Computational Analysis of Naturally Occurring Aristolochic Acid Analogues and Their Biological Sources
Abstract
:1. Introduction
2. Materials and Methods
2.1. Virtual Screening of Naturally Occurring Aristolochic Acid Analogues
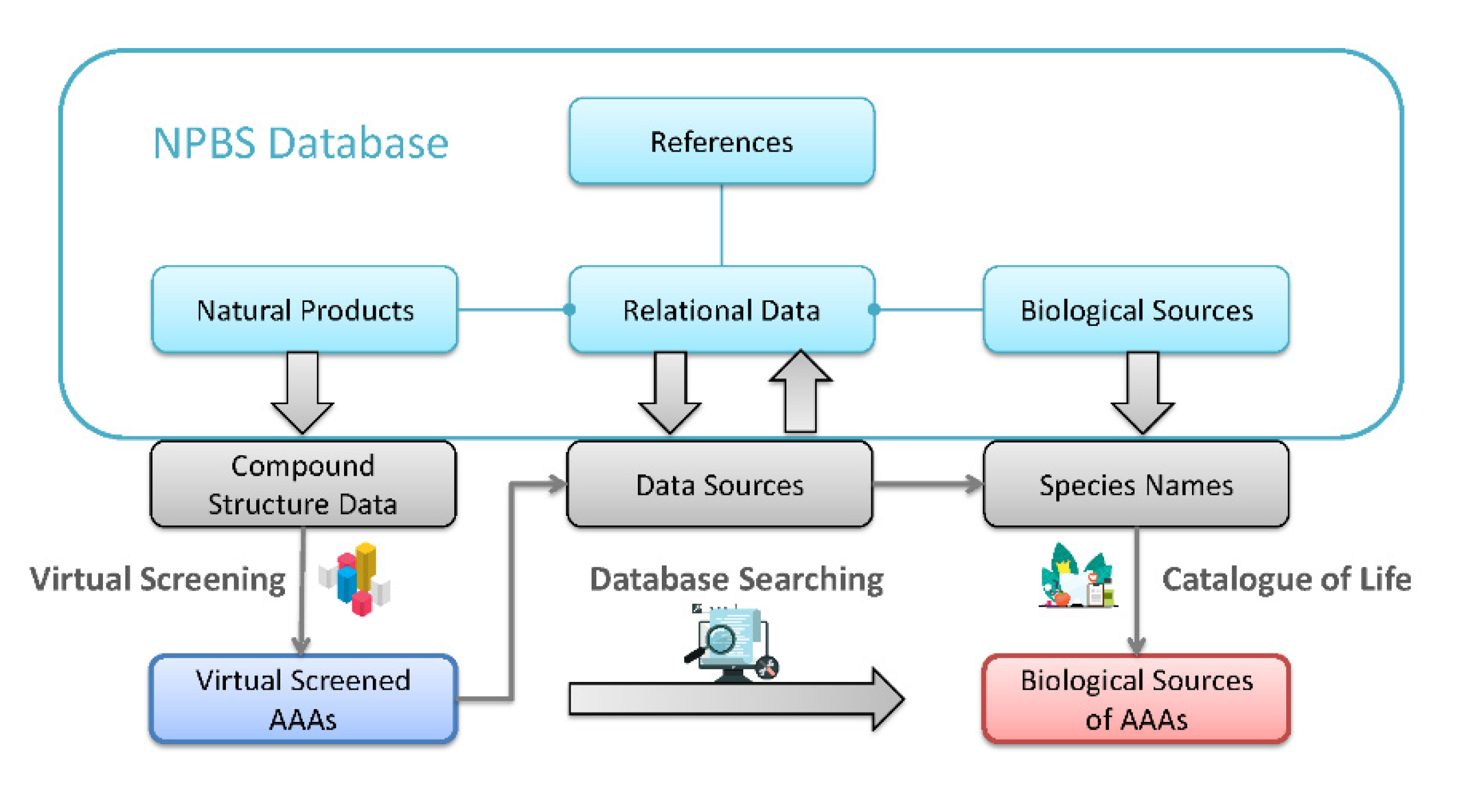
2.2. Obtaining Information Data of Biological Sources Containing Aristolochic Acid Analogues
2.3. Computational Toxicology Estimations of Aristolochic Acid Analogues
3. Results
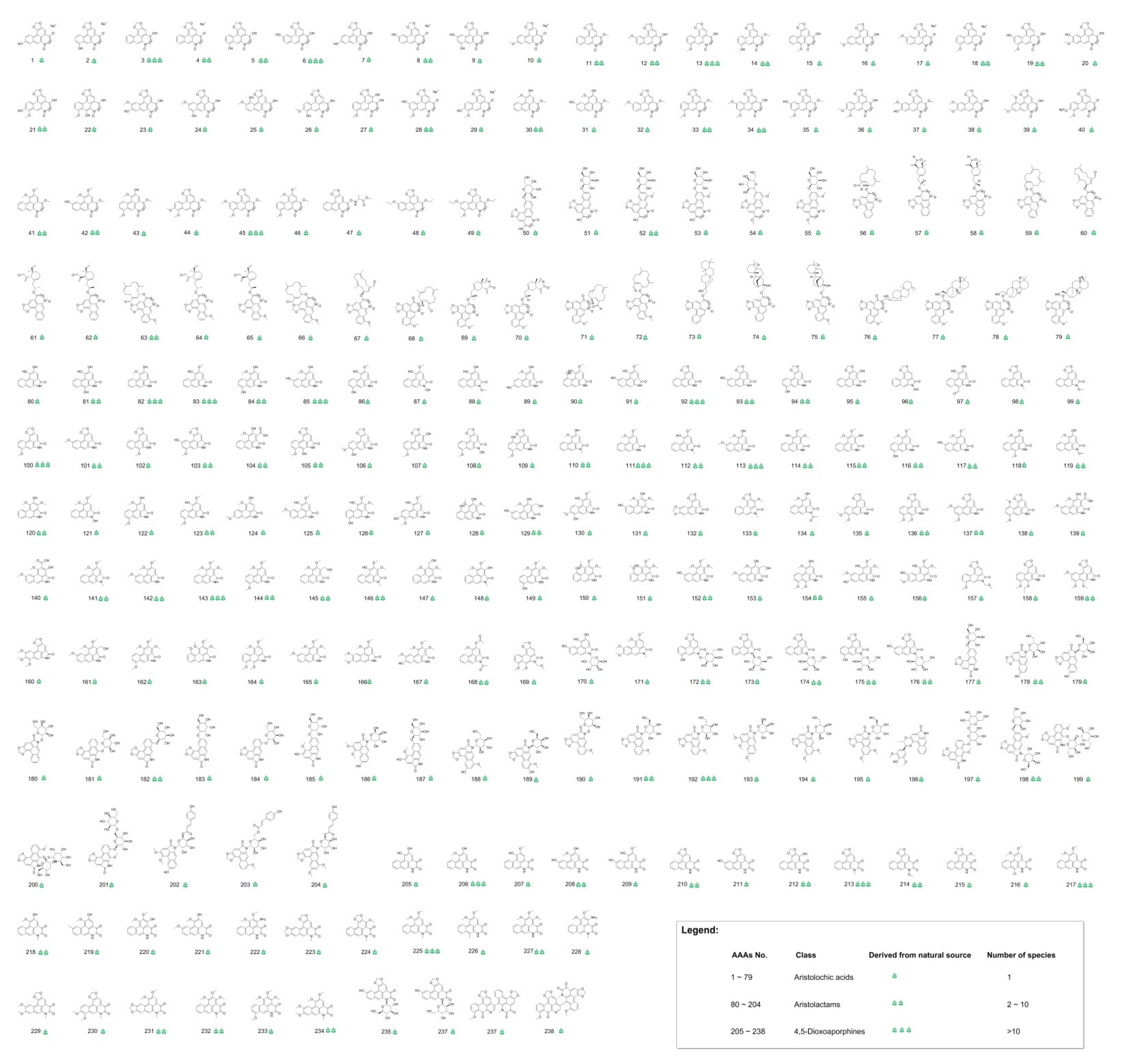
3.1. Chemical Space of Naturally Occurring Aristolochic Acid Analogues
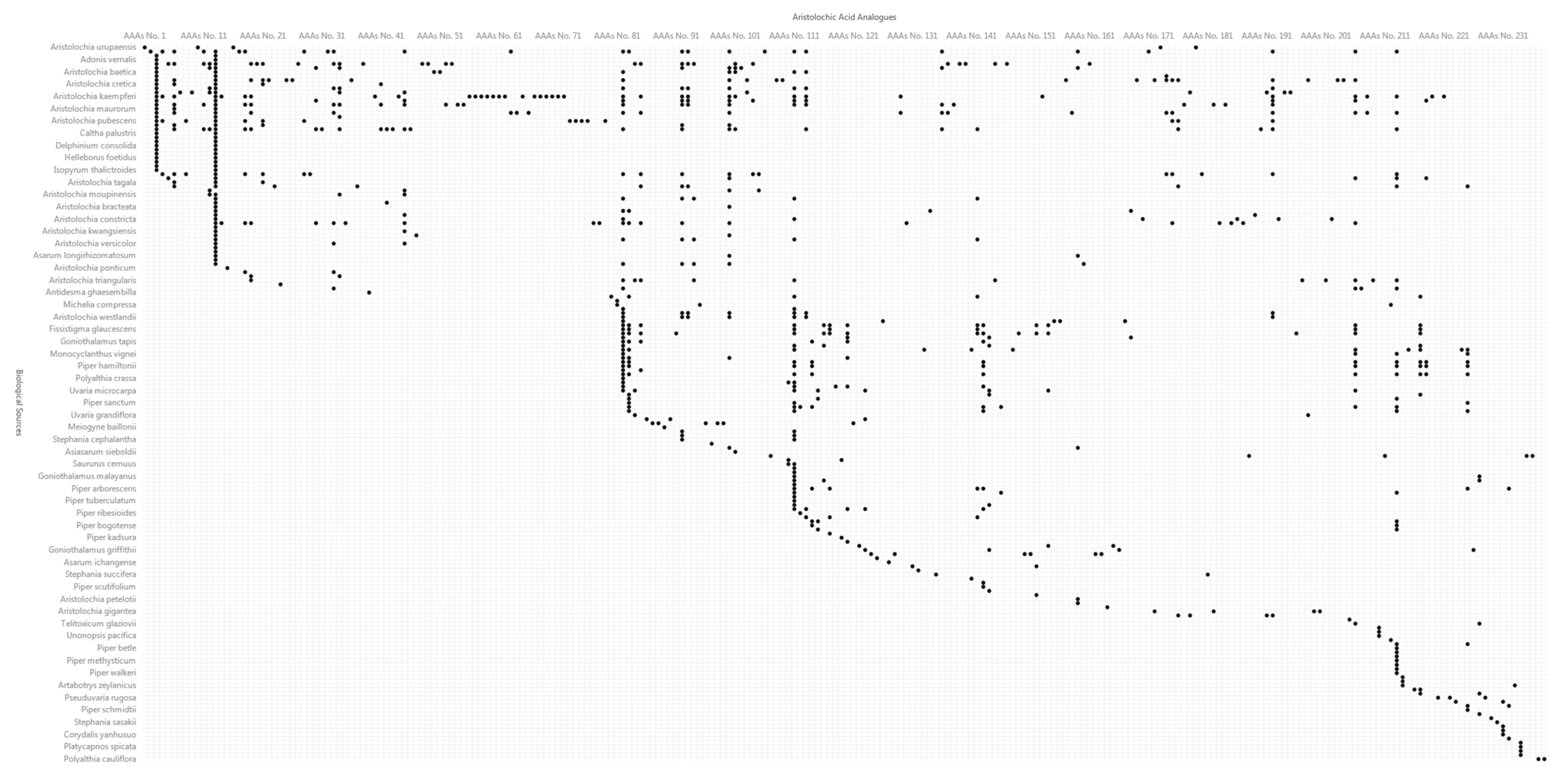
3.2. Coverage of Biological Sources Containing Aristolochic Acid Analogues
3.3. Relationship of Aristolochic Acid Analogues and Their Biological Sources
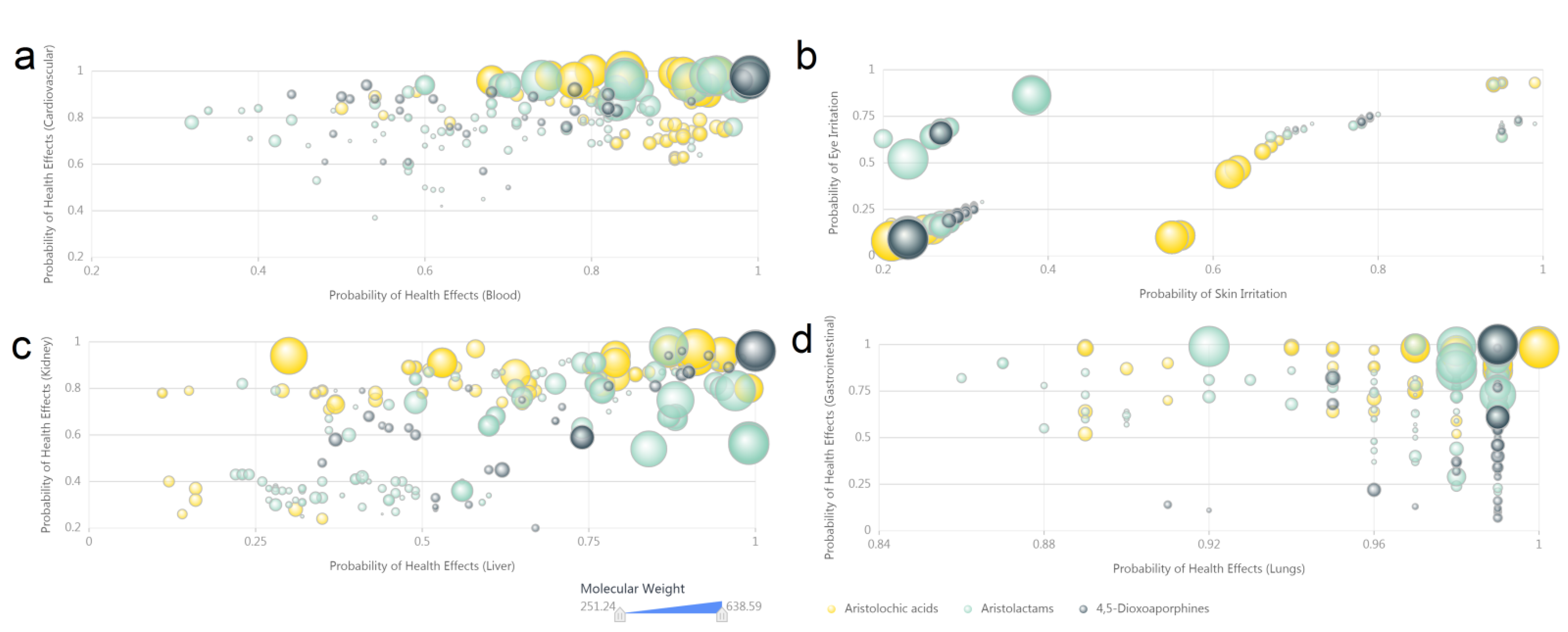
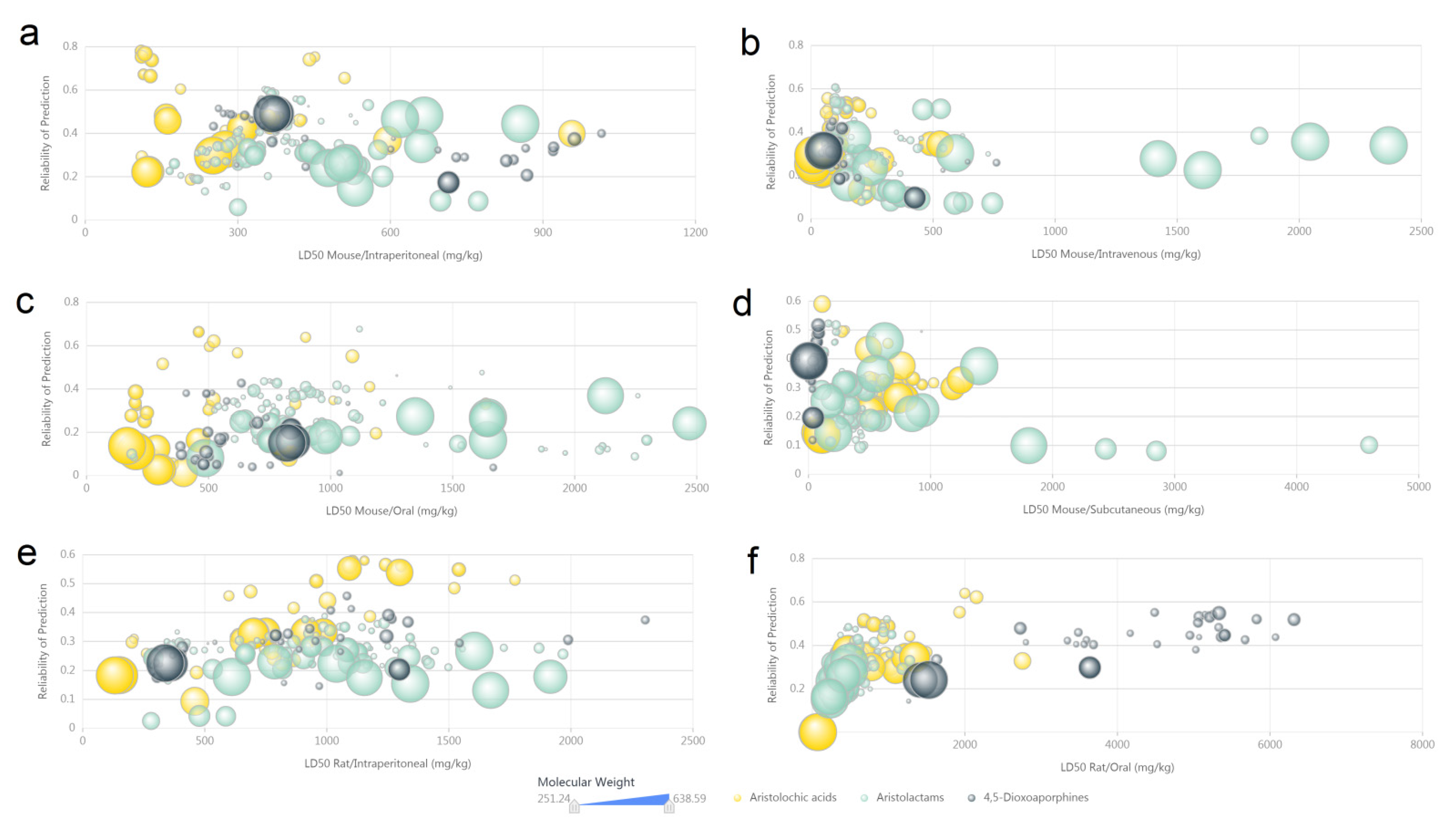
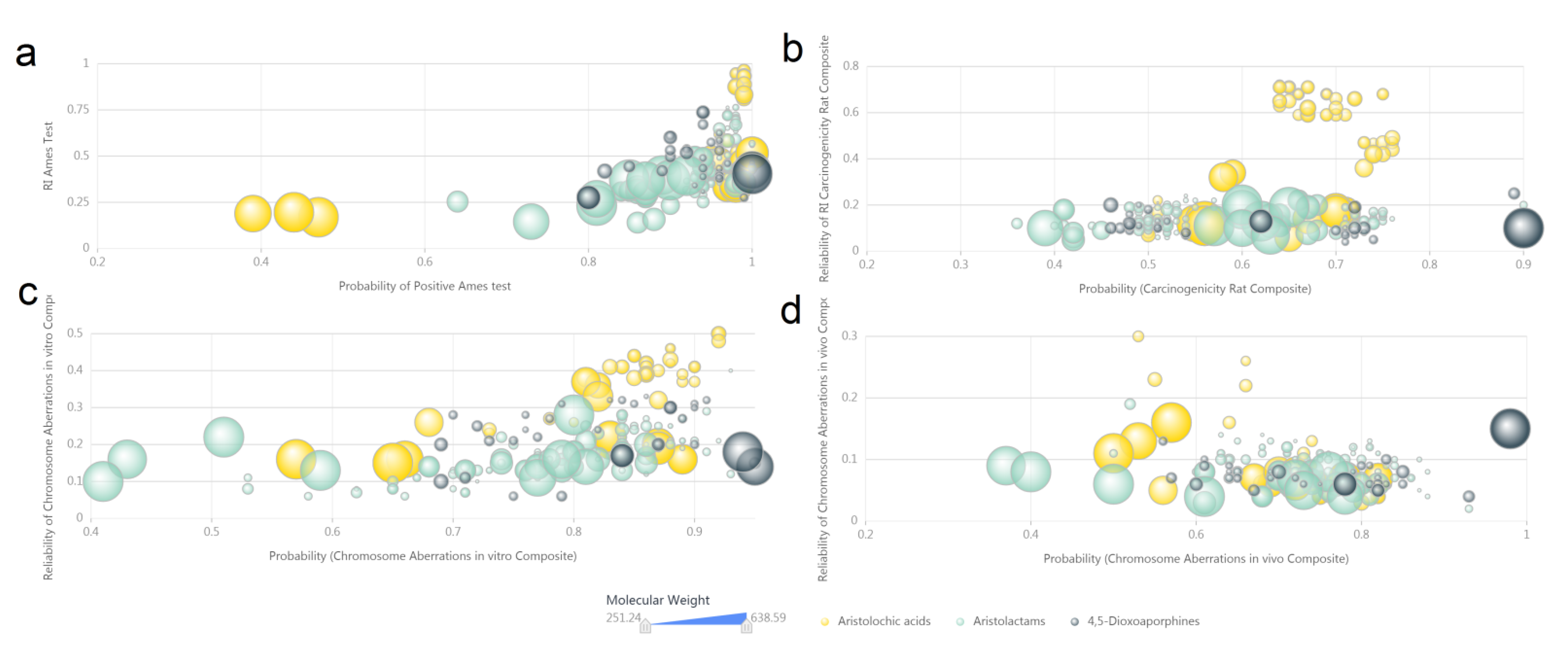
3.4. Computer Predicted Toxicity of Aristolochic Acid Analogues
3.5. Exclusive Aristolochic Acid Analogues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TCM | Traditional Chinese Medicine |
| AAAs | Aristolochic Acid Analogues |
| SAR | Structure Activity Relationship |
| QSAR | Quantitative Structure Activity Relationship |
| MS | Molecular Similarity |
| MCS | Maximum Common Substructure |
| NPBS | Natural Product and Biological Source database |
| COL | Catalogue of Life |
References
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Butler, M.S. The Role of Natural Product Chemistry in Drug Discovery. J. Nat. Prod. 2004, 67, 2141–2153. [Google Scholar] [CrossRef] [PubMed]
- The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat. Med. 2011, 17, 1217–1220. [CrossRef]
- Heinrich, M.; Chan, J.; Wanke, S.; Neinhuis, C.; Simmonds, M.S.J. Local uses of Aristolochia species and content of nephrotoxic aristolochic acid 1 and 2—A global assessment based on bibliographic sources. J. Ethnopharmacol. 2009, 125, 108–144. [Google Scholar] [CrossRef] [PubMed]
- Vanherweghem, J.L. A new form of nephropathy secondary to the absorption of Chinese herbs. Bull. Mem. Acad. R. Med. Belg. 1994, 149, 128–135. [Google Scholar]
- Vanherweghem, J.L. Misuse of herbal remedies: The case of an outbreak of terminal renal failure in Belgium (Chinese herbs nephropathy). J. Altern. Complement. Med. 1998, 4, 9–13. [Google Scholar] [CrossRef]
- Debelle, F.D.; Vanherweghem, J.L.; Nortier, J.L. Aristolochic acid nephropathy: A worldwide problem. Kidney Int. 2008, 74, 158–169. [Google Scholar] [CrossRef] [Green Version]
- De Broe, M.E. On a nephrotoxic and carcinogenic slimming regimen. Am. J. Kidney Dis. 1999, 33, 1171–1173. [Google Scholar] [CrossRef]
- Grollman, A.P.; Shibutani, S.; Moriya, M.; Miller, F.; Wu, L.; Moll, U.; Jelakovic, B. Aristolochic acid and the etiology of endemic (Balkan) nephropathy. Proc. Natl. Acad. Sci. USA 2007, 104, 12129–12134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nortier, J.L.; Martinez, M.-C.M.; Schmeiser, H.H.; Arlt, V.M.; Bieler, C.A.; Petein, M.; Vanherweghem, J.-L. Urothelial Carcinoma Associated with the Use of a Chinese Herb (Aristolochia fangchi). N. Engl. J. Med. 2000, 342, 1686–1692. [Google Scholar] [CrossRef] [Green Version]
- Ng, A.W.T.; Poon, S.L.; Huang, M.N.; Lim, J.Q.; Boot, A.; Yu, W.; Rozen, S.G. Aristolochic acids and their derivatives are widely implicated in liver cancers in Taiwan and throughout Asia. Sci. Transl. Med. 2017, 9, eaan6446. [Google Scholar] [CrossRef] [Green Version]
- Stiborová, M.; Frei, E.; Arlt, V.M.; Schmeiser, H.H. Metabolic activation of carcinogenic aristolochic acid, a risk factor for Balkan endemic nephropathy. Mutat. Res. Rev. Mutat. Res. 2008, 658, 55–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Zhang, L.; Jiang, Z.; Shu, B.; Li, F.; Bao, Q.; Zhang, L. Toxicities of aristolochic acid I and aristololactam I in cultured renal epithelial cells. Toxicol. Vitr. 2010, 24, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, P.; Wei, F.; Lin, R.-C.; Khan, I.A.; Pasco, D.S. Structure activity relationships of aristolochic acid analogues: Toxicity in cultured renal epithelial cells. Kidney Int. 2005, 67, 1797–1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoang, M.L.; Chen, C.-H.; Sidorenko, V.S.; He, J.; Dickman, K.G.; Yun, B.H.; Rosenquist, T.A. Mutational Signature of Aristolochic Acid Exposure as Revealed by Whole-Exome Sequencing. Sci. Transl. Med. 2013, 5, 197ra102. [Google Scholar] [CrossRef] [Green Version]
- Poon, S.L.; Pang, S.-T.; McPherson, J.R.; Yu, W.; Huang, K.K.; Guan, P.; Teh, B.T. Genome-Wide Mutational Signatures of Aristolochic Acid and Its Application as a Screening Tool. Sci. Transl. Med. 2013, 5, 197ra101. [Google Scholar] [CrossRef]
- Chen, C.-H.; Dickman, K.G.; Moriya, M.; Zavadil, J.; Sidorenko, V.S.; Edwards, K.L.; Grollman, A.P. Aristolochic acid-associated urothelial cancer in Taiwan. Proc. Natl. Acad. Sci. USA 2012, 109, 8241–8246. [Google Scholar] [CrossRef] [Green Version]
- Imielinski, M.; Berger, A.H.; Hammerman, P.S.; Hernandez, B.; Pugh, T.J.; Hodis, E.; Meyerson, M. Mapping the Hallmarks of Lung Adenocarcinoma with Massively Parallel Sequencing. Cell 2012, 150, 1107–1120. [Google Scholar] [CrossRef] [Green Version]
- Berger, M.F.; Hodis, E.; Heffernan, T.P.; Deribe, Y.L.; Lawrence, M.S.; Protopopov, A.; Garraway, L.A. Melanoma genome sequencing reveals frequent PREX2 mutations. Nature 2012, 485, 502–506. [Google Scholar] [CrossRef]
- Kumar, V.; Poonam; Prasad, A.K.; Parmar, V.S. Naturally occurring aristolactams, aristolochic acids and dioxoaporphines and their biological activities. Nat. Prod. Rep. 2003, 20, 565. [Google Scholar] [CrossRef]
- Michl, J.; Ingrouille, M.J.; Simmonds, M.S.J.; Heinrich, M. Naturally occurring aristolochic acid analogues and their toxicities. Nat. Prod. Rep. 2014, 31, 676–693. [Google Scholar] [CrossRef]
- Ji, H.; Li, J.; Wu, S.; Wu, W.; Yao, C.; Yao, S.; Zhang, J.; Guo, D. Two New Aristolochic Acid Analogues from the Roots of Aristolochia contorta with Significant Cytotoxic Activity. Molecules 2021, 26, 44. [Google Scholar] [CrossRef]
- Ma, H.; Dong, C.; Zhou, X.; Bu, M.; Yu, S. Aristololactam derivatives from the fruits of Aristolochia contorta Bunge. Nat. Prod. Res. 2018, 32, 2505–2509. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Wei, R.; Wang, Z.; Liu, W.; Sang, Z.; Li, Y.; Huang, H. Bioactive alkaloids from the aerial parts of Houttuynia cordata. J. Ethnopharmacol. 2017, 195, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Ang, L.P.; Ng, P.W.; Lean, Y.L.; Kotra, V.; Kifli, N.; Goh, H.P.; Lee, K.S.; Sarker, M.M.R.; Al-Worafi, Y.M.; Ming, L.C. Herbal products containing aristolochic acids: A call to revisit the context of safety. J. Herb. Med. 2021, 28, 100447. [Google Scholar] [CrossRef]
- Wang, L.; Cui, X.; Cheng, L.; Yuan, Q.; Li, T.; Li, Y.; Deng, S.; Shang, H.; Bian, Z. Adverse events to Houttuynia injection: A systematic review. J. Evid.-Based. Med. 2010, 3, 168–176. [Google Scholar] [CrossRef]
- Cheung, T.P.; Xue, C.; Leung, K.; Chan, K.; Li, C.G. Aristolochic Acids Detected in Some Raw Chinese Medicinal Herbs and Manufactured Herbal Products—A Consequence of Inappropriate Nomenclature and Imprecise Labelling? Clin. Toxicol. 2006, 44, 371–378. [Google Scholar] [CrossRef]
- Santibáñez-Morán, M.G.; López-López, E.; Prieto-Martínez, F.D.; Sánchez-Cruz, N.; Medina-Franco, J.L. Consensus virtual screening of dark chemical matter and food chemicals uncover potential inhibitors of SARS-CoV-2 main protease. RSC Adv. 2020, 10, 25089–25099. [Google Scholar] [CrossRef]
- Xu, T.; Chen, W.; Zhou, J.; Dai, J.; Li, Y.; Zhao, Y. Virtual Screening for Reactive Natural Products and Their Probable Artifacts of Solvolysis and Oxidation. Biomolecules 2020, 10, 1486. [Google Scholar] [CrossRef]
- Maggiora, G.; Vogt, M.; Stumpfe, D.; Bajorath, J. Molecular similarity in medicinal chemistry. J. Med. Chem. 2013, 57, 3186–3204. [Google Scholar] [CrossRef]
- Riniker, S.; Landrum, G.A. Similarity Maps—A Visualization Strategy for Molecular Fingerprints and Machine-Learning Methods. J. Cheminf. 2013, 5, 43. [Google Scholar] [CrossRef]
- Vogt, M.; Stumpfe, D.; Geppert, H.; Bajorath, J. Scaffold hopping using two-dimensional fingerprints: True potential, black magic, or a hopeless endeavor? guidelines for virtual screening. J. Med. Chem. 2010, 12, 5707–5715. [Google Scholar] [CrossRef]
- Hu, Y.; Stumpfe, D.; Bajorath, J. Recent advances in scaffold hopping. J. Med. Chem. 2017, 60, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Kunimoto, R.; Vogt, M.; Bajorath, J. Maximum common substructure-based Tversky index: An asymmetric hybrid similarity measure. J. Comput. Aided Mol. Des. 2016, 30, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Antelo-Collado, A.; Carrasco-Velar, R.; García-Pedrajas, N.; Cerruela-García, G. Maximum common property: A new approach for molecular similarity. J. Cheminform. 2020, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Chen, W.; Zhou, J.; Dai, J.; Li, Y.; Zhao, Y. NPBS database: A chemical data resource with relational data between natural products and biological sources. Database 2020, 2020, baaa102. [Google Scholar] [CrossRef]
- Durán-Iturbide, N.A.; Díaz-Eufracio, B.I.; Medina-Franco, J.L. In Silico ADME/Tox Profiling of Natural Products: A Focus on BIOFACQUIM. ACS Omega 2020, 5, 16076–16084. [Google Scholar] [CrossRef]
- Glatt, H. Use of genetically manipulated Salmonella typhimurium strains to evaluate the role of sulfotransferases and acetyltransferases in nitrofen mutagenicity. Carcinogenesis 2003, 25, 779–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westwood, I.M.; Holton, S.J.; Rodrigues-Lima, F.; Dupret, J.-M.; Bhakta, S.; Noble, M.E.M.; Sim, E. Expression, purification, characterization and structure of Pseudomonas aeruginosa arylamine N-acetyltransferase. Biochem. J. 2005, 385, 605–612. [Google Scholar] [CrossRef]
- Morais, A.B.B.; Brown, K.S.; Stanton, M.A.; Massuda, K.F.; Trigo, J.R. Are Aristolochic Acids Responsible for the Chemical Defence of Aposematic Larvae of Battus polydamas (L.) (Lepidoptera: Papilionidae)? Neotrop. Entomol. 2013, 42, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Priestap, H.A.; Velandia, A.E.; Johnson, J.V.; Barbieri, M.A. Secondary metabolite uptake by the Aristolochia-feeding papilionoid butterfly Battus polydamas. Biochem. Syst. Ecol. 2012, 40, 126–137. [Google Scholar] [CrossRef]
- Cachet, X.; Langrand, J.; Bottai, C.; Dufat, H.; Locatelli-Jouans, C.; Nossin, E.; Boucaud-Maitre, D. Detection of aristolochic acids I and II in “Chiniy-trèf”, a traditional medicinal preparation containing caterpillars feeding on Aristolochia trilobata L. in Martinique, French West Indies. Toxicon 2016, 114, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Nishida, R. Sequestration of Defensive Substances from Plants by Lepidoptera. Annu. Rev. Entomol. 2002, 47, 57–92. [Google Scholar] [CrossRef] [PubMed]
- Klitzke, C.F.; Brown, K.S., Jr. The occurrence of aristolochic acids in neotropical troidine swallowtails (Lepidoptera: Papilionidae). Chemoecology 2000, 10, 99–102. [Google Scholar] [CrossRef]
- Capon, R.J. Extracting value: Mechanistic insights into the formation of natural product artifacts–case studies in marine natural products. Nat. Prod. Rep. 2020, 37, 55–79. [Google Scholar] [CrossRef]
- Hegde, V.R.; Borges, S.; Pu, H.; Patel, M.; Gullo, V.P.; Wu, B.; Kirschmeier, P.; Michael, J.W.; Madison, V.; Fischmann, T.; et al. Semi-synthetic aristolactams—Inhibitors of CDK2 enzyme. Bioorg. Med. Chem. Lett. 2010, 20, 1384–1387. [Google Scholar] [CrossRef]
- Stiborová, M.; Frei, E.; Wiessler, M.; Schmeiser, H.H. Human enzymes involved in the metabolic activation of carcinogenic aristolochic acids: Evidence for reductive activation by cytochromes P450 1A1 and 1A2. Chem. Res. Toxicol. 2001, 14, 1128–1137. [Google Scholar] [CrossRef]
- Wijeratne, E.K.; Hatanaka, Y.; Kikuchi, T.; Tezuka, Y.; Gunatilaka, A.L. A dioxoaporphine and other alkaloids of two annonaceous plants of Sri Lanka. Phytochemistry 1996, 42, 1703–1706. [Google Scholar] [CrossRef]
- Liao, J.C.; Lin, K.H.; Ho, H.Y.; Peng, W.H.; Yao, X.; Kitanaka, S.; Wu, J.B. Inhibitory effects of 87 species of traditional Chinese herbs on nitric oxide production in RAW264. 7 macrophages, activated with lipopolysaccharide and interferon-γ. Pharm. Biol. 2005, 43, 158–163. [Google Scholar] [CrossRef]
- Xu, X.L.; Yang, L.J.; Jiang, J.G. Renal toxic ingredients and their toxicology from traditional Chinese medicine. Expert Opin. Drug Metab. Toxicol. 2016, 12, 149–159. [Google Scholar] [CrossRef]
- Xue, X.; Xiao, Y.; Gong, L.; Guan, S.; Liu, Y.; Lu, H.; Qi, X.; Zhang, Y.; Li, Y.; Wu, X.; et al. Comparative 28-day repeated oral toxicity of Longdan Xieganwan, Akebia trifoliate (Thunb.) koidz., Akebia quinata (Thunb.) Decne. and Caulis aristolochiae manshuriensis in mice. J. Ethnopharmacol. 2008, 119, 87–93. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, T.; Chen, W.; Zhou, J.; Dai, J.; Li, Y.; Zhao, Y. Computational Analysis of Naturally Occurring Aristolochic Acid Analogues and Their Biological Sources. Biomolecules 2021, 11, 1344. https://doi.org/10.3390/biom11091344
Xu T, Chen W, Zhou J, Dai J, Li Y, Zhao Y. Computational Analysis of Naturally Occurring Aristolochic Acid Analogues and Their Biological Sources. Biomolecules. 2021; 11(9):1344. https://doi.org/10.3390/biom11091344
Chicago/Turabian StyleXu, Tingjun, Weiming Chen, Junhong Zhou, Jingfang Dai, Yingyong Li, and Yingli Zhao. 2021. "Computational Analysis of Naturally Occurring Aristolochic Acid Analogues and Their Biological Sources" Biomolecules 11, no. 9: 1344. https://doi.org/10.3390/biom11091344
APA StyleXu, T., Chen, W., Zhou, J., Dai, J., Li, Y., & Zhao, Y. (2021). Computational Analysis of Naturally Occurring Aristolochic Acid Analogues and Their Biological Sources. Biomolecules, 11(9), 1344. https://doi.org/10.3390/biom11091344





