Repurposing Small Molecules to Target PPAR-γ as New Therapies for Peripheral Nerve Injuries
Abstract
1. Introduction
2. Signaling Pathways Involved in PNI
3. Proliferator-Activated Receptor Gamma (PPAR-γ) Activation
4. PPAR-γ in Peripheral Nerves
5. Repurposing Drugs and Small Molecules to Target PPAR-γ in PNS
6. PPAR-γ in the Central Nervous System
7. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| 3D | 3-dimensional |
| AF2 | Activation function 2 |
| Akt | Serine/threonine protein kinase B |
| AP-1 | Activator protein 1 |
| cAMP | Cyclic adenosine monophosphate |
| CNS | Central nervous system |
| COX-2 | Cyclooxygenase-2 |
| CREB | cAMP-response Element-Binding Protein |
| CRMP2 | Collapsin response mediator protein 2 |
| CSPG | Chondroitin sulphate proteoglycans |
| GAP | GTPase activating proteins |
| GDI | Guanine nucleotide dissociation inhibitors |
| GDP | Guanosine diphosphate |
| GEF | Guanine nucleotide exchange factor |
| GTP | Guanosine triphosphate |
| H | Helix |
| IL-6 | Interleukin-6 |
| iNOS | Inducible nitric oxide synthase |
| IP2 | Inositol biphosphate |
| IP3 | Inositol triphosphate |
| LIMK | LIM kinase |
| MAG | Myelin-associated glycoprotein |
| MLC | Myosin light chain |
| NFkB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| Ngr | Nogo receptor |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| p75 NTR | p75 neurotropic receptor |
| PIPK5 | Phosphatidylinositol 4-Phosphate-5 kinase |
| PKA | Protein kinase A |
| PKN | Protein kinase N |
| PNI | Peripheral nerve injury |
| PNS | Peripheral nervous system |
| PPAR-γ | Peroxisome proliferator-activated receptor gamma |
| PTEN | Phosphatase and tensin homolog |
| PTP | Protein tyrosine phosphatase |
| RhoA | Ras homolog family member A |
| ROCK | Rho-associated kinase |
| SHP-2 | Src homology region 2–containing protein tyrosine phosphatase-2 |
| STAT3 | Signal transducer and activator of transcription 3 |
| TFI | Tibial functional index |
| Tyr473 | Tyrosine residue 473 |
References
- Fex Svennigsen, A.; Dahlin, L.B. Repair of the Peripheral Nerve-Remyelination that Works. Brain Sci. 2013, 3, 1182–1197. [Google Scholar] [CrossRef]
- Nectow, A.R.; Marra, K.G.; Kaplan, D.L. Biomaterials for the development of peripheral nerve guidance conduits. Tissue Eng. Part B, Rev. 2012, 18, 40–50. [Google Scholar] [CrossRef]
- Jones, S.; Eisenberg, H.M.; Jia, X. Advances and Future Applications of Augmented Peripheral Nerve Regeneration. Int. J. Mol. Sci 2016, 17, 1494. [Google Scholar] [CrossRef]
- Isaacs, J. Major peripheral nerve injuries. Hand Clin. 2013, 29, 371–382. [Google Scholar] [CrossRef]
- Faroni, A.; Mobasseri, S.A.; Kingham, P.J.; Reid, A.J. Peripheral nerve regeneration: Experimental strategies and future perspectives. Adv. Drug Deliv. Rev. 2015, 82–83, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Ubogu, E.E. Translational strategies in peripheral neuroinflammation and neurovascular repair. Transl. Neurosci. 2012, 3, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Hall, S. The response to injury in the peripheral nervous system. J. Bone Joint Surg. 2005, 87, 1309–1319. [Google Scholar] [CrossRef]
- Hoke, A.; Brushart, T. Introduction to special issue: Challenges and opportunities for regeneration in the peripheral nervous system. Exp. Neurol. 2010, 223, 1–4. [Google Scholar] [CrossRef]
- Burnett, M.G.; Zager, E.L. Pathophysiology of peripheral nerve injury: A brief review. Neurosurg. Focus 2004, 16, E1. [Google Scholar] [CrossRef] [PubMed]
- Poppler, L.H.; Ee, X.; Schellhardt, L.; Hoben, G.M.; Pan, D.; Hunter, D.A.; Yan, Y.; Moore, A.M.; Snyder-Warwick, A.K.; Stewart, S.A.; et al. Axonal Growth Arrests after an Increased Accumulation of Schwann Cells Expressing Senescence Markers and Stromal Cells in Acellular Nerve Allografts. Tissue Eng. Part A 2016, 22, 949–961. [Google Scholar] [CrossRef]
- Wagstaff, L.J.; Gomez-Sanchez, J.A.; Fazal, S.V.; Otto, G.W.; Kilpatrick, A.M.; Michael, K.; Wong, L.Y.N.; Ma, K.H.; Turmaine, M.; Svaren, J.; et al. Failures of nerve regeneration caused by aging or chronic denervation are rescued by restoring Schwann cell c-Jun. Elife 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.L.; Yu, W.M.; Strickland, S. Peripheral regeneration. Annu. Rev. Neurosci. 2007, 30, 209–233. [Google Scholar] [CrossRef] [PubMed]
- Zochodne, D.W. The challenges and beauty of peripheral nerve regrowth. J. Peripher. Nerv. Syst. 2012, 17, 1–18. [Google Scholar] [CrossRef]
- Dubovy, P.; Jancalek, R.; Kubek, T. Role of inflammation and cytokines in peripheral nerve regeneration. Int. Rev. Neurobiol. 2013, 108, 173–206. [Google Scholar] [CrossRef]
- Fu, S.Y.; Gordon, T. Contributing factors to poor functional recovery after delayed nerve repair: Prolonged axotomy. J. Neurosci. 1995, 15, 3876–3885. [Google Scholar] [CrossRef]
- Hiraga, A.; Kuwabara, S.; Doya, H.; Kanai, K.; Fujitani, M.; Taniguchi, J.; Arai, K.; Mori, M.; Hattori, T.; Yamashita, T. Rho-kinase inhibition enhances axonal regeneration after peripheral nerve injury. J. Peripher. Nerv. Syst. 2006, 11, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, A. Tuning the orchestra: Transcriptional pathways controlling axon regeneration. Front. Mol. Neurosci. 2011, 4, 60. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.D.; Mackinnon, S.E. Pathways regulating modality-specific axonal regeneration in peripheral nerve. Exp. Neurol. 2015, 265, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.M.; Gordon, T.; Zochodne, D.W.; Power, H.A. Improving peripheral nerve regeneration: From molecular mechanisms to potential therapeutic targets. Exp. Neurol. 2014, 261, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Madura, T.; Yamashita, T.; Kubo, T.; Fujitani, M.; Hosokawa, K.; Tohyama, M. Activation of Rho in the injured axons following spinal cord injury. EMBO Rep. 2004, 5, 412–417. [Google Scholar] [CrossRef]
- Kubo, T.; Yamaguchi, A.; Iwata, N.; Yamashita, T. The therapeutic effects of Rho-ROCK inhibitors on CNS disorders. Ther. Clin. Risk Manag. 2008, 4, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Longo, F.M.; Zhou, H.; Massa, S.M.; Chen, Y.H. Signaling through Rho GTPase pathway as viable drug target. Curr. Med. Chem. 2009, 16, 1355–1365. [Google Scholar] [CrossRef]
- Van Aelst, L.; Cline, H.T. Rho GTPases and activity-dependent dendrite development. Curr. Opin. Neurobiol. 2004, 14, 297–304. [Google Scholar] [CrossRef] [PubMed]
- DeGeer, J.; Lamarche-Vane, N. Rho GTPases in neurodegeneration diseases. Exp. Cell Res. 2013, 319, 2384–2394. [Google Scholar] [CrossRef]
- Hodge, R.G.; Ridley, A.J. Regulating Rho GTPases and their regulators. Nat. Rev. Mol. Cell Biol. 2016, 17, 496–510. [Google Scholar] [CrossRef]
- Shang, X.; Marchioni, F.; Sipes, N.; Evelyn, C.R.; Jerabek-Willemsen, M.; Duhr, S.; Seibel, W.; Wortman, M.; Zheng, Y. Rational design of small molecule inhibitors targeting RhoA subfamily Rho GTPases. Chem. Biol. 2012, 19, 699–710. [Google Scholar] [CrossRef]
- Amin, E.; Dubey, B.N.; Zhang, S.C.; Gremer, L.; Dvorsky, R.; Moll, J.M.; Taha, M.S.; Nagel-Steger, L.; Piekorz, R.P.; Somlyo, A.V.; et al. Rho-kinase: Regulation, (dys)function, and inhibition. Biol. Chem. 2013, 394, 1399–1410. [Google Scholar] [CrossRef]
- Tang, B.L. Inhibitors of neuronal regeneration: Mediators and signaling mechanisms. Neurochem. Int. 2003, 42, 189–203. [Google Scholar] [CrossRef]
- Lin, Y.; Zheng, Y. Approaches of targeting Rho GTPases in cancer drug discovery. Expert Opin. Drug Discov. 2015, 10, 991–1010. [Google Scholar] [CrossRef]
- Amano, M.; Nakayama, M.; Kaibuchi, K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton 2010, 67, 545–554. [Google Scholar] [CrossRef]
- Schmandke, A.; Schmandke, A.; Strittmatter, S.M. ROCK and Rho: Biochemistry and neuronal functions of Rho-associated protein kinases. Neuroscientist 2007, 13, 454–469. [Google Scholar] [CrossRef] [PubMed]
- Wakino, S.; Hayashi, K.; Kanda, T.; Tatematsu, S.; Homma, K.; Yoshioka, K.; Takamatsu, I.; Saruta, T. Peroxisome proliferator-activated receptor gamma ligands inhibit Rho/Rho kinase pathway by inducing protein tyrosine phosphatase SHP-2. Circ. Res. 2004, 95, e45–e55. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.R.; Bobylev, I.; Zhang, G.; Sheikh, K.A.; Lehmann, H.C. Inhibition of Rho-kinase differentially affects axon regeneration of peripheral motor and sensory nerves. Exp. Neurol. 2015, 263, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.C.; Khodarahmi, K.; Liu, J.; Sutherland, D.; Oschipok, L.W.; Steeves, J.D.; Tetzlaff, W. Dose-dependent beneficial and detrimental effects of ROCK inhibitor Y27632 on axonal sprouting and functional recovery after rat spinal cord injury. Exp. Neurol. 2005, 196, 352–364. [Google Scholar] [CrossRef]
- Cheng, C.; Webber, C.A.; Wang, J.; Xu, Y.; Martinez, J.A.; Liu, W.Q.; McDonald, D.; Guo, G.F.; Nguyen, M.D.; Zochodne, D.W. Activated RHOA and peripheral axon regeneration. Exp. Neurol. 2008, 212, 358–369. [Google Scholar] [CrossRef]
- Fuentes, E.O.; Leemhuis, J.; Stark, G.B.; Lang, E.M. Rho kinase inhibitors Y27632 and H1152 augment neurite extension in the presence of cultured Schwann cells. J. Brachial Plexus Peripher. Nerve Inj. 2008, 3, 19. [Google Scholar] [CrossRef][Green Version]
- Lie, M.; Grover, M.; Whitlon, D.S. Accelerated neurite growth from spiral ganglion neurons exposed to the Rho kinase inhibitor H-1152. Neuroscience 2010, 169, 855–862. [Google Scholar] [CrossRef]
- Quintanilla, R.A.; Utreras, E.; Cabezas-Opazo, F.A. Role of PPAR gamma in the Differentiation and Function of Neurons. PPAR Res. 2014, 2014, 768594. [Google Scholar] [CrossRef]
- Barr, A.J. Protein tyrosine phosphatases as drug targets: Strategies and challenges of inhibitor development. Future Med. Chem. 2010, 2, 1563–1576. [Google Scholar] [CrossRef]
- Puhl, A.C.; Milton, F.A.; Cvoro, A.; Sieglaff, D.H.; Campos, J.C.; Bernardes, A.; Filgueira, C.S.; Lindemann, J.L.; Deng, T.; Neves, F.A.; et al. Mechanisms of peroxisome proliferator activated receptor gamma regulation by non-steroidal anti-inflammatory drugs. Nucl. Recept. Signal. 2015, 13. [Google Scholar] [CrossRef]
- Nolte, R.T.; Wisely, G.B.; Westin, S.; Cobb, J.E.; Lambert, M.H.; Kurokawa, R.; Rosenfeld, M.G.; Willson, T.M.; Glass, C.K.; Milburn, M.V. Ligand binding and co-activator assembly of the peroxisome proliferator-activated receptor-gamma. Nature 1998, 395, 137–143. [Google Scholar] [CrossRef]
- Iannotti, F.A.; Vitale, R.M. The Endocannabinoid System and PPARs: Focus on Their Signalling Crosstalk, Action and Transcriptional Regulation. Cells 2021, 10, 586. [Google Scholar] [CrossRef]
- Yamagishi, S.; Ogasawara, S.; Mizukami, H.; Yajima, N.; Wada, R.; Sugawara, A.; Yagihashi, S. Correction of protein kinase C activity and macrophage migration in peripheral nerve by pioglitazone, peroxisome proliferator activated-gamma-ligand, in insulin-deficient diabetic rats. J. Neurochem. 2008, 104, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, Q.; Zhou, Z.; Wang, Y.; Liu, Y.; Ji, Y.; Liu, F. Changes of peroxisome proliferator-activated receptor-gamma on crushed rat sciatic nerves and differentiated primary Schwann cells. J. Mol. Neurosci. 2012, 47, 380–388. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, F.; Yan, M.; Ji, H.; Hu, L.; Li, X.; Qian, J.; He, X.; Zhang, L.; Shen, A.; et al. Peroxisome proliferator-activated receptor-gamma agonists suppress iNOS expression induced by LPS in rat primary Schwann cells. J. Neuroimmunol. 2010, 218, 36–47. [Google Scholar] [CrossRef]
- Meyer Zu Reckendorf, S.; Brand, C.; Pedro, M.T.; Hegler, J.; Schilling, C.S.; Lerner, R.; Bindila, L.; Antoniadis, G.; Knoll, B. Lipid metabolism adaptations are reduced in human compared to murine Schwann cells following injury. Nat. Commun. 2020, 11, 2123. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The Success and Failure of the Schwann Cell Response to Nerve Injury. Front. Cell Neurosci. 2019, 13, 33. [Google Scholar] [CrossRef]
- Lezana, J.P.; Dagan, S.Y.; Robinson, A.; Goldstein, R.S.; Fainzilber, M.; Bronfman, F.C.; Bronfman, M. Axonal PPARgamma promotes neuronal regeneration after injury. Dev. Neurobiol. 2016, 76, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Han, S.W.; Greene, M.E.; Pitts, J.; Wada, R.K.; Sidell, N. Novel expression and function of peroxisome proliferator-activated receptor gamma (PPARgamma) in human neuroblastoma cells. Clin. Cancer Res. 2001, 7, 98–104. [Google Scholar]
- Quintanilla, R.A.; Godoy, J.A.; Alfaro, I.; Cabezas, D.; von Bernhardi, R.; Bronfman, M.; Inestrosa, N.C. Thiazolidinediones promote axonal growth through the activation of the JNK pathway. PLoS ONE 2013, 8, e65140. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shafi, S.; Gupta, P.; Khatik, G.L.; Gupta, J. PPARgamma: Potential Therapeutic Target for Ailments beyond Diabetes and its Natural Agonism. Curr. Drug Targets 2019, 20, 1281–1294. [Google Scholar] [CrossRef]
- Daynes, R.A.; Jones, D.C. Emerging roles of PPARs in inflammation and immunity. Nat. Rev. Immunol. 2002, 2, 748–759. [Google Scholar] [CrossRef]
- Esposito, E.; Cuzzocrea, S. Targeting the peroxisome proliferator-activated receptors (PPARs) in spinal cord injury. Expert Opin. Ther. Targets 2011, 15, 943–959. [Google Scholar] [CrossRef]
- O’Sullivan, S.E. Cannabinoids go nuclear: Evidence for activation of peroxisome proliferator-activated receptors. Br. J. Pharmacol. 2007, 152, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Scuderi, C.; Steardo, L.; Esposito, G. Cannabidiol promotes amyloid precursor protein ubiquitination and reduction of beta amyloid expression in SHSY5YAPP+ cells through PPARgamma involvement. Phytother. Res. 2014, 28, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Ruiz, J.; Moro, M.A.; Martinez-Orgado, J. Cannabinoids in Neurodegenerative Disorders and Stroke/Brain Trauma: From Preclinical Models to Clinical Applications. Neurotherapeutics 2015, 12, 793–806. [Google Scholar] [CrossRef]
- Mirza, A.Z.; Althagafi, C., II; Shamshad, H. Role of PPAR receptor in different diseases and their ligands: Physiological importance and clinical implications. Eur. J. Med. Chem. 2019, 166, 502–513. [Google Scholar] [CrossRef]
- Avarachan, J.A.; Pallavi Mahadev, S.; Venkatesh, G. A Mechanistic approach of Peroxisome Proliferator-Activated Receptors and its subtypes on Clinical and preclinical model of Neurodegenerative disorders. Res. J. Pharm. Technol. 2021, 14, 3967–3975. [Google Scholar] [CrossRef]
- Padhy, B.M.; Gupta, Y.K. Drug repositioning: Re-investigating existing drugs for new therapeutic indications. J. Postgrad. Med. 2011, 57, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Madura, T.; Tomita, K.; Terenghi, G. Ibuprofen improves functional outcome after axotomy and immediate repair in the peripheral nervous system. J. Plast. Reconstr. Aesthetic Surg. 2011, 64, 1641–1646. [Google Scholar] [CrossRef] [PubMed]
- Rayner, M.L.D.; Laranjeira, S.; Evans, R.E.; Shipley, R.J.; Healy, J.; Phillips, J.B. Developing an In Vitro Model to Screen Drugs for Nerve Regeneration. Anat. Rec. (Hoboken) 2018, 301, 1628–1637. [Google Scholar] [CrossRef]
- Rayner, M.L.D.; Grillo, A.; Williams, G.R.; Tawfik, E.; Zhang, T.; Volitaki, C.; Craig, D.Q.M.; Healy, J.; Phillips, J.B. Controlled local release of PPARgamma agonists from biomaterials to treat peripheral nerve injury. J. Neural. Eng. 2020, 17, 046030. [Google Scholar] [CrossRef]
- Mohammadi, R.; Hirsaee, M.A.; Amini, K. Improvement of functional recovery of transected peripheral nerve by means of artery grafts filled with diclofenac. Int. J. Surg. 2013, 11, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Eto, M.; Sumi, H.; Fujimura, H.; Yoshikawa, H.; Sakoda, S. Pioglitazone promotes peripheral nerve remyelination after crush injury through CD36 upregulation. J. Peripher. Nerv. Syst. 2008, 13, 242–248. [Google Scholar] [CrossRef]
- Katz, E.G.; Moustafa, A.A.; Heidenberg, D.; Haney, N.; Peak, T.; Lasker, G.F.; Knoedler, M.; Rittenberg, D.; Rezk, B.M.; Abd Elmageed, Z.Y.; et al. Pioglitazone Enhances Survival and Regeneration of Pelvic Ganglion Neurons after Cavernosal Nerve Injury. Urology 2016, 89, 76–82. [Google Scholar] [CrossRef]
- Chiang, M.C.; Cheng, Y.C.; Chen, H.M.; Liang, Y.J.; Yen, C.H. Rosiglitazone promotes neurite outgrowth and mitochondrial function in N2A cells via PPARgamma pathway. Mitochondrion 2014, 14, 7–17. [Google Scholar] [CrossRef]
- Kopp, M.A.; Liebscher, T.; Niedeggen, A.; Laufer, S.; Brommer, B.; Jungehulsing, G.J.; Strittmatter, S.M.; Dirnagl, U.; Schwab, J.M. Small-molecule-induced Rho-inhibition: NSAIDs after spinal cord injury. Cell Tissue Res. 2012, 349, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Budel, S.; Baughman, K.; Gould, G.; Song, K.H.; Strittmatter, S.M. Ibuprofen enhances recovery from spinal cord injury by limiting tissue loss and stimulating axonal growth. J. Neurotrauma 2009, 26, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Dill, J.; Patel, A.R.; Yang, X.L.; Bachoo, R.; Powell, C.M.; Li, S. A molecular mechanism for ibuprofen-mediated RhoA inhibition in neurons. J. Neurosci. 2010, 30, 963–972. [Google Scholar] [CrossRef]
- Fu, Q.; Hue, J.; Li, S. Nonsteroidal anti-inflammatory drugs promote axon regeneration via RhoA inhibition. J. Neurosci. 2007, 27, 4154–4164. [Google Scholar] [CrossRef]
- Sharp, K.G.; Yee, K.M.; Stiles, T.L.; Aguilar, R.M.; Steward, O. A re-assessment of the effects of treatment with a non-steroidal anti-inflammatory (ibuprofen) on promoting axon regeneration via RhoA inhibition after spinal cord injury. Exp. Neurol. 2013, 248, 321–337. [Google Scholar] [CrossRef]
- Xing, B.; Li, H.; Wang, H.; Mukhopadhyay, D.; Fisher, D.; Gilpin, C.J.; Li, S. RhoA-inhibiting NSAIDs promote axonal myelination after spinal cord injury. Exp. Neurol. 2011, 231, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Que, L.; Lv, X.; Li, Q.; Yin, H.; Zhang, L. Tolerance of neurite outgrowth to Rho kinase inhibitors decreased by cyclooxygenase-2 inhibitor. Neural Regen. Res. 2012, 7, 2705–2712. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.L.; Chen, Y.; Yin, H.; Duan, W.G. Combination of fasudil and celecoxib promotes the recovery of injured spinal cord in rats better than celecoxib or fasudil alone. Neural Regen. Res. 2015, 10, 1836–1840. [Google Scholar] [CrossRef] [PubMed]
- Li, X.G.; Lin, X.J.; Du, J.H.; Xu, S.Z.; Lou, X.F.; Chen, Z. Combination of methylprednisolone and rosiglitazone promotes recovery of neurological function after spinal cord injury. Neural Regen. Res. 2016, 11, 1678–1684. [Google Scholar] [CrossRef] [PubMed]
- Mannan, A.; Garg, N.; Singh, T.G.; Kang, H.K. Peroxisome Proliferator-Activated Receptor-Gamma (PPAR-): Molecular Effects and Its Importance as a Novel Therapeutic Target for Cerebral Ischemic Injury. Neurochem. Res. 2021. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, W.; Xu, F.; Dai, X.; Shi, L.; Cai, W.; Mu, H.; Hitchens, T.K.; Foley, L.M.; Liu, X.; et al. The interleukin-4/PPARgamma signaling axis promotes oligodendrocyte differentiation and remyelination after brain injury. PLoS Biol. 2019, 17, e3000330. [Google Scholar] [CrossRef]
- Cai, W.; Yang, T.; Liu, H.; Han, L.; Zhang, K.; Hu, X.; Zhang, X.; Yin, K.J.; Gao, Y.; Bennett, M.V.L.; et al. Peroxisome proliferator-activated receptor gamma (PPARgamma): A master gatekeeper in CNS injury and repair. Prog. Neurobiol. 2018, 163–164, 27–58. [Google Scholar] [CrossRef] [PubMed]
- Villapol, S. Roles of Peroxisome Proliferator-Activated Receptor Gamma on Brain and Peripheral Inflammation. Cell Mol. Neurobiol. 2018, 38, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Yi, J.H.; Miranpuri, G.; Satriotomo, I.; Bowen, K.; Resnick, D.K.; Vemuganti, R. Thiazolidinedione class of peroxisome proliferator-activated receptor gamma agonists prevents neuronal damage, motor dysfunction, myelin loss, neuropathic pain, and inflammation after spinal cord injury in adult rats. J. Pharmacol. Exp. Ther. 2007, 320, 1002–1012. [Google Scholar] [CrossRef]
- Han, L.; Cai, W.; Mao, L.; Liu, J.; Li, P.; Leak, R.K.; Xu, Y.; Hu, X.; Chen, J. Rosiglitazone Promotes White Matter Integrity and Long-Term Functional Recovery after Focal Cerebral Ischemia. Stroke 2015, 46, 2628–2636. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Q.; Yang, X.; Wang, L. PPAR-gamma agonist rosiglitazone reduces autophagy and promotes functional recovery in experimental traumaticspinal cord injury. Neurosci. Lett. 2017, 650, 89–96. [Google Scholar] [CrossRef]
- McTigue, D.M.; Tripathi, R.; Wei, P.; Lash, A.T. The PPAR gamma agonist Pioglitazone improves anatomical and locomotor recovery after rodent spinal cord injury. Exp. Neurol. 2007, 205, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.J.; Sun, X.H.; Wang, S.W.; Chen, J.L.; Bi, Y.H.; Jiang, D.X. Mifepristone alleviates cerebral ischemia-reperfusion injury in rats by stimulating PPAR gamma. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5688–5696. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, K.T.; Kuplicki, R.; Savitz, J.; Burrows, K.; Simmons, W.K.; Khalsa, S.S.; Teague, T.K.; Aupperle, R.L.; Paulus, M.P. Impact of ibuprofen and peroxisome proliferator-activated receptor gamma on emotion-related neural activation: A randomized, placebo-controlled trial. Brain Behav. Immun. 2021, 96, 135–142. [Google Scholar] [CrossRef] [PubMed]
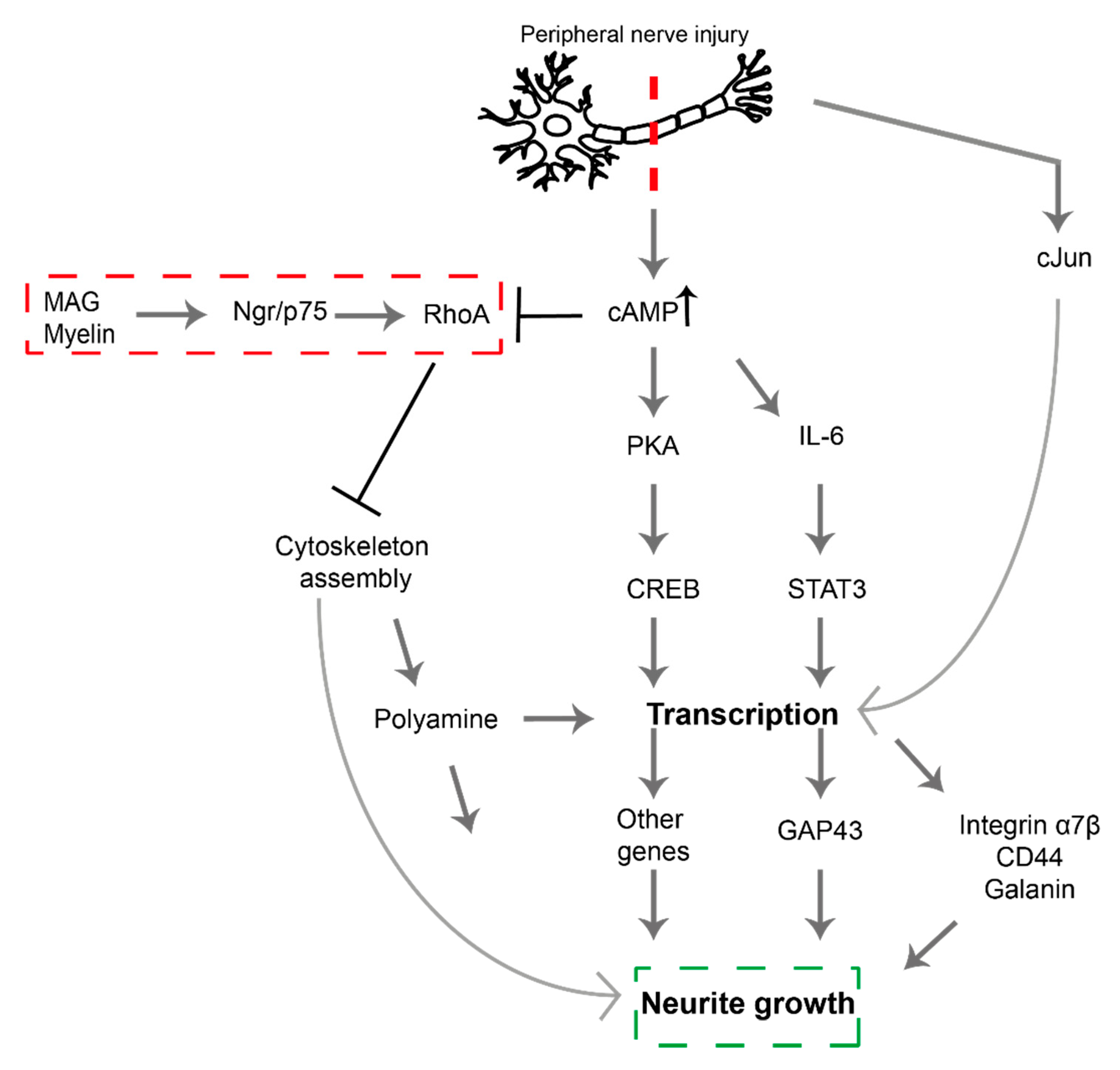

| Compound | Chemical Structure | Model | Effect on Nerve Regeneration | Reference |
|---|---|---|---|---|
| Ibuprofen | 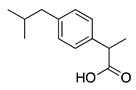 | In vivo: Interpositional graft on adult rat tibial nerve; treated through osmotic pumps. In vitro: NG108-15, DRG and 3D co-culture. In vivo: Transection with primary repair in sciatic nerve treated through osmotic pump. In vivo: Transection with primary repair or crush injury in sciatic nerve treated through biomaterials. | Recovery of TFI and increase of area of axon and myelin. In vitro: Elongation of neurites In vivo: Increase in axon number. Increase in axon number and functional recovery. | [61,62,63] |
| Diclofenac |  | In vivo: Sciatic nerve transection with artery graft filled with diclofenac. | Improved functional recovery and faster recovery of regenerated axons. | [64] |
| Sulindac sulfide |  | In vivo: Transection with primary repair or crush injury in sciatic nerve treated through biomaterials. | Improved functional recovery. | [63] |
| Pioglitazone | 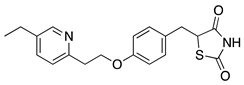 | In vivo: Crush injury on sciatic nerve in CD36-deficient mice. In vivo: Bilateral cavernosal nerve crush injury. | Improved re-myelination. Protective effect on pelvic ganglion neurons. | [65,66] |
| Rosiglitazone | 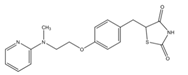 | In vitro: N2A cell culture. | Promoted neurite outgrowth and increased population of neurite-bearing cells. | [67] |
| Compound | Clinical Indication | Reference |
|---|---|---|
| Ibuprofen | CNS injury | [70] |
| CNS injury | [61] | |
| Spinal cord injury | [69] | |
| Spinal cord injury | [72] | |
| CNS injury | [61] | |
| Indomethacin | CNS injury | [61] |
| Spinal cord injury | [72] | |
| Rosiglitazone | CNS injury | [49] |
| Spinal cord injury | [80] | |
| Cerebral Ischemia injury | [81] | |
| Spinal cord injury | [82] | |
| Pioglitazone | Spinal cord injury | [80] |
| Spinal cord injury | [83] | |
| Mifepristone | Cerebral ischemia-reperfusion | [84] |
| injury |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rayner, M.L.D.; Healy, J.; Phillips, J.B. Repurposing Small Molecules to Target PPAR-γ as New Therapies for Peripheral Nerve Injuries. Biomolecules 2021, 11, 1301. https://doi.org/10.3390/biom11091301
Rayner MLD, Healy J, Phillips JB. Repurposing Small Molecules to Target PPAR-γ as New Therapies for Peripheral Nerve Injuries. Biomolecules. 2021; 11(9):1301. https://doi.org/10.3390/biom11091301
Chicago/Turabian StyleRayner, Melissa L. D., Jess Healy, and James B. Phillips. 2021. "Repurposing Small Molecules to Target PPAR-γ as New Therapies for Peripheral Nerve Injuries" Biomolecules 11, no. 9: 1301. https://doi.org/10.3390/biom11091301
APA StyleRayner, M. L. D., Healy, J., & Phillips, J. B. (2021). Repurposing Small Molecules to Target PPAR-γ as New Therapies for Peripheral Nerve Injuries. Biomolecules, 11(9), 1301. https://doi.org/10.3390/biom11091301






