Role of Lipopolysaccharide, Derived from Various Bacterial Species, in Pulpitis—A Systematic Review
Abstract
1. Introduction
1.1. Cells in Dental Pulp
1.2. Pulpitis
1.3. Lipopolysaccharide
2. Materials and Methods
2.1. Study Selection
2.2. Data Extraction and Analyses
3. Results
3.1. Escherichia coli LPS
3.1.1. hDPCs
3.1.2. Fibroblasts
3.1.3. Odontoblasts
3.1.4. DPSCs
Differentiation
Senescence
3.2. Porphyromonas gingivalis LPS
3.2.1. hDPCs
3.2.2. Fibroblasts
3.2.3. DPSCs
3.3. Other Groups
3.3.1. hDPCs
3.3.2. Fibroblasts
3.3.3. Odontoblasts
3.3.4. DPSCs
3.4. Non-Specific
3.4.1. hDPCs
3.4.2. Fibroblasts
3.4.3. Odontoblasts
3.4.4. DPSCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Park, S.H.; Ye, L.; Love, R.M.; Farges, J.C.; Yumoto, H. Inflammation of the Dental Pulp. Mediat. Inflamm. 2015, 2015, 980196. [Google Scholar] [CrossRef] [PubMed]
- Ghannam, M.G.; Alameddine, H.; Bordoni, B. Anatomy, Head and Neck, Pulp (Tooth); StatPearls [Internet]; Updated 11 August 2021; StatPearls Publishing: Treasure Island, FL, USA, January 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537112/ (accessed on 29 October 2021).
- Zargar, N.; Ashraf, H.; Marashi, S.M.A.; Sabeti, M.; Aziz, A. Identification of microorganisms in irreversible pulpitis and primary endodontic infections with respect to clinical and radiographic findings. Clin. Oral Investig. 2020, 24, 2099–2108. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Marcos, J.F. Aetiology, classification and pathogenesis of pulp and periapical disease. Med. Oral Patol. Oral Cir. Bucal 2004, 9 (Suppl. 58–62), 52–57. [Google Scholar]
- Farhana, A.; Khan, Y.S. Biochemistry, Lipopolysaccharide; StatPearls [Internet]; Updated 29 April 2021; StatPearls Publishing: Treasure Island, FL, USA, January 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554414 (accessed on 29 October 2021).
- Panagakos, F.S. Regulation of pulp cell matrix metalloproteinase production by cytokines and lipopolysaccharides. J. Endod. 1996, 22, 358–361. [Google Scholar] [CrossRef]
- Wang, X.X.; Feng, Z.H.; Li, Q.M.; Yi, B.C.; Xu, Q. DNA methylcytosine dioxygenase ten-eleven translocation 2 enhances lipopolysaccharide-induced cytokine expression in human dental pulp cells by regulating MyD88 hydroxymethylation. Cell Tissue Res. 2018, 373, 477–485. [Google Scholar] [CrossRef]
- Kong, Q.; Liu, L.; Huang, Y.; Zhang, F.; Wei, X.; Ling, J. The effect of octamer-binding transcription factor 4B1 on microRNA signals in human dental pulp cells with inflammatory response. J. Endod. 2014, 40, 101–108. [Google Scholar] [CrossRef]
- Liu, L.; Huang, R.; Yang, R.; Wei, X. OCT4B1 regulates the cellular stress response of human dental pulp cells with inflammation. BioMed Res. Int. 2017, 2017, 2756891. [Google Scholar] [CrossRef]
- Cai, L.; Zhan, M.; Li, Q.; Li, D.; Xu, Q. DNA methyltransferase DNMT1 inhibits lipopolysaccharide-induced inflammatory response in human dental pulp cells involving the methylation changes of IL-6 and TRAF6. Mol. Med. Rep. 2020, 21, 959–968. [Google Scholar] [CrossRef]
- Feng, Z.; Zhan, M.; Meng, R.; Wang, X.; Xu, Q. 5-Aza-2’-deoxycytidine enhances lipopolysaccharide-induced inflammatory cytokine expression in human dental pulp cells by regulating TRAF6 methylation. Bioengineered 2019, 10, 197–206. [Google Scholar] [CrossRef]
- Liu, M.; Chen, L.; Wu, J.; Lin, Z.; Huang, S. Long noncoding RNA MEG3 expressed in human dental pulp regulates LPS-Induced inflammation and odontogenic differentiation in pulpitis. Exp. Cell Res. 2021, 400, 112495. [Google Scholar] [CrossRef]
- Wang, X.; Sun, H.; Hu, Z.; Mei, P.; Wu, Y.; Zhu, M. NUTM2A-AS1 silencing alleviates LPS-induced apoptosis and inflammation in dental pulp cells through targeting let-7c-5p/HMGB1 axis. Int. Immunopharmacol. 2021, 96, 107497. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, H.; Gong, Q.; Fan, C.; Huang, Y.; Ling, J. Expression of high mobility group box 1 in inflamed dental pulp and its chemotactic effect on dental pulp cells. Biochem. Biophys. Res. Commun. 2014, 450, 1547–1552. [Google Scholar] [CrossRef]
- Nara, K.; Kawashima, N.; Noda, S.; Fujii, M.; Hashimoto, K.; Tazawa, K.; Okiji, T. Anti-inflammatory roles of microRNA 21 in lipopolysaccharide-stimulated human dental pulp cells. J. Cell. Physiol. 2019, 234, 21331–21341. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Kim, J.C.; Lee, Y.H.; Yu, M.K.; Lee, N.H.; Park, J.D.; Bhattarai, G.; Yi, H.K. Anti-inflammatory mechanism of PPARγ on LPS-induced pulp cells: Role of the ROS removal activity. Arch. Oral Biol. 2012, 57, 392–400. [Google Scholar] [CrossRef]
- Yu, M.K.; Lee, J.C.; Kim, J.H.; Lee, Y.H.; Jeon, J.G.; Jhee, E.C.; Yi, H.K. Anti-inflammatory Effect of Peroxisome Proliferator Activated Receptor Gamma on Human Dental Pulp Cells. J. Endod. 2009, 35, 524–528. [Google Scholar] [CrossRef]
- Wu, H.; He, M.; Yang, R.; Zuo, Y.; Bian, Z. Astrocyte elevated gene-1 participates in the production of pro-inflammatory cytokines in dental pulp cells via NF-κB signalling pathway. Int. Endod. J. 2018, 51, 1130–1138. [Google Scholar] [CrossRef]
- Fujii, M.; Kawashima, N.; Tazawa, K.; Hashimoto, K.; Nara, K.; Noda, S.; Nagai, S.; Okiji, T. Hypoxia-inducible factor 1α promotes interleukin 1β and tumour necrosis factor α expression in lipopolysaccharide-stimulated human dental pulp cells. Int. Endod. J. 2020, 53, 636–646. [Google Scholar] [CrossRef]
- Fujii, M.; Kawashima, N.; Tazawa, K.; Hashimoto, K.; Nara, K.; Noda, S.; Kuramoto, M.; Orikasa, S.; Nagai, S.; Okiji, T. HIF1α inhibits LPS-mediated induction of IL-6 synthesis via SOCS3-dependent CEBPβ suppression in human dental pulp cells. Biochem. Biophys. Res. Commun. 2020, 522, 308–314. [Google Scholar] [CrossRef]
- Zhang, L.; Bai, L.; Ren, Q.; Sun, G.; Si, Y. Protective effects of SIRT6 against lipopolysaccharide (LPS) are mediated by deacetylation of Ku70. Mol. Immunol. 2018, 101, 312–318. [Google Scholar] [CrossRef]
- Takanche, J.S.; Kim, J.S.; Kim, J.E.; Han, S.H.; Yi, H.K. Schisandrin C enhances odontoblastic differentiation through autophagy and mitochondrial biogenesis in human dental pulp cells. Arch. Oral Biol. 2018, 88, 60–66. [Google Scholar] [CrossRef]
- Lee, N.H.; Lee, Y.H.; Bhattari, G.; Lee, I.K.; Yun, B.S.; Jeon, J.G.; Hwang, P.H.; Yi, H.K. Reactive oxygen species removal activity of davallialactone reduces lipopolysaccharide-induced pulpal inflammation through inhibition of the extracellular signal-regulated kinase 1/2 and nuclear factor kappa B pathway. J. Endod. 2011, 37, 491–495. [Google Scholar] [CrossRef]
- Lee, I.K.; Jung, J.Y.; Seok, S.J.; Kim, W.G.; Yun, B.S. Free radical scavengers from the medicinal mushroom Inonotus xeranticus and their proposed biogenesis. Bioorg. Med. Chem. Lett. 2006, 16, 5621–5624. [Google Scholar] [CrossRef]
- Liu, X.; Cao, Y.; Zhang, Y.; Sun, B.; Liang, H. Teneligliptin inhibits lipopolysaccharide-induced cytotoxicity and inflammation in dental pulp cells. Int. Immunopharmacol. 2019, 73, 57–63. [Google Scholar] [CrossRef]
- Weekate, K.; Chuenjitkuntaworn, B.; Chuveera, P.; Vaseenon, S.; Chompu-inwai, P.; Ittichaicharoen, J.; Chattipakorn, S.; Srisuwan, T. Alterations of mitochondrial dynamics, inflammation and mineralization potential of lipopolysaccharide-induced human dental pulp cells after exposure to N-acetyl cysteine, Biodentine or ProRoot MTA. Int. Endod. J. 2021, 54, 951–965. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, T.; Wang, X.; Wang, Y. Fluocinolone acetonide partially restores the mineralization of LPS-stimulated dental pulp cells through inhibition of NF-κB pathway and activation of AP-1 pathway. Br. J. Pharmacol. 2013, 170, 1262–1271. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Zhu, N.X.; Zhao, Y.M.; Ge, L.H.; Qin, M. Mineralisation Influence of Betamethasone on Lipopolysaccharide-Stimulated Dental Pulp Cells. Chin. J. Dent. Res. 2019, 22, 123–129. [Google Scholar] [CrossRef]
- Jung, J.Y.; Woo, S.M.; Kim, W.J.; Lee, B.N.; Nör, J.E.; Min, K.S.; Choi, C.H.; Koh, J.T.; Lee, K.J.; Hwang, Y.C. Simvastatin inhibits the expression of inflammatory cytokines and cell adhesion molecules induced by LPS in human dental pulp cells. Int. Endod. J. 2017, 50, 377–386. [Google Scholar] [CrossRef]
- Cao, R.; Wang, Q.; Wu, J.; Liu, M.; Han, Q.; Wang, X. Nell-1 attenuates lipopolysaccharide-induced inflammation in human dental pulp cells. J. Mol. Histol. 2021, 52, 671–680. [Google Scholar] [CrossRef]
- Pavan, R.; Jain, S.; Shraddha; Kumar, A. Properties and therapeutic application of bromelain: A review. Biotechnol. Res. Int. 2012, 2012, 976203. [Google Scholar] [CrossRef]
- Hong, J.H.; Kim, M.R.; Lee, B.N.; Oh, W.M.; Min, K.S.; Im, Y.G.; Hwang, Y.C. Anti-inflammatory and mineralization effects of bromelain on lipopolysaccharide-induced inflammation of human dental pulp cells. Medicina 2021, 57, 591. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Yu, M.K.; Lee, R.; Lee, Y.H.; Jeon, J.G.; Lee, M.H.; Jhee, E.C.; Yoo, I.D.; Yi, H.K. Terrein Reduces Pulpal Inflammation in Human Dental Pulp Cells. J. Endod. 2008, 34, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Jeong, H.J.; Lee, K.M.; Myung, N.Y.; An, N.H.; Yang, W.M.; Park, S.K.; Lee, H.J.; Hong, S.H.; Kim, H.M.; et al. Epigallocatechin-3-gallate suppresses NF-kappaB activation and phosphorylation of p38 MAPK and JNK in human astrocytoma U373MG cells. J. Nutr. Biochem. 2007, 18, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Mukai, K.; Yumoto, H.; Hirao, K.; Hosokawa, Y.; Matsuo, T. Anti-inflammatory effect of catechin on cultured human dental pulp cells affected by bacteria-derived factors. Eur. J. Oral Sci. 2010, 118, 145–150. [Google Scholar] [CrossRef]
- Nakanishi, T.; Mukai, K.; Hosokawa, Y.; Takegawa, D.; Matsuo, T. Catechins inhibit vascular endothelial growth factor production and cyclooxygenase-2 expression in human dental pulp cells. Int. Endod. J. 2015, 48, 277–282. [Google Scholar] [CrossRef]
- Songsiripradubboon, S.; Kladkaew, S.; Trairatvorakul, C.; Sangvanich, P.; Soontornvipart, K.; Banlunara, W.; Thunyakitpisal, P. Stimulation of Dentin Regeneration by Using Acemannan in Teeth with Lipopolysaccharide-induced Pulp Inflammation. J. Endod. 2017, 43, 1097–1103. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, H.; Gong, Q.; Li, X.; Ling, J. Lipopolysaccharide stimulation improves the odontoblastic differentiation of human dental pulp cells. Mol. Med. Rep. 2015, 11, 3547–3552. [Google Scholar] [CrossRef]
- Sugiuchi, A.; Sano, Y.; Furusawa, M.; Abe, S.; Muramatsu, T. Human Dental Pulp Cells Express Cellular Markers for Inflammation and Hard Tissue Formation in Response to Bacterial Information. J. Endod. 2018, 44, 992–996. [Google Scholar] [CrossRef]
- Colombini-Ishikiriama, B.L.; Dionisio, T.J.; Garbieri, T.F.; da Silva, R.A.; Machado, M.; de Oliveira, S.H.P.; Lara, V.S.; Greene, A.S.; Santos, C.F. What is the response profile of deciduous pulp fibroblasts stimulated with E. coli LPS and E. faecalis LTA? BMC Immunol. 2020, 21, 38. [Google Scholar] [CrossRef]
- Coil, J.; Tam, E.; Waterfield, J.D. Proinflammatory cytokine profiles in pulp fibroblasts stimulated with lipopolysaccharide and methyl mercaptan. J. Endod. 2004, 30, 88–91. [Google Scholar] [CrossRef]
- Jiang, W.; Lv, H.; Wang, H.; Wang, D.; Sun, S.; Jia, Q.; Wang, P.; Song, B.; Ni, L. Activation of the NLRP3/caspase-1 inflammasome in human dental pulp tissue and human dental pulp fibroblasts. Cell Tissue Res. 2015, 361, 541–555. [Google Scholar] [CrossRef]
- Zhang, A.; Wang, P.; Ma, X.; Yin, X.; Li, J.; Wang, H.; Jiang, W.; Jia, Q.; Ni, L. Mechanisms that lead to the regulation of NLRP3 inflammasome expression and activation in human dental pulp fibroblasts. Mol. Immunol. 2015, 66, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Kantrong, N.; Jit-Armart, P.; Arayatrakoollikit, U. Melatonin antagonizes lipopolysaccharide-induced pulpal fibroblast responses. BMC Oral Health 2020, 20, 91. [Google Scholar] [CrossRef]
- Nagaoka, S.; Tokuda, M.; Sakuta, T.; Taketoshi, Y.; Tamura, M.; Takada, H.; Kawagoe, M. Interleukin-8 gene expression by human dental pulp fibroblast in cultures stimulated with Prevotella intermedia lipopolysaccharide. J. Endod. 1996, 22, 9–12. [Google Scholar] [CrossRef]
- Takahashi, K.; Nakanishi, T.; Yumoto, H.; Adachi, T.; Matsuo, T. CCL20 production is induced in human dental pulp upon stimulation by Streptococcus mutans and proinflammatory cytokines. Oral Microbiol. Immunol. 2008, 23, 320–327. [Google Scholar] [CrossRef]
- Song, J.; Wu, Q.; Jiang, J.; Sun, D.; Wang, F.; Xin, B.; Cui, Q. Berberine reduces inflammation of human dental pulp fibroblast via miR-21/KBTBD7 axis. Arch. Oral Biol. 2020, 110, 104630. [Google Scholar] [CrossRef]
- Botero, T.M.; Shelburne, C.E.; Holland, G.R.; Hanks, C.T.; Nör, J.E. TLR4 Mediates LPS-Induced VEGF Expression in Odontoblasts. J. Endod. 2006, 32, 951–955. [Google Scholar] [CrossRef]
- Levin, L.G.; Rudd, A.; Bletsa, A.; Reisner, H. Expression of IL-8 by cells of the odontoblast layer in vitro. Eur. J. Oral Sci. 1999, 107, 131–137. [Google Scholar] [CrossRef]
- Choi, B.D.; Jeong, S.J.; Wang, G.; Kim, H.J.; Kim, B.O.; Hwang, H.K.; Lim, D.S.; Kim, S.H.; Jeong, M.J. Temporal Induction of Secretory Leukocyte Protease Inhibitor (SLPI) in Odontoblasts by Lipopolysaccharide and Wound Infection. J. Endod. 2009, 35, 997–1002. [Google Scholar] [CrossRef]
- Ma, L.; Wang, S.C.; Tong, J.; Hu, Y.; Zhang, Y.Q.; Yu, Q. Activation and dynamic expression of Notch signalling in dental pulp cells after injury in vitro and in vivo. Int. Endod. J. 2016, 49, 1165–1174. [Google Scholar] [CrossRef]
- Neiva, K.G.; Catalfamo, D.L.; Holliday, L.S.; Wallet, S.M.; Pileggi, R. Propolis decreases lipopolysaccharide-induced inflammatory mediators in pulp cells and osteoclasts. Dent. Traumatol. 2014, 30, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Wang, Y.; Ou, Y.; Wu, Y.; Zhou, Y.; Liang, S. Effects of sclerostin on lipopolysaccharide-induced inflammatory phenotype in human odontoblasts and dental pulp cells. Int. J. Biochem. Cell Biol. 2019, 117, 105628. [Google Scholar] [CrossRef] [PubMed]
- Bindal, P.; Ramasamy, T.S.; Kasim, N.H.A.; Gnanasegaran, N.; Chai, W.L. Immune responses of human dental pulp stem cells in lipopolysaccharide-induced microenvironment. Cell Biol. Int. 2018, 42, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Widbiller, M.; Eidt, A.; Wölflick, M.; Lindner, S.R.; Schweikl, H.; Hiller, K.A.; Buchalla, W.; Galler, K.M. Interactive effects of LPS and dentine matrix proteins on human dental pulp stem cells. Int. Endod. J. 2018, 51, 877–888. [Google Scholar] [CrossRef]
- Lee, S.; Zhang, Q.Z.; Karabucak, B.; Le, A.D. DPSCs from inflamed pulp modulate macrophage function via the TNF-α/IDO axis. J. Dent. Res. 2016, 95, 1274–1281. [Google Scholar] [CrossRef]
- He, W.; Wang, Z.; Zhou, Z.; Zhang, Y.; Zhu, Q.; Wei, K.; Lin, Y.; Cooper, P.R.; Smith, A.J.; Yu, Q. Lipopolysaccharide enhances Wnt5a expression through toll-like receptor 4, myeloid differentiating factor 88, phosphatidylinositol 3-OH kinase/AKT and nuclear factor kappa B pathways in human dental pulp stem cells. J. Endod. 2014, 40, 69–75. [Google Scholar] [CrossRef]
- Ning, T.; Shao, J.; Zhang, X.; Luo, X.; Huang, X.; Wu, H.; Xu, S.; Wu, B.; Ma, D. Ageing affects the proliferation and mineralization of rat dental pulp stem cells under inflammatory conditions. Int. Endod. J. 2020, 53, 72–83. [Google Scholar] [CrossRef]
- Yuan, H.; Zhao, H.; Wang, J.; Zhang, H.; Hong, L.; Li, H.; Che, H.; Zhang, Z. MicroRNA let-7c-5p promotes osteogenic differentiation of dental pulp stem cells by inhibiting lipopolysaccharide-induced inflammation via HMGA2/PI3K/Akt signal blockade. Clin. Exp. Pharmacol. Physiol. 2019, 46, 389–397. [Google Scholar] [CrossRef]
- He, W.X.; Wang, Z.H.; Luo, Z.R.; Yu, Q.; Jiang, Y.; Zhang, Y.Q.; Zhou, Z.Y.; Smith, A.J.; Cooper, P.R. LPS Promote the Odontoblastic Differentiation of Human Dental Pulp Stem Cells via MAPK Signaling Pathway. J. Cell. Physiol. 2015, 230, 554–561. [Google Scholar] [CrossRef]
- Chen, W.; Guan, Y.; Xu, F.; Jiang, B. 4-Methylumbelliferone promotes the migration and odontogenetic differentiation of human dental pulp stem cells exposed to lipopolysaccharide in vitro. Cell Biol. Int. 2021, 45, 1415–1422. [Google Scholar] [CrossRef]
- Gong, Q.M.; Quan, J.J.; Jiang, H.W.; Ling, J.Q. Regulation of the stromal cell-derived factor-1α-CXCR4 axis in human dental pulp cells. J. Endod. 2010, 36, 1499–1503. [Google Scholar] [CrossRef]
- Feng, X.; Feng, G.; Xing, J.; Shen, B.; Tan, W.; Huang, D.; Lu, X.; Tao, T.; Zhang, J.; Li, L.; et al. Repeated lipopolysaccharide stimulation promotes cellular senescence in human dental pulp stem cells (DPSCs). Cell Tissue Res. 2014, 356, 369–380. [Google Scholar] [CrossRef]
- Feng, G.; Zheng, K.; Cao, T.; Zhang, J.; Lian, M.; Huang, D.; Wei, C.; Gu, Z.; Feng, X. Repeated stimulation by LPS promotes the senescence of DPSCs via TLR4/MyD88-NF-κB-p53/p21 signaling. Cytotechnology 2018, 70, 1023–1035. [Google Scholar] [CrossRef]
- Huang, Y.; Qiao, W.; Wang, X.; Gao, Q.; Peng, Y.; Bian, Z.; Meng, L. Role of Ku70 in the apoptosis of inflamed dental pulp stem cells. Inflamm. Res. 2018, 67, 777–788. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Han, J.; Wang, Y.; Lei, S. Effects of epigallocatechin gallate (EGCG) on the biological properties of human dental pulp stem cells and inflammatory pulp tissue. Arch. Oral Biol. 2021, 123, 105034. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, N.X.; Qin, M.; Wang, Y.Y. Betamethasone suppresses the inflammatory response in LPS-stimulated dental pulp cells through inhibition of NF-κB. Arch. Oral Biol. 2019, 98, 156–163. [Google Scholar] [CrossRef]
- Soares, D.G.; Zhang, Z.; Mohamed, F.; Eyster, T.W.; de Souza Costa, C.A.; Ma, P.X. Simvastatin and nanofibrous poly(L-lactic acid) scaffolds to promote the odontogenic potential of dental pulp cells in an inflammatory environment. Acta Biomater. 2018, 68, 190–203. [Google Scholar] [CrossRef]
- Mo, Z.; Li, Q.; Cai, L.; Zhan, M.; Xu, Q. The effect of DNA methylation on the miRNA expression pattern in lipopolysaccharide-induced inflammatory responses in human dental pulp cells. Mol. Immunol. 2019, 111, 11–18. [Google Scholar] [CrossRef]
- Sipert, C.R.; Morandini, A.C.; Dionisio, T.J.; Trachtenberg, A.J.; Kuo, W.P.; Santos, C.F. MicroRNA-146a and microRNA-155 show tissue-dependent expression in dental pulp, gingival and periodontal ligament fibroblasts in vitro. J. Oral Sci. 2014, 56, 157–164. [Google Scholar] [CrossRef]
- Feng, Z.; Li, Q.; Meng, R.; Yi, B.; Xu, Q. METTL3 regulates alternative splicing of MyD88 upon the lipopolysaccharide-induced inflammatory response in human dental pulp cells. J. Cell. Mol. Med. 2018, 22, 2558–2568. [Google Scholar] [CrossRef]
- Tian, X.X.; Li, R.; Liu, C.; Liu, F.; Yang, L.J.; Wang, S.P.; Wang, C.L. NLRP6-caspase 4 inflammasome activation in response to cariogenic bacterial lipoteichoic acid in human dental pulp inflammation. Int. Endod. J. 2021, 54, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Sun, X.; Ren, Q.; Liu, J. Protective effect of SIRT6 against LPS-induced human dental pulp cell apoptosis via regulating Ku70 deacetylation. Int. J. Clin. Exp. Pathol. 2017, 10, 72–79. [Google Scholar]
- Shin, M.R.; Kang, S.K.; Kim, Y.S.; Lee, S.Y.; Hong, S.C.; Kim, E.C. TNF-α and LPS activate angiogenesis via VEGF and SIRT1 signalling in human dental pulp cells. Int. Endod. J. 2015, 48, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.I.; Kang, S.K.; Jung, H.J.; Chun, Y.H.; Kwon, Y.D.; Kim, E.C. Muramyl dipeptide activates human beta defensin 2 and pro-inflammatory mediators through Toll-like receptors and NLRP3 inflammasomes in human dental pulp cells. Clin. Oral Investig. 2015, 19, 1419–1428. [Google Scholar] [CrossRef]
- Lee, S.A.; Kim, B.R.; Kim, B.K.; Kim, D.W.; Shon, W.J.; Lee, N.R.; Inn, K.S.; Kim, B.J. Heat shock protein-mediated cell penetration and cytosolic delivery of macromolecules by a telomerase-derived peptide vaccine. Biomaterials 2013, 34, 7495–7505. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.J.; Kwon, K.Y.; Kum, K.Y.; Lee, W.C.; Baek, S.H.; Kang, M.K.; Shon, W.J. The Anti-Inflammatory Effect of Human Telomerase-Derived Peptide on P. gingivalis Lipopolysaccharide-Induced Inflammatory Cytokine Production and Its Mechanism in Human Dental Pulp Cells. Mediat. Inflamm. 2015, 2015, 385127. [Google Scholar] [CrossRef]
- Wang, F.; Han, Y.; Xi, S.; Lu, Y. Catechins reduce inflammation in lipopolysaccharide-stimulated dental pulp cells by inhibiting activation of the NF-κB pathway. Oral Dis. 2020, 26, 815–821. [Google Scholar] [CrossRef]
- Kim, D.S.; Shin, M.R.; Kim, Y.S.; Bae, W.J.; Roh, D.H.; Hwang, Y.S.; Kim, E.C. Anti-inflammatory effects of glutamine on LPS-stimulated human dental pulp cells correlate with activation of MKP-1 and attenuation of the MAPK and NF-κB pathways. Int. Endod. J. 2015, 48, 220–228. [Google Scholar] [CrossRef]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Dohan, D.M. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part IV: Clinical effects on tissue healing. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e56–e60. [Google Scholar] [CrossRef]
- Kim, J.H.; Woo, S.M.; Choi, N.K.; Kim, W.J.; Kim, S.M.; Jung, J.Y. Effect of Platelet-rich Fibrin on Odontoblastic Differentiation in Human Dental Pulp Cells Exposed to Lipopolysaccharide. J. Endod. 2017, 43, 433–438. [Google Scholar] [CrossRef]
- Galicia, J.C.; Naqvi, A.R.; Ko, C.C.; Nares, S.; Khan, A.A. MiRNA-181a regulates Toll-like receptor agonist-induced inflammatory response in human fibroblasts. Genes Immun. 2014, 15, 333–337. [Google Scholar] [CrossRef]
- Sipert, C.R.; Morandini, A.C.F.; Modena, K.C.S.; Dionisio, T.J.; Machado, M.A.A.M.; de Oliveira, S.H.P.; Campanelli, A.P.; Santos, C.F. CCL3 and CXCL12 production in vitro by dental pulp fibroblasts from permanent and deciduous teeth stimulated by Porphyromonas gingivalis LPS. J. Appl. Oral Sci. 2013, 21, 99–105. [Google Scholar] [CrossRef]
- Chang, J.; Zhang, C.; Tani-Ishii, N.; Shi, S.; Wang, C.Y. NF-κB activation in human dental pulp stem cells by TNF and LPS. J. Dent. Res. 2005, 84, 994–998. [Google Scholar] [CrossRef]
- Nakane, A.; Yoshida, T.; Nakata, K.; Horiba, N.; Nakamura, H. Effects of lipopolysaccharides on human dental pulp cells. J. Endod. 1995, 21, 128–130. [Google Scholar] [CrossRef]
- Hosoya, S.; Matsushima, K. Stimulation of interleukin-1β production of human dental pulp cells by Porphyromonas endodontalis lipopolysaccharide. J. Endod. 1997, 23, 39–42. [Google Scholar] [CrossRef]
- Lu, H.X.; Xiao, M.Z.; Niu, Z.Y.; Guo, X.M.; Zhao, S.L.; Wang, H.G.; Guo, H.Y. Effect of IL-1ra on human dental pulp cells and pulpal inflammation. Int. Endod. J. 2002, 35, 807–811. [Google Scholar] [CrossRef]
- Tokuda, M. Regulation of interleukin-6 expression in human dental pulp cell cultures stimulated with prevotella intermedia lipopolysaccharide. J. Endod. 2001, 27, 273–277. [Google Scholar] [CrossRef]
- Tokuda, M.; Miyamoto, R.; Nagaoka, S.; Torii, M. Substance P enhances expression of lipopolysaccharide-induced inflammatory factors in dental pulp cells. J. Endod. 2004, 30, 770–774. [Google Scholar] [CrossRef]
- Matsushita, K.; Motani, R.; Sakuta, T.; Nagaoka, S.; Matsuyama, T.; Abeyama, K.; Maruyama, I.; Takada, H.; Torii, M. Lipopolysaccharide enhances the production of vascular endothelial growth factor by human pulp cells in culture. Infect. Immun. 1999, 67, 1633–1639. [Google Scholar] [CrossRef]
- Modena, K.C.S.; Calvo, A.M.; Sipert, C.R.; Dionísio, T.J.; Navarro, M.F.L.; Atta, M.T.; Dos Santos, C.F. Dental pulp fibroblasts response after stimulation with hema and adhesive system. Braz. Dent. J. 2018, 29, 419–426. [Google Scholar] [CrossRef][Green Version]
- Tancharoen, S.; Tengrungsun, T.; Suddhasthira, T.; Kikuchi, K.; Vechvongvan, N.; Tokuda, M.; Maruyama, I. Overexpression of receptor for advanced glycation end products and high-mobility group box 1 in human dental pulp inflammation. Mediat. Inflamm. 2014, 2014, 754069. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, F.; Kitamura, C.; Nagayoshi, M.; Chen, K.K.; Terashita, M.; Nishihara, T. Ozonated Water Improves Lipopolysaccharide-Induced Responses of an Odontoblast-like Cell Line. J. Endod. 2009, 35, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.C.; Tsao, J.T.; Lew, W.Z.; Chan, Y.H.; Lee, L.W.; Lin, C.T.; Huang, Y.K.; Huang, H.M. Static magnetic field attenuates lipopolysaccharide-induced inflammation in pulp cells by affecting cell membrane stability. Sci. World J. 2015, 2015, 492683. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Huang, Y.; Bian, Z.; Sun, X.; Wang, X.; Gao, Q.; Peng, Y.; Meng, L. Lipopolysaccharide-induced DNA damage response activates nuclear factor κB signalling pathway via GATA4 in dental pulp cells. Int. Endod. J. 2019, 52, 1704–1715. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, N.; Li, J.; Zhao, L.; Gao, L.; Zhang, Y.; Ruan, J. RNA-binding protein Lin28 is associated with injured dentin-dental pulp complex in Sprague-Dawley rats. Int. J. Clin. Exp. Pathol. 2018, 11, 4385–4394. [Google Scholar]
- Jun-Hao, E.T.; Gupta, R.R.; Shyh-Chang, N. Lin28 and let-7 in the Metabolic Physiology of Aging. Trends Endocrinol. Metab. 2016, 27, 132–141. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, L.; Shen, Z.; Li, J.; Huang, S.; Wang, R.; Lin, Z.; Song, Z. Expression of nucleotide-binding oligomerization domain-like receptor family pyrin domain containing 6 in human dental pulp tissues and cells. Arch. Oral Biol. 2020, 117, 104794. [Google Scholar] [CrossRef]
- Liu, M.; Mu, H.; Peng, W.; Zhao, L.; Hu, W.; Jiang, Z.; Gao, L.; Cao, X.; Li, N.; Han, J. Time-dependent C5a and C5aR expression in dental pulp cells following stimulation with LTA and LPS. Int. J. Mol. Med. 2019, 44, 823–834. [Google Scholar] [CrossRef]
- Kawai, S.; Harada, K.; Daito, K.; Arita, K.; Ohura, K. Tnf-α and LPS enhance MMP production in human dental pulp cells of deciduous teeth. J. Hard Tissue Biol. 2012, 21, 151–156. [Google Scholar] [CrossRef]
- Shen, S.; Shang, L.; Liu, H.; Liang, Q.; Liang, W.; Ge, S. AGGF1 inhibits the expression of inflammatory mediators and promotes angiogenesis in dental pulp cells. Clin. Oral Investig. 2021, 25, 581–592. [Google Scholar] [CrossRef]
- Sun, G.; Ren, Q.; Bai, L.; Zhang, L. Phoenixin-20 suppresses lipopolysaccharide-induced inflammation in dental pulp cells. Chem.-Biol. Interact. 2020, 318, 108971. [Google Scholar] [CrossRef]
- Yosten, G.L.; Lyu, R.M.; Hsueh, A.J.; Avsian-Kretchmer, O.; Chang, J.K.; Tullock, C.W.; Dun, S.L.; Dun, N.; Samson, W.K. A novel reproductive peptide, phoenixin. J. Neuroendocr. 2013, 25, 206–215. [Google Scholar] [CrossRef]
- Huang, Y.; Li, X.; Liu, Y.; Gong, Q.; Tian, J.; Jiang, H. LPS-induced autophagy in human dental pulp cells is associated with p38. J. Mol. Histol. 2021, 5, 919–928. [Google Scholar] [CrossRef]
- Gao, Y.; You, X.; Liu, Y.; Gao, F.; Zhang, Y.; Yang, J.; Yang, C. Induction of autophagy protects human dental pulp cells from lipopolysaccharide-induced pyroptotic cell death. Exp. Med. 2020, 19, 2202–2210. [Google Scholar] [CrossRef]
- Hu, J.; Chen, W.; Qiu, Z.; Lv, H. Robust expression of SIRT6 inhibits pulpitis via activation of the TRPV1 channel. Cell Biochem. Funct. 2020, 38, 676–682. [Google Scholar] [CrossRef]
- Paudel, U.; Lee, Y.H.; Kwon, T.H.; Park, N.H.; Yun, B.S.; Hwang, P.H.; Yi, H.K. Eckols reduce dental pulp inflammation through the ERK1/2 pathway independent of COX-2 inhibition. Oral Dis. 2014, 20, 827–832. [Google Scholar] [CrossRef]
- Liu, L.; Shu, S.; Cheung, G.S.; Wei, X. Effect of miR-146a/bFGF/PEG-PEI Nanoparticles on Inflammation Response and Tissue Regeneration of Human Dental Pulp Cells. BioMed Res. Int. 2016, 2016, 3892685. [Google Scholar] [CrossRef]
- Guo, X.; Chen, J. The protective effects of saxagliptin against lipopolysaccharide (LPS)-induced inflammation and damage in human dental pulp cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1288–1294. [Google Scholar] [CrossRef]
- Lai, W.Y.; Kao, C.T.; Hung, C.J.; Huang, T.H.; Shie, M.Y. An evaluation of the inflammatory response of lipopolysaccharide-treated primary dental pulp cells with regard to calcium silicate-based cements. Int. J. Oral Sci. 2014, 6, 94–98. [Google Scholar] [CrossRef]
- Wang, M.C.; Tu, H.F.; Chang, K.W.; Lin, S.C.; Yeh, L.Y.; Hung, P.S. The molecular functions of Biodentine and mineral trioxide aggregate in lipopolysaccharide-induced inflamed dental pulp cells. Int. Endod. J. 2021, 54, 1317–1327. [Google Scholar] [CrossRef]
- Yang, G.; Ju, Y.; Liu, S.; Zhao, S. Lipopolysaccharide upregulates the proliferation, migration, and odontoblastic differentiation of NG2+ cells from human dental pulp in vitro. Cell Biol. Int. 2019, 43, 1276–1285. [Google Scholar] [CrossRef]
- Chmilewsky, F.; Jeanneau, C.; Laurent, P.; About, I. LPS induces pulp progenitor cell recruitment via complement activation. J. Dent. Res. 2015, 94, 166–174. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Li, W.; Tian, S. PECAM1 Combines With CXCR4 to Trigger Inflammatory Cell Infiltration and Pulpitis Progression Through Activating the NF-κB Signaling Pathway. Front. Cell Dev. Biol. 2020, 8, 1694. [Google Scholar] [CrossRef]
- Wang, D.; Sun, S.; Xue, Y.; Qiu, J.; Ye, T.; Zhang, R.; Song, B.; He, W.; Zhang, Y.; Jiang, W. MicroRNA-223 negatively regulates LPS-induced inflammatory responses by targeting NLRP3 in human dental pulp fibroblasts. Int. Endod. J. 2021, 54, 241–254. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Pei, F.; Chen, Z.; Zhang, L. FoxO3a Regulates Inflammation-induced Autophagy in Odontoblasts. J. Endod. 2018, 44, 786–791. [Google Scholar] [CrossRef]
- Wang, H.S.; Yang, F.H.; Wang, Y.J.; Pei, F.; Chen, Z.; Zhang, L. Odontoblastic Exosomes Attenuate Apoptosis in Neighboring Cells. J. Dent. Res. 2019, 98, 1271–1278. [Google Scholar] [CrossRef]
- He, Q.; Wang, H.; Fan, M.; Zhang, L.; Huang, S.; Li, Y. Activation of autophagy in pulpitis is associated with TLR4. Int. J. Clin. Exp. Pathol. 2017, 10, 4488–4496. [Google Scholar]
- Yuan, H.; Zhang, H.; Hong, L.; Zhao, H.; Wang, J.; Li, H.; Che, H.; Zhang, Z. MicroRNA let-7c-5p suppressed lipopolysaccharide-induced dental pulp inflammation by inhibiting dentin matrix protein-1-mediated nuclear factor kappa b (NF-κb) pathway in vitro and in vivo. Med. Sci. Monit. 2018, 24, 6656–6665. [Google Scholar] [CrossRef]
- Huang, X.; Qiu, W.; Pan, Y.; Li, J.; Chen, Z.; Zhang, K.; Luo, Y.; Wu, B.; Xu, W. Exosomes from LPS-Stimulated hDPSCs Activated the Angiogenic Potential of HUVECs in Vitro. Stem Cells Int. 2021, 2021, 6685307. [Google Scholar] [CrossRef]
- Wang, J.; Du, Y.; Deng, J.; Wang, X.; Long, F.; He, J. MicroRNA-506 is involved in regulation of the occurrence of lipopolysaccharides (LPS)-induced pulpitis by sirtuin 1 (SIRT1). Med. Sci. Monit. 2019, 25, 10008–10015. [Google Scholar] [CrossRef]
- Zhai, Y.; Yuan, X.; Zhao, Y.; Ge, L.; Wang, Y. Potential Application of Human β-Defensin 4 in Dental Pulp Repair. Front. Physiol. 2020, 11, 1077. [Google Scholar] [CrossRef] [PubMed]
- Zhong, T.Y.; Zhang, Z.C.; Gao, Y.N.; Lu, Z.; Qiao, H.; Zhou, H.; Liu, Y. Loss of Wnt4 expression inhibits the odontogenic potential of dental pulp stem cells through JNK signaling in pulpitis. Am. J. Transl. Res. 2019, 11, 1819–1826. [Google Scholar] [PubMed]
- Chen, W.J.; Xie, J.; Lin, X.; Ou, M.H.; Zhou, J.; Wei, X.L.; Chen, W.X. The Role of Small Extracellular Vesicles Derived from Lipopolysaccharide-preconditioned Human Dental Pulp Stem Cells in Dental Pulp Regeneration. J. Endod. 2021, 47, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Meng, L.; Qiao, W.; Yang, R.; Gao, Q.; Peng, Y.; Bian, Z. Vascular endothelial growth factor A/Vascular endothelial growth factor receptor 2 axis promotes human dental pulp stem cell migration via the FAK/PI3K/Akt and p38 MAPK signalling pathways. Int. Endod. J. 2019, 52, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Bindal, P.; Gnanasegaran, N.; Bindal, U.; Haque, N.; Ramasamy, T.S.; Chai, W.L.; Kasim, N.H.A. Angiogenic effect of platelet-rich concentrates on dental pulp stem cells in inflamed microenvironment. Clin. Oral Investig. 2019, 23, 3821–3831. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Gong, Z.; Zhu, F.; Qiu, Y.; Li, X. Simvastatin increases cell viability and suppresses the expression of cytokines and vascular endothelial growth factor in inflamed human dental pulp stem cells in vitro. Adv. Clin. Exp. Med. 2018, 27, 1615–1623. [Google Scholar] [CrossRef]
- Kyoto Encyclopedia of Genes and Genomes. Available online: https://www.genome.jp/kegg/pathway.html (accessed on 29 October 2021).

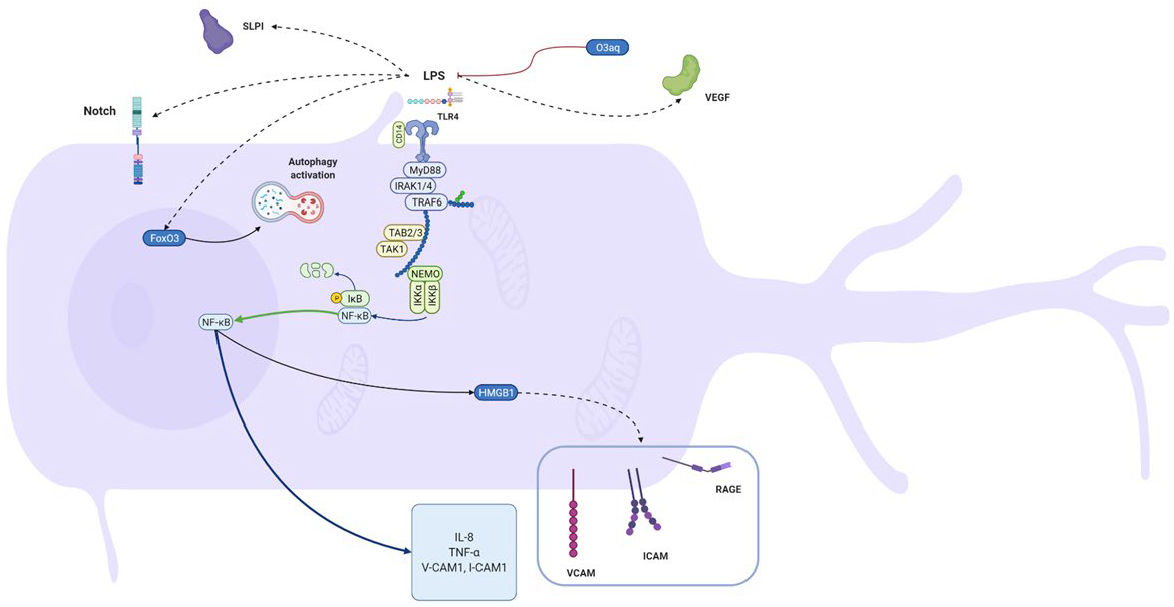
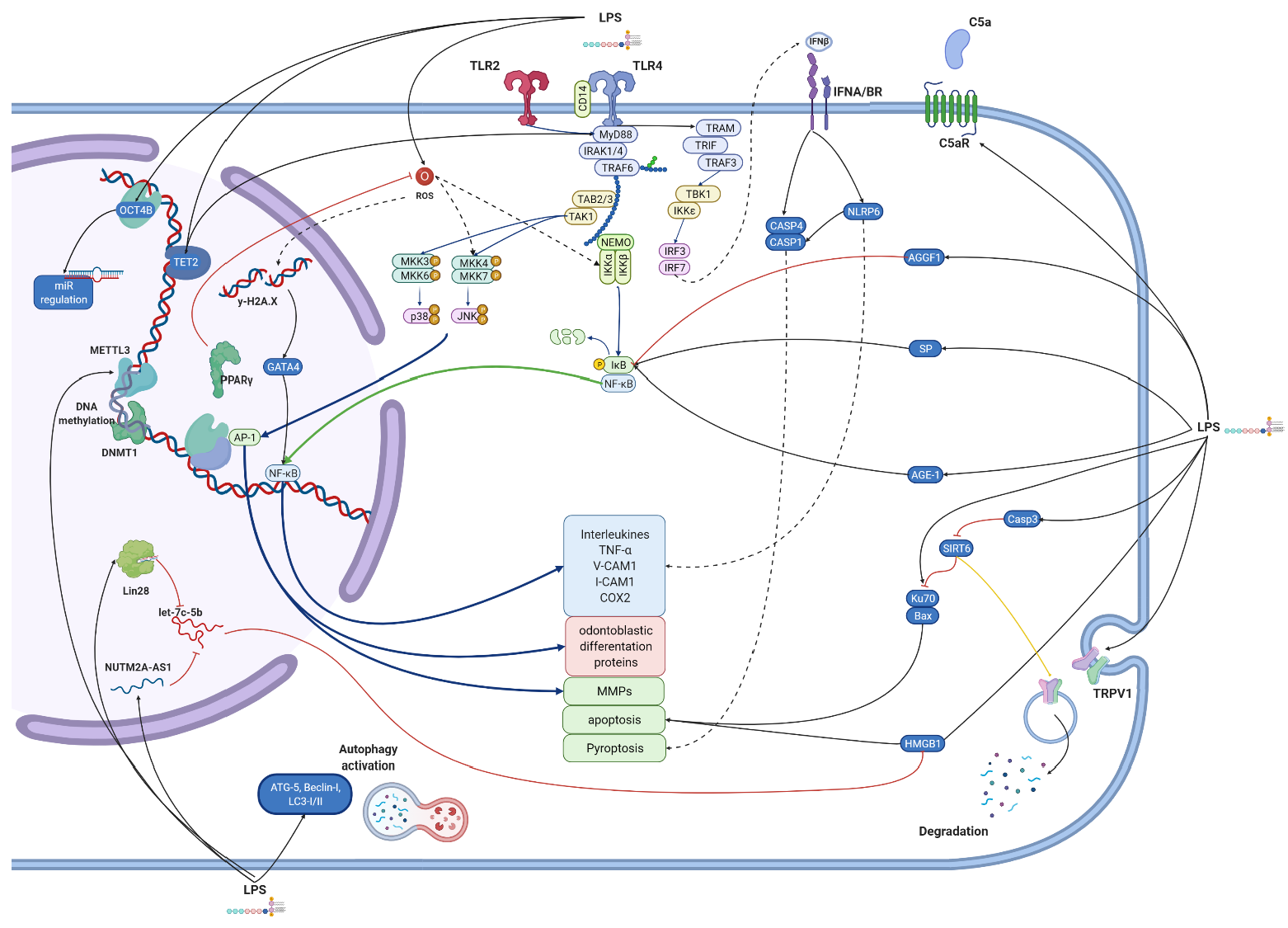
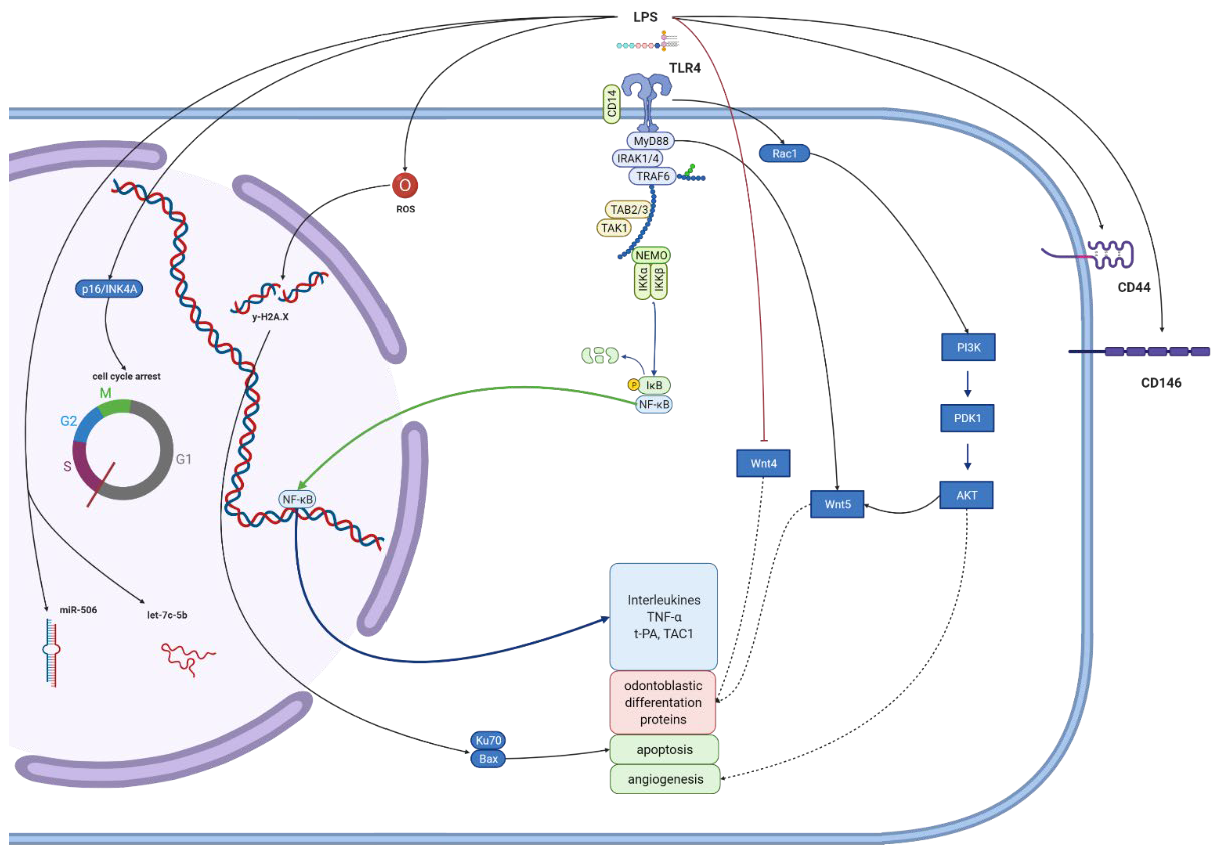

| Cells | LPS | Effect in LPS-Stimulated Cells | Author |
|---|---|---|---|
| hDPCs from healthy molar tooth | E. coli LPS 0111:B4(L5293) | Higher NLRP6 expression. Knockdown of NLRP6 inhibits IL-1B expression. | [99] |
| hDPCs from healthy molar tooth | E. coli 055:B5 | Upregulated HMGB1. Increased DPCs mobility. | [14] |
| hDPCs from healthy molar tooth | E. coli 055:B5 | SITR6 promotes Ku70 deacetylation in LPS-stimulated cells; suppresses Bax protein release and apoptosis. | [22] |
| hDPCs from healthy molar tooth | E. coli O111:B4 | High level/activation of PPARγ inhibits expression of MMP2, MMP9, VCAM-1 and ICAM-1 | [18] |
| hDPCs and NG2+ from healthy premolar tooth | unspecified | Upregulated IL-1β, IL-6, IL-8, and TNF-α in hDPCs and NG2+. The latter show enhanced proliferation, migration, odontoblastic differentiation. | [113] |
| hDPCs | E. coli 0127:B8 | Augmented AGE-1 protein expression. Silencing AGE-1 attenuates expression of IL-1β, IL-6, TNF-α; decreases nuclear translocation of p65 from NF-κB pathway | [19] |
| hDPCs from healthy molar tooth | E. coli | Betamethasone reduces expression of IL-1β, IL-6, TNF-α in LPS induced cells; upregulates ALP, OCN and DSPP proteins | [29] |
| hDPCs—homogeneous, spindle-shaped fibroblasts | E. coli | Elevated TET2 mRNA and protein levels. TET2 knockdown inhibits LPS-induced inflammatory response in hDPCs by downregulating MyD88 hydroxymethylation. | [7] |
| hDPCs from American Tissue Culture Collection (ATCC, Manassas, VA, USA) | E. coli 055:B5 | Increased NUTM2A-AS1 levels; lowered expression of let-7c-5p. High level of HMGB-1 attenuates hDPCs vitality, induces apoptosis and expression of IL-6 and IL-8. | [13] |
| hDPCs from canine, premolar and molar healthy teeth | unspecified | Biodentine and MTA did not decrease level of inflammation related miR-146a but enhanced dentinogenesis, proliferation and adhesion features of cells. | [112] |
| hDPCs from healthy permanent teeth | P. gingivalis | ECG and EGCG reduce mRNA expression and IL-1β, IL-6, TNF-α secretion by interfering with NF-κB pathway and reducing p65 levels. | [79] |
| hDPCs—dental-pulp fibroblasts | P. intermedia | Upregulated mRNA expression for Substance P and its receptor. Substance P enhances LPS-induced expression of IL-1α and COX-2; increases LPS-induced NF-κB binding activity. | [90] |
| hDPCs—dental-pulp fibroblasts | P. intermedia, Salmonella abortusequi, E. coli | CD14 is costimulatory in IL-6 expression in dental-pulp fibroblasts. Higher IL-6 activity in P. intermedia culture than in Salmonella. | [89] |
| hDPCs from healthy molar tooth | P. gingivalis | Increased levels of IFN-β1 (after 3h), NLRP6, CASP1, CASP4 (after 6h). Blocking IFNAR1 lowers expression of those particles. Higher expression of IL-1β. | [73] |
| hDPCs from healthy molar tooth | E. coli 0111:B4 | Decreased ATG-5, Beclin-I and LC3-I/II (autophagy), HO-1 and PGC-1α (mitochondria). Schisandrin C treatment recovered expression of all mentioned proteins. | [23] |
| hDPCs from healthy molar tooth | unspecified | Inhibited GPR173 mRNA and protein expression -maximal effect at 24h. Phoenixin20 inhibits expression of MMP2 and MMP9; suppresses TLR4 and MyD88. The effect of phoenixin20 depends on GPR173. | [103] |
| hDPCs immortalized by transfection with a human telomerase transcriptase gene (HPD-hTERTs) | E. coli 0111:B4 | Higher IL-1β, IL-6 expression. Induced Wnt5a, Runx2, and ALP (markers of dentinogenesis). Box-5 (Wnt5a antagonist) treatment mitigates expression of Runx2 and ALP. | [40] |
| hDPCs immortalized by transfection with a human telomerase transcriptase gene (HPD-hTERTs) | P. gingivalis | LPS with TNF-α upregulates VEGF and SIRT1 with subsequent upregulation of MMP-2 and MMP-9. Pathway that leads to this activation involves PI3K, p38, ERK, JNK and NF-κB. | [75] |
| hDPCs and from healthy premolar tooth | unspecified | Upregulated AGGF1 protein inhibits phosphorylation of NF-κB and its transfer into the nucleus. | [102] |
| hDPCs from healthy molar tooth | unspecified | Upregulated expression of GATA4, γ-H2A.X and p65. Higher ROS levels cause double-strand breaks. Subsequently, γ-H2A.X upregulates GATA4 and intensifies p65 translocation into nucleus. Elevated IL-1β, IL-6, TNF-α levels. | [96] |
| hDPCs | unspecified | EB1 and EB5 lower ICAM-1 and VCAM-1 expression, inhibiting ERK and JNK kinases and blocking translocation of NF-κB into nucleus. EB1 mitigates COX-2 expression. | [108] |
| hDPCs from healthy molar tooth | E. coli | Increased production of MMPs. | [6] |
| hDPCs from healthy molar tooth | E. coli 0111:B4 | Overexpressed miR-21-5p decreases NF-κB p65 phosphorylation, expression of IL-6, interfering with TRAF6 mRNA, part of TLR4 transduction complex. | [15] |
| hDPCs from healthy molar tooth | E. coli | EGCG and ECG reduce IL-6, IL-8, VCAM-1 and ICAM-1 protein expression. | [36] |
| hDPCs from healthy tooth | E. coli | EGCG and ECG reduce LPS-mediated VEGF production. Catechins attenuated COX-2 expression. | [37] |
| hDPCs from healthy tooth | P. gingivalis AEI2, P. endodontalis AE51, and F. nucleatum | E. coli LPS inhibits cell protein production more potently than LPS from other types. Unlike others, the E. coli LPS induces DNA production by dental pulp cells. | [86] |
| hDPCs from healthy premolar tooth | P. gingivalis | Downregulation of mRNA for DNMT3B and protein level of DNMT1. Inhibition of those protein results in upregulation of miR-146a-5p. | [70] |
| hDPCs from healthy tooth | P. intermedia | Enhanced VEGF production | [91] |
| hDPCs from healthy tooth | F. nucleatum | Both LPS and IL-1ra reduce IL-1β synthesis. | [88] |
| hDPCs from healthy premolar tooth | E. coli | Fluocinolone acetonide upregulates DSPP, RUNX2, inhibiting phosphorylated NF-κB p65 and activating AP-1. FA may restore expression of ALP, RUNX2 and DSPP. | [28] |
| hDPCs from healthy premolar or molar teeth | unspecified | Overexpression of Lin28 RNA binding protein lowers let-7b, let-7g and miR98 levels. | [97] |
| Primary human dental pulp cells were purchased from American Type Culture Collection (ATCC). | E. coli and P. gingivalis | Tenegliptin reduces production of 4-HNE from lipid peroxidation as the result of ROS activity in LPS stimulated cells. In addition, suppresses TLR4 mRNA and protein levels after LPS treatment. | [26] |
| hDPCs from healthy molar tooth | unspecified | Upregulated C5a and C5aR mRNA and protein expression | [100] |
| hDPCs from healthy premolar tooth | E. coli 0111:B4 | Upregulated lncMEG3; its knock-down inhibits the secretion of IL-1β, IL-6, TNF-α and promotes odontogenic differentiation through Wnt/β-catenin pathway. | [12] |
| hDPCs from healthy molar tooth | unspecified | Specific miR-146a/PEG-PEI nanoparticles combined with alginate hydrogel with basic fibroblast growth factor stimulate cell proliferation and expression of DMP-1 and DSP protein. | [109] |
| hDPCs from healthy molar tooth | E. coli 0111:B4 | Higher levels of OCT4B1 leading to decreased percentage of apoptotic cells in viability tests. | [9] |
| hDPCs from healthy molar tooth | E. coli | Induced expression of sclerostin; subsequent increase of proinflammatory cytokines through NF-κB pathway. Sclerostin induces expression of VCAM-1 and ICAM-1 and inhibits odontoblastic differentiation. LPS upregulates VEGF, VEGFR and PlGF expression; effect aggravated by sclerostin. | [54] |
| hDPCs immortalized by transfection with a human telomerase transcriptase gene (HPD-hTERTs) | E. coli and P. gingivalis | MDP + TLR ligands treatment increases NOD2 and hBD2 mRNA and protein levels compared with MDP + TLR2/TLR4. | [76] |
| hDPCs from healthy molar tooth? | E. coli 0111:B4 | Davallialactone eliminates ROS from LPS-induced cells. Decreased expression of ICAM-1, VCAM-1, MMP-2, MMP-9, iNOS and COX-2, caused by inhibiting inflammation at the ERK1/2 and NF-κB stages. | [24] |
| hDPCs from healthy molar tooth | E. coli 0111:B4 | Terrein reduces ICAM-1, VCAM-1 levels by blocking Akt-1 action and suppressing NF-κB activation. | [34] |
| hDPCs from healthy molar tooth | E. coli | Upregulated Oct-4B1 and Oct-4B mRNA levels. Oct-4B1 knock-down downregulates Oct-4B and increases number of apoptotic cells. Absence of Oct-4B1 change expression pattern of 38 microRNA. | [8] |
| hDPCs from healthy molar tooth | P. gingivalis | GV1001 peptide inhibits LPS-induced IL-6, TNF-α expression. Effect mediated by reduced phosphorylation of ERK and p38 MAP kinases. | [78] |
| hDPCs from maxillary supernumerary incisors and molar teeth | P. gingivalis | PRFe attenuate IL-1β, IL-6, IL-8, VCAM-1, ICAM-1 expression; enhances dentin sialophosphoprotein, dentin matrix acidic phosphoprotein 1 expression and increases ALP activity | [82] |
| hDPCs from healthy molar tooth | E. coli 0111:B4 | Decreased PPARγ levels, ERK1/2 activities and NF-κB translocation. Rosiglitazone decreases proinflammatory stimulation. Activity of PPARγ mediated by removal of ROS formation. | [17] |
| hDPCs immortalized by transfection with a human telomerase transcriptase gene (HPD-hTERTs) | P. gingivalis | Glutamine reduces iNOS and COX-2 expression, inhibits IL-1β, IL-8, TNF-α production and attenuates MAP kinases phosphorylation, NF-κB-p65 nuclear translocation; induces MKP-1 expression. | [80] |
| hDPCs from healthy deciduous tooth | unspecified | TNF-α and LPS stimulation increases MMP-1 and MMP-3 mRNA levels, but only MMP-3 protein. | [101] |
| hDPCs from healthy deciduous tooth | unspecified | Simvastatin reduces LPS-stimulated IL-1β, IL-6, VCAM-1, ICAM-1 production, attenuating phosphorylation and translocation of p-65 and I-κB. | [30] |
| hDPCs from healthy molar tooth | unspecified | Induced expression of beclin-1 and LC3II (autophagy) via MAP kinases phosphorylation and translocation of NF-κB into the nucleus. | [105] |
| hDPCs from healthy premolar or molar teeth | unspecified | In odontogenic induction medium: increased ALP activity, DSPP-1 and DMP-1 expression. Enhanced NF-κB translocation into nucleus. | [39] |
| hDPCs from healthy premolar or molar teeth | unspecified | SIRT6 overexpression downregulates IL-1β, IL-6, TNF-α and inhibits NF-κB pathway; reduces expression of TRPV1, whose activation by capsaicin upregulates expression of proinflammatory cytokines. | [107] |
| hDPCs from healthy permanent teeth | P. endodontalis | Increased IL-1β mRNA and protein level in a dose-dependent manner. | [87] |
| hDPCs from human supernumerary teeth | E. coli | Bromelain reduces IL-1β, IL-6, IL-8, VCAM-1 and ICAM-1 expression by inhibiting phosphorylation of p65 protein, ERK and p38 kinases; increases ALP activity. | [33] |
| hDPCs from human supernumerary teeth | unspecified | Promotion of pyroptotic cell death, with increased levels of IL 1β, IL 18 and caspase 1. Promotion of autophagy inhibits pyroptotic cell death. | [106] |
| hDPCs from healthy molar tooth | E. coli 0111:B4 | The antioxidant effect of sole N-acetylcysteine (NAC) not evident. In combination with Biodentine or MTA, improved LPS-induced hDPCs survival at 24 h. NAC+MTA promoted mineralization. | [27] |
| hDPCs from healthy molar tooth | P. gingivalis | Elevated cell apoptosis (higher caspase 3 activity) inhibited cell proliferation and survival, lower SIRT6 expression. SIRT6 has anti-apoptotic effect via regulating Ku70 protein deacetylation. | [74] |
| hDPCs from healthy premolar and molar teeth | E. coli | Upregulated secretion of IL-6 and IL-8. 5-Aza-CdR can promote LPS-induced inflammation by upregulating proinflammatory cytokines expression and activating NF-κB and MAPK signaling pathways by decreasing the methylation level of TRAF6 promoter. | [11] |
| Primary human dental pulp cells were purchased from American Type Culture Collection (ATCC). | unspecified | Upregulated DPP-4 expression. Saxagliptin ameliorated LPS-induced oxidative stress, mitochondrial dysfunction, apoptosis, and reduced LPS-induced production of TNF-a, IL-1b and IL-8; inhibited p38 activation and NF-κB signaling pathway. | [110] |
| hDPCs from healthy premolar tooth | unspecified | MTA stimulates IL-1β expression and apoptosis. CS cement enhances cellular proliferation. | [111] |
| hDPCs from healthy premolar and molar teeth | E. coli | Decreased expression of methyltransferase DNMT1, leading to increased cytokine secretion and activating NF-κB and MAPK signaling. Silencing DNMT1 contributes to downregulating methylation levels at the promoters of IL-6 and TRAF6. | [10] |
| hDPCs from healthy premolar and molar teeth | E. coli | Nell-1 may attenuate LPS-induced inflammation acting via p38 and ERK MAPK, but not JNK MAPK signaling pathway. | [31] |
| hDPCs from healthy premolar and molar teeth | unspecified | Upregulated expression of m6A and METTL3. METTL3 depletion decreased the accumulation of inflammatory cytokines and suppressed the NF-κB and MAPK signaling pathways. METTL3 modulated the alternative splicing of MyD88. | [72] |
| hDPCs from healthy molar teeth | E. coli 0111:B4 | HIF1a suppressed IL-6 expression via SOCS3-dependent downregulation of CEBPb. | [21] |
| hDPCs from healthy molar teeth | E. coli 0111:B4 | HIF1a promotes pro-inflammatory mediator synthesis via NF-κB signaling—increased levels of IL1b and TNFα. HIF-1α also upregulates phosphorylation of NF-κB p65 and activation of NF-κB signaling. LPS stimulation induced HIF1a expression. | [20] |
| hDPCs from healthy deciduous tooth | E. coli | Acemannan from Aloe vera, induces proliferation, differentiation, growth factor and extracellular matrix components synthesis in LPS stimulated cells. | [38] |
| Human pulp fibroblasts | unspecified | Induced membrane attack complex labeling on the cell surface. Increased C5a levels in LPS—treated fibroblasts. | [114] |
| Human pulp fibroblasts | E. coli 055B5 | Enhanced IL6 and CH3SH production. LPS doesn’t stimulate IL-1β and TNFβ production | [42] |
| Human pulp fibroblasts | E. coli | Upregulated production of CCL2, IL-8 and Il-6. Less pronounced elevation in IL-4, GCFS, GM-CSF and CCL5, followed by IL-1β, IL-10, IL12p70, IL-17A, TNF- α and INF-λ. Most cytokines stimulated during the first 6 h. | [41] |
| Human pulp fibroblasts | P. gingivalis W83 | IL-8 detectable after LPS stimulation. | [83] |
| Human pulp fibroblasts | E. coli O111:B4 | Induced release of IL-1β, no effect on NLRP3/caspase-1 inflammasome. ATP + LPS stimulate the pyrogenic P2X7 ATP-gated ion channel, activating the NLRP3/caspase-1 pathway and inducing the maturation and release of IL-1β. | [43] |
| Human pulp fibroblasts | E. coli | COX-2, but not COX-1 was induced by E. coli LPS after stimulation for 3 h. Exogenous melatonin suppresses COX-2 and IL-1β. | [45] |
| Human pulp fibroblasts | unspecified | Increased mRNA and protein expression of MEF2C, PECAM1 and CXCR4. | [115] |
| Human pulp fibroblasts | P. intermedia ATCC 25611 | P. intermedia LPS expressed IL-8 mRNA and released IL-8 in human dental pulp fibroblast cultures, expression peaked after 12 h. | [92] |
| Human pulp fibroblasts | E. coli | Increased expression of miR-146a in dental pulp cells (also in gingiva and periodontal ligament fibroblasts). Decreased expression of miR-155 in gingival fibroblasts. | [46] |
| Human pulp fibroblasts from permanent and deciduous teeth | P. gingivalis | Increased CCL3 production in cells from both permanent and deciduous teeth. Pulp fibroblasts from deciduous teeth had elevated production of CXCL12. | [48] |
| Human pulp fibroblasts | E. coli 055:B5, L2880 | Downregulated miR-21 and upregulated KBTBD7. Berberine reduced expressions of IL-1β, IL-6 and TNF-α, as well as enhanced cell proliferation and miR-21 expression. | [47] |
| Human pulp fibroblasts | unspecified | ATP + LPS reduced miR-223 expression in human dental pulp cells. miR-223 negatively regulated production and secretion of IL-1β and IL-18, acting via the NLRP3/CASP1 inflammasome pathway by targeting NLRP3. | [116] |
| Human pulp fibroblasts from premolars | E. coli 0111:B4 | LPS + ATP increases IL-1β secretion via NLRP3 inflammasome activating caspase-1. The TLR4/MyD88/NF-κB mediates up-regulation of NLRP3 and pro-IL-1β genes expression. ATP promotes ROS production which serves as the second signal for the activation of NLRP3 inflammasome. | [44] |
| hDPSCs from healthy molar tooth | E. coli | DPSCs/NF-PLLA scaffold constructs with simvastatin reversed LPS induced TNF-α, IL-1β and MMP-9 mRNA expression on day 28. | [69] |
| Adult human dental pulp stem cells | unspecified | 20% HPL stimulates angiogenesis by increasing levels of pro-angiogenic factors’ mRNA and protein | [127] |
| Adult human dental pulp stem cells from first premolars | E. coli | Induced expression of IL-6, IL-8, tPA and TAC1 levels. Levels of IL-1α were increasing proportionally to LPS concentrations. TNFα showed increased expression with no changes at gene expression level. This could be attributed to initiation of inflammation via TLR4 and TLR2. The viability was reduced in LPS treated cells. | [55] |
| human dental pulp stem cells | P. gingivalis, P. endodontalis | LPS and TNF activated the NF-κB signaling pathway. Stimulation by the latter lasted longer. TNF induced the phosphorylation and degradation of IκBα more potently than LPS. LPS did not induce phosphorylation of p65 transactivation domain, while TNF only weakly stimulated p65 phosphorylation. | [85] |
| human dental pulp stem cells from third mandibular molars | E. coli 0111:B4L2880 | 4-MU may facilitate cell differentiation by downregulating the expression of inflammatory cytokines. CD44 expression was downregulated upon 4-MU treatment or with LPS. CD44 plays a primary role in HA-induced cytokine release via the formation of the LMW HA-TLR4-CD44 complex. 4-MU could accelerate the LPS-induced migration of hDPSCs. | [62] |
| human dental pulp stem cells | unspecified | Altered content of dental pulp cells-derived small extracellular vesicles (sEVs). sEVs carry biologically active molecules from parental cells, which can mediate intercellular communication, induce MSC differentiation, and ultimately promote the healing. | [125] |
| human dental pulp stem cells from third molars | E. coli 0111:B4 | More pronounced SA-b-gal-positive signal (a maker of senescence), likely resulting from TLR4/MyD88-NF-jB-p53/p21 signaling pathway activation. Knockdown of p65 reversed the senescence of enhanced proliferation and the increased the total number of DPSCs with more organized F-actin. | [65] |
| human dental pulp stem cells from third molars | E. coli 0111:B4 | LPS binding with TLR4 generates ROS. The DDR and p16INK4A pathways might be the main mediators of DPSC LPS-induced senescence. ROS production may promote DDR and p16INK4A expression and then cell cycle arrest. | [64] |
| human dental pulp stem cells from premolars or third molars | E. coli 055:B5 | SDF-1a secretion largely suppressed, increased production of CXCR4. | [63] |
| human dental pulp stem cells from third molars | Ultrapure E. coli | LPS can enhance Wnt5a expression preincubation with TLR4 neutralizing antibodies reduced LPS-induction Wnt5a expression (mainly activated through the MyD88-dependent pathway). NF-kB activation and PI3K/AKT signal pathways regulate Wnt5a expression. | [58] |
| human dental pulp stem cells | Ultrapure E. coli | Induced odontogenic differentiation by increasing ALP, OCN, DMP1 and DSPP expression and mineralized nodules formation. TLR4 expression maximal after 7 days. Inhibiting or blocking TLR4 decreased the LPS-mediated expression of mineralized tissue markers and nodule formation. NF-kB signaling activated by LPS in a time-dependent manner, but not involved in cellular differentiation. Activation of p38 and ERK MAPK, not JNK MAPK signaling pathways contribute to LPS-induced differentiation of hDPSCs. | [61] |
| human dental pulp stem cells from wisdom teeth/premolars | P. aeruginosa | Dose-dependent toxic effect. (SMF) stimulation inhibits inflammatory response, can enhance proliferation. | [95] |
| human dental pulp stem cells from third molars | unspecified | Exosomes from LPS-stimulated hDPSCs exert a stronger pro-angiogenic effect on HUVECs than normal hDPSCs—LPS-dose dependently. Expression of 7 microRNAs were increased (miR-146a-5p, miR-92b-5p, miR-218-5p, miR-23b-5p, miR-2110, miR-27a-5p, and miR-200b-3p) and 3 decreased (miR-223-3p, miR-1246 and miR-494-3p). Five of them play important roles in inflammation and HUVEC function and angiogenesis (miR-223-3p being the strongest candidate). | [121] |
| human dental pulp stem cells from premolars | E. coli 0111:B4 | High expression of γ-H2A.X. marker of DSB. The mRNA and protein expression levels of Ku70 and Xrcc4 involved in NHEJ, and Rad51 in HR, significantly increased in DPSCs. Ku70 knockdown reduces the expression of XRCC4 and promotes apoptosis of DPSCs during inflammation, thereby Ku70 serves as a link between DNA damage and apoptosis. | [66] |
| human dental pulp stem cells from third molars | E. coli | Upregulated CD146 expression levels. Partially blocked expression of the NF-κB subunit p65 leading to reduced TNF-α production by macrophages. The innate immune response dependent on the TNF-α/IDO axis. | [57] |
| human dental pulp stem cells from third molars | E. coli | EGCG exerted an anti-inflammatory effect on hDPSCs without affecting cell proliferation or differentiation. EGCG inhibits hypoxia-induced apoptosis. | [67] |
| rat dental pulp stem cells | E. coli 055:B5 | LPS at low concentrations upregulated mRNA expression of mineralization-related genes (OCN, DSPP, ALP and BSP) in JDPSCs and ADPSCs, LPS effects declined with age. Enhanced proliferation by increasing TLR4 expression and through PI3K/Akt signaling. | [59] |
| human dental pulp stem cells from third molars | unspecified | VEGFA promoted the migration of hDPSCs in a concentration-dependent manner. VEGFA/VEGFR2 axis interacted with the FAK/PI3K/Akt and p38 MAPK signaling pathways in mediating hDPSCs migration. | [126] |
| human dental pulp stem cells from premolars (DPSCs) and stem cells from human exfoliated deciduous teeth (SHED) | E. coli | Betamethasone blocks NF-κB activation and exhibits an osteo-/odonto-inductive effect on DPSCs and SHED. It also displays an osteoclast effect on SHED, not on the DPSCs. | [68] |
| rat dental pulp stem cells | unspecified | The expression of miR-506 was high while that of SIRT1 was low, which was associated with pro-inflammatory cytokines upregulation and activation of the TLR4-NFkB pathway. | [122] |
| human dental pulp stem cells from third molars | E. coli O55:B5 | Decreased cell survival and more frequent necrosis. Reduced COL1A1 expression after 21 d. Promoted production of IL-6 in the late phase. | [56] |
| human dental pulp stem cells from third molars | unspecified | Simvastatin promoted cell proliferation, cell cycling and apoptosis in LPS-induced DPSCs. Expression of cytokines and VEGF (via MAPK signaling blockade) was inhibited. | [128] |
| rat dental pulp stem cells | unspecified | Inhibited expression of let-7c-5p both in vivo and in vitro. The overexpression of let-7c-5p suppressed the production of pro-inflammatory cytokines, restoring viability; also inhibited the LPS-induced activation of NF-kB signaling by inhibiting the phosphorylation of IκBa and IKKb and increasing total IκBa expression, hence suppressing the nuclear translocation of NF-kB p65. let-7c-5p action depends on the inhibition of DMP1 function. | [120] |
| rat dental pulp stem cells | E. coli 055:B5 | Induced expression of let-7c-5p could suppress inflammation and restored the osteogenic differentiation potential of inflamed DPSCs. The effect depended on the repression on HMGA2 function by let-7c-5p, leading to inhibiting PI3K/Akt pathway. | [60] |
| human dental pulp stem cells | unspecified | Human β-Defensin 4 (HBD4) shows anti-inflammatory activity in vitro, by reduction of IL-1α, IL-1β, IL-6 and TNF-α expression and promotes mineralizing cell phenotype differentiation in DPSC. Similar effects are noted in vivo. | [123] |
| human dental pulp stem cells—normal pulp derived from the mandibular third molar and inflamed pulps derived from pulps of patients with irreversible pulpitis | unspecified | Decreased Wnt4 expression, impairing the odontogenic differentiation of DPSCs. Restoration of Wnt4 was able to rehabilitate the impaired odontogenic differentiation potential. Wnt4 may function through its effect on JNK signaling pathways. | [124] |
| odontoblast-like cells (MDPC-23), undifferentiated dental pulp cells (OD-21), macrophages, and gingival fibroblasts and human embryonic kidney cells (293T, ATCC) | E. coli | Upregulated VEGF expression (TLR4-dependent signaling pathway). Therapeutic blockade of the LPS-TLR4-VEGF pathway might be beneficial for the treatment of teeth with reversible pulpitis. | [49] |
| odontoblast-like cells (MDPC-23) | E. coli k-235 strain | Odontoblast-like cells produce secretory leukocyte protease inhibitor (SLPI) in response to LPS, inhibiting the activation of NF-kB. | [51] |
| Human odontoblasts from third molars | E. coli 055:B5 | Upregulated IL-8 mRNA and protein levels. | [50] |
| self-established pre-odontoblastic cell line from third molars | unspecified | During the early stage of inflammation, FoxO3a might regulate autophagy activation for odontoblast survival. | [117] |
| Mouse odontoblast-like cells | E. coli L-2880 | Notch signaling activation by LPS stimulation is similar to that caused by mechanical injury in vivo. | [52] |
| rat clonal dental pulp cell line with odontoblastic properties (KN-3) | A. actinomycetemcomitans ATCC29524 | O3aq directly suppresses the biological effects of LPS on calcification and immunologic responses of odontoblast-like cells and its ability to demolish cell walls and cytoplasmic membranes. O3aq effects may be achieved through the direct inhibition of lipid A. | [94] |
| Human Dental Pulp Tissues; Odontoblast-like cells, OLC-1, obtained from mouse tooth germs | P. intermedia | Upregulated danger signals HMGB1 and RAGE. In response to HMGB1 stimulation, human microvascular endothelial cells increase expression of RAGE, cell adhesion molecules, e.g., ICAM-1 and VCAM-1, and the secretion of TNFα and IL-8. The increase of HMGB1 in the cells was blocked by an inhibitor of IKK-β (TPCA-1). P. intermedia LPS-mediated HMGB1 translocation involves NF-κB activation in OLC-1. | [93] |
| Odontoblast-like cells from human dental stem cells from the apical papilla (SLMhSCAP) were primarily cultured from extracted third molar dental papilla | unspecified | Increased exosome production in odontoblast-like cells generated from mineralization medium-treated hSCAPs. The exosomes showed anti-apoptosis functions. These exosome-dependent intercellular pathways may protect cells from LPS-induced apoptosis. | [118] |
| Mouse odontoblast-like cells | E. coli | Propolis decreased production of IL-1α, MIP-1a, IL-12(p70), and IL-15 in odontoblast-like cells. | [53] |
| Preodontoblastic cell line | unspecified | Levels of TLR4, NOD2 IL-1β and autophagy proteins (LC3II, beclin1) increased. | [119] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brodzikowska, A.; Ciechanowska, M.; Kopka, M.; Stachura, A.; Włodarski, P.K. Role of Lipopolysaccharide, Derived from Various Bacterial Species, in Pulpitis—A Systematic Review. Biomolecules 2022, 12, 138. https://doi.org/10.3390/biom12010138
Brodzikowska A, Ciechanowska M, Kopka M, Stachura A, Włodarski PK. Role of Lipopolysaccharide, Derived from Various Bacterial Species, in Pulpitis—A Systematic Review. Biomolecules. 2022; 12(1):138. https://doi.org/10.3390/biom12010138
Chicago/Turabian StyleBrodzikowska, Aniela, Monika Ciechanowska, Michał Kopka, Albert Stachura, and Paweł K. Włodarski. 2022. "Role of Lipopolysaccharide, Derived from Various Bacterial Species, in Pulpitis—A Systematic Review" Biomolecules 12, no. 1: 138. https://doi.org/10.3390/biom12010138
APA StyleBrodzikowska, A., Ciechanowska, M., Kopka, M., Stachura, A., & Włodarski, P. K. (2022). Role of Lipopolysaccharide, Derived from Various Bacterial Species, in Pulpitis—A Systematic Review. Biomolecules, 12(1), 138. https://doi.org/10.3390/biom12010138






