2020, 88, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Cinar, R.; Hu, X.; Lin, Y.; Luo, G.; Lovinger, D.M. Spinal astrocyte aldehyde dehydrogenase-2 mediates ethanol metabolism and analgesia in mice. Br. J. Anaesth. 2021, 1–14. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, B.B.; Hösch, N.G.; Alcantara, Q.A.; Budas, G.R.; Chen, C.-H.; Mochly-Rosen, D.; Ferreira, J.C.B.; Zambelli, V.O. Activation of PKCε-ALDH2 Axis Prevents 4-HNE-Induced Pain in Mice. Biomolecules 2021, 11, 1798. https://doi.org/10.3390/biom11121798
Martins BB, Hösch NG, Alcantara QA, Budas GR, Chen C-H, Mochly-Rosen D, Ferreira JCB, Zambelli VO. Activation of PKCε-ALDH2 Axis Prevents 4-HNE-Induced Pain in Mice. Biomolecules. 2021; 11(12):1798. https://doi.org/10.3390/biom11121798
Chicago/Turabian StyleMartins, Bárbara B., Natália G. Hösch, Queren A. Alcantara, Grant R. Budas, Che-Hong Chen, Daria Mochly-Rosen, Julio C. B. Ferreira, and Vanessa O. Zambelli. 2021. "Activation of PKCε-ALDH2 Axis Prevents 4-HNE-Induced Pain in Mice" Biomolecules 11, no. 12: 1798. https://doi.org/10.3390/biom11121798
APA StyleMartins, B. B., Hösch, N. G., Alcantara, Q. A., Budas, G. R., Chen, C.-H., Mochly-Rosen, D., Ferreira, J. C. B., & Zambelli, V. O. (2021). Activation of PKCε-ALDH2 Axis Prevents 4-HNE-Induced Pain in Mice. Biomolecules, 11(12), 1798. https://doi.org/10.3390/biom11121798