Gastroprotection against Rat Ulcers by Nephthea Sterol Derivative
Abstract
:1. Introduction
2. Materials and Methods
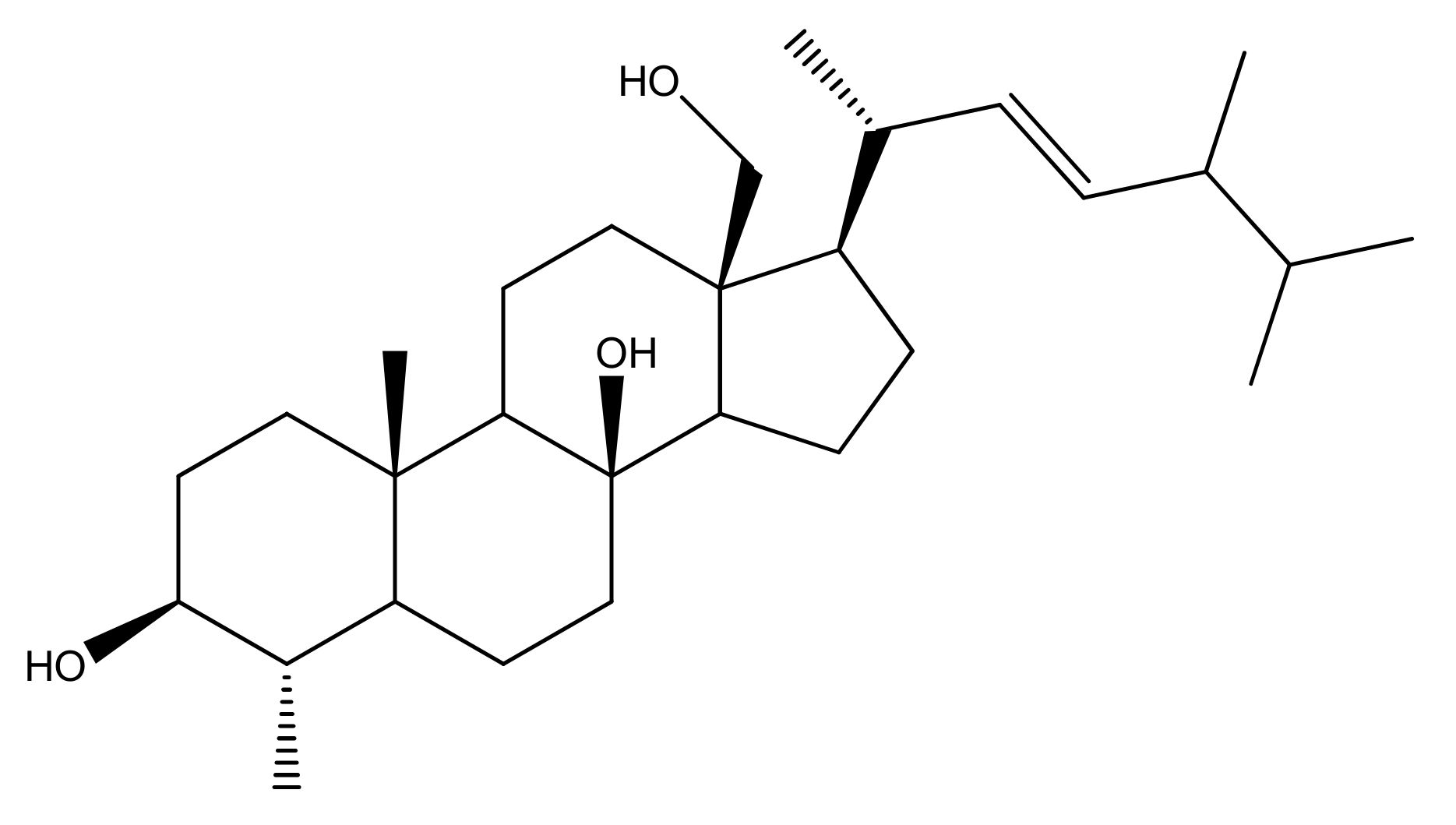
2.1. Soft Coral Material, Extraction, Isolation, Purification and NMR Spectroscopy
2.2. Experimental Animals, Ethical Statement, Ulcer Induction, and Grouping
2.3. Galactin-3 and TNF-α Determination
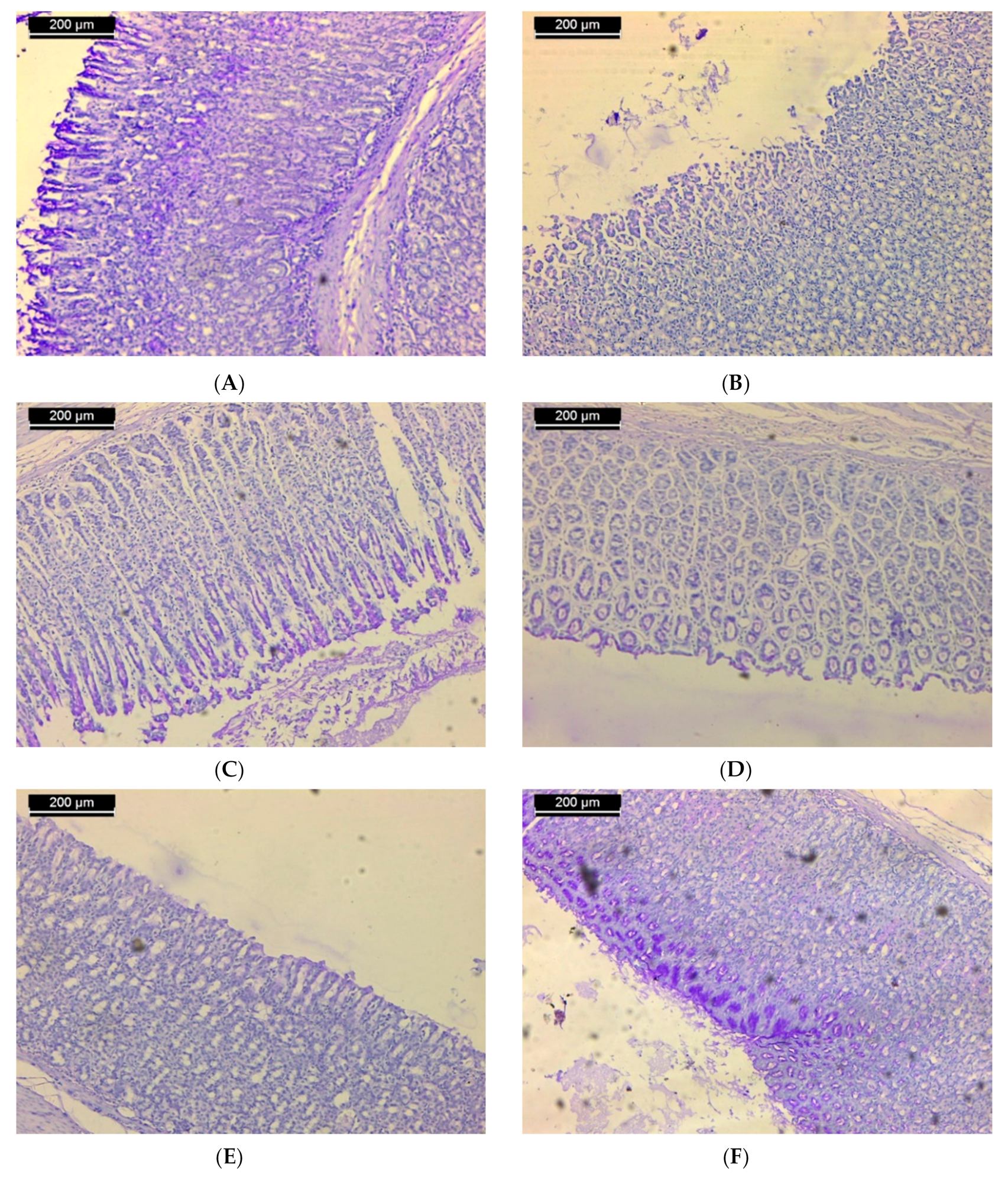
2.3.1. Histopathology Study
2.3.2. Gastric Mucosal Glycoprotein Evaluation
2.3.3. Statistical Analysis
2.4. Molecular Docking
2.5. Protein–Protein Interaction
3. Results
3.1. Biochemical Results
3.2. Histopathological Results
3.3. Histochemical Results
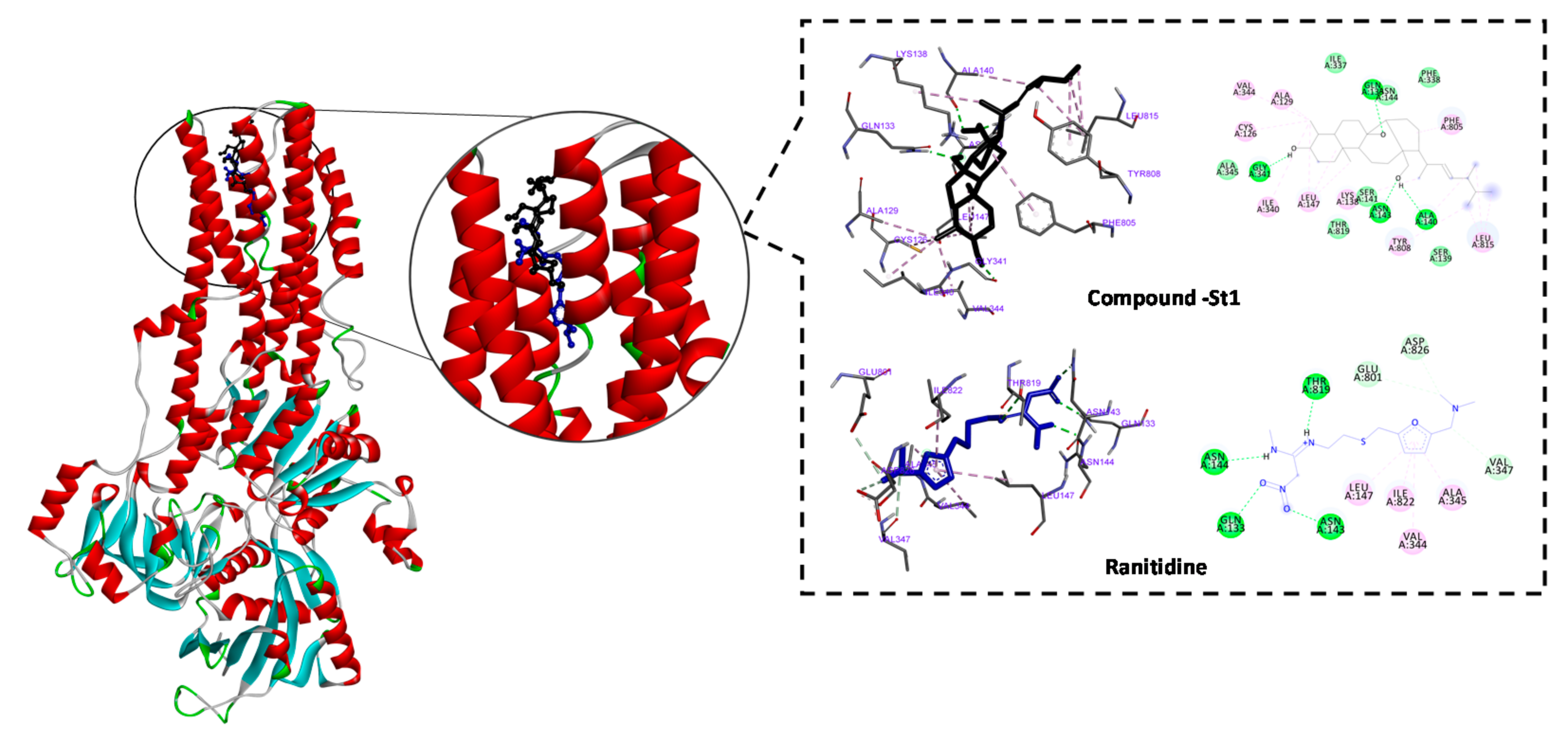
3.4. In Silico Inhibitory Effect of ST-1 on H+/K+-ATPase
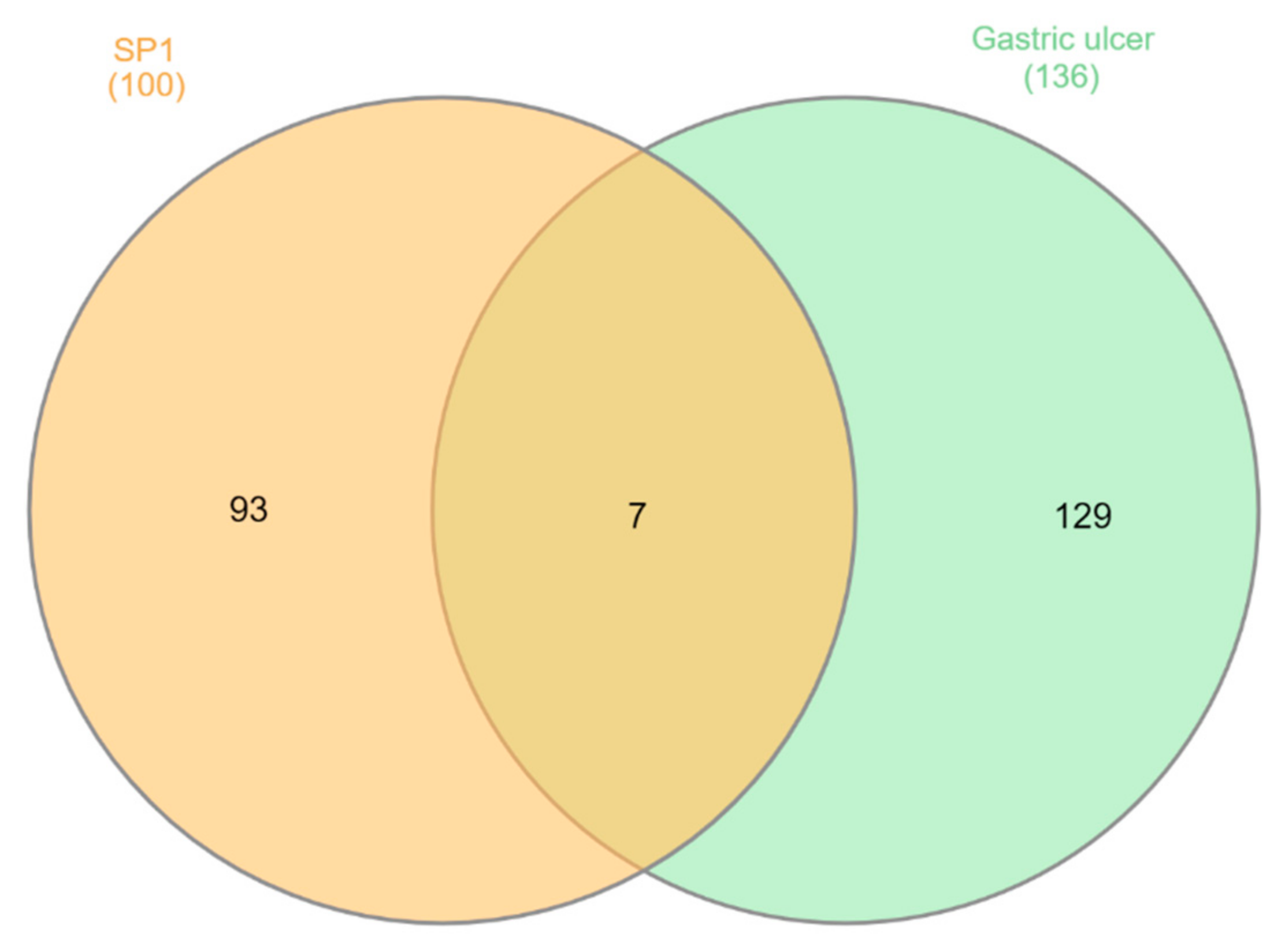
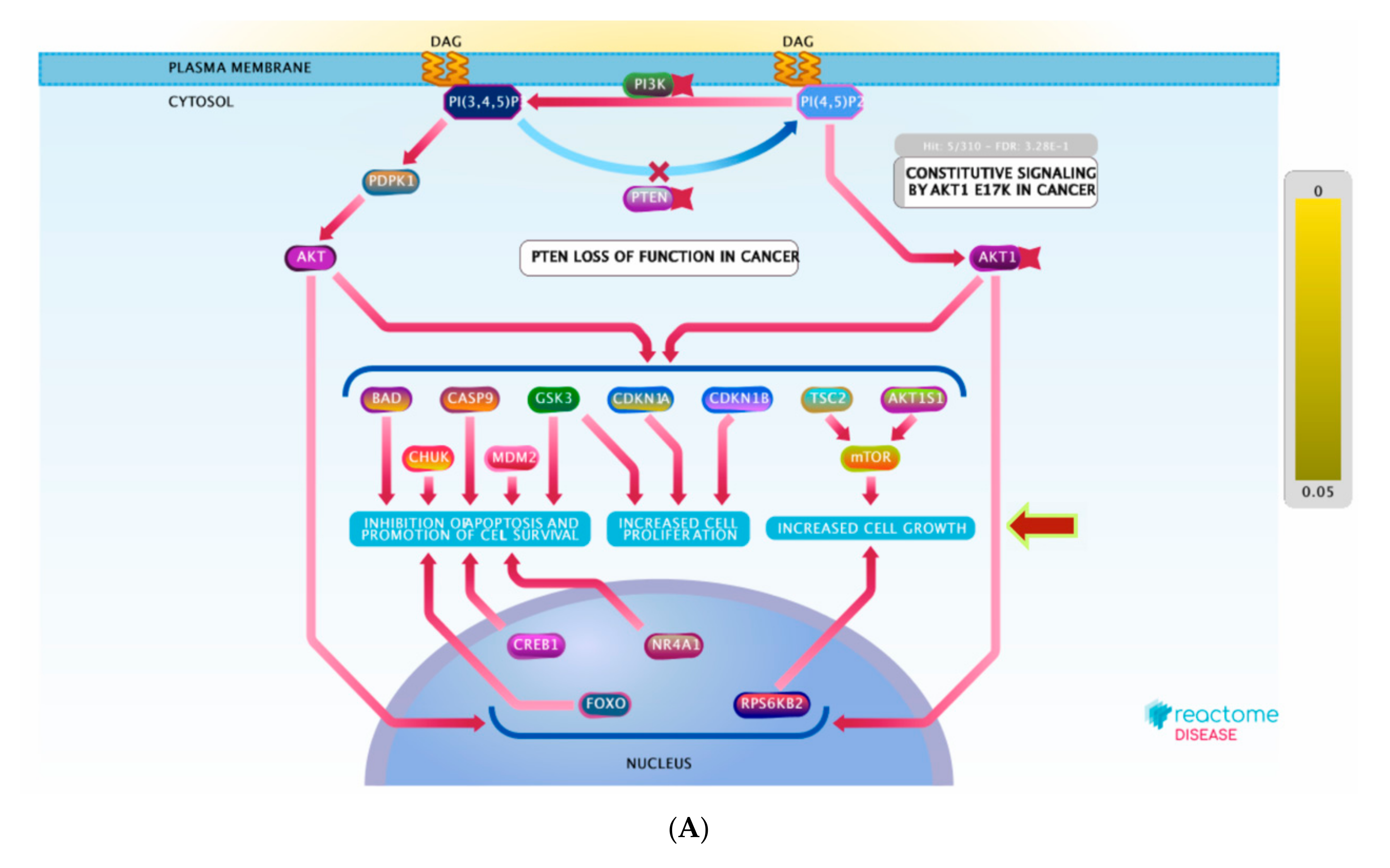
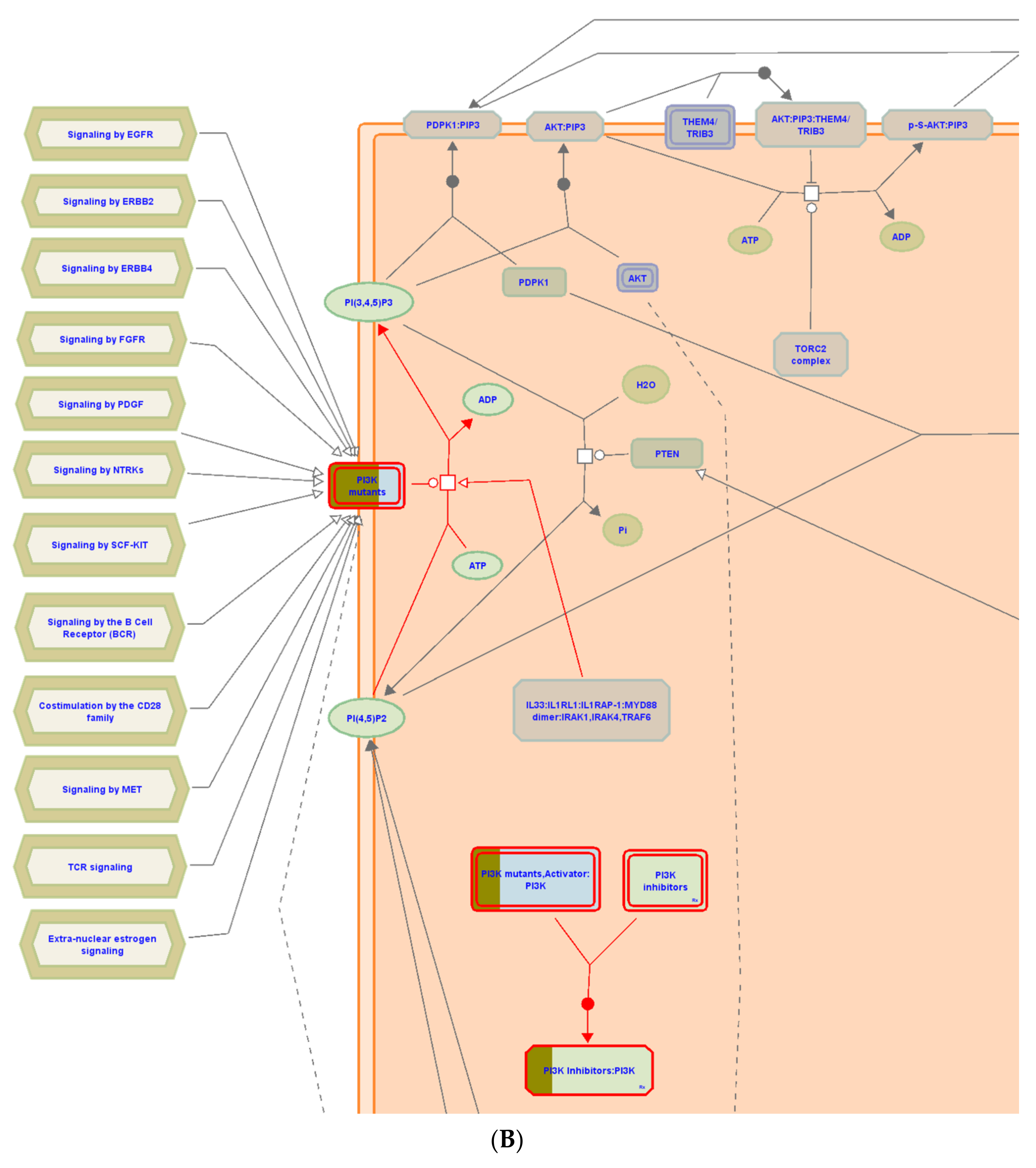
3.5. Molecular Target Prediction and Network Analysis
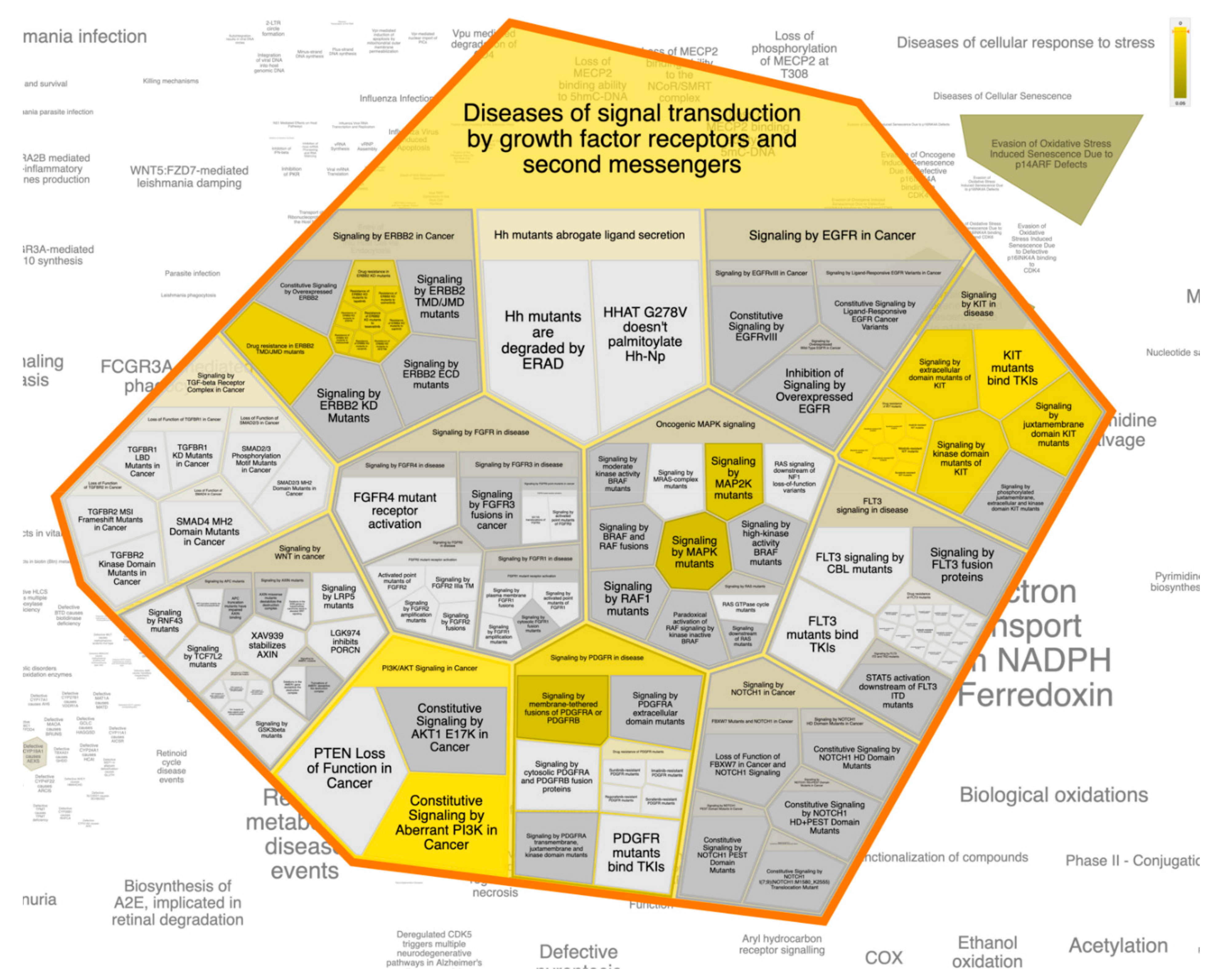
3.6. Pathway Enrichment Analysis (PEA)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grossman, M.I. Peptic Ulcer: A Guide for the Practicing Physician; Year Book Medical Chicago: Chicago, IL, USA, 1981. [Google Scholar]
- Sang, V.T.; Dat, T.T.H.; Vinh, L.B.; Cuong, L.C.V.; Oanh, P.T.T.; Ha, H.; Kim, Y.H.; Anh, H.L.T.; Yang, S.Y. Coral and coral-associated microorganisms: A prolific source of potential bioactive natural products. Mar. Drugs 2019, 17, 468. [Google Scholar] [CrossRef] [Green Version]
- Hossen, M.A.; Reza, A.S.M.A.; Ahmed, A.M.A.; Islam, M.K.; Jahan, I.; Hossain, R.; Khan, M.F.; Maruf, M.R.A.; Haque, M.A.; Rahman, M.A. Pretreatment of Blumea lacera leaves ameliorate acute ulcer and oxidative stress in ethanol-induced long-evan rat: A combined experimental and chemico-biological interaction. Biomed. Pharmacother. 2021, 135, 111211. [Google Scholar] [CrossRef] [PubMed]
- Manonmani, S.; Vishwanathan, V.P.; Subramanian, S.; Govindasamy, S. Biochemical studies on the antiulcerogenic activity of cauvery 100, an ayurvedic formulation in experimental ulcers. Indian J. Pharmacol. 1995, 27, 101. [Google Scholar]
- Anoop, A.; Jegadeesan, M. Biochemical studies on the anti-ulcerogenic potential of Hemidesmus indicus R. Br. var. indicus. J. Ethnopharmacol. 2003, 84, 149–156. [Google Scholar] [CrossRef]
- Dharmani, P.; Mishra, P.K.; Maurya, R.; Chauhan, V.S.; Palit, G. Allophylus serratus: A plant with potential anti-ulcerogenic activity. J. Ethnopharmacol. 2005, 99, 361–366. [Google Scholar] [CrossRef]
- Park, A.-M.; Khadka, S.; Sato, F.; Omura, S.; Fujita, M.; Hsu, D.K.; Liu, F.-T.; Tsunoda, I. Galectin-3 as a therapeutic target for NSAID-induced intestinal ulcers. Front. Immunol. 2020, 11, 2432. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, C.; Liu, F.-T.; Gipson, I.K.; Panjwani, N. Galectin-3 promotes lamellipodia formation in epithelial cells by interacting with complex N-glycans on α3β1 integrin. J. Cell Sci. 2009, 122, 3684–3693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, A.-M.; Hagiwara, S.; Hsu, D.K.; Liu, F.-T.; Yoshie, O. Galectin-3 plays an important role in innate immunity to gastric infection by Helicobacter pylori. Infect. Immun. 2016, 84, 1184–1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, D.; Liang, B.; Li, Y. Serum galectin-3 as a potential marker for gastric cancer. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015, 21, 755. [Google Scholar]
- Idriss, H.T.; Naismith, J.H. TNFα and the TNF receptor superfamily: Structure-Function relationship (s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef]
- Borrelli, F.; Izzo, A.A. The plant kingdom as a source of anti-ulcer remedies. Phytother. Res. 2000, 14, 581–591. [Google Scholar] [CrossRef]
- Dharmani, P.; Palit, G. Exploring Indian medicinal plants for antiulcer activity. Indian J. Pharmacol. 2006, 38, 95. [Google Scholar]
- Kath, R.K.; Gupta, R.K. Antioxidant activity of hydroalcoholic leaf extract of Ocimum sanctum in animal models of peptic ulcer. Indian J. Physiol. Pharmacol. 2006, 50, 391. [Google Scholar] [PubMed]
- Malairajan, P.; Gopalakrishnan, G.; Narasimhan, S.; Veni, K.J.K.; Kavimani, S. Anti-Ulcer activity of crude alcoholic extract of Toona ciliata Roemer (heart wood). J. Ethnopharmacol. 2007, 110, 348–351. [Google Scholar] [CrossRef]
- Siti, Z.M.; Tahir, A.; Farah, A.I.; Fazlin, S.M.A.; Sondi, S.; Azman, A.H.; Maimunah, A.H.; Haniza, M.A.; Haslinda, M.D.S.; Zulkarnain, A.K. Use of traditional and complementary medicine in Malaysia: A baseline study. Complement. Ther. Med. 2009, 17, 292–299. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Hegazy, M.-E.F.; Hassan, N.M.; Wojcinska, M.; Karchesy, J.; Pare, P.W.; Mabry, T.J. Constituents of Chrysothamnus viscidiflorus. Phytochemistry 2006, 67, 1547–1553. [Google Scholar] [CrossRef]
- Duh, C.-Y.; Wang, S.-K.; Weng, Y.-L.; Chiang, M.Y.; Dai, C.-F. Cytotoxic terpenoids from the Formosan soft coral Nephthea brassica. J. Nat. Prod. 1999, 62, 1518–1521. [Google Scholar] [CrossRef] [PubMed]
- Elkhateeb, A.; El-Beih, A.A.; Gamal-Eldeen, A.M.; Alhammady, M.A.; Ohta, S.; Paré, P.W.; Hegazy, M.-E.F. New terpenes from the Egyptian soft coral Sarcophyton ehrenbergi. Mar. Drugs 2014, 12, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.E.F.; Mohamed, T.A.; Alhammady, M.A.; Shaheen, A.M.; Reda, E.H.; Elshamy, A.I.; Aziz, M.; Paré, P.W. Molecular architecture and biomedical leads of terpenes from red sea marine invertebrates. Mar. Drugs 2015, 13, 3154–3181. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.-E.F.; Moustafa, M.S.; El-Wahed, A.; Al-Mousawi, S.M.; Musharraf, S.G. Marine natural products: A source of novel anticancer drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef] [Green Version]
- Hegazy, M.-E.F.; Gamal-Eldeen, A.M.; Mohamed, T.A.; Alhammady, M.A.; Hassanien, A.A.; Shreadah, M.A.; Abdelgawad, I.I.; Elkady, E.M.; Paré, P.W. New cytotoxic constituents from the Red Sea soft coral Nephthea sp. Nat. Prod. Res. 2016, 30, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Januar, H.I.; Chasanah, E.; Motti, C.A.; Tapiolas, D.M.; Liptrot, C.H.; Wright, A.D. Cytotoxic cembranes from Indonesian specimens of the soft coral Nephthea sp. Mar. Drugs 2010, 8, 2142–2152. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.-H.; Chou, T.-H.; Yang, C.-C.; Hung, W.J.; Chang, L.-C.; Cheng, D.-L.; Wang, G.-H. Cytotoxic effect of Discosoma sp., Isis hippuris and Nephthea chabrolii on human oral SCC25 cells. J. Taiwan Inst. Chem. Eng. 2010, 41, 333–337. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Huang, Y.-C.; Wen, Z.-H.; Hsu, C.-H.; Wang, S.-K.; Dai, C.-F.; Duh, C.-Y. New 19-oxygenated and 4-methylated steroids from the Formosan soft coral Nephthea chabroli. Steroids 2009, 74, 543–547. [Google Scholar] [CrossRef]
- Chung, T.-W.; Su, J.-H.; Lin, C.-C.; Li, Y.-R.; Chao, Y.-H.; Lin, S.-H.; Chan, H.-L. 24-Methyl-Cholesta-5, 24 (28)-Diene-3β, 19-diol-7β-Monoacetate inhibits human small cell lung cancer growth In Vitro and In Vivo via apoptosis induction. Mar. Drugs 2017, 15, 210. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-S.; Duh, T.-H.; Siao, S.-S.; Chang, R.-C.; Wang, S.-K.; Duh, C.-Y. New cytotoxic terpenoids from soft corals Nephthea chabroli and Paralemnalia thyrsoides. Mar. Drugs 2017, 15, 392. [Google Scholar] [CrossRef] [Green Version]
- Tsai, T.-C.; Huang, Y.-T.; Chou, S.-K.; Shih, M.-C.; Chiang, C.-Y.; Su, J.-H. Cytotoxic oxygenated steroids from the soft coral Nephthea erecta. Chem. Pharm. Bull. 2016, 64, 1519–1522. [Google Scholar] [CrossRef] [Green Version]
- Whuang, T.-Y.; Tsai, W.-C.; Chen, N.-F.; Chen, Z.-C.; Tsui, K.-H.; Wen, Z.-H.; Su, Y.-D.; Chang, Y.-C.; Chen, Y.-H.; Lu, M.-C. Columnaristerol A, a novel 19-norsterol from the Formosan octocoral Nephthea columnaris. Bioorganic Med. Chem. Lett. 2016, 26, 4966–4969. [Google Scholar] [CrossRef]
- Benvenutti, R.C.; Dalla Vecchia, C.A.; Locateli, G.; Serpa, P.Z.; Lutinski, J.A.; Junior, S.A.R.; Corralo, V.; Gutiérrez, M.V.; Vilegas, W.; Somensi, L.B. Gastroprotective activity of hydroalcoholic extract of the leaves of Urera baccifera in rodents. J. Ethnopharmacol. 2020, 250, 112473. [Google Scholar] [CrossRef]
- Park, J.U.; Kang, J.H.; Rahman, M.A.A.; Hussain, A.; Cho, J.S.; Lee, Y.I. Gastroprotective effects of plants extracts on gastric mucosal injury in experimental sprague-dawley rats. BioMed Res. Int. 2019, 2019, 8759708. [Google Scholar]
- Farrag, A.R.H.; Abdallah, H.M.I.; Khattab, A.R.; Elshamy, A.I.; El Gendy, A.E.-N.G.; Mohamed, T.A.; Farag, M.A.; Efferth, T.; Hegazy, M.-E.F. Antiulcer activity of Cyperus alternifolius in relation to its UPLC-MS metabolite fingerprint: A mechanistic study. Phytomedicine 2019, 62, 152970. [Google Scholar] [CrossRef] [PubMed]
- Elshamy, A.I.; Farrag, A.-R.H.; Mohamed, S.H.; Ali, N.A.; Mohamed, T.A.; Menshawy, M.M.; Zaglool, A.W.; Efferth, T.; Hegazy, M.-E.F. Gastroprotective effects of ursolic acid isolated from Ochrosia elliptica on ethanol-induced gastric ulcer in rats. Med. Chem. Res. 2020, 29, 113–125. [Google Scholar] [CrossRef]
- Boume, L.D. Theory and Practice of Histological Technique; Bancroft, J.D., Steven, A., Turner, D.R., Eds.; Churchill Livingstone: London, UK, 1990; pp. 465–492. [Google Scholar]
- McManus, J.F.A. The demonstration of certain fatty substances in paraffin sections. J. Pathol. Bacteriol. 1946, 58, 93–95. [Google Scholar] [CrossRef]
- Nordin, N.; Salama, S.M.; Golbabapour, S.; Hajrezaie, M.; Hassandarvish, P.; Kamalidehghan, B.; Majid, N.A.; Hashim, N.M.; Omar, H.; Fadaienasab, M. Anti-ulcerogenic effect of methanolic extracts from Enicosanthellum pulchrum (King) Heusden against ethanol-induced acute gastric lesion in animal models. PLoS ONE 2014, 9, e111925. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, T.A.; Elshamy, A.I.; Ibrahim, M.A.A.; Zellagui, A.; Moustafa, M.F.; Abdelrahman, A.H.M.; Ohta, S.; Pare, P.W.; Hegazy, M.-E.F. Carotane sesquiterpenes from Ferula vesceritensis: In silico analysis as SARS-CoV-2 binding inhibitors. RSC Adv. 2020, 10, 34541–34548. [Google Scholar] [CrossRef]
- Ibrahim, M.A.A.; Abdelrahman, A.H.M.; Hegazy, M.-E.F. In-Silico drug repurposing and molecular dynamics puzzled out potential SARS-CoV-2 main protease inhibitors. J. Biomol. Struct. Dyn. 2020, 1–12. [Google Scholar] [CrossRef]
- Ibrahim, M.A.A.; Abdeljawaad, K.A.A.; Abdelrahman, A.H.M.; Hegazy, M.-E.F. Natural-Like products as potential SARS-CoV-2 Mpro inhibitors: In-Silico drug discovery. J. Biomol. Struct. Dyn. 2020, 1–13. [Google Scholar] [CrossRef]
- Ibrahim, M.A.A.; Abdelrahman, A.H.M.; Mohamed, T.A.; Atia, M.A.M.; Al-Hammady, M.A.M.; Abdeljawaad, K.A.A.; Elkady, E.M.; Moustafa, M.F.; Alrumaihi, F.; Allemailem, K.S. In silico mining of terpenes from Red-Sea invertebrates for SARS-CoV-2 main protease (Mpro) inhibitors. Molecules 2021, 26, 2082. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 169–175. [Google Scholar] [CrossRef]
- Li, R.; Ma, X.; Song, Y.; Zhang, Y.; Xiong, W.; Li, L.; Zhou, L. Anti-Colorectal cancer targets of resveratrol and biological molecular mechanism: Analyses of network pharmacology, human and experimental data. J. Cell Biochem. 2019, 120, 11265–11273. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Blucher, A.S.; McWeeney, S.K.; Stein, L.; Wu, G. Visualization of drug target interactions in the contexts of pathways and networks with ReactomeFIViz. F1000Res 2019, 8, 908. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Liu, Y.; Wang, C.; Li, J.; Xiong, L.; Wang, Z.; Liu, J.; Li, P. Evaluation of the gastroprotective effects of 20 (S)-ginsenoside Rg3 on gastric ulcer models in mice. J. Ginseng Res. 2019, 43, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.K.; Sairam, K. Anti-Ulcer drugs from indigenous sources with emphasis on Musa sapientum, Tamrabhasma, Asparagus racemosus and Zingiber officinale. Indian J. Pharmacol. 2002, 34, 100. [Google Scholar]
- Al Batran, R.; Al-Bayaty, F.; Ameen Abdulla, M.; Jamil Al-Obaidi, M.M.; Hajrezaei, M.; Hassandarvish, P.; Fouad, M.; Golbabapour, S.; Talaee, S. Gastroprotective effects of Corchorus olitorius leaf extract against ethanol-induced gastric mucosal hemorrhagic lesions in rats. J. Gastroenterol. Hepatol. 2013, 28, 1321–1329. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Wang, G. Computer-aided targeting of the PI3K/Akt/mTOR pathway: Toxicity reduction and therapeutic opportunities. Int. J. Mol. Sci. 2014, 15, 18856–18891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoke, G.D.; Ramos, C.; Hoke, N.N.; Crossland, M.C.; Shawler, L.G.; Boykin, J.V. Atypical diabetic foot ulcer keratinocyte protein signaling correlates with impaired wound healing. J. Diabetes Res. 2016, 2016, 1586927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Squarize, C.H.; Castilho, R.M.; Bugge, T.H.; Gutkind, J.S. Accelerated wound healing by mTOR activation in genetically defined mouse models. PLoS ONE 2010, 5, e10643. [Google Scholar] [CrossRef]
- Peiris, T.H.; Ramirez, D.; Barghouth, P.G.; Oviedo, N.J. The Akt signaling pathway is required for tissue maintenance and regeneration in planarians. BMC Dev. Biol. 2016, 16, 7. [Google Scholar] [CrossRef] [Green Version]
- Xiao, W.; Tang, H.; Wu, M.; Liao, Y.; Li, K.; Li, L.; Xu, X. Ozone oil promotes wound healing by increasing the migration of fibroblasts via PI3K/Akt/mTOR signaling pathway. Biosci. Rep. 2017, 37, BSR20170658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khorami, S.A.H.; Movahedi, A.; Huzwah, K.; Sokhini, A.M.M. PI3K/AKT pathway in modulating glucose homeostasis and its alteration in diabetes. Ann. Med. Biomed. Sci. 2015, 1, 46–55. [Google Scholar]
- Jere, S.W.; Houreld, N.N.; Abrahamse, H. Role of the PI3K/AKT (mTOR and GSK3β) signalling pathway and photobiomodulation in diabetic wound healing. Cytokine Growth Factor Rev. 2019, 50, 52–59. [Google Scholar] [CrossRef]
- Hsu, D.K.; Yang, R.-Y.; Pan, Z.; Yu, L.; Salomon, D.R.; Fung-Leung, W.-P.; Liu, F.-T. Targeted disruption of the galectin-3 gene results in attenuated peritoneal inflammatory responses. Am. J. Pathol. 2000, 156, 1073–1083. [Google Scholar] [CrossRef] [Green Version]
- Fermino, M.L.; Polli, C.D.; Toledo, K.A.; Liu, F.-T.; Hsu, D.K.; Roque-Barreira, M.C.; Pereira-da-Silva, G.; Bernardes, E.S.; Halbwachs-Mecarelli, L. LPS-induced galectin-3 oligomerization results in enhancement of neutrophil activation. PLoS ONE 2011, 6, e26004. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Galactin-3 (ng/mL) | TNF-α Pg/ml | ||
|---|---|---|---|---|
| Groups | Mean ± S.E | % Change | Mean ± S.E | % Change |
| Control | 1.64 ± 0.11 | 0 | 29.82 ± 0.56 | 0 |
| Ethanol (1 mL) | 13.99 ± 0.14 a | +753 a | 209.37 ± 2.91 a | +602 a |
| Ranitidine | 2.25 ± 0.09 ab | −83.9 b | 42.58± 0.84 ab | −79.7 b |
| ST-1 (50 mg) | 12.68 ± 0.32 ab | −9.4 b | 193.44 ± 2.55 ab | −7.6 b |
| ST-1 (100 mg) | 9.52 ± 0.32 ab | −32 b | 168.81 ± 6.32 ab | −19.4 b |
| Name | Betweenness Centrality a | Closeness Centrality b | Degree c |
|---|---|---|---|
| MAPK1 | 0.12998495 | 0.585987261 | 34 |
| MTOR | 0.05082444 | 0.531791908 | 31 |
| PIK3CA | 0.04725076 | 0.51396648 | 30 |
| HSP90AA1 | 0.057040046 | 0.519774011 | 28 |
| ESR1 | 0.03944331 | 0.525714286 | 28 |
| MAPK8 | 0.096876023 | 0.541176471 | 26 |
| AR | 0.033861461 | 0.508287293 | 25 |
| CYP3A4 | 0.066526563 | 0.508287293 | 23 |
| KDR | 0.03944376 | 0.50273224 | 22 |
| NR3C1 | 0.059041577 | 0.528735632 | 21 |
| MDM2 | 0.015376587 | 0.476683938 | 20 |
| MAPK14 | 0.01274391 | 0.484210526 | 18 |
| HPGDS | 0.03677825 | 0.489361702 | 18 |
| PRKCD | 0.015162524 | 0.446601942 | 17 |
| CNR1 | 0.082987497 | 0.469387755 | 17 |
| CYP19A1 | 0.031696473 | 0.471794872 | 17 |
| PIK3CB | 0.004766941 | 0.433962264 | 16 |
| ABL1 | 0.006113177 | 0.444444444 | 15 |
| PIK3CD | 0.013300606 | 0.427906977 | 15 |
| KIT | 0.006066222 | 0.46 | 14 |
| Pathway Name | # Entities Found | # Interactors Found | Entities p-Value | # Reactions Found | Submitted Entities Hit Interactor |
|---|---|---|---|---|---|
| Diseases of signal transduction by growth factor receptors and second messengers | 18 | 22 | 0.62086893 | 269 | MTOR; KIT; PIK3CA; MAPK8; NR3C1; MAPK1; MAPK8; MAPK14; PIK3CD; HSP90AA1; MAPK14; PRKCD; KDR;ABL1; ESR1; PIK3CD; MDM2; ESR1; AR; PIK3CB; PIK3CA; KDR |
| Disease | 12 | 17 | 0.0033075 | 197 | MTOR; KIT;MAPK8; NR3C1; MAPK1; PIK3CD; HSP90AA1; PRKCD; ABL1; ESR1; PIK3CD; MDM2; AR; ESR1; PIK3CB; KDR;PIK3CA |
| Immune system | 12 | 18 | 0.96689721 | 150 | MTOR; KIT; MAPK8; MAPK1; NR3C1; PIK3CD; MAPK14; HSP90AA1; PRKCD; ABL1; PIK3CD; ESR1; MDM2; AR; PIK3CB; PIK3CA; HPGDS; KDR |
| Nuclear receptor transcription pathway | 11 | 2 | 3.75 × 10−14 | 2 | ESR1; MDM2 |
| Intracellular signaling by second messengers | 11 | 7 | 0.00100386 | 17 | MTOR; HSP90AA1; KIT; MAPK8; NR3C1; MDM2; ESR1 |
| PIP3 activates AKT signaling | 10 | 6 | 0.00133626 | 13 | MTOR; HSP90AA1; MAPK8; NR3C1; MDM2; ESR1 |
| Cellular responses to stress | 10 | 13 | 0.09103042 | 83 | MTOR; MAPK8; NR3C1; MAPK1; MAPK14; HSP90AA1; MDM2; ABL1; MDM2; ESR1; AR; KDR; PIK3CA |
| Cellular responses to external stimuli | 10 | 13 | 0.10148968 | 83 | MTOR; MAPK8; NR3C1; MAPK1; MAPK14; HSP90AA1; MDM2; ABL1; MDM2; ESR1; AR; KDR; PIK3CA |
| Signaling by receptor tyrosine kinases | 10 | 20 | 0.3190745 | 227 | MTOR; KIT;MAPK8; NR3C1; MAPK1; CNR1; PIK3CD; MAPK14; HSP90AA1; PRKCD; KDR;ABL1; ESR1; PIK3CD; ESR1; MDM2; PIK3CB; KDR;PIK3CA; HPGDS |
| Cytokine signaling in the immune system | 10 | 17 | 0.63708745 | 79 | KIT; MAPK8; MAPK1; NR3C1; PIK3CD; MAPK14; HSP90AA1; PRKCD; ABL1; PIK3CD; MDM2; ESR1; AR; PIK3CB; PIK3CA; HPGDS; KDR |
| PI3K/AKT signaling in cancer | 9 | 5 | 4.44 × 10−6 | 18 | MTOR; HSP90AA1; NR3C1; MDM2; ESR1 |
| PI5P, PP2A and IER3 regulate PI3K/AKT signaling | 8 | 3 | 2.68 × 10−6 | 4 | MTOR; NR3C1; MDM2 |
| Negative regulation of the PI3K/AKT network | 8 | 3 | 5.31 × 10−6 | 4 | MTOR; NR3C1; MDM2 |
| Axon guidance | 8 | 12 | 0.31029712 | 31 | KIT; HSP90AA1; MAPK8; MAPK14; PRKCD; ABL1; ESR1; NR3C1; MAPK1; MDM2; ESR1; KDR |
| Nervous system development | 8 | 12 | 0.39349828 | 32 | KIT; HSP90AA1; MAPK8; MAPK14; PRKCD; ABL1; ESR1; NR3C1; MAPK1; ESR1; MDM2; KDR |
| Signaling by interleukins | 8 | 12 | 0.4667549 | 51 | HSP90AA1; KIT; MAPK8; PRKCD; ABL1; ESR1; MDM2; MAPK14; PIK3CB; PIK3CA; KDR; HPGDS |
| Innate immune system | 8 | 11 | 0.73193686 | 66 | HSP90AA1; KIT; MAPK8; PRKCD; ABL1; MAPK1; ESR1; MDM2; AR; MAPK14; PIK3CA |
| Developmental biology | 8 | 15 | 0.92886656 | 60 | KIT; MAPK8; NR3C1; MAPK1; NR3C1; HSP90AA1; ESR1; MAPK14; PRKCD; ABL1; ESR1; ESR1; MDM2; AR; KDR |
| Metabolism | 8 | 12 | 0.99897572 | 71 | MTOR; NR3C1; HSP90AA1; PIK3CA; MDM2; ESR1; NR3C1; MAPK1; AR; MDM2; ESR1; MAPK14 |
| Constitutive signaling by aberrant PI3K in cancer | 7 | 0 | 1.40 × 10−6 | 2 | MTOR; KIT;PIK3CA; MAPK8; NR3C1; MAPK1; MAPK8; MAPK14; PIK3CD; HSP90AA1; MAPK14; PRKCD; KDR;ABL1; ESR1; PIK3CD; MDM2; ESR1; AR; PIK3CB; PIK3CA; KDR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, T.A.; Elshamy, A.I.; Ibrahim, M.A.A.; Atia, M.A.M.; Ahmed, R.F.; Ali, S.K.; Mahdy, K.A.; Alshammari, S.O.; Al-Abd, A.M.; Moustafa, M.F.; et al. Gastroprotection against Rat Ulcers by Nephthea Sterol Derivative. Biomolecules 2021, 11, 1247. https://doi.org/10.3390/biom11081247
Mohamed TA, Elshamy AI, Ibrahim MAA, Atia MAM, Ahmed RF, Ali SK, Mahdy KA, Alshammari SO, Al-Abd AM, Moustafa MF, et al. Gastroprotection against Rat Ulcers by Nephthea Sterol Derivative. Biomolecules. 2021; 11(8):1247. https://doi.org/10.3390/biom11081247
Chicago/Turabian StyleMohamed, Tarik A., Abdelsamed I. Elshamy, Mahmoud A. A. Ibrahim, Mohamed A. M. Atia, Rania F. Ahmed, Sherin K. Ali, Karam A. Mahdy, Shifaa O. Alshammari, Ahmed M. Al-Abd, Mahmoud F. Moustafa, and et al. 2021. "Gastroprotection against Rat Ulcers by Nephthea Sterol Derivative" Biomolecules 11, no. 8: 1247. https://doi.org/10.3390/biom11081247
APA StyleMohamed, T. A., Elshamy, A. I., Ibrahim, M. A. A., Atia, M. A. M., Ahmed, R. F., Ali, S. K., Mahdy, K. A., Alshammari, S. O., Al-Abd, A. M., Moustafa, M. F., Farrag, A. R. H., & Hegazy, M.-E. F. (2021). Gastroprotection against Rat Ulcers by Nephthea Sterol Derivative. Biomolecules, 11(8), 1247. https://doi.org/10.3390/biom11081247











